Abstract
Veterans who deployed in support of Operation Enduring Freedom (OEF), Iraqi Freedom (OIF), and New Dawn (OND) commonly experience severe psychological trauma, often accompanied by physical brain trauma resulting in mild traumatic brain injury (mTBI). Prior studies of individuals with posttraumatic stress disorder (PTSD) have revealed alterations in brain structure, accelerated cellular aging, and impacts on cognition following exposure to severe psychological trauma and potential interactive effects of military‐related mTBI. To date, however, little is known how such deployment‐related trauma changes with time and age of injury of the affected veteran. In this study, we explored changes in cortical thickness, volume, and surface area after an average interval of approximately 2 years in a cohort of 254 OEF/OIF/OND Veterans ranging in age from 19 to 67 years. Whole‐brain vertex‐wise analyses revealed that veterans who met criteria for severe PTSD (Clinician‐Administered PTSD Scale ≥60) at baseline showed greater negative longitudinal changes in cortical thickness, volume, and area over time. Analyses also revealed a significant severe‐PTSD by age interaction on cortical measures with severe‐PTSD individuals exhibiting accelerated cortical degeneration with increasing age. Interaction effects of comorbid military‐related mTBI within the severe‐PTSD group were also observed in several cortical regions. These results suggest that those exhibiting severe PTSD symptomatology have accelerated atrophy that is exacerbated with increasing age and history of mTBI.
Keywords: aging, cortical thickness, FreeSurfer, longitudinal, mild traumatic brain injury, posttraumatic stress disorder
1. INTRODUCTION
Posttraumatic stress disorder (PTSD), a psychiatric disorder marked by trauma re‐experiencing, avoidance behaviors, hyperarousal, and negative alterations in cognition and mood (American Psychiatric Association, 2013), has been diagnosed in approximately 20% of returning veterans from Operations Enduring Freedom (OEF), Iraqi Freedom (OIF), and New Dawn (OND) (Fulton et al., 2015). Over the last decade it has become increasingly clear that many individuals who receive a diagnosis of PTSD are also diagnosed with mild traumatic brain injury (mTBI). In some cases, both diagnoses are caused by the same combat‐related event, while in other cases, the conditions arise from largely independent circumstances. Previous work has suggested the occurrence of TBI in the often horrific circumstances of combat directly leads to a more severe form of psychological trauma (Vasterling et al., 2018), or perhaps the two sequelae co‐occur as the conditions simply have parallel physical and psychiatric consequences. Given the high prevalence of comorbid TBI and PTSD at 17%–35% (Fortier et al., 2014; Lindquist et al., 2017), the question of whether or not these two diagnoses have overlapping or similar neurobiological consequences becomes particularly salient and clinically relevant. An understanding of the neuropathological causes and consequences of PTSD and TBI is of great interest to researchers and clinicians given the high rates of associated disability (Lippa et al., 2015; Thomas et al., 2010), substance abuse (Petrakis et al., 2011), and suicide (Fonda et al., 2017; Kaplan et al., 2012).
Neuroimaging studies have reported associations between PTSD symptoms and structural integrity of a variety of brain regions (Crombie et al., 2021; Lindemer et al., 2013; Meng et al., 2014; O'Doherty et al., 2015; Sadeh et al., 2015; Sherin & Nemeroff, 2011; Wolf, Sadeh, et al., 2016; Wrocklage et al., 2017). Overall PTSD burden has also been demonstrated to be correlated with structural brain measures, with greater symptom severity associated with lower hippocampal and amygdala volumes (Akiki et al., 2017; Averill et al., 2017; Crombie et al., 2021; Hayes et al., 2017), reduced tissue microstructure in limbic and paralimbic brain regions (Sydnor et al., 2020), and widespread reductions in cortical thickness (Lindemer et al., 2013; Sadeh et al., 2015, 2016; Wrocklage et al., 2017). Although the mechanisms of these relationships are not clear, PTSD symptomatology has also been associated with accelerated age at the cellular level (Sadeh et al., 2016; Wolf, Logue, et al., 2016), and elevated risk for physical health conditions (Mysliwiec et al., 2013; Wolf & Morrison, 2017), which may manifest in brain atrophy and contribute to reported cognitive deficits in afflicted individuals (Mattson et al., 2019; Riley et al., 2019).
Despite compelling evidence of PTSD and TBI‐related structural brain alterations and accelerated aging, prior neuroimaging studies have been limited to cross‐sectional explorations. Furthermore, few studies have investigated the neural correlates and long‐term functional impairments associated with complex and severe PTSD subtypes and it remains unclear to what extent these marked alterations may manifest over time. More severe forms of PTSD have been associated with significant psychiatric comorbidities, worse physical and psychosocial health outcomes, worse interpersonal problems, quality of life at later time points, and neurobiological alterations (Harned et al., 2018; Hou et al., 2007; Mitchell et al., 2021; Mueser et al., 2015; Van Woudenberg et al., 2018; You et al., 2020). This suggests that elevated stress responses could accelerate PTSD‐related cortical alterations. These findings highlight the potential role of severe PTSD and comorbid mental illness in maintaining functional impairments and accelerating brain‐aging trajectories. As prospective studies aim to identify those at risk for PTSD‐related disorders to develop interventions, a longitudinal approach can help to elucidate brain‐aging trajectories with respect to chronicity and severity of trauma observed at baseline. The present study of a relatively young veteran population sought to identify spatial patterns of differential rates of change in brain morphometry in individuals with severe PTSD compared to less afflicted individuals, as well as to understand the further moderating effects of military‐related mTBI. The main goals of this study were to (a) examine how severe PTSD may alter neural health over a longitudinal period as measured by cortical thickness, volume, and surface area, and to (b) explore the impact of comorbid mTBI on the PTSD–brain relationships in a large sample of OEF/OIF/OND veterans.
We hypothesized that individuals with severe PTSD would show greater reductions in cortical integrity at follow‐up compared to less severe PTSD and no‐PTSD individuals, and further predicted that this cortical atrophy would show stronger negative associations when regressed against age. Although no prior study, to our knowledge, has examined the impact of comorbid PTSD and mTBI on neural health in a large well‐characterized veteran sample across a longitudinal interval, we predicted that a history of military‐related mTBI would further interact with stress to exacerbate the age‐related decline in cortical integrity.
2. MATERIALS AND METHODS
2.1. Participants
The participant sample included US veterans and service members recruited from the Translational Research Center for TBI and Stress Disorders (TRACTS) prospective longitudinal cohort study, a Department of Veterans Affairs (VA) Rehabilitation Research and Development supported TBI National Network Research Center based at VA Boston Healthcare System and the Michael E. DeBakey VA Medical Center in Houston,TX. The current analysis consisted of VA Boston participants only. Detailed information about recruitment, inclusion/exclusion criteria, and measures in the battery has been previously described (McGlinchey et al., 2017). Briefly, participants had been or would be deployed service members of OEF, OIF, and/or OND. Participants were recruited throughout the Boston Metropolitan area and nationwide through outreach at military‐associated events and media. At the time of this report, the study has characterized a sample of over 650 service members from the Boston cohort for a baseline evaluation, and over 400 of those veterans have returned for a follow‐up evaluation, which averages 24 months (standard deviation [SD] = 11; range = 10–94 months) post‐baseline visit. The data presented in this study reflect the baseline and follow‐up assessments for the first 650 participants who also performed an imaging session at both baseline and follow‐up, resulting in a sample size of 281. For those with imaging data, their return for follow‐up averaged 24 months (SD = 11; range 12–94 months).
Exclusion criteria included: (i) history of seizures unrelated to head injury; (ii) history of neurological illness other than TBI (Huntington's, Parkinson's, dementia etc.); (iii) current diagnosis of schizophrenia, bipolar, or other psychotic disorder; (iv) current active homicidal and/or suicidal ideation or intent requiring crisis intervention; (v) cognitive disorder due to general medical condition other than TBI; (vi) and unstable psychological diagnosis (e.g., suspected psychotic or personality disorder) that would interfere with accurate data collection, determined by consensus of at least three licensed clinical psychologists.
This sample was drawn from participants enrolled into the TRACTS National Network Research Center who were eligible for an MRI and successfully underwent a neuroimaging session at two time points including anatomical scans used to calculate cortical measures (n = 281). Participants were then excluded if they had sustained a moderate or severe traumatic brain injury during or post‐deployment (n = 4), or were missing any covariate used in the final model, including those without measures of combat exposure (n = 20) or anxiety (n = 12) resulting in a final sample size of 254 (mean interval = 24 months, SD = 11 months).
