Summary
Clinically relevant animal models are crucial for effective development of therapeutics for peritoneal carcinomatosis (PC). This protocol describes the generation of patient-derived ascites-dependent xenograft (PDADX) models from the cellular component of ascites. The use of routine intraperitoneal injection of the fluid component of ascites is analogous to the biological events occurring intra-abdominally in patients with PC. By serving as a proxy, PDADX models represent a valuable tool for preclinical testing of new therapeutics for PC.
For complete details on the use and execution of this protocol, please refer to Hendrikson et al. (2022).
Subject areas: Cancer, Health Sciences, Model Organisms, Molecular Biology
Graphical abstract
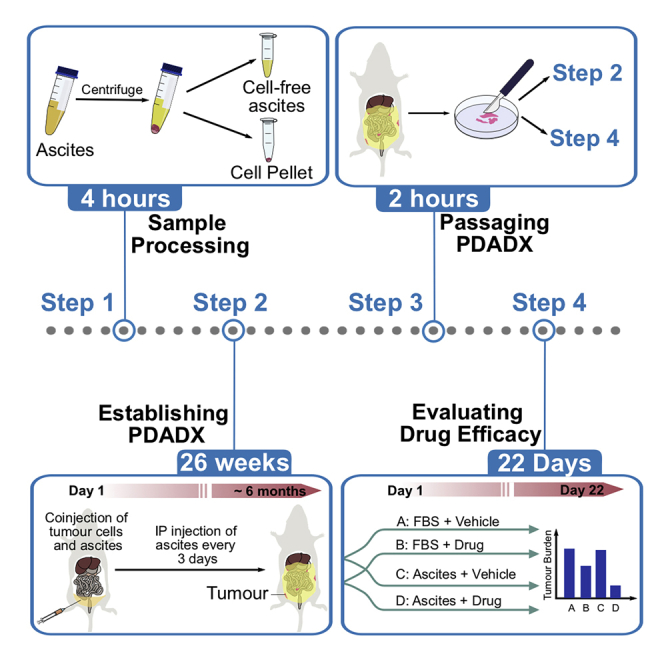
Highlights
-
•
Generation of patient-derived ascites-dependent xenograft (PDADX) models
-
•
Serial passages of PDADX tumors in mice
-
•
Evaluation of patient-specific drug efficacy utilizing PDADX models
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Clinically relevant animal models are crucial for effective development of therapeutics for peritoneal carcinomatosis (PC). This protocol describes the generation of patient-derived ascites-dependent xenograft (PDADX) models from the cellular component of ascites. The use of routine intraperitoneal injection of the fluid component of ascites is analogous to the biological events occurring intra-abdominally in patients with PC. By serving as a proxy, PDADX models represent a valuable tool for preclinical testing of new therapeutics for PC.
Before you begin
The protocol below details the process of establishing PDADX mouse models using malignant ascites from patients with PC as well as the utility of the model to evaluate drug efficacy. For example, we generated PDADX models of colorectal PC origin to evaluate susceptibility to the inhibition of a paracrine factor in ascites (Hendrikson et al., 2022). This protocol can be adapted for the establishment of mouse models of any histological subtype of PC (primary, colorectal, gastric, ovarian, etc.), should sufficient volume of ascites be available for collection.
Institutional permissions
-
1.
Tissue samples should only be collected from patients who have provided informed consent prior to tissue use according to the study protocol as approved by the appropriate institutional approving committee. Patient samples used in this protocol were obtained from patients who provided written informed consent as approved by the SingHealth Centralised Institutional Review Board.
-
2.
All murine experiments should be performed according to protocols as approved by the appropriate institutional approving committee. Experiments described in this protocol were performed as approved by the SingHealth Institutional Animal Care and Use Committee.
Sample collection and processing of specimens
Patient blood (at least 1 mL), tumor (at least 0.5 cm × 0.5 cm × 0.5 cm) and ascites (at least 500 mL) are transferred to the laboratory at 20°C–24°C within 10 min or are placed on ice if a longer duration is required. All samples are kept at 4°C until processing. All samples should be processed within 2 h of collection. Processing of patient blood, patient tumor, patient ascites and PDADX tumor should be performed in a Class II Biological Safety Cabinet.
Note: In the scenario where, insufficient patient material is obtained, the sample can be used for other experimental plans such as target validation or screening assays. This falls outside the scope of this protocol and will not be described in detail.
Patient blood
-
3.
Patient blood can be collected in sterile EDTA blood collection tubes. We used 3 mL EDTA blood collection tubes with 5.4 mg K2EDTA (BD, Catalog No.: 367856).
-
4.
Transfer 1 mL of patient blood specimen to a cryogenic vial (Corning, Catalog No.: 430488).
-
5.
Snap-freeze using liquid nitrogen.
-
6.
Store at −80°C for sequencing analysis.
Pause point: The snap-frozen patient blood sample can be kept at −80°C for 6 months or longer.
Patient tumor
-
7.
Patient tumor should be collected during surgery. Tumor (at least 0.5 cm × 0.5 cm × 0.5 cm) can be collected in a sterile polypropylene (PP) container (DELTALAB, Catalog No.: 409526G).
-
8.
Transfer patient tumor specimen (approximately 0.5 cm × 0.5 cm × 0.5 cm) to a cryogenic vial.
-
9.
Snap-freeze using liquid nitrogen.
-
10.
Store at −80°C for sequencing analysis.
Pause point: The snap-frozen patient tumor specimen can be kept at −80°C for 6 months or longer.
Patient ascites
-
11.
Ascites should be collected from the patient’s peritoneal cavity at the beginning of surgery or during routine paracentesis. Ascites can be collected in a sterile suction canister (MedQuest, Catalog No.: 1131-03-009-A). At least 500 mL ascites should be collected.
-
12.
Centrifuge at 2,000 g for 10 min at 20°C–24˚C using Eppendorf benchtop centrifuge 5810 affixed with Eppendorf A-4-62 swing bucket rotor.
