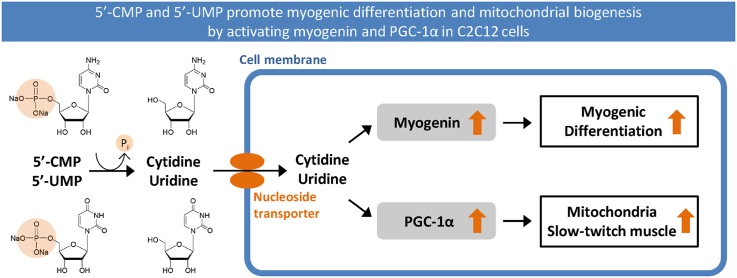
Abstract
Ribonucleotides are basic monomeric building blocks for RNA considered as conditionally essential nutrients. They are normally produced in sufficient quantity, but can become insufficient upon stressful challenges. The administration of pyrimidine nucleotides, such as cytidine-5′-monophosphate (5′-CMP) and uridine-5′-monophosphate (5′-UMP), enables rats to endure prolonged exercise. However, the underlying mechanisms have remained elusive. To investigate these mechanisms, we studied the effect of 5′-CMP and 5′-UMP on muscular differentiation and mitochondrial biogenesis in myoblast C2C12 cells. 5′-CMP and 5′-UMP were found to increase the mRNA levels of myogenin, which is a myogenic regulatory protein expressed during the final differentiation step and fusion of myoblasts into myotubes. 5′-CMP and 5′-UMP also promoted myoblast differentiation into myotube cells. 5′-CMP and 5′-UMP further increased the mRNA levels of PGC-1α which regulates mitochondrial biogenesis and skeletal muscle fiber type. In addition, 5′-CMP and 5′-UMP increased mitochondrial DNA copy number and enhanced mRNA levels of slow-muscle myosin heavy chains. Moreover, cytidine and uridine, nucleosides corresponding to 5′-CMP and 5′-UMP, markedly promoted myotube formation in C2C12 cells. Considering the metabolism and absorption of nucleotides, the active bodies underlying the effects observed with 5′-CMP and 5′-UMP could be cytidine and uridine. In conclusion, our results indicate that 5′-CMP and 5′-UMP can promote myogenic differentiation and mitochondrial biogenesis, as well as increase slow-twitch fiber via the activation of myogenin and PGC-1α. In addition, 5′-CMP and 5′-UMP may be considered as safe and effective agents to enhance muscle growth and improve the endurance in skeletal muscles.
Keywords: Cytidine-5′-monophosphate, Uridine-5′-monophosphate, Nutrition, Endurance, Mitochondria, Myogenic differentiation
Abbreviations: 5'-CMP, Cytidine-5′-monophosphate; 5'-UMP, Uridine-5′-monophosphate; FBS, Fetal bovine serum; BS, Bovine serum; MRF, Myogenic regulatory factor; MyHC, Myosin heavy chanin
Graphical abstract
Highlights
-
•
5′-CMP and 5′-UMP enhanced myogenin expression and myotube formation in C2C12 cells.
-
•
5′-CMP and 5′-UMP enhanced the expression of PGC-1α and slow-muscle myosin heavy chains in C2C12 cells.
-
•
5′-CMP and 5′-UMP promoted mitochondrial biogenesis in C2C12 cells.
-
•
Corresponding nucleosides promoted myotube formation in C2C12 cells.
1. Introduction
Skeletal muscles represent a highly regenerative organ. Injuries to skeletal muscles trigger the proliferation of satellite cells. These proliferating satellite cells and their progeny are often referred to as myogenic precursor cells or myoblasts. After several rounds of cell divisions, myoblasts fuse with each other to form multinucleated myotubes. Myotubes repair skeletal muscle by forming new muscle fibers or by fusing with pre-existing muscle fibers [1].
The satellite cell differentiation process is regulated by myogenic regulatory factors (MRFs), including MyoD, myogenic factor 5 (Myf5), MRF4, and myogenin. MyoD and Myf5 allow for the differentiation of satellite cells into myoblasts. Myogenin is essential for myoblast differentiation, and it acts in the late stages of myogenesis to regulate myoblast fusion [2].