The VA Boston Healthcare System Institutional Review Board approved this study and participants provided written informed consent.
2.2. Clinical assessment
2.2.1. Posttraumatic stress disorder
Current (previous 30 days) and/or lifetime (pre‐military and post‐military deployment) diagnosis for PTSD and symptom severity were assessed using the Clinician‐Administered PTSD Scale for DSM‐IV (CAPS‐IV; Blake et al., 1995), the gold standard PTSD structured diagnostic interview corresponding to DSM‐IV criteria for PTSD. To allow for a better characterization of the severity of symptoms, a global score was computed by summing the frequency and intensity of clusters of symptoms B (Intrusion), C (Avoidance), and D (Hyperarousal) with a minimum possible score of 0 and maximum of 136. Diagnosis of PTSD was confirmed via consensus of at least three clinically trained doctoral‐level psychologists.
Severity score ranges have been proposed for interpreting the CAPS total: 0–19 = asymptomatic/few symptoms, 20–39 = mild PTSD/subthreshold, 40–59 = moderate PTSD, 60–79 = severe PTSD symptomatology, and >80 = extreme PTSD (Weathers et al., 2001). Participants in this study were divided into two groups based on their symptom severity score at their baseline visit. The severe‐PTSD group consisted of 88 individuals who had a CAPS current symptom severity score of 60 or greater (indicating severe PTSD symptomatology) at the time of their baseline visit. The nonsevere‐PTSD group was composed of 166 individuals with a CAPS score of less than 60. Note that some participants in the nonsevere‐PTSD group had a lifetime history of PTSD (pre‐ or post‐deployment; n = 43), and/or met DSM‐IV criteria at baseline (n = 52), but none had a current symptom severity score of at least 60. Individuals in lower score ranges were heterogenous in their ultimate PTSD diagnosis and those who did meet DSM‐IV criteria with CAPS under 60 were more variable in their current reported PTSD severity. Therefore, rather than exclude these subjects and potentially underpower the study, they were included in the nonsevere‐PTSD group. Follow‐up analyses were performed comparing the severe‐PTSD group to no‐PTSD to validate findings.
2.2.2. Military blast exposure and mTBI
TBI and blast history were evaluated by a clinical psychologist using the Boston Assessment of TBI‐Lifetime (BAT‐L; Fortier et al., 2014). The BAT‐L queries participants about blast exposure (regardless of TBI diagnosis) and TBIs that occurred before, during, and after military experience. TBI diagnosis was determined via consensus according to Department of Defense and the American Congress of Rehabilitation Medicine standard criteria (Fortier et al., 2014). The majority of military TBIs experienced were mild, defined as a loss‐of‐consciousness lasting less than 30 min; and/or altered mental status; and/or posttraumatic amnesia lasting less than 24 h. Three participants had moderate TBIs that occurred prior to their military deployment and were included in the final sample. For the purpose of this study, only moderate TBIs that occurred during or post‐military deployment were excluded.
2.2.3. Demographic and other assessments
Demographic and deployment information was collected using self‐report questionnaires. Several secondary analyses were conducted to assess the effects of potential confounders. These were defined as follows: premorbid IQ was estimated with the Wechsler Test of Adult Reading (Wechsler, 2001); combat exposure was assessed using the Deployment Risk and Resilience Inventory Combat Exposure Scale (King et al., 2006); depression and anxiety severity scores were measured using the short form version of the Depression Anxiety Stress Scale (DASS‐21; Nieuwenhuijsen et al., 2003); influence of alcohol consumption was examined using the Lifetime Drinking History Scale (LDH; Skinner & Sheu, 1982). A number of secondary analyses were conducted to assess the effects of potential confounders related to conditions that are often comorbid in individuals with a history of PTSD and TBI.
2.3. Image acquisition, processing, analysis
2.3.1. Acquisition
Neuroimaging data were acquired on a 3T Siemens TIM Trio scanner at baseline and follow‐up and after scanner upgrade with a Magnetom Prismafit scanner (Trio‐upgrade). One hundred forty‐three subjects' data were acquired with the TIM Trio for both time points (severe‐PTSD+ = 47, severe‐PTSD− = 96), 46 subjects' were acquired with Magnetom Prismafit scanner for both time points (severe‐PTSD+ = 17, severe‐PTSD− = 29), and 65 subjects' data were acquired across scanner upgrade (severe‐PTSD+ = 24, severe‐PTSD− = 41). The TIM Trio data were collected with a 12‐channel‐phased array head coil, and the Prismafit data were collected with a 20‐channel head coil. The acquisition on both scanners included two‐high resolution whole‐brain T1‐weighted images using Magnetization‐Prepared Rapid Gradient Echo (MP‐RAGE) volumes with approximately matched parameters across upgrade (Trio: TR/TE = 2.53 s/3.32 ms, Prismafit: TR/TE = 2.53 s/3.35 ms, flip angle = 7°, 1 mm isotropic). Scans were 3D sagittal acquisitions with 176 contiguous slices (imaging matrix = 256 × 176, in‐plane resolution = 1 mm, slice thickness = 1 mm). The two MP‐RAGE volumes were averaged to create a single high‐contrast‐to‐noise image. A second MP‐RAGE acquisition was unavailable for four individuals at baseline, and 10 individuals at follow‐up, and therefore structural measures were quantified from a single MP‐RAGE scan for those participants.
2.3.2. Processing
All T1‐weighted images were visually inspected for motion artifact and gray–white contrast. Cortical reconstruction and volumetric segmentation was performed with version 7.1 of the FreeSurfer image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu). The technical details of these procedures are described in prior publications (Dale et al., 1999; Dale & Sereno, 1993; Fischl & Dale, 2000; Fischl et al., 2001, 2002; Fischl, Salat, et al., 2004; Fischl et al., 1999; Fischl, van der Kouwe, et al., 2004; Han et al., 2006; Jovicich et al., 2006; Reuter et al., 2010, 2012; Ségonne et al., 2004). Briefly, this processing includes motion correction and averaging of multiple volumetric T1‐weighted images (when more than one is available) (Reuter et al., 2010), removal of nonbrain tissue using a hybrid watershed/surface deformation procedure automated Talairach transformation (Ségonne et al., 2004), segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) (Fischl et al., 2002; Fischl, Salat, et al., 2004), intensity normalization (Sled et al., 1998), tessellation of the gray matter white matter boundary, automated topology correction (Fischl et al., 2001; Ségonne et al., 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al., 1999; Dale & Sereno, 1993; Fischl & Dale, 2000). FreeSurfer morphometric procedures have been demonstrated to show good test–retest reliability across scanner manufacturers and across field strengths (Han et al., 2006; Reuter et al., 2012). After all subjects were run through the standard processing stream, the data were manually inspected and edited for accuracy of the gray/white and gray/pial surfaces. Images were then run through second reconstruction, beginning at the point where edits were applied.
To extract reliable longitudinal cortical volume, thickness, and area change estimates, images were run through the longitudinal stream of FreeSurfer version 7.1 (Reuter et al., 2012). Specifically, an unbiased within‐subject template space and image is created based on the two cross‐sectional images created for each participant using robust, inverse consistent registration (Reuter et al., 2010). Using this method, a within‐subject template is referenced to enforce consistent segmentation results across time points, thereby reducing the confounding effects associated with longitudinal analysis (Reuter & Fischl, 2011). Surface maps were resampled, transformed via spherical registration to a common surface space, and smoothed using a circulatory symmetric Gaussian kernel with a full width half maximum (FWHM) of 20 mm (Fischl et al., 1999).
2.3.3. Statistical analysis
Vertex‐wise general linear models were performed using FreeSurfer 7.1 (http://surfer.nmr.mgh.harvard.edu), and hierarchical regression modeling and plots were produced with R (R Core Team, 2018). To limit the interindividual variability in scan intervals, longitudinal change in cortical thickness, volume, and area for each hemisphere was calculated as an annualized measure of the symmetrized percent change (i.e., the annual rate of change with respect to the average thickness/volume/area measure across the two time points). Statistical correction for multiple comparisons was performed using a Monte Carlo simulation‐based cluster‐wise procedure to determine the distribution of the maximum cluster size under the null hypothesis as described previously (Hagler et al., 2006). Five‐thousand iterations of simulations were performed, using a threshold of p = .05 and a FWHM of 20 for each analysis.
