Abstract
Background
Type 1 diabetes (T1D) is a complex autoimmune disorder whose pathogenesis involves an intricate interplay between β cells of the pancreatic islet, other islet cells, and cells of the immune system. Direct intercellular communication within the islet occurs via cell surface proteins and indirect intercellular communication has traditionally been seen as occurring via secreted proteins (e.g., endocrine hormones and cytokines). However, recent literature suggests that extracellular vesicles (EVs) secreted by β cells constitute an additional and biologically important mechanism for transmitting signals to within the islet.
Scope of review
This review summarizes the general mechanisms of EV formation, with a particular focus on how lipids and lipid signaling pathways influence their formation and cargo. We review the implications of EV release from β cells for T1D pathogenesis, how EVs and their cargo might be leveraged as biomarkers of this process, and how EVs might be engineered as a therapeutic candidate to counter T1D outcomes.
Major conclusions
Islet β cells have been viewed as initiators and propagators of the cellular circuit giving rise to autoimmunity in T1D. In this context, emerging literature suggests that EVs may represent a conduit for communication that holds more comprehensive messaging about the β cells from which they arise. As the field of EV biology advances, it opens the possibility that intervening with EV formation and cargo loading could be a novel disease-modifying approach in T1D.
Keywords: Islet, Diabetes, Extracellular vesicles, Lipids
1. Introduction
Diabetes is a global epidemic affecting over 300 million people worldwide and is expected to increase by 50% by 2030 [1]. Clinically, diabetes has been classified as either emanating from autoimmunity against the insulin-producing β cell (type 1 diabetes, T1D) or from an inability of the β cell to compensate for peripheral tissue insulin resistance (type 2 diabetes, T2D). It is now clear that molecular and phenotypic diversity exists across these two forms such that this simplistic categorization has necessitated more granular refinement. Nevertheless, a common denominator across virtually all classifications of diabetes is the failure of sufficient insulin secretion from β cells, whether due to reduced β cell mass or β cell dysfunction. At the molecular level, extracellular signals (e.g. viruses, inflammatory cytokines, free fatty acids, hyperglycemia) trigger intracellular insults (e.g. endoplasmic reticulum stress, oxidative stress, mitochondrial decompensation, DNA damage) that ultimately lead to cellular senescence, dysfunction, and/or death [2]. Whereas this cell-autonomous perspective of β cell loss has garnered broad acceptance in the field, an increased focus on the islet microenvironment has led to an appreciation of intercellular communication serving to disseminate messages between neighboring cells [2]. Such communication between cells appears to be a means to propagate and amplify signaling that can regulate cellular mass, survival or function. To date, several modes of intercellular communication between β cells and other local cell types (endocrine cells, cells of the immune system, etc.) have been demonstrated, including the release of soluble factors (e.g. senescence-associated secretory phenotype) [3] and direct cell–cell communication (e.g. PD-L1/PD-1 interactions) [4]. More recently, the potential of extracellular vesicles (EVs) as conduits to deliver nucleic acid and protein cargo to nearby cells has gained momentum. In this review, we discuss the pathways that give rise to EVs, the nature of their cargo, and their potential for communication, as biomarkers of β cell stress, and for engineering them as therapeutic vehicles. Particular attention is paid to the role of lipids in these processes.