Skeletal muscles are broadly classified into slow-twitch (type I fibers) and fast-twitch (type II fibers) fibers. Type I fibers have a slower twitch speed but higher endurance, as well as contain more mitochondria. Type II fibers, symmetrically, have a faster twitch speed but a lower endurance. The fast-twitch fibers are further divided into subtypes including type IIa fibers, type IIb fibers, and type IIx fibers, according to their predominantly expressed isoform of myosin heavy chain (MyHC). MyHC1 (encoded by MYH7), MyHC2A (MYH2), MyHC2X (MYH1), and MyHC2B (MYH4) are expressed in Type I, IIa, type IIx, IIb fibers, respectively [3]. Myh7 is expressed not only in developing skeletal muscles, but also in adult skeletal muscles [4].
The peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a metabolic regulator involved in mitochondrial biogenesis and skeletal muscle fiber type determination. PGC-1α is highly expressed in skeletal muscles. Its expression levels are higher in slow-muscle fibers versus fast muscle fibers, and increased by endurance exercise. Mice presenting with muscle-specific increased PGC-1α have a significantly increased proportion of slow-muscle fibers compared to wild-type mice [[5], [6], [7]].
Ribonucleotides are basic monomeric building blocks for RNA that is essential for all life activities. Nucleotides are normally biosynthesized from amino acids and glycolytic metabolites, but they easily become deficient under stressful conditions such as trauma, infectious diseases, fatigue, and rapid growth in infants. Dietary nucleotides play a prominent role in mediating the rapid proliferation of cells required for optimal function. The human breast milk contains a higher concentration of nucleotides compared to bovine milk or bovine milk powder, while nucleotides are supplemented in many infant formulas. Therefore, dietary nucleotides are considered as safe and conditionally essential in the presence of a variety of physiological stresses [8,9].
Nucleotides are not only used for RNA synthesis, but were also claimed to exert various biological activities such as enhancement of immune function, liver protection, and brain activity promotion [[10], [11], [12]]. Cytidine-5′-monophosphate (5′-CMP) and uridine-5′-monophosphate (5′-UMP) belong to the class of pyrimidine ribonucleotides. Pyrimidine ribonucleotides consist of a phosphate group, a pentose sugar ribose, and the pyrimidine nucleobase. 5′-CMP and 5′-UMP include cytosine and uracil as the pyrimidine nucleobase, respectively. As a biological activity for muscles, it has been reported that rats treated intramuscularly with a mixture of 5′-CMP and 5′-UMP (3.0 mg/kg 5′-UMP, 2.5 mg/kg 5′-CMP) during 10 consecutive days were able to endure long-term exercise on a treadmill [13]. However, the underlying mechanisms were not yet clearly established.
Here, we investigated the effect of 5′-CMP and 5′-UMP on muscular differentiation and mitochondrial biogenesis to clarify its possible mechanisms in myoblast C2C12 cells.
2. Materials and methods
2.1. Cell culture
Mouse C2C12 myoblast cells (RIKEN BRC, RCB0987) were provided by the RIKEN BRC. C2C12 cells were grown in Dulbecco's Modified Eagle Medium (DMEM), low glucose supplemented with 10% fetal bovine serum (FBS), 100 units/ml of penicillin, and 100 μg/ml of streptomycin. The cells were incubated at 37 °C in a humidified atmosphere with 5% of CO2. The cells were plated at 25,000 cells/well in 24-well plates and incubated until they reached a 70–90% confluence. To initiate differentiation, the media was replaced by DMEM supplemented with 2% of heat-inactivated bovine serum (BS), 100 units/ml of penicillin, and 100 μg/ml of streptomycin. In parallel with induction of differentiation, 5′-CMP disodium salt, 5′-UMP disodium salt, cytidine and uridine (YAMASA, Chiba, Japan) were added to the medium to give a final concentration of 1–5 mM. The controls were left untreated. The medium was changed at least every other day.
2.2. Total RNA isolation and quantitative RT–PCR
Total RNA was extracted from the cells using an RNeasy Mini kit (QIAGEN, Hilden, Germany). In brief, mRNA was reverse transcribed using a ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan). The mRNA levels were quantified using a Thermal Cycler Dice Real Time System (Takara Bio, Shiga, Japan) with a GoTaq qPCR Master Mix (Promega, Madison, USA). The PCR amplification consisted of an activation of polymerase at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min. The expression levels of the target genes were normalized to β-actin. The primer sequences used are listed in Table 1.
Table 1.