In addition to whole‐brain vertex‐wise analyses, regions of interest (ROIs) were created based on regions showing a significant effect in the whole‐brain analyses comparing cortical change between groups and group‐by‐age interactions. Maps were corrected for multiple comparisons and with a threshold of p < 0.05. For ROI‐based analyses, mean annual percent change of the thickness, volume, and area, within the ROI from each analysis was calculated for all participants and used as a dependent variable. The whole‐brain voxel‐wise analyses with the following covariates are included in the final results: education, combat exposure, lifetime estimated alcohol use (LDH total weight corrected), anxiety severity, history of military TBI, and gender.
2.4. Data availability
Data will be made available via controlled public access mechanisms, primarily through data use/transfer agreements. The requester and data custodian will comply with all VA requirements regarding HIPAA, The Privacy Act of 1974, Records Management, and Data Use/Transfer directives. Code will be made available upon request.
3. RESULTS
3.1. Group differences
Demographics for the sample are reported in Table 1. Unpaired two‐sample t‐tests revealed no group differences in age (t(182) = 1.04, p = .30), time interval between scans (t(195) = 0.9, p = .37), IQ (t(185) = 1.57, p = .118), or total number or duration of deployments (t(159) = −0.66, p = .508; t(170) = −0.98, p = .327). There were no group differences in age, interval between scans, or combat exposure for group‐by‐scanner pairings (i.e., Trio‐to‐Trio, Prismafit‐to‐Prismafit, and Trio‐to‐Prismafit). Pearson's χ 2 tests revealed no significant group differences in gender ( 2(1) = 0.92, p = .337), race ( 2(1) = 2.92, p = .087), scanner at baseline ( 2(1) = 0.04, p = .847) or scanner at follow‐up ( 2(1) = 0.3, p = .587). Gender and race were not significantly different between groups for scanner pairings. As expected, groups differed on CAPS score at both baseline t(237) = −25.28, p < .001) and follow‐up (t(170) = −11.27, p < .001), and groups also differed significantly on education (t(230) = 2.71, p = .007), anxiety (t(112) = −8.93, p < .001), depression (t(126) = −7.99, p < .001), and stress (t(147) = −10.49, p < .001), combat exposure t(166) = −4.25, p < .001), presence of military TBI (χ 2(1) = 26.13, p < .001), and lifetime estimated alcohol consumption (LDH total weight corrected; t(167) = −2.57, p = .011). Education, IQ, and frequency of deployments were significantly different between groups for those scanned within Trio for both time points, and across scanner upgrade, but not within Prismafit for both time points. Occurrence of military mTBI was not significantly different between groups for those scanned on the Prismafit for both time points.
TABLE 1.
Demographic and clinical participant characteristics
| Severe‐PTSD− (N = 166) | Severe‐PTSD+ (N = 88) | Total (N = 254) | Statistic | |
|---|---|---|---|---|
| Age T2, M (SD) | 35.5 (8.63) | 34.4 (8.40) | 35.1 (8.55) | t(182) = 1.04, p = .30 |
| Years since baseline, M (SD) | 2.05 (0.96) | 1.94 (0.86) | 2.01 (0.93) | t(195) = 0.9, p = .37 |
| Male, n (%) | 156 (94.0%) | 79 (89.8%) | 235 (92.5%) | 2(1) = 0.92, p = .337 |
| Caucasian, n (%) | 116 (69.9%) | 71 (80.7%) | 187 (73.6%) | 2(1) = 2.92, p = .087 |
| Education, M (SD) | 14.2 (2.13) | 13.6 (1.53) | 14.0 (1.96) | t(230) = 2.71, p = .007** |
| IQ, M (SD) a | 105 (12.0) | 102 (11.5) | 104 (11.9) | t(185) = 1.57, p = .118 |
| Number of deployments, M (SD) | 1.51 (0.94) | 1.65 (0.99) | 1.56 (0.96) | t(159) = −0.66, p = .508 |
| Duration of deployments (months), M (SD) | 14.7 (9.12) | 15.9 (9.58) | 15.1 (9.28) | t(170) = −0.98, p = .327 |
| CAPS T1, M (SD) | 29.8 (18.4) | 79.2 (12.5) | 47.0 (28.8) | t(237) = −25.28, p < .001** |
| CAPS T2, M (SD) | 30.6 (23.1) | 66.1 (24.2) | 42.9 (28.9) | t(170) = −11.27, p < .001** |
| DASS anxiety total, M (SD) | 3.47 (4.64) | 12.6 (8.99) | 6.64 (7.80) | t(112) = −8.93, p < .001** |
| DASS depression total, M (SD) | 5.46 (6.59) | 15.1 (10.3) | 8.81 (9.28) | t(126) = −7.99, p < .001** |
| DASS stress total, M (SD) | 8.60 (7.57) | 20.9 (9.50) | 12.9 (10.1) | t(147) = −10.49, p < .001** |
| Combat exposure, M (SD) | 13.8 (11.1) | 20.4 (12.0) | 16.1 (11.9) | t(166) = −4.25, p < .001** |
| Presence of military mTBI, n (%) | 56 (33.7%) | 60 (68.2%) | 116 (45.7%) | 2(1) = 26.13, p < .001** |
| LDH total weight corrected, M (SD) | 1860 (2350) | 2690 (2530) | 2150 (2440) | t(167) = −2.57, p = .011* |
| Baseline Scanner (Trio), n (%) | 137 (82.5%) | 71 (80.7%) | 208 (81.9%) | 2(1) = 0.04, p = .847 |
| Follow‐up Scanner (Trio), n (%) | 96 (57.8%) | 47 (53.4%) | 143 (56.3%) | 2(1) = 0.3, p = .587 |
Abbreviations: CAPS, Clinician‐Administered PTSD Scale; DASS, Depression Anxiety Stress Scales; LDH, Lifetime Drinking History; mTBI, mild traumatic brain injury; PTSD, posttraumatic stress disorder; WTAR, Wechsler Test of Adult Reading.
IQ data were not available for one subject (severe‐PTSD− = 165, severe‐PTSD+ = 88).
p < .05.
p < .01.
3.2. Whole‐brain vertex‐wise analyses
General linear model analysis across the entire cohort revealed significant regional annual percent change between time points for the three cortical measures: thickness, volume, and area (see Figure S1) demonstrating expected longitudinal reductions in all three measures. Analyses revealed a significant group difference in severe‐PTSD on cortical measures, with the severe‐PTSD group exhibiting greater negative change in cortical measures at follow‐up, however, no regions survived correction for multiple comparisons. An uncorrected significance map of these group differences can be found in Figure S2. Additional analyses revealed a significant bilateral severe‐PTSD and age interaction on annual cortical change, of which the ROIs that survived multiple comparisons correction are presented in Figure 1. Uncorrected results can be found in Figure S3. ROIs of mean annualized cortical change were created encompassing regions that survived correction, including the left rostral middle frontal gyrus and precuneus, and right superior frontal and paracentral gyri. Figure 2 presents scatterplots of interactions between age and mean annual percent change for each region. Follow‐up analyses of the severe‐PTSD group (n = 88) against individuals who did not meet criteria for PTSD at baseline (n = 114) yielded statistically similar results.
FIGURE 1.
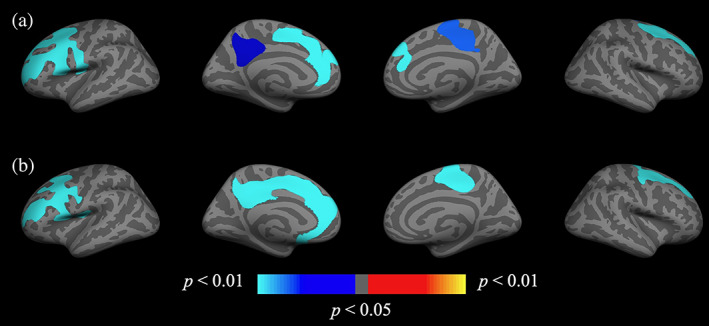
Group (severe‐PTSD+ and severe‐PTSD−) by age interaction on cortical change. Whole‐brain vertex‐wise analysis of the severe‐PTSD × age interaction on annual percent change in (a) cortical thickness and (b) volume. Clusters remained significant following cluster correction (p < .05). Maps are presented on the inflated cortical surface of an average brain. Blue indicates a more negative association between age and annual percent change in the severe‐PTSD+ group, red/yellow indicates a positive relationship. Uncorrected maps can be found in the supplemental information (Figure S3). PTSD, posttraumatic stress disorder
FIGURE 2.