2. EV subtypes and lipid pathways leading to EV formation
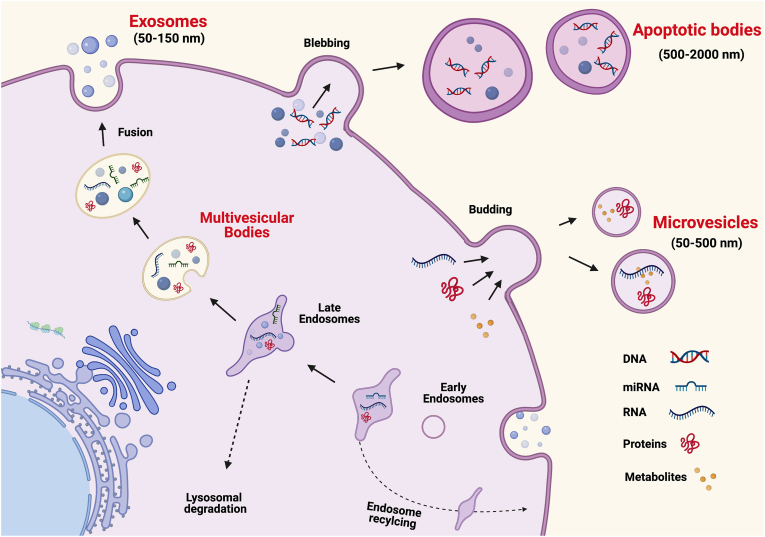
Extracellular vesicles (EVs) are broadly classified into 3 major categories: exosomes, microvesicles, and apoptotic bodies, each of which has distinct intracellular origins (see Figure 1). Exosomes (30–200 nm diameter) and microvesicles (100–1000 nm diameter) are often distinguished based upon size as well as mechanism of formation, which occurs via orchestrated intracellular pathways. Apoptotic bodies (>1000 nm diameter) are derived from the blebbing of cells undergoing apoptosis [5]. Exosomes are generated via an endocytic pathway in which intracellular multivesicular bodies fuse with the plasma membrane and release their intraluminal vesicles. Microvesicles, by contrast, are generated from direct budding of the plasma membrane [6]. The discrete distinction based upon formation may be an over-simplification, as overlapping mechanisms can regulate the formation of both exosomes and microvesicles, with some proposing to categorize only on size (and not mechanism of formation) by labeling the vesicles as “small EVs” (usually <100 nm-200 nm) or “large EVs” (usually >200 nm) [5,7,8].
Figure 1.
Biogenesis and secretion of extracellular vesicles (EVs). Extracellular vesicles consist of at least three subtypes, exosomes, microvesicles, and apoptotic bodies. The figure shows in the intracellular biogenesis of each subtype and the potential cargo contents of each.
2.1. Formation of exosomes via the endosome pathway
The overall fate of the endosomal pathway can be divided into either reutilization/recycling or degradation [9]. Endosomes are formed by endocytosis, the process whereby extracellular and plasma membrane components are internalized into the cell [9]. These “early endosomes” are then directed to different cellular components such as the trans-golgi network, lysosomes for degradation and recycling, to form late endosomes, or back to the plasma membrane (Figure 1) [10]. The majority of endosomes are recycled back to the plasma membrane but a small number are targeted for autophagy, a degradative process that clears defective proteins and organelles to maintain intracellular homeostasis [11]. The endosomal sorting complex required for transport (ESCRT) protein complexes 0-III are fundamental in the targeting of the endosomes for one pathway or another.
Endosomes that do not quickly recycle back to the plasma membrane or are not targeted to lysosomes for degradation form multivesicular bodies containing intraluminal vesicles [12]. When the multivesicular bodies fuse with the plasma membrane, the released intraluminal vesicles become exosomes [13]. Exosome biogenesis is a dynamic process whereby the contents of the original early endosome may be distinct from the contents released as exosomes. Exosome generation and autophagy pathways overlap with multiple layers of regulation and complexity that are only beginning to be understood [[11], [12], [13]]. Overlapping pathways are speculated to exist, but depending upon cellular conditions, the intraluminal vesicles are shuttled either for release (exosomes) or to lysosomes for autophagy [14]. For example, RAS GTPase superfamily members, specifically Rab GTPases transport and target vesicles intracellularly for endosomal recycling, to lysosomes for degradation, or to secretion as exosomes [9]. Each Rab protein is involved in a specific transport step, with greater than 28 Rab isoforms characterized in exosomes. Most of these are involved in the endocytic pathway, although some are involved in endoplasmic reticulum (ER)-Golgi transport [10]. The three Rab proteins most frequently linked to exosome biogenesis and secretion are: Rab11, Rab35 and Rab27, whose presence direct the multivesicular body to plasma membrane fusion and exosome release. In contrast, Rab7 targets the multivesicular body to lysosomes and subsequent degradation [11]. Table 1 summarizes key proteins that have been described and their functions in extracellular vesicle biogenesis.