The primers used in this paper.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| Myogenin | 5′- CCTTGCTCAGCTCCCTCA -3′ | 5′- TGGGAGTTGCATTCACTGG -3′ |
| Pgc-1α | 5′- TATGGAGTGACATAGAGTGTGCT -3′ | 5′- CCACTTCAATCCACCCAGAAAG -3′ |
| Myh7 | 5′- ACTGTCAACACTAAGAGGGTCA -3′ | 5′- TTGGATGATTTGATCTTCCAGGG -3′ |
| β-Actin | 5′- CATCCGTAAAGACCTCTATGCCAAC -3′ | 5′- ATGGAGCCACCGATCCACA -3′ |
| Ppia | 5′- ACACGCCATAATGGCACTGG -3′ | 5′- CAGTCTTGGCAGTGCAGAT -3′ |
| Cox2 | 5′- CCATAGGGCACCAATGATACTG -3′ | 5′- AGTCGGCCTGGGATGGCATC -3′ |
2.3. Mitochondrial DNA analysis
Mitochondrial DNA (mtDNA) analysis was performed as previously described [14]. Briefly, total DNA was extracted from the cells using a DNeasy Blood & Tissue Kit (QIAGEN). Then, MtDNA copy number in total DNA was quantified by measuring expression levels of a mitochondrial-encoded gene (Cox2) by quantitative RT–PCR. MtDNA copy number was normalized to the expression level of a nuclear-encoded gene (Cyclophilin A [Ppia]). The primer sequences used are listed in Table 1.
2.4. Measurement of myotube diameter
Myotubes from five culture fields located near the center of the wells were photographed using an optical microscope (100× magnification). In each field, the top 10 thickest myotubes were selected visually and their diameter was measured using the ImageJ software. In a total of 50 myotubes for each well, the average diameter was calculated.
2.5. Statistical analysis
All the experimental data were presented as the mean ± standard error of the mean. Student's t-test was used for the comparison of two means. One-way analysis of variance, followed by Dunnett's test was used for the comparison of three or more means. All statistical analyses were performed with the JMP14 software (SAS Institute, Cary, USA). P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. 5′-CMP and 5′-UMP induce myogenin expression and promote myogenic differentiation
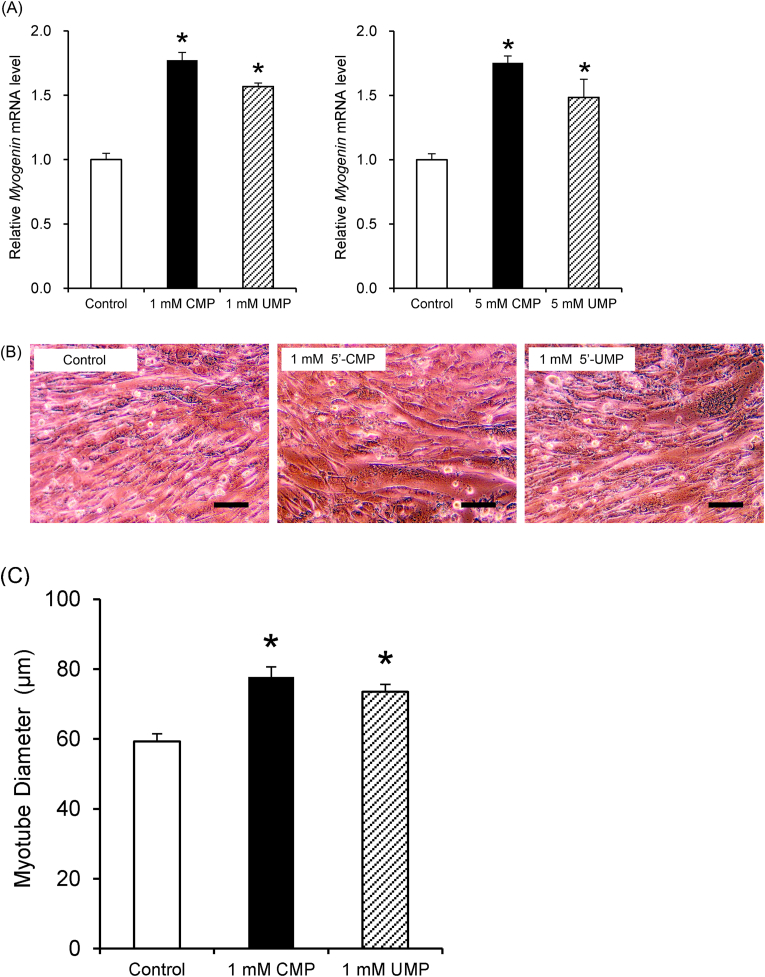
Considering that 5′-CMP and 5′-UMP enhanced the effect of endurance training in rats, we hypothesized that 5′-CMP and 5′-UMP may promote muscle differentiation and muscle repair. We evaluated the effect of 5′-CMP and 5′-UMP on the expression of myogenin, a myogenic regulatory factor expressed following the differentiation of myoblasts into multinucleated myotubes. We found that 5′-CMP (1 and 5 mM) and 5′-UMP (1 and 5 mM) significantly increased myogenin mRNA levels, compared to the control (Fig. 1A).