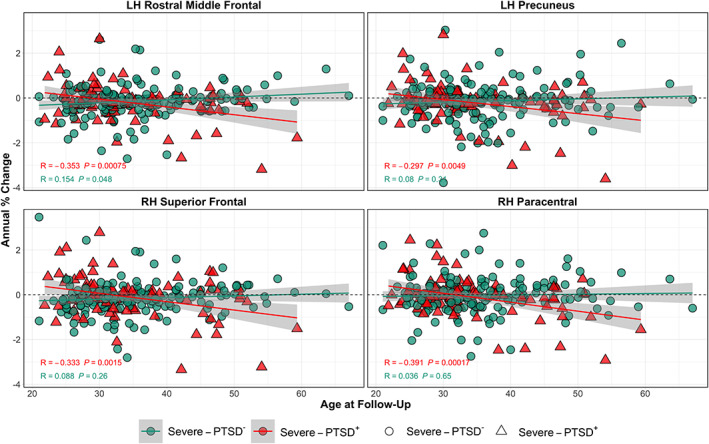
Regions in which the annual percent change of cortical measures with age shows more rapid decline with severe‐PTSD. Plots of annual percent change in cortical thickness in the left rostral middle frontal gyrus and precuneus, and right superior frontal and paracental gyri by age. Scatter plots are color coded with red triangles and green circles corresponding to severe‐PTSD+ and severe‐PTSD−, respectively. PTSD, posttraumatic stress disorder
In addition, changes were made to the model to account for other potential confounders. The interaction of severe‐PTSD and age accounted for a significant amount of variance for measures of cortical change in thickness and volume after accounting for covariates including education, combat exposure, alcohol use, anxiety severity, military mTBI history, and gender. Education and history of military‐related mTBI accounted for some variance in observed cortical changes, however, results were largely insensitive to inclusion of covariates that were included in the final results. Neither the substitution of depression nor stress severity for anxiety could account for the results presented. Standard least squares regression models were performed for the association of age by PTSD group on cortical change in each of the corrected regions depicted in Figure 2, these statistics can be found in Table S1. Follow‐up analyses were performed comparing the severe PTSD group to the nonsevere‐PTSD and no‐PTSD groups separately and results were statistically similar. Rather than exclude the nonsevere‐PTSD individuals and underpower the study, these individuals were included in the final model.
3.3. Interactions with mTBI
To determine whether military‐related mTBI influenced associations between age and annual percent change in cortical measures, the severe‐PTSD group (n = 88) was divided into two subgroups based on a history of mTBI; PTSD‐only (n = 28) and comorbid PTSD/mTBI (n = 60). Demographics for the subgroups are reported in Table 2. These groups did not differ significantly in age (t(45) = 1.66, p = .103), time interval between scans (t(79) = −1.25, p = .215), education (t(51) = 1.55, p = .128), IQ (t(41) = −0.09, p = .927), deployment frequency/duration (t(76) = −1.23, p = .221; t(72) = −1.05, p = .295), CAPS score at baseline or follow‐up (t(54) = −0.59, p = .556; t(51) = −0.07, p = .945), anxiety (t(54) = −1.16, p = .25), depression (t(46) = 0.97, p = .337), or stress (t(56) = −0.07, p = .945), or LDH total lifetime consumption weight corrected (t(41) = 0.4, p = .689). They did, however, differ significantly on combat exposure (t(54) = −3.61, p < .001), race ( 2(1) = 5.62, p = .018), gender ( 2(1) = 12.26, p = .001), and scanner at baseline ( 2(1) = 5.62, p = .018), but not scanner at follow‐up ( 2(1) = 0.5, p = .478. The whole‐brain voxel‐wise analyses were performed with the following covariates: education, combat exposure, alcohol use, anxiety severity, and gender.
TABLE 2.
Demographic and clinical characteristics for subgroup by military mTBI history
| PTSD‐only (N = 28) | PTSD + mTBI (N = 60) | Total (N = 88) | Statistic | |
|---|---|---|---|---|
| Age T2, M (SD) | 36.7 (9.37) | 33.3 (7.75) | 34.4 (8.40) | t(45) = 1.66, p = .103 |
| Years since baseline, M (SD) | 1.80 (0.597) | 2.01 (0.956) | 1.94 (0.860) | t(79) = −1.25, p = .215 |
| Male, n (%) | 20 (71.4%) | 59 (98.3%) | 79 (89.8%) | 2(1) = 12.26, p < .001** |
| Caucasian, n (%) | 18 (64.3%) | 53 (88.3%) | 71 (80.7%) | 2(1) = 5.62, p = .018* |
| Education, M (SD) | 13.9 (1.56) | 13.4 (1.50) | 13.6 (1.53) | t(51) = 1.55, p = .128 |
| IQ, M (SD) | 102 (14.0) | 102 (10.3) | 102 (11.5) | t(41) = −0.09, p = .927 |
| Number of deployments, M (SD) | 1.50 (0.745) | 1.75 (1.13) | 1.67 (1.03) | t(76) = −1.23, p = .221 |
| Duration of deployments (months), M (SD) | 14.5 (7.41) | 16.6 (10.4) | 15.9 (9.58) | t(72) = −1.05, p = .295 |
| CAPS T1, M (SD) | 78.1 (12.3) | 79.8 (12.6) | 79.2 (12.5) | t(54) = −0.59, p = .556 |
| CAPS T2, M (SD) | 65.9 (24.9) | 66.3 (24.1) | 66.1 (24.2) | t(51) = −0.07, p = .945 |
| DASS anxiety total, M (SD) | 11.0 (8.84) | 13.4 (9.04) | 12.6 (8.99) | t(54) = −1.16, p = .25 |
| DASS depression total, M (SD) | 16.8 (11.4) | 14.4 (9.76) | 15.1 (10.3) | t(46) = 0.97, p = .337 |
| DASS stress total, M (SD) | 20.8 (9.18) | 20.9 (9.72) | 20.9 (9.50) | t(56) = −0.07, p = .945 |
| Combat exposure, M (SD) | 14.1 (11.1) | 23.4 (11.4) | 20.4 (12.0) | t(54) = −3.61, p < .001** |
| LDH total weight corrected, M (SD) | 2870 (3050) | 2610 (2260) | 2690 (2530) | t(41) = 0.4, p = .689 |
| Baseline Scanner (Trio), n (%) | 18 (64.3%) | 53 (88.3%) | 71 (80.7%) | 2(1) = 5.62, p = .018* |
| Follow‐up Scanner (Trio), n (%) | 17 (60.7%) | 30 (50.0%) | 47 (53.4%) | 2(1) = 0.5, p = .478 |
Abbreviations: CAPS, Clinician‐Administered PTSD Scale; DASS, Depression Anxiety Stress Scales; LDH, Lifetime Drinking History; mTBI, mild traumatic brain injury; PTSD, posttraumatic stress disorder; WTAR, Wechsler Test of Adult Reading.
p < .05.
p < .01.
A whole‐brain vertex‐wise analysis revealed interactive effects between subgroup and age on cortical measures. An uncorrected significance map of this analysis, and statistics of a least squares regression model can be found in Figure S4 and Table S2, respectively. Regions surviving multiple comparisons correction are presented in Figure 3. Although slightly overlapping with regions in the previous analysis including all participants, significantly greater longitudinal reduction with age within the PTSD + mTBI group in thickness was observed in the left rostral middle frontal gyrus and isthmus cingulate. ROIs of annualized percent change in thickness were created encompassing these regions. Figure 4 presents scatterplots for this analysis.
FIGURE 3.
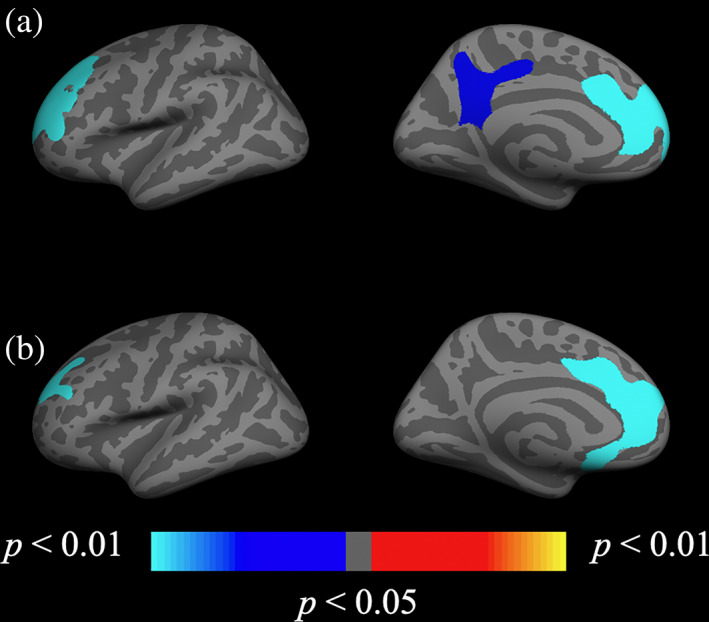
Subgroup (severe‐PTSD with mTBI and severe‐PTSD only) by age interaction on cortical change significance maps. Whole‐brain vertex‐wise analysis of the severe‐PTSD + mTBI subgroup × age interaction on annual percent change in (a) cortical thickness and (b) volume. Clusters remained significant following cluster correction (p < .05). Maps are presented on the inflated cortical surface of an average brain. Blue indicates a more negative association between age and annual percent change in the severe‐PTSD+ group, red/yellow indicates a positive relationship. Uncorrected maps can be found in the supplemental information (Figure S4). mTBI, mild traumatic brain injury; PTSD, posttraumatic stress disorder
FIGURE 4.