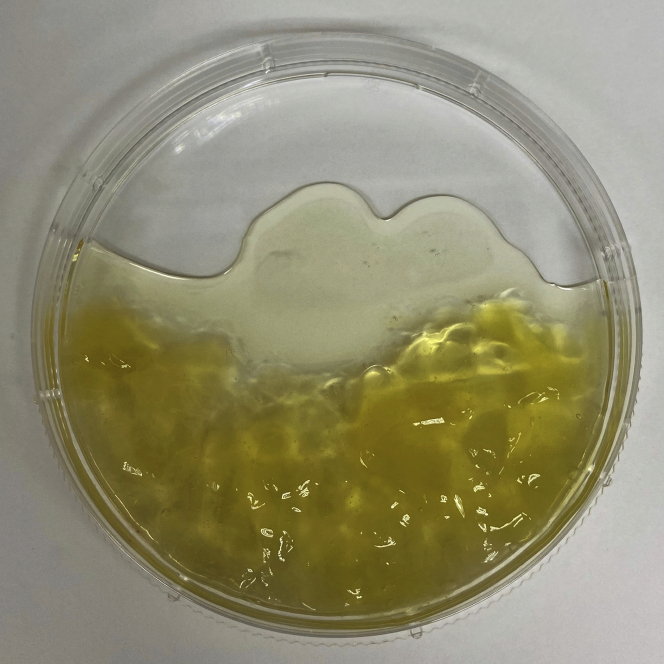
Note: Mucinous material may be found in some ascites specimens and should be removed after centrifugation. Mucinous material will appear like white or yellow jelly-like substances (Figure 1).
Figure 1.
Representative image of mucinous material that may be found in patient ascites
Fluid component
-
13.
Transfer fluid component to 2 sterile 50 mL PP centrifuge tubes (Greiner, Catalog No.: 227261) for easy thawing and the remaining into multiple sterile 50 mL PP centrifuge tubes or 500 mL polycarbonate bottles (Corning, Catalog No.: 431432).
Note: As it is tedious and time-consuming to filter-sterilize large volumes of the fluid component of ascites, non-sterilized fluid component can be stored at −80°C and subsequently filter-sterilized before use.
Pause point: The non-sterilized fluid component can be kept at −80°C for 2 years or longer.
-
14.
Sterilize fluid component using a 0.22 μm filter (Sartorius, Catalog No.: 16532K) to obtain filter-sterilized cell-free ascites.
-
15.
Store in 1.5 mL PP tubes (Axygen, Catalog No.: MCT-150-C) at −80°C for further use.
Pause point: The filter-sterilized cell-free ascites can be kept at −80°C for 2 years or longer.
Cellular component for storage
Approximately 5–10 mL of cell pellet can be obtained from 500 mL of ascites. Troubleshooting 1.
-
16.
Add 4 pellet volumes of cell-free ascites obtained from the same patient (subsequently referred to as “matched” specimens in the rest of the protocol) to the cell pellet in the tube and resuspend until a homogenous cell suspension is achieved. For example, add 16 mL of cell-free ascites to 4 mL of cell pellet.
-
17.Transfer 500 μL of homogenous cell suspension to a new 1.5 mL PP tube.
-
a.Centrifuge at 2,000 g for 10 min. This will result in approximately 100 μL of cell pellet.
-
b.Aspirate off the supernatant.
-
c.Snap-freeze the cell pellet using liquid nitrogen.
-
d.Store at −80°C for sequencing analysis.
-
a.
Pause point: The snap-frozen cell pellet can be kept at −80°C for 6 months or longer.
-
18.Prepare cryopreserved stock by transferring 2 mL of homogenous cell suspension to a new 2 mL PP tube (Eppendorf, Catalog No.: 0030.120.094).
-
a.Centrifuge at 2,000 g for 10 min. This will result in approximately 400 μL of cell pellet.
-
b.Aspirate off the supernatant.
-
c.Mix the cell pellet with 1 mL prepared freezing medium.Note: Freezing medium should be filter-sterilized with a 0.22 μm filter and stored at 4°C for a maximum of 5 days prior to use.
-
d.Transfer the mixture to a cryogenic vial and store in a freezing container (Corning, Catalog No.: 432002) at −80°C for 12–18 h.
-
e.Transfer the frozen vial to liquid nitrogen for long-term storage.
 Pause point: The frozen mixture can be kept in liquid nitrogen for at least 8 years.
Pause point: The frozen mixture can be kept in liquid nitrogen for at least 8 years. CRITICAL: At least 10 vials of cryopreserved stocks are suggested to be stored for further implantation.
CRITICAL: At least 10 vials of cryopreserved stocks are suggested to be stored for further implantation.
-
a.
Cellular component for generation of PDADX models
CRITICAL: A minimum of 5 mice are suggested to be implanted to increase success rates of establishing the PDADX model. Please refer to the section on establishing PDADX mouse models.
-
19.
Transfer 1 mL of homogenous cell suspension to a new sterile tube.
-
20.
Centrifuge at 2,000 g for 10 min. This will result in approximately 200 μL of cell pellet.
-
21.
Aspirate off the supernatant.
-
22.
Add 200 μL matched filter-sterilized cell-free ascites to the cell pellet in the tube.
CRITICAL: The cell-free ascites used in this protocol should be matched with the same patient’s cellular component to create a surrogate tumor microenvironment of the patient.
-
23.
Resuspend the cell pellet in the filter-sterilized matched cell-free ascites (1:1 ratio) by passing through a 23 G syringe needle (B.Braun, Catalog No.: 465667) repeatedly.
Note: As a minimum of 5 mice are recommended to be implanted, a master mix of resuspended cells corresponding to volumes necessary for 5 mice, i.e., 5 mL of homogenous cell suspension (equivalent to approximately 1 mL of cell pellet) and 1 mL matched filter-sterilized cell-free ascites, will be useful to reduce variations between cell suspensions injected into each mouse.
Note: The resuspended cells are ready for implantation when a homogenous mixture if formed. Due to inherent differences across cell pellets, at least 10 needle passes may be required to obtain a homogenous mixture.
Animals
-
24.
Consider appropriateness of mice gender for the study. We used 6–8 weeks old female BALB/c nude mice (C.Cg/AnNTac-Foxn1nuNE9, RRID: IMSR_Tac:balbnu) in this protocol as we are interested to determine the spread of PC to all intra-abdominal organs, including the ovaries.
Note: Investigators can choose to utilize male mice if they are interested to investigate tumor development in the male reproductive system. However, the authors have not explored this and optimization of the protocol may be necessary.
-
25.
Mice should have free access to food and water. We recommend food with 18% protein composition and autoclaved acidified reverse osmosis water with pH 2.6–3.1 ad libitum.