Fig. 1.
5′-CMP and 5′-UMP activate myogenin expression and promote myotube formation
(A) Relative myogenin mRNA levels. Total RNA was extracted from C2C12 cells after 6 days of differentiation in culture with 5′-CMP and 5′-UMP. Myogenin mRNA levels were evaluated using real-time PCR. Myogenin mRNA levels were normalized to β-actin mRNA levels. Each bar represents the relative mRNA levels to untreated controls. Data are expressed as mean ± standard error of the mean (SEM) (n = 6) *P < 0.05 compared to the untreated controls. (B) Images of differentiated C2C12 cells. Cells were treated with 5′-CMP or 5′-UMP for 5 days, then photographed under a phase contrast microscopy. Scale bar, 100 μm. (C) Diameter of C2C12 myotubes. The myotubes were photographed using a phase contrast microscope (100× magnification) within five culture fields near the center of the well. The top 10 thickest myotubes in the photograph were selected visually and their diameter was measured with the ImageJ software. The average diameter of 50 myotubes for each well was then determined. Data are expressed as mean ± SEM (n = 6) *P < 0.05 compared to the untreated controls.
In addition, 5′-CMP and 5′-UMP promoted myogenin expression, and they appeared to accelerate myotube formation upon microscopic observation (Fig. 1B). Therefore, the effect of 5′-CMP and 5′-UMP on myogenic differentiation was evaluated by measuring myotube cell diameter. C2C12 cells were cultured in a differentiation medium and treated with 1 mM 5′-CMP or 1 mM 5′-UMP for 5 days. We found that 1 mM 5′-CMP or 1 mM 5′-UMP significantly increased myotube diameter (Fig. 1C). Taken together, these results suggest that 5′-CMP and 5′-UMP promote myotube formation by enhancing myogenin expression in C2C12 cells.
3.2. 5′-CMP and 5′-UMP activate the expression of PGC-1α and Myh7, as well as enhance mitochondrial biogenesis
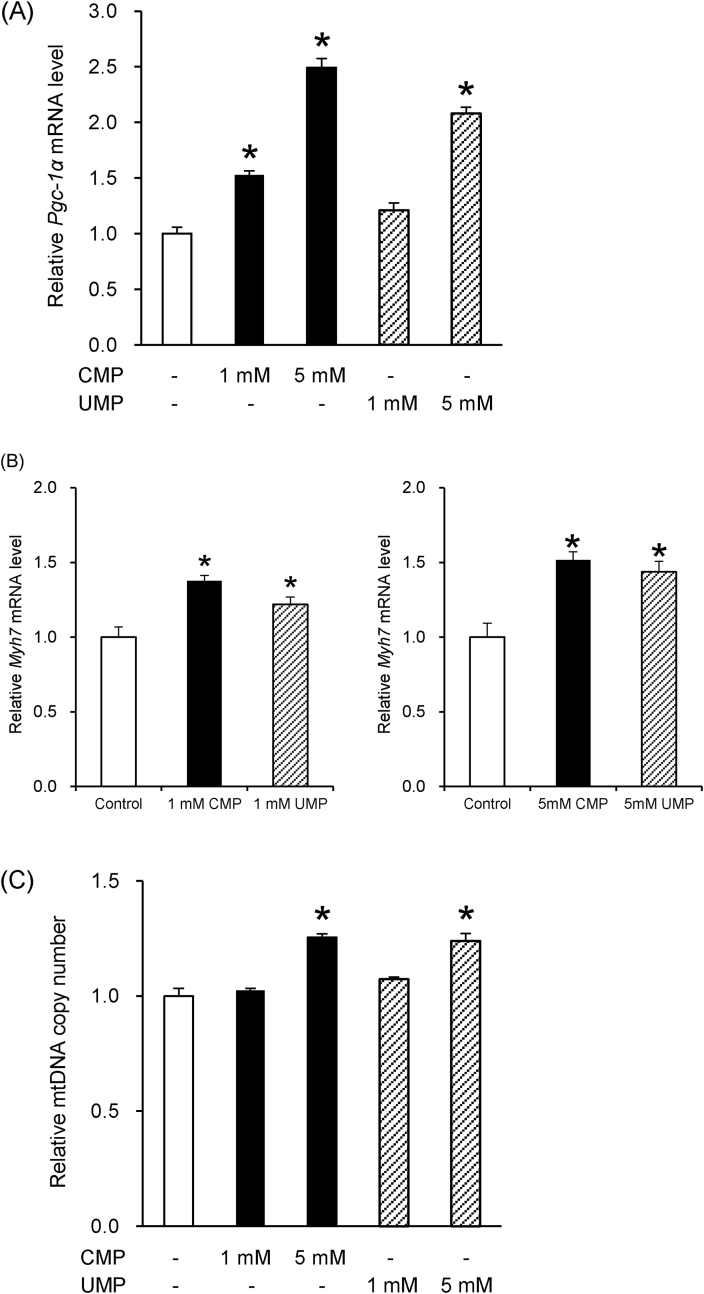
It has been reported that 5′-CMP and 5′-UMP increase the effects of endurance training in rats. Therefore, we hypothesized that 5′-CMP and 5′-UMP may promote mitochondrial biogenesis and the synthesis of slow-twitch fibers. We evaluated the effects of 5′-CMP and 5′-UMP on the mRNA levels of PGC-1α, a master regulator of mitochondrial biogenesis, and Myh7, the predominant isoform of MyHC in slow-twitch type I fibers. 