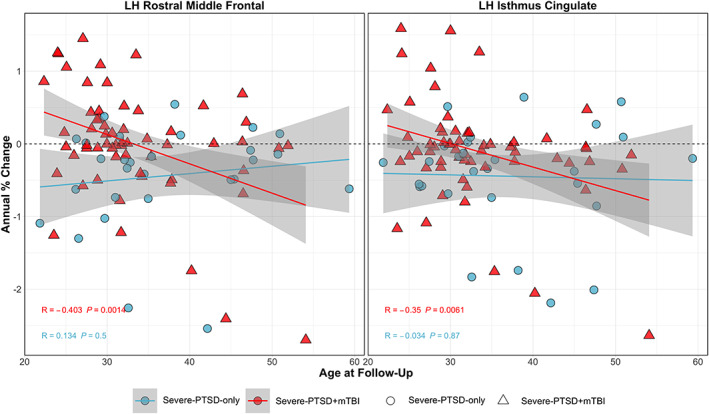
Regions in which the annual percent change of cortical measures with age shows more rapid decline with comorbid severe‐PTSD and military mTBI. Plots of annual percent change in cortical thickness in the left rostral middle frontal gyrus and isthmus cingulate by age. Scatter plots are color coded with red triangles and blue circles corresponding to comorbid PTSD + mTBI and PTSD‐only, respectively. Linear models of annual percent change versus age within each group are corrected (p < .05). mTBI, mild traumatic brain injury; PTSD, posttraumatic stress disorder
4. DISCUSSION
The current study of a cohort of post‐9/11 veterans found that severe PTSD and a history of military mTBI were important factors impacting cortical changes over time. Even in this sample of predominately young individuals, those who reported greater PTSD symptom severity at their baseline visit showed statistically significant bilateral negative annual change in cortical thickness, volume, and surface area across several regions. Broader reductions in all cortical measures were observed in severe‐PTSD when regressed against age, and these were strongest in frontal and cingulate regions. Furthermore, individuals with comorbid PTSD and military mTBI showed stronger associations between age and cortical change compared to those with PTSD alone, which may suggest a dose–response relationship, wherein history of mTBI interacts with PTSD burden, contributing to greater stress‐induced neurodegeneration. Results highlight the need for longitudinal assessments to evaluate the impact of traumatic stress and further modulating effects of mTBI on cortical changes.
To our knowledge, this is the first study to explore the impact of PTSD and mTBI on cortical structural integrity over a longitudinal period in a large veteran sample. However, the prominence of frontal region alterations observed in this study is consistent with prior cross‐sectional studies showing PTSD severity to be associated with reductions in frontal region thickness and microstructural integrity via DNA methylation and other metabolic factors (Sadeh et al., 2016; Wolf, Logue, et al., 2016; Wolf, Sadeh, et al., 2016), widespread cortical thinning associated with chronicity and severity of lifetime PTSD (Clausen et al., 2020; Geuze et al., 2008; Lindemer et al., 2013; Woodward et al., 2009; Wrocklage et al., 2017), and the further impact of comorbid mTBI on these neurobiological alterations (Clausen et al., 2020; Lindemer et al., 2013; Savjani et al., 2017; Sydnor et al., 2020).
Although findings show strong evidence for the impact of severe PTSD and mTBI on cortical integrity over a longitudinal period, we also observed a clinically significant reduction in current reported PTSD symptom severity within the severe‐PTSD group at follow‐up (15‐point change in CAPS total severity; Weathers et al., 2001). The majority of those in the severe‐PTSD group, however, still met DSM‐IV criteria for PTSD (n = 71; 80.68%) despite reduction in symptom severity and remained above the severe/extreme symptomatology threshold (n = 53; 60.22%). Despite possible improvement of PTSD symptom severity in these individuals, we observed significant reductions in morphometry over time. This suggests that an elevated stress response may be sufficient to initiate neurobiological consequences analogous to accelerated aging. However, the degree to which fluctuations in symptom severity are reflected in differential rates of cortical change remain unclear, and additional longitudinal data is needed to assess this relationship directly. Additional time points will also be necessary to determine a threshold at which PTSD symptom severity precipitates these mechanistic cortical changes. Future research would benefit from long‐term treatment tracking and PTSD symptom measurement to determine cortical markers of remission associated with varying levels of persistence of PTSD symptomatology.
Furthermore, mTBI in this study was defined as the experience of loss‐of‐consciousness and/or altered mental status and/or a period of posttraumatic amnesia at the time of injury. A significant number of service members, however, experience multiple head injuries and/or military‐associated blast exposures that did not meet diagnostic criteria for mTBI diagnosis, which have yet to be examined longitudinally. Therefore, future work would benefit from exploring the potentially interactive effects of blast and other subconcussive head injuries on brain structure. Finally, the observed effects indicate that history of mTBI may be an important consideration for determining appropriate course of treatment and predicting clinical outcomes among veterans with severe PTSD.
The findings in this study contribute to the literature of PTSD‐related accelerated cortical aging by demonstrating enhanced age‐related decline in cortical integrity due to the burden of PTSD, which is further exacerbated by mTBI‐related stress in a longitudinal cohort. These findings also suggest that there are additional health factors to consider. PTSD and mTBI have been linked to other physical health conditions, therefore future work must consider how various other behavioral health factors confer risk for neurodegeneration and cognitive decline (Mysliwiec et al., 2013; Wolf & Morrison, 2017; Wolf, Sadeh, et al., 2016). Previous studies have also found the complex and dissociative PTSD subtypes present with higher PTSD severity (Roca et al., 2006; Wolf et al., 2017). Although we did not have sufficient information to determine the subtypes in this cohort, the results presented here could inform future studies directly investigating the neural correlates of various forms of PTSD (e.g., severe, complex, dissociative PTSD). Future work would require exploring relationships between longitudinal cortical changes and functional outcomes in patients with varying severity of PTSD to assess the impact of chronic and severe trauma, complex trauma histories, and multiple comorbidities. Additional studies with larger sample sizes are also necessary to validate these early findings and assess their generalizability.
4.1. Limitations
Although these results are consistent with cross‐sectional findings in the literature, the following limitations should be considered. The time interval between scans ranged from 1 year to 8 years. Groups were approximately matched for follow‐up interval and the interval duration was annualized to reduce variability, however, we cannot rule out that high interval variability may have influenced the results. Furthermore, as we only have two time points available and no prior study has reported data from more than two time points, we can only speculate about the impact of the interval variability. Although our results are consistent with previous work showing decreased brain structural integrity with PTSD, this is caveated by the fact that these results are based on data from generally young combat veterans and may not reflect the general population. Future work would benefit from longitudinal meta‐analyses to identify the genetic and neurodevelopmental factors that predispose individuals to develop PTSD, as well as the timing and etiology of the other neurobiological factors of PTSD. In addition, this study examined severe PTSD, which does not consider the potentially heterogeneous nature of the condition and symptom expression. A more dimensional approach to PTSD symptoms will be important for populations with subdiagnostic threshold PTSD symptoms that may still be associated with intermediate levels of PTSD‐related neurological changes. Finally, we cannot rule out the impact of scanner upgrade and the potential influence of interscanner variation on cortical change estimations.
5. CONCLUSION
This study demonstrated the longitudinal neural impact of severe PTSD symptomatology. Results indicate that history of mTBI may further exacerbate the associations between brain alterations and stress‐induced neurodegeneration in individuals with PTSD. Our findings highlight the need for identifying those at risk for PTSD and mTBI‐related morbidities to develop effective early treatment methods to prevent and/or reverse the neurobiological and medical consequences of elevated stress. This study also supports the need for further research employing longitudinal design, as it could be utilized in the identification of neuroimaging biomarkers as well as in long‐term monitoring of treatment mechanisms.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Figure S1 Significant regions of annual percent change in thickness, volume, and area between time points. Regions of a significant (p < 0.05) annual percent change in cortical measures (thickness, volume, and area) across the entire cohort presented on the semi‐folded cortical surface. Blue indicates a more negative annual percent change at follow‐up, red/yellow indicates a positive change.