-
26.
Mice should be maintained in individually ventilated cages with sterile bedding. We typically use autoclaved corn cob bedding (BioCob, Catalog No.: 1090678(1)) and ALPHA-twist enrichment (LBS Biotechnology, Catalog No.: NA). A rodent dome (Bio-Serv, Catalog No.: K3327) can be provided in cages housing a single mouse.
-
27.
Mice should be maintained under pathogen-free conditions and a controlled environment with temperature at 20°C–24°C, humidity at 55%–65%, pressure of 4–15 kPa, and a 12 h light/dark cycle.
-
28.
Mice should be anesthetized with 2% isoflurane (Henry Schein, Catalog No.: 900-8931) for 5 min followed by anesthetic depth assessments, such as toe pinch and tail pinch, before intraperitoneal injection or cervical dislocation. The E-Z Anesthesia Auto Flow System (Catalog No.: EX-AF9000) is used in this protocol.
Characterization of PDADX tumor before evaluation of drug efficacy
-
29.Verification of the cancerous nature of the established PDADX tumor should be performed prior to performing experiments to evaluate drug efficacy. This can be done in different ways, including:
-
a.Whole exome / genome sequencing of the PDADX tumor and subsequent comparison to the genetic profiles of parental patient tumor tissue, patient ascites cell pellet and patient blood;
-
b.Immunohistochemistry analysis of known tumor markers could be performed on PDADX tumors. For instance, in the case of PC originating from colorectal cancer, staining of cytokeratin 7 (CK7), cytokeratin 20 (CK20) and caudal-type homeobox 2 (CDX2) could be performed because CK20+/CK7- and CDX2 expressions are observed in the vast majority of colorectal cancers (Kaimaktchiev et al., 2004; Lugli et al., 2008; Rubin et al., 2001).
-
a.
Note: This falls outside the scope of this protocol and will not be described in detail.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human blood samples | (Hendrikson et al., 2022) | |
| Human ascites samples | (Hendrikson et al., 2022) | |
| Human tumor samples | (Hendrikson et al., 2022) | |
| Chemicals, peptides, and recombinant proteins | ||
| Isothesia (Isoflurane) | Henry Schein | 900-8931 |
| Dimethyl sulfoxide, DMSO | Sigma | D2650 |
| HyClone™ Fetal bovine serum, FBS | Cytiva | SV30160.03 |
| Phosphate-buffered saline, PBS | Thermo Scientific | AM9624 |
| Experimental models: Organisms/strains | ||
| BALB/c nude mouse, 6–8 weeks old, female | Taconic Biosciences | C.Cg/AnNTac-Foxn1nuNE9 (RRID: IMSR_Tac:balbnu) |
| Software and algorithms | ||
| GraphPad Prism, version 7.0 | n/a | https://www.graphpad.com/scientific-software/prism/ |
| Inkscape, version 1.1 | n/a | https://inkscape.org/ |
| Other | ||
| Teklad global diet | Envigo | 2918 |
| Corn cob bedding | BioCob | 1090678(1) |
| ALPHA-twist | LBS Biotechnology | n/a |
| Rodent dome, Mouse Igloo® and Fast Tracs | Bio-Serv | K3327 |
| EDTA tube, K2EDTA 5.4 mg | BD | 367856 |
| Polypropylene containers (38 × 65 mm), 60 mL | DELTALAB | 409526G |
| Suction canister, 3000 mL | MedQuest | 1131-03-009-A |
| Cryogenic vial, 2 mL | Corning | 430488 |
| Polypropylene tube, 50 mL | Greiner | 227270 (227261) |
| Polycarbonate storage bottle, 500 mL | Corning | 431432 |
| Minisart® Syringe Filters, 0.22 μm | Sartorius | 16532K |
| Nalgene™ Rapid-Flow™ Sterile Single Use Vacuum Filter Units | Thermo Scientific | 567-0020 |
| Corning® CoolCell® LX Cell Freezing Container | Corning | 432002 |
| Polypropylene tube, 1.5 mL | Axygen | MCT-150-C |
| Polypropylene tube, 2 mL | Eppendorf | 0030.120.094 |
| Disposable hypodermic needle, 23 G | B.Braun | 4657667 |
| Omnifix Luer Syringe, 1 mL | B.Braun | 9161406V |
| Insulin syringe 0.5 mL with needle, 31 G | Terumo | SS∗05M3108KS |
| Carbon steel surgical blade, #10 | Swann-Morton | 0201 |
| Cell culture dish, 60 × 15 mm | Nunc | 150288 |
| Anesthesia Auto Flow System | E-Z Systems | EZ-AF9000 |
| Class II Biosafety Cabinet | NuAire | NU-425-400 |
| Benchtop centrifuge | Eppendorf | 5810 |
Materials and equipment
Preparation of freezing medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMSO | 10% | 5 mL |
| FBS | 90% | 45 mL |
| Total | n/a | 50 mL |
Alternatives: DMSO (Sigma, Catalog No.: D2650) and FBS (Cytiva, Catalog No.: SV30160.03) were used in this protocol. Any other cell culture-grade DMSO and FBS could be used.
Preparation of 1× PBS solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 10× PBS | 1× | 100 mL |
| Autoclaved Milli-Q water | n/a | 900 mL |
| Total | n/a | 1,000 mL |
Alternatives: Any other filter-sterilized PBS buffer with a 1× working concentration containing 136.9 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, and 1.5 mM KH2PO4 (pH7.4) could be used.