Table 1.
Selection of Key Proteins in Extracellular Vesicle Biogenesis and Lipid Signaling.
| Protein Complex or Protein | Role | References |
|---|---|---|
| ESCRT | Targets endosomes to a pathway | [11,194] |
| ESCRT-0 (with Hrs) | Recognizes ubiquitinylated cargo and sorts to PI3P rich vesicles | [9] |
| ESCRT-1 | With ESCRT-2, generates membrane budding inward in endosomes to form intraluminal vesicles, recruits ESCRT-0-ubiquitin domains to buds | [195] |
| ESCRT-2 | With ESCRT-1, generates membrane budding inward in endosomes to form intraluminal vesicles, recruits ESCRT-0-ubiquitin domains to buds | [195] |
| ESCRT-3 | Cleaves membrane buds to form intraluminal vesicles | [195] |
| Vacuolar protein sorting (Vps)4 | Interacts with ESCRT I-III and oxysterol-binding protein; may regulate facilitate miRNAs in exosomes that modulate PI3K/Akt pathway | [22,23,196] |
| TSG101 | ESCRT machinery protein, interacts with ARRDC1, participates in multivesicular body sorting via binding with ALIX, mediates the release of microvesicles containing TSG101, ARRDC1 and other cellular proteins | [15,197] |
| Arrestin domain-containing protein 1 (ARRDC1) | Interacts with TSG101, mediates the release of microvesicles containing TSG101, ARRDC1, mitochondrial proteins and other cellular proteins; mediates release of exosomes containing proteins implicated in apoptosis | [15,198] |
| Charged multivesicular body protein (CHMP) | Participates in multivesicular body sorting via binding with ALIX | [197] |
| Rab GTPases | Transport of endosomes | [9] |
| Rab11 | Targets multivesicular body to plasma membrane for fusion and exosome release | [10,199] |
| Rab27 | Targets multivesicular body to plasma membrane for fusion and exosome release | [10,200] |
| Rab35 | Targets multivesicular body to plasma membrane for fusion and exosome release | [10,201] |
| Rab22A | Selective recruitment of proteins to microvesicles under hypoxic conditions | [16] |
| Alg2-interacting protein X (Alix) | Recruited to endosomal membrane via domain that binds BMP; with LBPA, induces formation of endosome membrane invaginations; participates in multivesicular body sorting via binding with TSG101 and CHMP4, interacts with syntenin to support intraluminal vesicle budding | [197,202,203] |
| Acid ceramidase | Converts ceramide to sphingosine and fatty acids in lysosomes; inhibition increases exosome release | [38,204] |
| ADP-Ribosylation Factor 6 (ARF6) | GTPase; activates phospholipase D2 & PIP5K; interacts with syntenin; regulates intraluminal vesicle budding and exosome production; regulates selective recruitment of proteins into microvesicles | [14,203,205] |
| ATP-binding cassette transporter A3 | Reduces exosome formation; mediates transport of choline-phospholipids into intracellular vesicles (possible connection to exosome formation?) | [206,207] |
| Diacyl glycerol (DAG) kinase a | Catalyzes conversion of DAG to phosphatidic acid; inhibits exosome secretion (by reducing DAG levels) | [208] |
| Flotillins | Modify exosomal cargo | [34,41] |
| Phosphatidylinositol 4-phosphate 5-kinase (PIP5K) | Catalyzes phosphatidylinositol 4-phosphate (PI4P) phosphorylation to form phosphatidylinositol 4,5-bisphosphonate (PI4,5P2), Activated by ARF6 and phosphatidic acid | [209] |
| Phospholipase D2 | Catalyzes hydrolysis of phosphatidylcholine to produce phosphatidic acid; involved in formation of exosomes with syntenin, ALIX, CD63, regulator of intraluminal vesicle budding and exosome production | [205,210,211] |
| PIKfyve | converts PI3P to either PI(3,5)P2 or PI5P | [20] |
| Sorting nexin proteins (SNX) | Interact with PI3P and enable transport to trans-golgi or plasma membrane | [20] |
| Sphingomyelinases (SMase) | Hydrolyze sphingomyelins to produce ceramide | [24] |