Figure S2. Group difference significance maps (severe‐PTSD + and severe‐PTSD − ). Regions of significant (p < 0.05) group difference of severe‐PTSD on annual percent change of cortical measures [a) thickness, b) volume, and c) area]. Maps are presented on the inflated cortical surface of an average brain. The bottom color scale represents the statistical significance of the correlation with light blue and yellow indicating the most significant regions; blue indicates a more negative annual percent change in severe‐PTSD individuals, red/yellow indicates a positive change.
Figure S3. Group by age interaction significance maps (severe‐PTSD + and severe‐PTSD − ). Regions of a significant (p < 0.05) severe‐PTSD × age interaction on annual percent change in cortical measures [a) thickness, b) volume, and c) area]. Maps are presented on the inflated cortical surface of an average brain. Blue indicates a more negative association between age and annual percent change in the severe‐PTSD+ group, red/yellow indicates a positive relationship.
Figure S4. Subgroup by age interaction significance maps. Regions of a significant (p < 0.05) comorbid military mTBI and PTSD × age interaction of annual percent change in cortical measures [a) thickness, b) volume, and c) area]. Blue indicates a more negative association between age and annual percent change in the comorbid mTBI/PTSD group, red/yellow indicates a positive relationship.
Table S1. Statistics for standard least squares regressions run with interactions between PTSD and age on annual percent change in the 4 ROIs depicted in Figure 2 of the main text. B values are effect sizes and SE values are standard error for the effect sizes. Reported F values are Fischer's statistics and R2 values are coefficients of determination. DASS = Depression Anxiety Stress Scales; LDH = lifetime drinking history. *p < 0.05, **p < 0.01, ***p < 0.001
Table S2. Statistics for standard least squares regressions run with interactions between PTSD+mTBI subgroup and age on annual percent change in the 2 ROIs depicted in Figure 4 of the main text. B values are effect sizes and SE values are standard error for the effect sizes. Reported F values are Fischer's statistics and R2 values are coefficients of determination. DASS = Depression Anxiety Stress Scales; LDH = lifetime drinking history. *p < 0.05, **p < 0.01, ***p < 0.001
ACKNOWLEDGMENTS
The authors would like to thank Walter Musto CMSgt (Ret), RING for his tireless efforts in recruiting participants on our behalf and the team of investigators at TRACTS for their assistance with data collection and management. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. This research was supported by the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B3001‐C). This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System
Brown, E. M. , Salat, D. H. , Milberg, W. P. , Fortier, C. B. , & McGlinchey, R. E. (2022). Accelerated longitudinal cortical atrophy in OEF/OIF/OND veterans with severe PTSD and the impact of comorbid TBI . Human Brain Mapping, 43(12), 3694–3705. 10.1002/hbm.25877
Funding information U.S. Department of Veterans Affairs, Grant/Award Number: B3001‐C
DATA AVAILABILITY STATEMENT
Data will be made available via controlled public access mechanisms, primarily through data use/transfer agreements. The requester and data custodian will comply with all VA requirements regarding HIPAA, The Privacy Act of 1974, Records Management, and Data Use/Transfer directives. Code will be made available upon request.
REFERENCES
- Akiki, T. J. , Averill, C. L. , Wrocklage, K. M. , Schweinsburg, B. , Cobb Scott, J. , Martini, B. , Averill, L. A. , Southwick, S. M. , Krystal, J. H. , & Abdallah, C. G. (2017). The association of PTSD symptom severity with localized hippocampus and amygdala abnormalities. Chronic Stress, 1, 2470547017724069. 10.1177/2470547017724069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders: DSM‐5. American Psychiatric Association. [Google Scholar]
- Averill, L. A. , Abdallah, C. G. , Pietrzak, R. H. , Averill, C. L. , Southwick, S. M. , Krystal, J. H. , & Harpaz‐Rotem, I. (2017). Combat exposure severity is associated with reduced cortical thickness in combat veterans: A preliminary report. Chronic Stress, 1, 247054701772471. 10.1177/2470547017724714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. D. , Weathers, F. W. , Nagy, L. M. , Kaloupek, D. G. , Gusman, F. D. , Charney, D. S. , & Keane, T. M. (1995). The development of a clinician‐administered PTSD scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/bf02105408 [DOI] [PubMed] [Google Scholar]
- Clausen, A. N. , Clarke, E. , Phillips, R. D. , Haswell, C. , VA Mid‐Atlantic MIRECC Workgroup , & Morey, R. A. (2020). Combat exposure, posttraumatic stress disorder, and head injuries differentially relate to alterations in cortical thickness in military veterans. Neuropsychopharmacology, 45(3), 491–498. 10.1038/s41386-019-0539-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie, K. M. , Ross, M. C. , Letkiewicz, A. M. , Sartin‐Tarm, A. , & Cisler, J. M. (2021). Differential relationships of PTSD symptom clusters with cortical thickness and grey matter volumes among women with PTSD. Scientific Reports, 11, 1825. 10.1038/s41598-020-80776-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, A. M. , Fischl, B. , & Sereno, M. I. (1999). Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Dale, A. M. , & Sereno, M. I. (1993). Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience, 5(2), 162–176. 10.1162/jocn.1993.5.2.162 [DOI] [PubMed] [Google Scholar]
- Fischl, B. , & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Liu, A. , & Dale, A. M. (2001). Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70–80. 10.1109/42.906426 [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , van der Kouwe, A. , Killiany, R. , Kennedy, D. , Klaveness, S. , Montillo, A. , Makris, N. , Rosen, B. , & Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , van der Kouwe, A. J. W. , Makris, N. , Ségonne, F. , Quinn, B. T. , & Dale, A. M. (2004). Sequence‐independent segmentation of magnetic resonance images. NeuroImage, 23(Suppl 1), S69–S84. 10.1016/j.neuroimage.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Sereno, M. I. , & Dale, A. M. (1999). Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. NeuroImage, 9(2), 195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Fischl, B. , van der Kouwe, A. , Destrieux, C. , Halgren, E. , Ségonne, F. , Salat, D. H. , Busa, E. , Seidman, L. J. , Goldstein, J. , Kennedy, D. , Caviness, V. , Makris, N. , Rosen, B. , & Dale, A. M. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fonda, J. R. , Fredman, L. , Brogly, S. B. , McGlinchey, R. E. , Milberg, W. P. , & Gradus, J. L. (2017). Traumatic brain injury and attempted suicide among veterans of the wars in Iraq and Afghanistan. American Journal of Epidemiology, 186(2), 220–226. 10.1093/aje/kwx044 [DOI] [PubMed] [Google Scholar]
- Fortier, C. B. , Amick, M. M. , Grande, L. , McGlynn, S. , Kenna, A. , Morra, L. , Clark, A. , Milberg, W. P. , & McGlinchey, R. E. (2014). The Boston Assessment of Traumatic Brain Injury‐Lifetime (BAT‐L) semistructured interview: Evidence of research utility and validity. The Journal of Head Trauma Rehabilitation, 29(1), 89–98. 10.1097/HTR.0b013e3182865859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton, J. J. , Calhoun, P. S. , Wagner, H. R. , Schry, A. R. , Hair, L. P. , Feeling, N. , Elbogen, E. , & Beckham, J. C. (2015). The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans: A meta‐analysis. Journal of Anxiety Disorders, 31, 98–107. 10.1016/j.janxdis.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Geuze, E. , Westenberg, H. G. M. , Heinecke, A. , de Kloet, C. S. , Goebel, R. , & Vermetten, E. (2008). Thinner prefrontal cortex in veterans with posttraumatic stress disorder. NeuroImage, 41(3), 675–681. 10.1016/j.neuroimage.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Hagler, D. J. , Saygin, A. P. , & Sereno, M. I. (2006). Smoothing and cluster thresholding for cortical surface‐based group analysis of fMRI data. NeuroImage, 33(4), 1093–1103. 10.1016/j.neuroimage.2006.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X. , Jovicich, J. , Salat, D. , van der Kouwe, A. , Quinn, B. , Czanner, S. , Busa, E. , Pacheco, J. , Albert, M. , Killiany, R. , Maguire, P. , Rosas, D. , Makris, N. , Dale, A. , Dickerson, B. , & Fischl, B. (2006). Reliability of MRI‐derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Harned, M. S. , Wilks, C. R. , Schmidt, S. C. , & Coyle, T. N. (2018). Improving functional outcomes in women with borderline personality disorder and PTSD by changing PTSD severity and post‐traumatic cognitions. Behaviour Research and Therapy, 103, 53–61. 10.1016/j.brat.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, J. P. , Hayes, S. , Miller, D. R. , Lafleche, G. , Logue, M. W. , & Verfaellie, M. (2017). Automated measurement of hippocampal subfields in PTSD: Evidence for smaller dentate gyrus volume. Journal of Psychiatric Research, 95, 247–252. 10.1016/j.jpsychires.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, C. , Liu, J. , Wang, K. , Li, L. , Liang, M. , He, Z. , Liu, Y. , Zhang, Y. , Li, W. , & Jiang, T. (2007). Brain responses to symptom provocation and trauma‐related short‐term memory recall in coal mining accident survivors with acute severe PTSD. Brain Research, 1144, 165–174. 10.1016/j.brainres.2007.01.089 [DOI] [PubMed] [Google Scholar]