5′-CMP and 5′-UMP significantly increased PGC-1α mRNA levels in a dose-dependent manner (Fig. 2A). In addition, 5′-CMP and 5′-UMP significantly increased Myh7 mRNA levels (Fig. 2B). The effect of 5′-CMP and 5′-UMP on mitochondrial biogenesis was next evaluated by measuring mtDNA copy number. We found that 5′-CMP and 5′-UMP significantly increased mtDNA copy number in a dose-dependent manner (Fig. 2C). Together, these results suggest that 5′-CMP and 5′-UMP promote mitochondrial biogenesis and increase slow-twitch fiber by activating the expression of PGC-1α and Myh7 in C2C12 cells.
Fig. 2.
5′-CMP and 5′-UMP enhance the expression of PGC-1α and Myh7, as well as promote mitochondrial biogenesis
(A) Relative PGC-1α mRNA levels. (B) Relative Myh7 mRNA levels. Total RNA was extracted from the cells after 6 days of differentiation in culture with 5′-CMP and 5′-UMP. The mRNA levels were normalized to β-actin. Each bar represents relative mRNA levels to untreated controls. Data are expressed as mean ± standard error of the mean (SEM) (n = 6) *P < 0.05 compared to untreated controls. (C) Relative mtDNA copy number. Total DNA was extracted from the cells after 6 days of differentiation in culture with 5′-CMP and 5′-UMP. Relative mtDNA copy number was calculated as the ratio of Cox2 to Ppia levels measured by real-time quantitative PCR. Each bar represents the relative value to untreated control. Data are expressed as mean ± SEM (n = 3) *P < 0.05 compared to the untreated controls.
3.3. Corresponding nucleosides promote myogenic differentiation
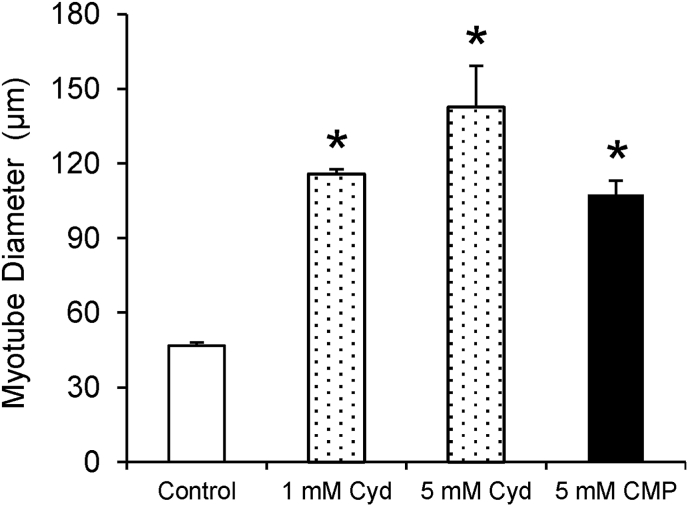
The orally ingested nucleotides are hydrolyzed to nucleosides by alkaline phosphatases and nucleotidases, which are then absorbed primarily as nucleosides via nucleoside transporters within the gastrointestinal tract [15,16]. Considering that 5′-nucleotidases are present in the serum, nucleotides are rapidly degraded to nucleosides in the serum [17]. We evaluated cytidine and uridine, which are nucleosides corresponding to 5′-CMP and 5′-UMP, respectively. Cytidine significantly promoted myotube formation (Fig. 3). Similarly, uridine increased myotube formation (Fig. S1). These findings indicate that nucleosides corresponding to 5′-CMP and 5′-UMP induce myogenic differentiation.