| Syndecans | Ubiquitous transmembrane proteins, enriched in CD63+, flotillin-1- exosomes, bind with syntenin, likely recruit syntenin-ALIX and support membrane budding | [203] |
| Syntenin | Interacts with ALIX, syndecans to support formation of intraluminal vesicles | [203] |
| Tetraspanins | Participate in exosome biogenesis; interact with other transmembrane receptors, within themselves and with integrins and other proteins; often form tetraspanin-enriched microdomains | [40] |
| Vps24 | Binds with PI(3,5)P2 to target endosomes to EVs | [20] |
2.2. Pathways leading to release of microvesicles
Localized changes in the plasma membrane that cause direct outward blebbing of the membrane lead to the generation of a microvesicle [12]. Similar to exosomes, microvesicle biogenesis is a regulated process with selected cargo that differs based on the cellular microenvironment [13]. One regulator is the small GTP-binding protein ADP-Ribosylation Factor 6 (ARF6), which initiates a phosphorylation cascade required for microvesicle shedding [14]. Another regulator, TSG101 (tumor susceptibility gene), is an ESCRT machinery protein that interacts with arrestin domain-containing protein 1 (ARRDC1) in order to mediate the release of microvesicles containing TSG101, ARRDC1 and other cellular proteins [15]. Rab GTPases are also involved in microvesicle shedding, especially under hypoxic conditions. Specifically, Rab22A expression increases after the activation of hypoxia-inducible factors and co-localizes with budding microvesicles [16]. TNFα treatment also changes the miRNA cargo of microvesicles released from endothelial cells [17]. While the molecular mechanisms of microvesicle biogenesis are not as well-characterized as exosome biogenesis [18], multiple layers of regulation are clearly involved and a significant degree of overlap in protein families and pathways are likely at play.
2.3. Lipids in ESCRT-dependent and ESCRT-independent EV formation pathways
Lipids are an integral component of the ESCRT-dependent system whereby they both recruit appropriate proteins as well as characterize the type of endosome. The phosphatidylinositol phosphates (PIPs) and derivatives recruit cytosolic proteins with key membrane recognition domains, which then participate in transport and possible exosome secretion [19]. The hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) of the ESCRT-0 complex sorts ubiquitinylated cargo to phosphatidylinositol-3-phosphate (PI3P)-rich endosomal compartments [9]. PI3P interacts with sorting nexin proteins (SNX) to facilitate transfer of the endosome to the trans-Golgi network or plasma membrane [20]. PI3P in turn is converted to either PI(3,5)P2 or PI5P by phosphatidylinositol-3-phosphate 5-kinase (PIKfyve). PI(3,5)P2 is a major component of late endosomes and autophagosomes whereas PI5P is a negative regulator of endosomal maturation and blocks degradation of associated proteins [20,21]. The fate of PI(3,5)P2 containing endosomes seems to be related to whether PI(3,5)P2 binds to vacuolar protein sorting (Vps) 24 (targeting to EVs) or the transient receptor potential cation channel 1 (TRPML-1) (targeting to lysosomes/degradation) [20]. Another Vps (Vps4) associates with ESCRT complexes I-III and (in yeast) has been shown to interact with oxysterol binding proteins, suggesting that oxysterols are implicated in EV formation [22,23]. Alg2-interacting protein X (Alix) is involved in both ESCRT-dependent and –independent pathways. It is recruited to the endosomal membrane via a domain that binds the endosomal lipid bis(monoacylglycero)phosphate (BMP), an endosomal lipid [22]. Lipids relevant in EV biogenesis are summarized in Table 2.