Preparation of 5% cell-free ascites
| Reagent | Final concentration | Amount |
|---|---|---|
| Filter-sterilized cell-free ascites | 5% | 5 μL |
| 1× PBS | n/a | 95 μL |
| Total | n/a | 100 μL |
Preparation of 10% FBS solution with 1% vehicle for intraperitoneal injection
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 10% | 40 μL |
| 1× PBS | n/a | 356 μL |
| Appropriate vehicle of tested drug | 1% | 4 μL |
| Total | n/a | 400 μL |
Preparation of 10% FBS solution with tested drug for intraperitoneal injection
| Reagent | Final concentration | Amount |
|---|---|---|
| FBS | 10% | 40 μL |
| 1× PBS | n/a | 356 μL |
| 100× drug stock | To be optimized | 4 μL |
| Total | n/a | 400 μL |
Preparation of 5% ascites solution with 1% vehicle for intraperitoneal injection
| Reagent | Final concentration | Amount |
|---|---|---|
| Filter-sterilized cell-free ascites | 5% | 20 μL |
| 1× PBS | n/a | 376 μL |
| Appropriate vehicle of tested drug | 1% | 4 μL |
| Total | n/a | 400 μL |
Preparation of 5% ascites solution with tested drug for intraperitoneal injection
| Reagent | Final concentration | Amount |
|---|---|---|
| Filter-sterilized cell-free ascites | 5% | 20 μL |
| 1× PBS | n/a | 376 μL |
| 100× drug stock | To be optimized | 4 μL |
| Total | n/a | 400 μL |
Preparation of solution with 1% vehicle for oral administration
| Reagent | Final concentration | Amount |
|---|---|---|
| 1× PBS | n/a | 396 μL |
| Appropriate vehicle of tested drug | 1% | 4 μL |
| Total | n/a | 400 μL |
Preparation of solution with tested drug for oral administration
| Reagent | Final concentration | Amount |
|---|---|---|
| 1× PBS | n/a | 396 μL |
| 100× drug stock | To be optimized | 4 μL |
| Total | n/a | 400 μL |
Step-by-step method details
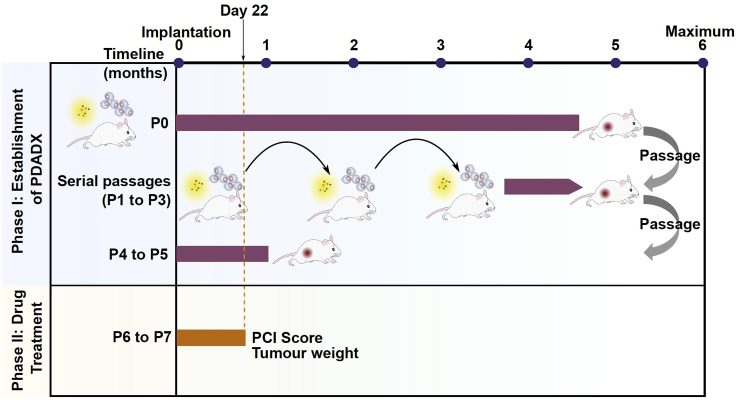
Figure 2 illustrates the schematic representation of the various stages of tumor growth in the PDADX mouse models, including establishment, serial passaging and experimental phase to determine drug efficacy.
Figure 2.
Schematic representation of the various stages of tumor growth in the patient-derived ascites-dependent xenograft (PDADX) mouse models, including establishment, serial passaging, and experimental phase to determine drug efficacy
Establishing PDADX mouse models
Timing: 26 weeks
The objective of this step is to establish PDADX models by implanting the cellular component of patient ascites into mice and creating a matched ascitic microenvironment within the intraperitoneal cavities of mice.
CRITICAL: The success rate of obtaining the first generation of PDADX tumor (P0) is approximately 50%. A minimum of 5 mice are suggested to be implanted to increase success rates of establishing the PDADX model.
-
1.
Implant 400 μL of 1:1 cellular component / filter-sterilized cell-free ascites mixture in each mouse via intraperitoneal injection using a 23 G syringe needle. The injection site is shown in Figure 3A.
-
2.
Inject 100 μL of 5% matched filter-sterilized cell-free ascites into each mouse via intraperitoneal injection using a 31 G syringe needle (Terumo, Catalog No.: SS∗05M3108KS), repeating the injection every three days.
Note: Following dilution, 5% ascites should be used immediately at 20°C–24°C, without storing.
CRITICAL: This step is crucial to recapitulate the tumor microenvironment in PC patients where tumor cells are growing in an ascitic microenvironment and are addicted to paracrine factors found within the ascites.
-
3.
Maintain implanted mice for PDADX tumor formation.
-
4.
Monitor mice during routine ascites injection and decide if tumor has developed by observing abdomen size or presence of tumor nodules for soft and hard tumors, respectively, or if no tumor has developed by 6 months. Examples of mice bearing soft PDADX tumor and hard PDADX tumor are shown in Figure 3B. Troubleshooting 2 and 3.
Note: PDADX tumor formation is generally observed 6-months post-implantation. As approved by our SingHealth IACUC protocol, the humane end-point for mouse maintenance is 6 months.
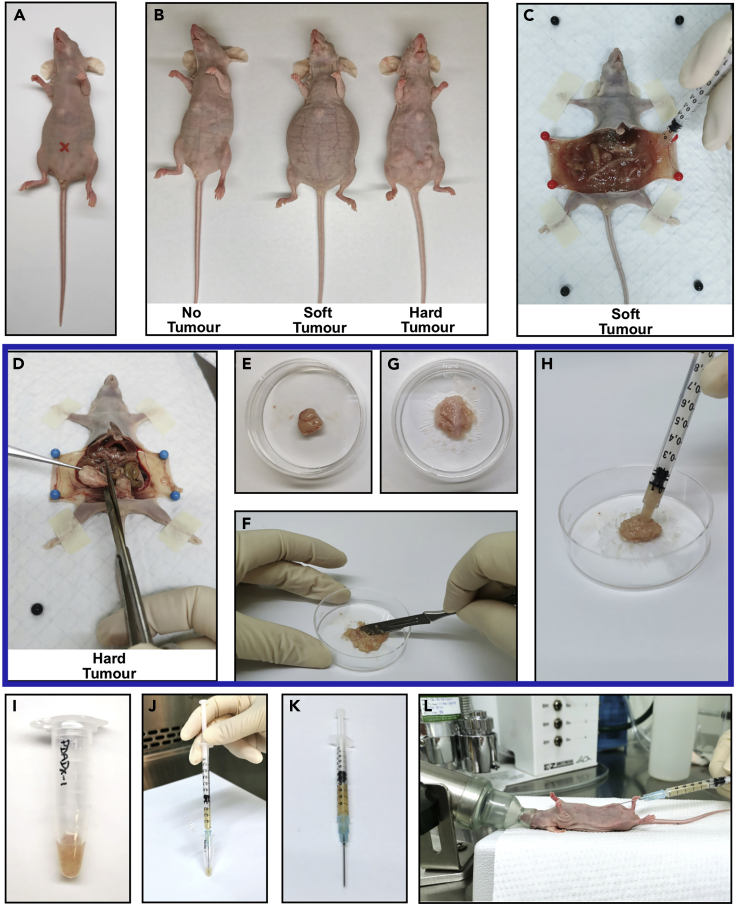
Figure 3.