Fig. 3.
Cytidine promotes myotube formation
Diameter of C2C12 myotubes. C2C12 cells were cultured in differentiation medium and treated with cytidine for 5 days. Data are expressed as mean ± standard error of the mean (n = 6) *P < 0.05 compared to untreated controls. Cyd, cytidine.
4. Discussion
Nucleotides are considered as conditionally essential nutrients because they tend to be deficient under stress conditions [9]. Intense and prolonged exercise also constitutes a form of physiological stress. Sublingual nucleotides formulations, containing 5′-CMP, 5′-UMP, guanosine-5′-monophosphate and adenosine-5′-monophosphate, prolong run time to exhaustion in young physically active men [18], while rats treated with a mixture of 5′-CMP and 5′-UMP can tolerate prolonged exercise [13]. In the human study described above, it is unclear which nucleotides contributed to the improvement of endurance. Furthermore, excessive consumption of purines increases the risk of gout, so pyrimidine nucleotides are preferred over purine nucleotides such as 5′-AMP, 5′-GMP for supplements. In this work, we report a novel role of 5′-CMP and 5′-UMP in regulating myogenic differentiation, mitochondrial biogenesis, and muscular fiber type in C2C12 cells.
Myogenin promotes the fusion of myoblasts into myotubes and developing myofibers [19]. Leucine is used as a sports nutrition to promote protein synthesis, which it also enhances myogenin expression and myoblast differentiation in vivo and in vitro [20,21]. Our data showed that 5′-CMP and 5′-UMP activate myogenin expression and promote myoblasts differentiation into myotubular cells.
Our data further indicated that 5′-CMP and 5′-UMP activate the expression of PGC-1α and Myh7 and promote mitochondrial biogenesis in C2C12 myotubes. PGC-1α is a metabolic regulator involved in determining mitochondrial biogenesis and skeletal muscle fiber differentiation. Myh7 is the predominant isoform of MyHC in slow-twitch type I. Alpha lipoic acid, a coenzyme that functions in the mitochondria and improves muscular endurance, also prevented metabolic anomalies through an upregulation of PGC-1α [22,23].
The orally ingested nucleotides are hydrolyzed to nucleosides by alkaline phosphatases and nucleotidases, which are then absorbed primarily as nucleosides through nucleoside transporters within the gastrointestinal tract. After absorption in the gastrointestinal tract, nucleosides reach the liver via the portal vein and enter the circulation to be utilized by various organs. Some absorbed nucleosides contribute to the biosynthesis of nucleic acid derivatives such as nucleoside monophosphate, nucleoside diphosphate, nucleoside triphosphate, nucleotide sugar, and CDP-choline [15,16]. We evaluated cytidine and uridine similarly to 5-‘CMP and 5′-UMP and found that cytidine and uridine markedly promote myotube formation. Since 5′-CMP, 5′-UMP, cytidine and uridine are highly polar compounds, they unable to diffuse across the cell membrane by passive diffusion. Carrier proteins are responsible for the diffusion of nucleosides and nucleotides across the plasma membranes of most cells. Nucleotides are rapidly degraded to nucleosides in blood, and nucleotide transporters are localized in mitochondria but rarely in the plasma membrane. Uptake of nucleosides or nucleobases via nucleoside transporters plays a major role in the salvage pathway of nucleotide biosynthesis. Considering the absorption, distribution, and metabolism of nucleotides, the active bodies underlying of the effects observed in 5′-CMP and 5′-UMP were identified as cytidine and uridine. Moreover, 5′-CMP and 5′-UMP are expected to be effective when taken orally. Because of the high oral bioavailability of nucleotides [24], oral intake is appropriate for clinical application of 5′-CMP and 5′-UMP.