Table 2.
Lipids Subtypes in Extracellular Vesicle Biogenesis.
| Stage of Biogenesis | Lipid | Main Function(s) | References |
|---|---|---|---|
| Formation | Bis(monoacylglycero)phosphate (BMP) [a.k.a lysobisphosphatidic acid (LBPA)] | Induces formation of multivesicular bodies, interacts with ALIX, enriched in late endosomes | [22,202,212] |
| Ceramides | Promotes formation of multivesicular bodies | [31] | |
| Cholesterol | Allows for membrane conditions permitting budding | [205] | |
| Diacylglycerol | Promotes formation of secretory vesicles in the trans-Golgi network; recruits soluble cell membrane proteins and interacts with cytoskeleton | [208] | |
| Phosphatidic Acid | Regulates membrane curvature and fission; interacts with syntenin and other proteins in intraluminal vesicle budding | [43,211,213,214] | |
| Phosphatidylinositol 3-phosphate (PI3P) | Feature of early endosomes and multivesicular bodies; interacts with Rabs, SNX's, HRS, PILfyve | [9,215,216] | |
| Transport and Cargo Sorting | Ceramides | May enrich for certain miRNA cargo | [33] |
| Cholesterol | Increases release of flotillin+ exosomes (oligodendroglial cells); controls movement of endosomes along microtubules | [41,217] | |
| Ether lipids | Changes protein composition of EVs | [218] | |
| Lysophosphatidylcholine | With palmitate increases proinflammatory EV cargo | [219] | |
| Palmitate | With lysophosphatidylcholine increases proinflammatory EV cargo | [219] | |
| Phosphatidylserine | Regulates retrograde transport | [215] | |
| PI3P | Interacts with SNX, facilitates endosomal transfer to the trans-Golgi network or plasma membrane | [20] | |
| Sphingosine 1-phosphate | Activates inhibitory G protein-coupled S1P receptors on multivesicular bodies (necessary to target CD63, CD81, flotillin to intraluminal vesicles destined for plasma membrane) | [220] | |
| Cholesterol | Increases exosome release, Induces fusion of multivesicular vesicles with cell membrane | [147,221] | |
| Ether Lipids | Increases exosome release | [218] | |
| Phosphatidylinositol-3,5-bisphosphonate [PI(3,5)P2] | Component of late endosomes; interacts with Vps24 to target vesicles to EVs; high levels may target vesicles to autophagy/lysosomes | [20,24,215,222] |
Prominent among the ESCRT-independent pathways is the ceramide-dependent pathway. Ceramides (Cer) are sphingolipids that are generated in the ER by hydrolysis of sphingomyelin by sphingomyelinases (SMases) or lysosomes [24]. Ceramides are the central precursors for a family of sphingolipids that includes sphingomyelins and sphingosine phosphate (S1P). Ceramides can be generated via multiple pathways: de novo [25,26], sphingomyelin hydrolysis by acid (A) or neutral (N) SMases [27], or salvage [28]. The major ceramides in exosomes are the C18:0-Cer and C24:1-Cer molecular species [29,30] and they are proposed to enable membrane curvature to facilitate inward budding of the vesicles.
In a mouse oligodendroglial cell line, exosome formation is decreased both by knockdown or chemical inhibition of NSMase [31]. The importance of ceramide in EV formation (via NSMase) has been confirmed in several other cell lines, although it does not appear to be a uniform trait, since reduced SMase does not seem to affect exosome numbers in prostate nor melanoma cells [[32], [33], [34], [35]]. In polarized epithelial cells, ceramides are involved in differential exosome secretion in which basolateral but not apical exosome release depends upon ceramides [36]. Notably, while neutral SMase plays a role in exosome biogenesis, acidic SMase triggers microvesicle release in glial cells [37].