Passaging of PDADX tumors for maintenance in mice models
(A) The site for intraperitoneal injection (red mark).
(B) Representative images of mice bearing no tumor, soft tumor, and hard tumor.
(C) Representative image of soft tumor in mouse collected by syringe.
(D) Representative image of hard tumor in mouse excised by scissors.
(E) Transfer of excised hard tumor to a culture dish.
(F) The excised hard tumor was minced into fine pieces using a scalpel.
(G) Representative image of minced hard tumor ready for resuspension.
(H) Transfer of minced hard tumor by a syringe.
(I) Transfer of soft tumor or minced hard tumor into a sterile tube.
(J) Resuspend tumor cells in its matched filter-sterilized cell-free ascites by passing through a 23 G syringe needle repeatedly.
(K) Representative image of syringe containing tumor mixture ready for injection.
(L) Implantation of tumor mixture via intraperitoneal injection.
Passaging PDADX tumors in mouse models
Timing: 2 h
Upon successful establishment of PDADX tumors in mice (P0), tumors will be passaged into the next generation of mice. The objective of this step is to maintain PDADX tumor in mice by serial passaging. A minimum of 5 mice are suggested to be implanted to harvest PDADX tumors for downstream activities.
-
5.
Sacrifice mouse bearing PDADX tumor through cervical dislocation under anesthesia.
-
6.Collect PDADX tumor from the sacrificed mouse.
-
a.For soft tumors that can pass through a 23 G syringe needle, transfer at least 600 mg tumor to a sterile tube or a culture dish (Figure 3C) and mix well using a syringe.
-
b.For solid tumors that are too large to pass through a 23 G syringe needle,
-
i.Excise a piece of tumor using an autoclaved pair of scissors and forceps (Figure 3D).
-
ii.Place tumor on a culture dish (Nunc, Catalog No.: 150288) (Figure 3E).
-
iii.Mince tumor into fine pieces using a sterile scalpel (Swann-Morton, Catalog No.: 0201) (Figures 3F and 3G).
-
iv.Transfer minced tumor to a sterile tube using a syringe (B.Braun, Catalog No.: 9161406V) (Figure 3H).
-
i.
-
a.
-
7.
Transfer 100 mg tumor into a sterile tube for each mouse (Figure 3I).
-
8.
Add 400 μL of matched filter-sterilized cell-free ascites to the tube.
-
9.
Resuspend tumor cells by passing through a 23 G syringe needle repeatedly (Figure 3J).
Note: As a minimum of 5 mice are recommended to be implanted, a master mix of soft / hard tumor corresponding to volumes necessary for 5 mice, i.e., 500 mg tumor and 2 mL matched filter-sterilized cell-free ascites, will be useful to reduce variations between PDADX tumors injected into each mouse. The resuspended tumor is ready for implantation when a homogenous mixture is formed (Figure 3K).
-
10.
Implant approximately 500 μL mixture (corresponding to 100 mg tumor) into each mouse via intraperitoneal injection using a 23 G syringe needle (Figure 3L).
CRITICAL: The success rate of obtaining PDADX tumor at the passaging stage is close to 100%.
-
11.
Inject 100 μL of 5% filter-sterilized cell-free ascites into each mouse via intraperitoneal injection using a 31 G syringe needle, repeating the injection every three days.
-
12.
Maintain implanted mice for PDADX tumor formation.
-
13.
Monitor mice during routine ascites injection and decide if tumor has developed by observing abdomen size or presence of tumor nodules for soft and hard tumors, respectively (Figure 3B).
Note: PDADX tumor formation takes approximately 4–5 serial passages before the tumor development rate stabilizes to approximately 3–4 weeks (P4 to 5), depending on the aggressiveness of patient cancer cells.
-
14.
Upon observable PDADX tumor formation, please follow steps 5–13 to perform serial passaging or proceed to collect the tumor for drug efficacy evaluation.
Harvesting of tumor for cryopreservation and subsequent revival of PDADX tumors
Timing: 2 days
The objective of this step is to harvest PDADX tumor specimens for cryopreserved stocks and subsequent analyses such as genomic sequencing, morphological characterization and implantation into mice for evaluation of drug efficacy.
To cryopreserve PDADX tumor.
-
15.
Transfer 100 mg tumor to a cryogenic vial.
Note: Different methods are applied to collect and process soft and hard tumors, please refer to step 6.
-
16.
Add 1 mL freezing medium to the vial.
-
17.
Resuspend tumor cells by passing through a 23 G syringe needle repeatedly.
-
18.
Store the cryogenic vials in a freezing container at −80°C for 12–18 h.
-
19.
Transfer the frozen vials to liquid nitrogen for long-term storage.
Pause point: The frozen tumor cells can be kept in liquid nitrogen for at least 8 years.
CRITICAL: This step is critical to revive early passages of the PDADX model. At least 10 vials of cryopreserved stocks for each passage are suggested to be stored for further implantation.
To revive PDADX tumor in mice.
-
20.
Thaw one cryopreserved stock of PDADX tumor in a 37°C water bath for 2 min.
-
21.
Transfer the mixture to a sterile 50 mL PP centrifuge tube.
-
22.
Wash with 20 mL 1× PBS.
Note: 1× PBS should be filter-sterilized with a 0.22 μm filter unit (Thermo Scientific, Catalog No.: 567-0020) and stored at 4°C to be used within 1 year.
-
23.
Centrifuge at 500 g for 5 min with maximum brake setting using Eppendorf benchtop centrifuge 5810 affixed with Eppendorf A-4-62 swing bucket rotor.
-
24.
Remove supernatant.
-
25.
Add 400 μL matched filter-sterilized cell-free ascites.
-
26.
Resuspend tumor cells by passing through a 23 G syringe needle repeatedly.
-
27.
Implant approximately 500 μL mixture (corresponding to 100 mg tumor) into each mouse via intraperitoneal injection using a 23 G syringe needle.
-
28.
Follow steps 11–14 to maintain the implanted mice.
Evaluation of drug efficiency utilizing PDADX mouse models
Timing: 22 days
The objective of this step is to evaluate efficiency of drugs in inhibiting PDADX tumor growth in the matched ascitic microenvironment, which recapitulates the tumor microenvironment in PC patients.