- Jovicich, J. , Czanner, S. , Greve, D. , Haley, E. , van der Kouwe, A. , Gollub, R. , Kennedy, D. , Schmitt, F. , Brown, G. , Macfall, J. , Fischl, B. , & Dale, A. (2006). Reliability in multi‐site structural MRI studies: Effects of gradient non‐linearity correction on phantom and human data. NeuroImage, 30(2), 436–443. 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- Kaplan, M. S. , McFarland, B. H. , Huguet, N. , & Newsom, J. T. (2012). Estimating the risk of suicide among US veterans: How should we proceed from here? American Journal of Public Health, 102(Suppl 1), S21–S23. 10.2105/AJPH.2011.300611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, L. A. , King, D. W. , Vogt, D. S. , Knight, J. , & Samper, R. E. (2006). Deployment Risk and Resilience Inventory: A collection of measures for studying deployment‐related experiences of military personnel and veterans. Military Psychology, 18(2), 89–120. 10.1207/s15327876mp1802_1 [DOI] [Google Scholar]
- Lindemer, E. R. , Salat, D. H. , Leritz, E. C. , McGlinchey, R. E. , & Milberg, W. P. (2013). Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF veterans and the impact of comorbid TBI. NeuroImage. Clinical, 2, 601–611. 10.1016/j.nicl.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, L. K. , Love, H. C. , & Elbogen, E. B. (2017). Traumatic brain injury in Iraq and Afghanistan veterans: New results from a National Random Sample Study. The Journal of Neuropsychiatry and Clinical Neurosciences, 29(3), 254–259. 10.1176/appi.neuropsych.16050100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa, S. M. , Fonda, J. R. , Fortier, C. B. , Amick, M. A. , Kenna, A. , Milberg, W. P. , & McGlinchey, R. E. (2015). Deployment‐related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. Journal of Traumatic Stress, 28(1), 25–33. 10.1002/jts.21979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, E. K. , Nelson, N. W. , Sponheim, S. R. , & Disner, S. G. (2019). The impact of PTSD and mTBI on the relationship between subjective and objective cognitive deficits in combat‐exposed veterans. Neuropsychology, 33(7), 913–921. 10.1037/neu0000560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey, R. E. , Milberg, W. P. , Fonda, J. R. , & Fortier, C. B. (2017). A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: The TRACTS longitudinal prospective cohort study. International Journal of Methods in Psychiatric Research, 26(3), e1556. 10.1002/mpr.1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, Y. , Qiu, C. , Zhu, H. , Lama, S. , Lui, S. , Gong, Q. , & Zhang, W. (2014). Anatomical deficits in adult posttraumatic stress disorder: A meta‐analysis of voxel‐based morphometry studies. Behavioural Brain Research, 270, 307–315. 10.1016/j.bbr.2014.05.021 [DOI] [PubMed] [Google Scholar]
- Mitchell, J. M. , Bogenschutz, M. , Lilienstein, A. , Harrison, C. , Kleiman, S. , Parker‐Guilbert, K. , Marcela Ot'alora, G. , Garas, W. , Paleos, C. , Gorman, I. , Nicholas, C. , Mithoefer, M. , Carlin, S. , Poulter, B. , Mithoefer, A. , Quevedo, S. , Wells, G. , Klaire, S. S. , van der Kolk, B. , … Doblin, R. (2021). MDMA‐assisted therapy for severe PTSD: A randomized, double‐blind, placebo‐controlled phase 3 study. Nature Medicine, 27(6), 1025–1033. 10.1038/s41591-021-01336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser, K. T. , Gottlieb, J. D. , Xie, H. , Lu, W. , Yanos, P. T. , Rosenberg, S. D. , Silverstein, S. M. , Duva, S. M. , Minsky, S. , Wolfe, R. S. , & McHugo, G. J. (2015). Evaluation of cognitive restructuring for post‐traumatic stress disorder in people with severe mental illness. The British Journal of Psychiatry, 206(6), 501–508. 10.1192/bjp.bp.114.147926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysliwiec, V. , McGraw, L. , Pierce, R. , Smith, P. , Trapp, B. , & Roth, B. J. (2013). Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep, 36(2), 167–174. 10.5665/sleep.2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen, K. , de Boer, A. G. E. M. , Verbeek, J. H. A. M. , Blonk, R. W. B. , & van Dijk, F. J. H. (2003). The Depression Anxiety Stress Scales (DASS): Detecting anxiety disorder and depression in employees absent from work because of mental health problems. Occupational and Environmental Medicine, 60(Suppl 1), i77–i82. 10.1136/oem.60.suppl_1.i77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty, D. C. M. , Chitty, K. M. , Saddiqui, S. , Bennett, M. R. , & Lagopoulos, J. (2015). A systematic review and meta‐analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Research, 232(1), 1–33. 10.1016/j.pscychresns.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Petrakis, I. L. , Rosenheck, R. , & Desai, R. (2011). Substance use comorbidity among veterans with posttraumatic stress disorder and other psychiatric illness. The American Journal on Addictions, 20(3), 185–189. 10.1111/j.1521-0391.2011.00126.x [DOI] [PubMed] [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
- Reuter, M. , & Fischl, B. (2011). Avoiding asymmetry‐induced bias in longitudinal image processing. NeuroImage, 57(1), 19–21. 10.1016/j.neuroimage.2011.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, M. , Rosas, H. D. , & Fischl, B. (2010). Highly accurate inverse consistent registration: A robust approach. NeuroImage, 53(4), 1181–1196. 10.1016/j.neuroimage.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter, M. , Schmansky, N. J. , Rosas, H. D. , & Fischl, B. (2012). Within‐subject template estimation for unbiased longitudinal image analysis. NeuroImage, 61(4), 1402–1418. 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, E. , Mitko, A. , Stumps, A. , Robinson, M. , Milberg, W. , McGlinchey, R. , Esterman, M. , & DeGutis, J. (2019). Clinically significant cognitive dysfunction in OEF/OIF/OND veterans: Prevalence and clinical associations. Neuropsychology, 33(4), 534–546. 10.1037/neu0000529 [DOI] [PubMed] [Google Scholar]
- Roca, V. , Hart, J. , Kimbrell, T. , & Freeman, T. (2006). Cognitive function and dissociative disorder status among veteran subjects with chronic posttraumatic stress disorder: A preliminary study. The Journal of Neuropsychiatry and Clinical Neurosciences, 18(2), 226–230. 10.1176/jnp.2006.18.2.226 [DOI] [PubMed] [Google Scholar]
- Sadeh, N. , Spielberg, J. M. , Logue, M. W. , Wolf, E. J. , Smith, A. K. , Lusk, J. , Hayes, J. P. , Sperbeck, E. , Milberg, W. P. , McGlinchey, R. E. , Salat, D. H. , Carter, W. C. , Stone, A. , Schichman, S. A. , Humphries, D. E. , & Miller, M. W. (2016). SKA2 methylation is associated with decreased prefrontal cortical thickness and greater PTSD severity among trauma‐exposed veterans. Molecular Psychiatry, 21(3), 357–363. 10.1038/mp.2015.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh, N. , Spielberg, J. M. , Miller, M. W. , Milberg, W. P. , Salat, D. H. , Amick, M. M. , Fortier, C. B. , & McGlinchey, R. E. (2015). Neurobiological indicators of disinhibition in posttraumatic stress disorder. Human Brain Mapping, 36(8), 3076–3086. 10.1002/hbm.22829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savjani, R. R. , Taylor, B. A. , Acion, L. , Wilde, E. A. , & Jorge, R. E. (2017). Accelerated changes in cortical thickness measurements with age in military service members with traumatic brain injury. Journal of Neurotrauma, 34(22), 3107–3116. 10.1089/neu.2017.5022 [DOI] [PubMed] [Google Scholar]
- Ségonne, F. , Dale, A. M. , Busa, E. , Glessner, M. , Salat, D. , Hahn, H. K. , & Fischl, B. (2004). A hybrid approach to the skull stripping problem in MRI. NeuroImage, 22(3), 1060–1075. 10.1016/j.neuroimage.2004.03.032 [DOI] [PubMed] [Google Scholar]