2.4. Lipid rafts
Lipid rafts, or lipid microdomains, are membrane sections enriched in sterols, sphingolipids and glycosylphosphatidylinositol (GPI)-anchored proteins. They generally function in forming membrane invaginations, either via structural changes induced by lipid raft components and/or by protein recruitment and complex formation [38,39]. Among these recruited proteins are the tetraspanins, (also known as transmembrane 4 superfamily (TM4SF) proteins) and flotillins. Tetraspanins, which are intimately associated with exosomes, are thought to participate in exosome biogenesis, although the exact mechanisms have yet to be elucidated. These proteins interact with other proteins, including other tetraspanins, transmembrane receptors, and integrins, often forming distinct membrane regions called tetraspanin-enriched microdomains [40]. Flotillins do not seem to be directly involved in exosome release, but rather in modifying exosomal cargo [34,41].
Within lipid rafts exist planar lipid rafts, with similar architecture as surrounding membranes, and invaginated (caveolae) lipid rafts, which have a distinct appearance from the rest of the membrane [39]. Caveolae lipid rafts are non-clathrin-coated pits rich in the membrane protein caveolin and participate in intracellular signaling, vesicle trafficking, and cell migration. The caveolin protein Cav1 has been described in exosomes from several cancer cell lines and in exosomes harvested from serum of melanoma patients [42]. Given the location of caveolin in lipid rafts and role in cellular signaling and endocytosis, it likely contributes to intraluminal vesicle sorting and exosome formation [39].
2.5. Other relevant lipids in EV biogenesis
In addition to the aforementioned lipids, multiple other lipids are known or suspected to participate in different stages of EV biogenesis. Table 1 outlines many of the proteins involved in the formation of these lipids and Table 2 details individual lipids contributing to EV biogenesis. Importantly, the role of a particular lipid may vary depending upon cell type or physiological state (i.e. healthy, stressed, diseased). Another complicating factor is that many studies use exosome number as the measured variable. Thus, while one can conclude that a compound inhibits or promotes total EV release, which stage of EV biogenesis and release is involved still needs to be elucidated.
2.6. Lipid composition of EVs
Lipid content (relative to proteins) in exosomes is enriched 8.4-fold as compared the cell from which they are derived [43]. The lipid composition of EVs depends both upon cell of origin and health or disease status (i.e. EVs from malignant cells may have different compositions than nonmalignant cells) and likely also differs between primary cells and immortal cell lines [19,44]. Mass spectrometry quantification of the lipidomes of a prostate cell line (PC-3) and its released exosomes reveals that exosomes are enriched in glycosphingolipids, sphingomyelin, cholesterol and phosphatidylserine [43]. Collation of exosome lipid composition from multiple studies shows that phosphatidylethanolamine content is similar in cells and exosomes whereas phosphatidylcholine and phosphatidylinositol contents are lower in exosomes compared to cells [19], perhaps to facilitate target cell uptake [45] or due to the role of these lipids in EV biogenesis (Table 2). In 3T3-L1 adipocytes, phospholipids, sphingolipids, and glycerolipids are similar between the smaller EVs (mostly <100 nm) and large EVs (100–200 nm). However, cholesterol is more enriched in smaller EVs and annexin V binding (marker of externalized phosphatidylserine) is more enriched in large EVs [46].