CRITICAL: Each drug treatment group should include at least 5 mice.
Day 1
-
29.
Sacrifice mouse bearing PDADX tumor through cervical dislocation under anesthesia.
-
30.
Collect sufficient tumor for the experiment. For this protocol, 2.5 g of tumor is collected for a 20-mice experiment.
Note: Different methods are applied to collect soft and hard tumors, please refer to step 6.
-
31.
Aliquot 100 mg ± 1 mg tumor in each sterile tube.
CRITICAL: Amount of tumor for implantation should be determined for each PDADX model with appropriate experiment schedule. The indicated amount is applied for a 22-day experiment using PDADX models with an average growth rate of 1 month / passage.
-
32.
Add 400 μL of 5% matched filter-sterilized cell-free ascites or 10% FBS treated with 1% appropriate vehicle or optimal concentration of tested drugs to each tube. The groups of a 20-mice experiment are listed in Table 1.
Note: As a minimum of 5 mice are recommended to be implanted, a master mix of soft / hard tumor corresponding to volumes necessary for 5 mice, i.e., 500 mg tumor and 2 mL appropriate solution, will be useful to reduce variation between PDADX tumors injected into each mouse.
Note: The optimal concentration of tested drug for each PDADX model can be determined by serial dilution of drugs in the presence of sterile cell-free ascites. Add 400 μL of 5% matched filter-sterilized cell-free ascites treated with 1% appropriate vehicle or a serial dilution of tested drugs to each tube. At the optimal concentration, a significant reduction in tumor burden should be observed with an absence of signs indicating toxicity in mice, for example, drastic reduction in body weight and inability to eat or drink etc.
Optional: Drug efficiency of oral administration can also be tested using the PDADX model. Resuspend 100 mg tumor with 400 μL of 5% filter-sterilized cell-free ascites or 10% FBS. Prepare 400 μL 1× PBS solution containing 1% appropriate vehicle or tested drug at an optimal concentration for oral gavage.
-
33.
Resuspend the tumor cells by passing through a 23 G syringe needle repeatedly.
-
34.
Implant approximately 500 μL mixture (corresponding to 100 mg tumor) into each mouse via intraperitoneal injection using a 23 G syringe needle.
Table 1.
Groups of drug efficacy evaluation in patient-derived ascites-dependent xenograft (PDADX) models
| Condition | Control and ascites treatment | Drug treatment |
|---|---|---|
| 1 | 10% FBS | 1% appropriate vehicle |
| 2 | 10% FBS | Tested drug |
| 3 | 5% matched ascites | 1% appropriate vehicle |
| 4 | 5% matched ascites | Tested drug |
Days 2–21
-
35.Inject 400 μL of 5% matched filter-sterilized cell-free ascites or 10% FBS treated with 1% appropriate vehicle or optimal concentration of drugs in each mouse via intraperitoneal injection using a 31 G syringe, repeating the injection every three days.
-
a.For a 22-day experiment with 7 doses, drug treatment should be performed on Day 4, Day 7, Day 10, Day 13, Day 16, and Day 19. Troubleshooting 4.
-
a.
CRITICAL: Drug injection schedule and duration of experiments can be customized.
Optional: For drug efficacy of oral administration, 400 μL of 5% filter-sterilized cell-free ascites or 10% FBS should be administered via intraperitoneal injection using 31 G syringe to maintain the ascitic or control microenvironment. 400 μL of 1× PBS solution containing 1% appropriate vehicle and tested drug at an optimal concentration should be administered by oral gavage.
Day 22
-
36.Sacrifice mice through cervical dislocation under anesthesia and evaluate the tumor burden. Troubleshooting 5 and 6.
-
a.For soft tumor.
-
i.Weigh a sterile culture dish.
-
ii.Collect all soft tumor on the culture dish.
-
iii.Weigh the culture dish with tumor.
-
iv.Calculate tumor weight using the following formula:
-
i.
-
b.For solid tumor.
-
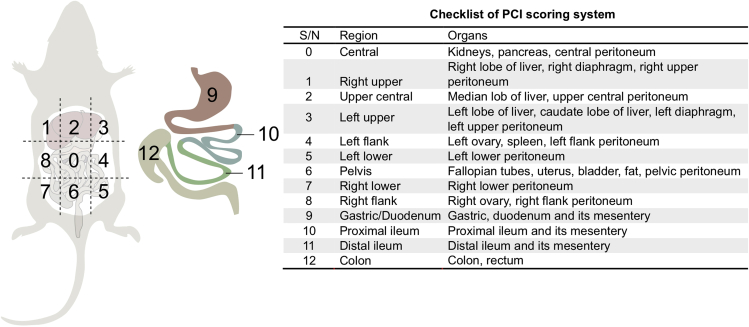
i.Examine presence of tumor on each organ. The list of organs is shown in Figure 4.Note: This scoring system is a modification of peritoneal carcinomatosis index (PCI) established previously (Klaver et al., 2010; Sugarbaker, 1998).
-
ii.Measure size of tumor and allocate scores of 0–3 for each organ. The criterion of scoring is listed in Table 2.
-
iii.Calculate a total score for each mouse using the modified PCI to quantify the tumor burden.
-
i.
-
a.
Figure 4.
Evaluation of solid tumor burden by modified peritoneal carcinomatosis index (PCI)
Schematic of modified PCI used to assess tumor burden in PDADX tumor models. This scoring system is a modification of PCI scoring adapted from (Sugarbaker, 1998) and (Klaver et al., 2010).
Table 2.
Modified peritoneal carcinomatosis index (PCI) score criterion
| PCI score | Size of tumor |
|---|---|
| 0 | No visible macroscopic tumor |
| 1 | < 1 mm nodule |
| 2 | 1–5 mm nodule |
| 3 | > 5 mm tumor or ≥ 10 nodules in one organ/region |
Expected outcomes
This protocol allows for the establishment and maintenance of patient-derived ascites-dependent xenograft (PDADX) mouse models of peritoneal carcinomatosis. The success rate of patient’s cancer cells growing into the first generation of PDADX tumor (P0) is approximately 50%. If tumor is established in the mouse, presence of an enlarged abdomen or tumor nodules should be observed 6-months post-implantation. The established PDADX tumors are expected to exhibit identical genomic features and morphological properties with matched patient peritoneal tumor and matched patient ascites cells. Regular intraperitoneal administration of matched ascites allows the PDADX tumor to grow in the ascitic microenvironment and to be addicted to key paracrine factors in its matched ascites, which capitulates the tumor microenvironment in a PC patient’s peritoneal cavity. Upon successful establishment of the first generation of PDADX tumor (P0), we will proceed to perform serial passages of the PDADX tumors into the next generation of mice. During the passaging stage, the success rate of growing PDADX tumors is close to 100% and the PDADX tumor formation takes approximately 4–5 serial passages before the tumor development rate stabilizes to approximately 3–4 weeks (P4 to 5), depending on the aggressiveness of the tumor.