These results suggest that 5’-CMP and 5’-UMP support muscle repair and strengthening by promoting muscle differentiation, as well as muscular endurance by activating on mitochondrial biogenesis. These functions of 5′-CMP and 5′-UMP may contribute to the improvement of endurance exercise capacity reported in rats and humans.
A limitation of the study is lacking of depth analysis. In vitro assays such as Western blot of PGC-1α and myogenin, immunostaining of MHC, mitochondrial staining, accurate estimation of nucleotides concentration in cultures have not been verified. Another limitation is that the mechanisms of action underlying the observed effects were not completely elucidated. 5-‘CMP and 5′-UMP are known to be absorbed into the gastrointestinal tract as cytidine and uridine, while some of them are metabolized into nucleic acid derivatives. Cytidine, uridine, or nucleic acid derivatives may induce the expression of myogenin and PGC-1α by acting on receptors or sensors, which remain to be identified. Besides, it has been reported that rats treated with a mixture of 5′-CMP and 5′-UMP were able to endure long-term exercise, however, the activation of myogenin and PGC-1 in animals ingested 5′-CMP and 5′-UMP have not been verified. Additional studies should be required to further support the hypothesis and to determine the detailed mechanisms of action.
Our study highlights the essential role of pyrimidine nucleotides, 5′-CMP and 5′-UMP, in the regulation of myogenic differentiation, mitochondrial biogenesis, and skeletal muscle fiber type. 5′-CMP and 5′-UMP promote not only myogenic differentiation but also mitochondrial biogenesis, as well as increase slow-twitch fiber via the activation of myogenin and PGC-1α. Considering the metabolism and absorption of nucleotides, and the myogenic effect of cytidine and uridine, the active bodies recruited could be cytidine and uridine. 5′-CMP and 5′-UMP are thus considered as safe and effective agents to enhance muscle growth and improve endurance in skeletal muscles.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
All authors are employees of YAMASA corp. All authors filed the patent related to this work. The patent has not been published and issued yet. YAMASA corp. provided the nucleotides and nucleosides.
Acknowledgments
The authors wish to thank Mr. Yoshihiro Nomura (Faculty of Agriculture, Tokyo University of Agriculture and Technology, Tokyo, Japan) for his kind advice on statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2022.101309.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Yin Hang, Price Feodor, Rudnicki Michael A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh Kulwant, Dilworth F Jeffrey. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS J. 2013;280:3991–4003. doi: 10.1111/febs.12188. [DOI] [PubMed] [Google Scholar]
- 3.Schiaffino Stefano, Reggiani Carlo. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 4.Schiaffino Stefano, Alberto C., Vika Smerdu Rossi, Leinwand Leslie A., Reggiani Carlo. Developmental myosins: expression patterns and functional significance. Skeletal Muscle. 2015;5:22. doi: 10.1186/s13395-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruas J.L., White J.P., Rao R.R., Kleiner S., Brannan K.T., Harrison B.C., Greene N.P., Wu J., Estall J.L., Irving B.A., Lanza I.R., Rasbach K.A., Okutsu M., Nair K.S., Yan Z., Leinwand L.A., Spiegelman B.M. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura Shinji, Kai Yuko, Ono Misaki, Ezaki Osamu. Overexpression of peroxisome proliferator-activated receptor gamma coactivator-1alpha down-regulates GLUT4 mRNA in skeletal muscles. J. Biol. Chem. 2003;278:31385–31390. doi: 10.1074/jbc.M304312200. [DOI] [PubMed] [Google Scholar]
- 7.Lin Jiandie, Wu Hai, Tarr Paul T., Zhang Chen-Yu, Wu Zhidan, Boss Olivier, Laura F Michael, Puigserver Pere, Isotani Eiji, Olson Eric N., Lowell Bradford B., Bassel-Duby Rhonda, Spiegelman Bruce M. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 8.Thorell L., Sjöberg L.B., Hernell O. Nucleotides in human milk: sources and metabolism by the newborn infant. Pediatr. Res. 1996;40:845–852. doi: 10.1203/00006450-199612000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Pozo A., Gil A. Nucleotides as semiessential nutritional components. Br. J. Nutr. 2002;87:S135–S137. doi: 10.1079/bjn2001467. [DOI] [PubMed] [Google Scholar]