In contrast to studies of lipid content in cell line-derived EVs, less information exists from EVs isolated from biofluids. One study examined the EVs obtained from the ejaculate of vasectomized men with EVs separated by differential centrifugation and size-exclusion chromatography into approximately 50 nm and 100 nm populations [47]. In contrast to studies in adipocyte cell lines, cholesterol content was shown not to vary significantly between smaller and larger EV populations; however, hexosylceramide is enriched in the smaller EVs and sphingomyelin species are enriched in larger EVs [47]. Exosomes harvested from urine have been shown to have significant cholesterol content and the lipidome characterized by mass spectrometry can be used to distinguish healthy controls from those with prostate or renal cell carcinoma [48,49]. Lipid content of EVs isolated from human serum was determined by an ultracentrifugation protocol in which large debris and apoptotic bodies were first removed, followed by ultracentrifugation of the supernatant. The lipid content was examined in a total of 7 fractions and varied depending upon the fraction, with the most buoyant fraction being lipid rich but lacking typical EV markers. The EV pellet was enriched with ceramides [50]. To our knowledge, the lipidome of β cell-derived EVs has not been characterized; however, it is possible that there may be a unique lipid signature of these EVs and/or a characteristic signature of stressed β cells.
3. Lipid cargo in EVs
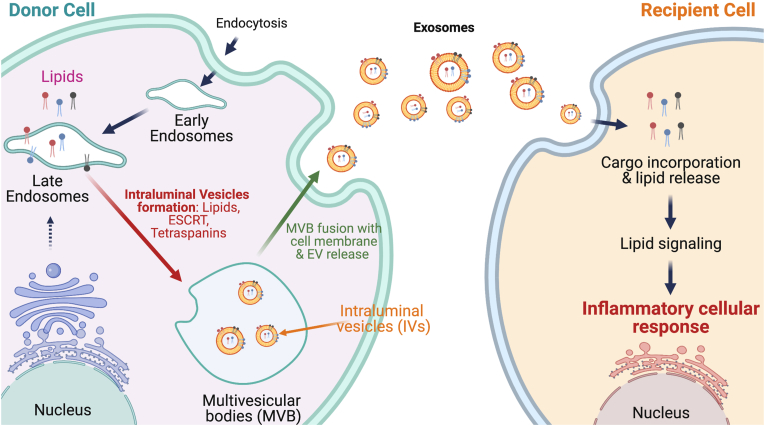
EVs, initially described as conduits for disposal of undesired cellular material, are now considered to be part of the secretome, serving as carriers of important cargo that can be delivered from a donor cell to a same or different recipient cell type (see Figure 2). Among the cargo includes proteins, enzymes, transcription factors, RNA, miRNA, DNA, and lipids [22,[51], [52], [53], [54], [55]]. Studies during the past decade have revealed that EVs are released from a variety of cells and their cargo participates in a variety of disorders, predominantly cancer, neurological, and metabolic disorders [56,57].
Figure 2.
Lipid incorporation into exosomes and their release to recipient cells. The figure illustrates incorporation of lipids into exosomes of “donor” cells (left), which release these exosomes that then communicate with the neighboring or distant “recipient” cells (right).
Recent attention to EVs in T1D has been directed at understanding the signaling transmitted by their protein or miRNA cargo. However, whereas the nature of EV-derived lipid signaling has been addressed in other disorders, it has been largely absent in the T1D literature. This section of the review will discuss the important roles of EV lipid cargo in propagating deleterious outcomes and extrapolate their importance in β-to-immune cell communication, which ultimately leads to T1D development.
3.1. Ceramides
A distinguishing feature of exosomal membranes, compared with plasma and microvesicle membranes, is the lipid composition, which, as noted previously, is enriched compared to their cell of origin. One of these, the ceramides, represents a class of sphingolipids that has generated much interest [58]. As signaling molecules, ceramides are implicated in inducing apoptosis in multiple systems [59,60]. As one example, treating oligodendroglioma cells with the Th1 pro-inflammatory cytokines TNFα and IFNγ can cause cell death via the formation, and exosomal release, of multiple ceramide molecular species [61,62]. Such shedding of ceramides was proposed to contribute to autoimmune responses that lead to neuronal demyelination. Consistently, TNFα and IFNγ are reported to be elevated in the brains of patients with multiple sclerosis (MS) [63], a disease associated with demyelination, and this is associated with increased accumulation of ceramide molecular species in the cerebrospinal fluid [64].