The established PDADX models can be used to identify tumor-specific and / or patient-specific therapeutic vulnerabilities via in vivo evaluation of drug efficacy. A lower tumor burden should be observed in the presence of efficacious drugs compared to vehicle control. Furthermore, the reduction of tumor burden should be greater in the presence of matched patient’s ascites compared to FBS control, demonstrating susceptibility of PDADX tumor to efficacious drugs in its matched ascitic microenvironment.
Quantification and statistical analysis
Statistical analysis of tumor burden between groups is performed using unpaired two-sided t-test in GraphPad Prism (version 7.0). The statistical significance level is set at P < 0.05.
Limitations
In this protocol, PDADX models are established from the cellular component of ascites with routine intraperitoneal injection of the fluid component of ascites. Success of establishing PDADX models is highly dependent on the quality of the starting material. The composition of cancer cells in the cellular component of ascites varies significantly among histological subtypes of PC and patients (Latifi et al., 2012), and can be as low as 0.1% of total cell count (Sheid, 1992). Low quantities of cancer cells in ascites may account for the relatively low success rates of establishing PDADX models (approximately 50%) in this protocol. Furthermore, the volume of ascites available for collection in the beginning is a limiting factor for the creation and utility of PDADX models. Cellular components of ascites are needed for implantation, genomic and morphological characterization, and cryopreserved stocks for further implantation, while the establishment, maintenance and drug efficiency evaluation processes require regular injection of cell-free ascites.
In addition, the cellular component of ascites contains a heterogenous mixture of cancer cells and stromal cells including epithelial / mesothelial cells, fibroblasts, adipocytes, and inflammatory cells (Hendrikson et al., 2022; Kim et al., 2016), which will form PDADX tumors with complex compositions. Although the cancer cells in PDADX tumors should exhibit similar genomic and morphological features to the matched patient’s cancer cells, variation in the compositions of different cell types generated in the process of tumor development are unavoidable among different PDADX passages and mice, which may lead to fluctuations in tumor burden when performing drug efficacy experiments.
Moreover, the establishment and maintenance of PDADX models are labor- and time-consuming. The establishment step (P0) takes up to 6 months post-implantation. During the passaging stage, PDADX tumor formation takes approximately 4–5 serial passages before the tumor development rate stabilizes to approximately 3–4 weeks (P4 to 5), depending on the aggressiveness of the tumor. Repeated intraperitoneal injection of 5% filter-sterilized cell-free ascites every three days is critical to create an ascitic microenvironment for PDADX tumors but is unrefutably a tedious process. Furthermore, the formation of PDADX tumor is determined by the subjective observation of changes in abdomen size and presence of tumor nodules (Figure 3B). Obvious symptoms are only visible when the PDADX tumors grow to substantial volumes over a long maintenance period.
Troubleshooting
Problem 1
Patient specimen is not sufficient for the establishment, maintenance and utility of PDADX models.
Potential solution
Collect a minimum of 500 mL of ascites at the beginning of surgery or during routine paracentesis. 500 mL of ascites contains approximately 5–10 mL of cellular component, which is sufficient for implantation into 5 mice (approximately 1 mL for 5 mice), genomic sequencing (100 μL), and cryopreserved stocks (at least 4 mL). The fluid component of 500 mL of ascites is sufficient for implantation (1 mL/specimen for 5 mice), serial passages of PDADX tumor (2 mL/passage for 5 mice), regular injection of cell-free ascites (3 mL/year for 5 mice), and drug efficacy evaluation experiments (1.4 mL/20-mice experiment, Table 1).
Problem 2
Success rates of establishing a PDADX model is relatively low.
Potential solution
Inject cellular components of ascites within 2 hours of specimen collection and implant into multiple mice. The successful growth of PDADX tumor depends on several factors, including but not limited to, cancer cell composition in the cellular component of ascites, number of viable cancer cells, growth ability of cancer cells in mice and aggressiveness of patient-specific tumor. Considering that intrinsic properties of patient cancer cells cannot be manipulated, success rates of PDADX establishment can only be improved by the immediate implantation to minimize loss of viable cancer cells over time.
Problem 3
There are no signs of tumor formation after implanting cellular components of ascites for 6 months.
Potential solution
Implant cellular components of ascites into multiple mice for each patient ascites samples. In the absence of any potential signs of an enlarged abdomen or presence of visible tumor nodules 6-months post-implantation, mice can be sacrificed to interrogate the existence of tumor. Depending on the different requirements of IACUC protocols, duration of maintaining implanted mice should be adjusted accordingly. In addition, adding fluorescent trackers and intravital imaging may potentially facilitate the process of establishing a PDADX model. These techniques have been reported in the literature (Christensen et al., 2015; Lee et al., 2018; Naumenko et al., 2016; Tanaka et al., 2012; Volpe et al., 2018; Winnard et al., 2006); however, the authors have not explored the utility of these techniques in the current protocol.
Problem 4
Experimental schedule of drug injection every 3 days of 7 doses is not suitable for some drugs.
Potential solution
Adjust drug injection schedule and duration of experiment to fit each drug’s appropriateness. For example, for drugs that remain efficacious after a long period of treatment, you may choose to reduce the implanted amount of PDADX tumors and elongate the duration of the experiment. For drugs requiring daily or weekly injections, you may choose to amend the injection schedule to the respective frequency. Although experimental designs can be customized, it is crucial to inject 10% FBS control or 5% matched filter-sterilized cell-free ascites every 3 days to create a control or ascitic microenvironment throughout the experiment.
Problem 5
A large standard deviation of tumor burden is observed in mice of the same group.