- 10.Hess Jennifer R., Greenberg Norman A. The role of nucleotides in the immune and gastrointestinal systems: potential clinical applications Nutr. Clin. Pract. 2012;27:281–294. doi: 10.1177/0884533611434933. [DOI] [PubMed] [Google Scholar]
- 11.José Pérez María, Sánchez-Medina Fermín, Torres Maribel, Gil Angel, Suárez Antonio. Dietary nucleotides enhance the liver redox state and protein synthesis in cirrhotic rats. J. Nutr. 2004;134:2504–2508. doi: 10.1093/jn/134.10.2504. [DOI] [PubMed] [Google Scholar]
- 12.Teather Lisa A., Wurtman Richard J. Chronic administration of UMP ameliorates the impairment of hippocampal-dependent memory in impoverished rats. J. Nutr. 2006;136:2834–2837. doi: 10.1093/jn/136.11.2834. [DOI] [PubMed] [Google Scholar]
- 13.Gella A., Ponce J., Cussó R., Durany N. Effect of the nucleotides CMP and UMP on exhaustion in exercise rats. J. Physiol. Biochem. 2008;64:9–17. doi: 10.1007/BF03168230. [DOI] [PubMed] [Google Scholar]
- 14.Shi Sally Yu, Lu Shun-Yan, Sivasubramaniyam Tharini, Revelo Xavier S., Cai Erica P., Cynthia T Luk, Stephanie A Schroer, Patel Prital, Kim Raymond H., Bombardier Eric, Joe Quadrilatero, Russell Tupling A., Mak Tak W., Winer Daniel A., Woo Minna. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Buren C.T., Kulkarni A.D., Rudolph F.B. The role of nucleotides in adult nutrition. J. Nutr. 1994;124:160S–164S. doi: 10.1093/jn/124.suppl_1.160S. [DOI] [PubMed] [Google Scholar]
- 16.Young James D., Yao Sylvia Y.M., Baldwin Jocelyn M., Cass Carol E., Baldwin Stephen A. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol. Aspect. Med. 2013;34:529–547. doi: 10.1016/j.mam.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Dixon T.F., Purdom M. Serum 5-nucleotidase. J. Clin. Pathol. 1954;7:341–343. doi: 10.1136/jcp.7.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostojic Sergej M., Idrizovic Kemal, Stojanovic Marko D. Sublingual nucleotides prolong run time to exhaustion in young physically active men. Nutrients. 2013;5:4776–4785. doi: 10.3390/nu5114776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meadows Eric, Cho Jang-Hyeon, Flynn Jesse M., Klein William H. Myogenin regulates a distinct genetic program in adult muscle stem cells. Dev. Biol. 2008;322:406–414. doi: 10.1016/j.ydbio.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Dai Jie-Min, Yu Mu-Xue, Shen Zhen-Yu, Guo Chu-Yi, Zhuang Si-Qi, Qiu Xiao-Shan. Leucine promotes proliferation and differentiation of primary preterm rat satellite cells in part through mTORC1 signaling pathway. Nutrients. 2015;7:3387–3400. doi: 10.3390/nu7053387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Minjung, Sung Bokyung, Kang Yong Jung, Kim Dong Hwan, Lee Yujin, Hwang Seong Yeon, Yoon Jeong-Hyun, Yoo Mi-Ae, Kim Cheol Min, Chung Hae Young, Kim Nam Deuk. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015;35:755–762. doi: 10.3892/ijmm.2014.2046. [DOI] [PubMed] [Google Scholar]
- 22.Trittel Lucas, Isenmann Eduard, Diel Patrick. The effects of alpha lipoic acid on muscle strength recovery after a single and a short-term chronic supplementation - a study in healthy well-trained individuals after intensive resistance and endurance training. J. Int. Soc. Sports Nutr. 2020;17:61. doi: 10.1186/s12970-020-00389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammed M.A., Mahmoud M.O., Awaad A.S., Gamal G.M., Abdelfatah D. vol. 144. 2019. pp. 1–7. (Alpha Lipoic Acid Protects against Dexamethasone-Induced Metabolic Abnormalities via APPL1 and PGC-1 α up Regulation Steroids). [DOI] [PubMed] [Google Scholar]
- 24.Sonoda T., Tatibana M. Metabolic fate of pyrimidines and purines in dietary nucleic acids ingested by mice. Biochim. Biophys. Acta. 1978;521:55–66. doi: 10.1016/0005-2787(78)90248-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.