- Ségonne, F. , Pacheco, J. , & Fischl, B. (2007). Geometrically accurate topology‐correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging, 26(4), 518–529. 10.1109/TMI.2006.887364 [DOI] [PubMed] [Google Scholar]
- Sherin, J. E. , & Nemeroff, C. B. (2011). Post‐traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues in Clinical Neuroscience, 13(3), 263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner, H. A. , & Sheu, W. J. (1982). Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of Studies on Alcohol, 43(11), 1157–1170. 10.15288/jsa.1982.43.1157 [DOI] [PubMed] [Google Scholar]
- Sled, J. G. , Zijdenbos, A. P. , & Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17(1), 87–97. 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- Sydnor, V. J. , Bouix, S. , Pasternak, O. , Hartl, E. , Levin‐Gleba, L. , Reid, B. , Tripodis, Y. , Guenette, J. P. , Kaufmann, D. , Makris, N. , Fortier, C. , Salat, D. H. , Rathi, Y. , Milberg, W. P. , McGlinchey, R. E. , Shenton, M. E. , & Koerte, I. K. (2020). Mild traumatic brain injury impacts associations between limbic system microstructure and post‐traumatic stress disorder symptomatology. NeuroImage: Clinical, 26, 102190. 10.1016/j.nicl.2020.102190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. L. , Wilk, J. E. , Riviere, L. A. , McGurk, D. , Castro, C. A. , & Hoge, C. W. (2010). Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Archives of General Psychiatry, 67(6), 614–623. 10.1001/archgenpsychiatry.2010.54 [DOI] [PubMed] [Google Scholar]
- Van Woudenberg, C. , Voorendonk, E. M. , Bongaerts, H. , Zoet, H. A. , Verhagen, M. , Lee, C. W. , van Minnen, A. , & De Jongh, A. (2018). Effectiveness of an intensive treatment programme combining prolonged exposure and eye movement desensitization and reprocessing for severe post‐traumatic stress disorder. European Journal of Psychotraumatology, 9(1), 1487225. 10.1080/20008198.2018.1487225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling, J. J. , Aslan, M. , Lee, L. O. , Proctor, S. P. , Ko, J. , Jacob, S. , & Concato, J. (2018). Longitudinal associations among posttraumatic stress disorder symptoms, traumatic brain injury, and neurocognitive functioning in Army soldiers deployed to the Iraq war. Journal of the International Neuropsychological Society, 24(4), 311–323. 10.1017/S1355617717001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers, F. W. , Keane, T. M. , & Davidson, J. R. (2001). Clinician‐administered PTSD scale: A review of the first ten years of research. Depression and Anxiety, 13(3), 132–156. 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2001). Wechsler test of adult reading: WTAR. Psychological Corporation. [Google Scholar]
- Wolf, E. J. , Logue, M. W. , Hayes, J. P. , Sadeh, N. , Schichman, S. A. , Stone, A. , Salat, D. H. , Milberg, W. , McGlinchey, R. , & Miller, M. W. (2016). Accelerated DNA methylation age: Associations with PTSD and neural integrity. Psychoneuroendocrinology, 63, 155–162. 10.1016/j.psyneuen.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, E. J. , Mitchell, K. S. , Sadeh, N. , Hein, C. , Fuhrman, I. , Pietrzak, R. H. , & Miller, M. W. (2017). The dissociative subtype of PTSD scale: Initial evaluation in a national sample of trauma‐exposed veterans. Assessment, 24(4), 503–516. 10.1177/1073191115615212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, E. J. , & Morrison, F. G. (2017). Traumatic stress and accelerated cellular aging: From epigenetics to cardiometabolic disease. Current Psychiatry Reports, 19(10), 75. 10.1007/s11920-017-0823-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, E. J. , Sadeh, N. , Leritz, E. C. , Logue, M. W. , Stoop, T. B. , McGlinchey, R. , Milberg, W. , & Miller, M. W. (2016). Posttraumatic stress disorder as a catalyst for the association between metabolic syndrome and reduced cortical thickness. Biological Psychiatry, 80(5), 363–371. 10.1016/j.biopsych.2015.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, S. H. , Schaer, M. , Kaloupek, D. G. , Cediel, L. , & Eliez, S. (2009). Smaller global and regional cortical volume in combat‐related posttraumatic stress disorder. Archives of General Psychiatry, 66(12), 1373–1382. 10.1001/archgenpsychiatry.2009.160 [DOI] [PubMed] [Google Scholar]
- Wrocklage, K. M. , Averill, L. A. , Cobb Scott, J. , Averill, C. L. , Schweinsburg, B. , Trejo, M. , Roy, A. , Weisser, V. , Kelly, C. , Martini, B. , Harpaz‐Rotem, I. , Southwick, S. M. , Krystal, J. H. , & Abdallah, C. G. (2017). Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. European Neuropsychopharmacology, 27(5), 515–525. 10.1016/j.euroneuro.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, D. S. , Ziadni, M. S. , Gilam, G. , Darnall, B. D. , & Mackey, S. C. (2020). Evaluation of candidate items for severe PTSD screening for patients with chronic pain: Pilot data analysis with the IRT approach. Pain Practice, 20(3), 262–268. 10.1111/papr.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Significant regions of annual percent change in thickness, volume, and area between time points. Regions of a significant (p < 0.05) annual percent change in cortical measures (thickness, volume, and area) across the entire cohort presented on the semi‐folded cortical surface. Blue indicates a more negative annual percent change at follow‐up, red/yellow indicates a positive change.
Figure S2. Group difference significance maps (severe‐PTSD + and severe‐PTSD − ). Regions of significant (p < 0.05) group difference of severe‐PTSD on annual percent change of cortical measures [a) thickness, b) volume, and c) area]. Maps are presented on the inflated cortical surface of an average brain. The bottom color scale represents the statistical significance of the correlation with light blue and yellow indicating the most significant regions; blue indicates a more negative annual percent change in severe‐PTSD individuals, red/yellow indicates a positive change.
Figure S3. Group by age interaction significance maps (severe‐PTSD + and severe‐PTSD − ). Regions of a significant (p < 0.05) severe‐PTSD × age interaction on annual percent change in cortical measures [a) thickness, b) volume, and c) area]. Maps are presented on the inflated cortical surface of an average brain. Blue indicates a more negative association between age and annual percent change in the severe‐PTSD+ group, red/yellow indicates a positive relationship.
Figure S4. Subgroup by age interaction significance maps. Regions of a significant (p < 0.05) comorbid military mTBI and PTSD × age interaction of annual percent change in cortical measures [a) thickness, b) volume, and c) area]. Blue indicates a more negative association between age and annual percent change in the comorbid mTBI/PTSD group, red/yellow indicates a positive relationship.
Table S1. Statistics for standard least squares regressions run with interactions between PTSD and age on annual percent change in the 4 ROIs depicted in Figure 2 of the main text. B values are effect sizes and SE values are standard error for the effect sizes. Reported F values are Fischer's statistics and R2 values are coefficients of determination. DASS = Depression Anxiety Stress Scales; LDH = lifetime drinking history. *p < 0.05, **p < 0.01, ***p < 0.001
Table S2. Statistics for standard least squares regressions run with interactions between PTSD+mTBI subgroup and age on annual percent change in the 2 ROIs depicted in Figure 4 of the main text. B values are effect sizes and SE values are standard error for the effect sizes. Reported F values are Fischer's statistics and R2 values are coefficients of determination. DASS = Depression Anxiety Stress Scales; LDH = lifetime drinking history. *p < 0.05, **p < 0.01, ***p < 0.001
Data Availability Statement
Data will be made available via controlled public access mechanisms, primarily through data use/transfer agreements. The requester and data custodian will comply with all VA requirements regarding HIPAA, The Privacy Act of 1974, Records Management, and Data Use/Transfer directives. Code will be made available upon request.
Data will be made available via controlled public access mechanisms, primarily through data use/transfer agreements. The requester and data custodian will comply with all VA requirements regarding HIPAA, The Privacy Act of 1974, Records Management, and Data Use/Transfer directives. Code will be made available upon request.