Potential solution
Prepare a master mix of PDADX tumors for all mice and implant into multiple mice. Slight variations in the composition of cancer cells and normal cells can be magnified during the growth of PDADX tumor in mice, which will lead to large standard deviations. To minimize these variations, PDADX tumors should be collected in 1 master mix tube, mixed well, aliquoted for each mouse, and subsequently resuspended in matched cell-free ascites. Additionally, more mice can be included for each group to achieve a more robust statistical analysis.
Problem 6
It is challenging to repeat drug efficacy evaluation because of varying growth rates between different passages of 1 PDADX model.
Potential solution
Prepare cryopreserved stocks of patient ascites cells and PDADX tumors of early passages as much as possible. Along with maintenance and serial passages, PDADX cancer cells may evolve and adapt to the environment of the intraperitoneal cavity of the mouse, which may affect aggressiveness and growth rates of tumors. To better reflect the properties of cancer cells in a patient’s abdomen and reduce the variations in tumor growth caused by long-term maintenance, early passages of PDADX tumors should be used for drug efficacy evaluation. Therefore, cryopreserved stock is of great importance to revive the early passages of PDADX tumors.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Chin-Ann Johnny Ong (johnny.ong.c.a@singhealth.com.sg).
Materials availability
This protocol did not generate new unique reagents.
Acknowledgments
This project is partially funded by the NCCS Cancer Fund (Research), NCC Research Fund as well as the SingHealth Duke-NUS Academic Medical Centre, facilitated by Joint Office of Academic Medicine (JOAM). As part of the Singapore Gastric Cancer Consortium, the study is also partially funded by the National Medical Research Council Open Fund-Large Collaborative Grant (OFLCG18May-0023). OCAJ is funded by the National Medical Research Council Clinician Scientist-Individual Research Grant (CIRG21jun-0038). All the funding sources had no role in the study design, data interpretation or writing of the manuscript.
Author contributions
Y.L., W.H.N., J.H., and W.K.L. designed and optimized the protocol. Y.L., W.H.N., H.-Y.Z., Q.X.T., J.W.-S.T., and G.N. wrote the manuscript. C.-A.J.O. supervised the research.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This protocol did not generate / analyze datasets.
References
- Christensen J., Vonwil D., Shastri V.P. Non-invasive in vivo imaging and quantification of tumor growth and metastasis in rats using cells expressing far-red fluorescence protein. PLoS One. 2015;10:e0132725. doi: 10.1371/journal.pone.0132725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikson J., Liu Y., Ng W.H., Lee J.Y., Lim A.H., Loh J.W., Ng C.C.Y., Ong W.S., Tan J.W.-S., Tan Q.X., et al. Ligand-mediated PAI-1 inhibition in a mouse model of peritoneal carcinomatosis. Cell Rep. Med. 2022;3:100526. doi: 10.1016/j.xcrm.2022.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimaktchiev V., Terracciano L., Tornillo L., Spichtin H., Stoios D., Bundi M., Korcheva V., Mirlacher M., Loda M., Sauter G., Corless C.L. The homeobox intestinal differentiation factor CDX2 is selectively expressed in gastrointestinal adenocarcinomas. Mod. Pathol. 2004;17:1392–1399. doi: 10.1038/modpathol.3800205. [DOI] [PubMed] [Google Scholar]
- Kim S., Kim B., Song Y.S. Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci. 2016;107:1173–1178. doi: 10.1111/cas.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver Y.L.B., Hendriks T., Lomme R.M.L.M., Rutten H.J.T., Bleichrodt R.P., de Hingh I.H.J.T. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery for peritoneal carcinomatosis in an experimental model. Br. J. Surg. 2010;97:1874–1880. doi: 10.1002/bjs.7249. [DOI] [PubMed] [Google Scholar]
- Latifi A., Luwor R.B., Bilandzic M., Nazaretian S., Stenvers K., Pyman J., Zhu H., Thompson E.W., Quinn M.A., Findlay J.K., Ahmed N. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One. 2012;7:e46858. doi: 10.1371/journal.pone.0046858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Park S.A., Zou Y., Seo S.U., Jun C.D., Lee W.J., Hyun Y.M., Cho N.H. Real-time monitoring of cancer cells in live mouse bone marrow. Front. Immunol. 2018;9:1681. doi: 10.3389/fimmu.2018.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli A., Tzankov A., Zlobec I., Terracciano L.M. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Mod. Pathol. 2008;21:1403–1412. doi: 10.1038/modpathol.2008.117. [DOI] [PubMed] [Google Scholar]
- Naumenko V., Jenne C., Mahoney D.J. Intravital microscopy for imaging the tumor microenvironment in live mice. Methods Mol. Biol. 2016;1458:217–230. doi: 10.1007/978-1-4939-3801-8_16. [DOI] [PubMed] [Google Scholar]
- Rubin B.P., Skarin A.T., Pisick E., Rizk M., Salgia R. Use of cytokeratins 7 and 20 in determining the origin of metastatic carcinoma of unknown primary, with special emphasis on lung cancer. Eur. J. Cancer Prev. 2001;10:77–82. doi: 10.1097/00008469-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Sheid B. Angiogenic effects of macrophages isolated from ascitic fluid aspirated from women with advanced ovarian cancer. Cancer Lett. 1992;62:153–158. doi: 10.1016/0304-3835(92)90186-Y. [DOI] [PubMed] [Google Scholar]
- Sugarbaker P.H. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin. Surg. Oncol. 1998;14:254–261. doi: 10.1002/(sici)1098-2388(199804/05)14:3<254::aid-ssu10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Morimoto Y., Toiyama Y., Matsushita K., Kawamura M., Koike Y., Okugawa Y., Inoue Y., Uchida K., Araki T., et al. In vivo time-course imaging of tumor angiogenesis in colorectal liver metastases in the same living mice using two-photon laser scanning microscopy. J. Oncol. 2012;2012:265487. doi: 10.1155/2012/265487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe A., Man F., Lim L., Khoshnevisan A., Blower J., Blower P.J., Fruhwirth G.O. Radionuclide-fluorescence reporter gene imaging to track tumor progression in rodent tumor models. JoVE. 2018:57088. doi: 10.3791/57088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnard P.T., Jr., Kluth J.B., Raman V. Noninvasive optical tracking of red fluorescent protein-expressing cancer cells in a model of metastatic breast cancer. Neoplasia. 2006;8:796–806. doi: 10.1593/neo.06304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This protocol did not generate / analyze datasets.