The neurodegenerative Alzheimer's disease is associated with the accumulation of amyloid-β peptides in the brain. During the progression of this disease, ceramide levels in the human brain increase [[65], [66], [67]]. Silencing NSMase2 using genetic approaches leads to reduced exosomes in the brain, reduced ceramide levels, and decreased plaque burden [68,69], suggesting that ceramide-rich exosomes contribute to Alzheimer's disease pathology. Similarly, Parkinson's disease is associated with aggregation of high molecular weight α-synuclein [70]. Reducing NSMase2 activity leads to decreased transfer of α-synculein aggregates between neuronal-like cells and less accumulation and aggregation of the high molecular weight α-synuclein [71]. While there are several reports of NSMase involvement in a variety of disorders [72], exosomes in the CSF of multiple sclerosis patients were found to contain high SMase activity, which correlated with exosome number and severity of the disease [73].
A recent comprehensive review [74] elegantly discusses the critical role of ceramides and S1P in the function and secretion of exosomes in a cancer milieu. For example, ceramides generated by the NSMase pathway in exosomes from a human multiple myeloma cell line increases exosomal secretion, decreases cell proliferation, and increases caspase-3/9/PARP-mediated cell death [75]. This finding is associated with increases in exosomal abundance of tumor-suppressive miRNAs. All of these outcomes are reversed with GW4869, an inhibitor of NSMase2. Consistently, lower expression of NSMase1 in exosomes has been reported in patients with hepatocellular carcinoma and is associated with poor long-term survival of those patients [76]. Utilizing hepatocellular carcinoma cell lines, the authors demonstrate that exosomal NSMase1 reduces hepatocellular carcinoma growth by decreasing the ratio of sphingomyelins to ceramides. Inflammation of the gut, promoted by bacterial infection, can lead to colon cancer. The enteropathogenic bacteria are proposed to stimulate intestinal epithelium to produce exosomes that are enriched in S1P and prostaglandin E2 (PGE2) lipids [77]. Through induction of inflammatory Th17 cells, S1P is thought to promote tumor cell growth, whereas PGE2 participates in recruitment and proliferation of Th17 cells.
Exosomal ceramides also are reported to play a role in mitigating atopic dermatitis, a systemic inflammatory disease that is associated with epidermal barrier disruption [78]. In this study, administration of exosomes from human adipose tissue-derived mesenchymal stem cells to mice treated with oxazolone to induce dermatitis was found to reduce inflammatory cytokine levels and restore epidermal barrier functions. These outcomes correlate with decreased ceramide production via the de novo pathway and increasing anti-apoptotic S1P via the salvage pathway. In addition to ceramides and S1P, other toxic sphingolipids like sulfatides and psychosine are under consideration as being shuttled from sick to healthy cells by exosomes, thereby spreading the disease [79].
3.2. Eicosanoids
Another class of lipids that are synthesized within and shuttled by EVs are eicosanoids, bioactive oxygenated lipid metabolites of arachidonic acid [54,80]. Arachidonic acid is hydrolyzed from membrane phospholipids by phospholipases A2 (PLA2) [81,82]. The most abundant eicosanoids in EVs are prostaglandins and leukotrienes, which are also among the most inflammatory lipids [81]. Early studies identified the involvement of secreted PLA2, PGE2, and lysophosphatidylcholine [83,84], the other product generated by PLA2 action, in maturation of dendritic cells. Subsequent studies revealed the presence of lysophosphatidylcholine and phospholipase D in exosomes from a basophilic leukemia cell line (RBL-2H3) [85,86]. Further analyses [87] showed that the cargo load of these exosomes includes prostaglandins, such as PGE2 and 15d-PGJ2, a PPARγ agonist. Moreover, the concentrations (micromolar) of PGs in the exosomes are sufficient to affect signaling. In another study, PGE2 delivered by IDENs (intestinal mucus-derived exosome-like nanoparticles) to the liver was shown to induce anergy in natural killer T cells and reduce their ability to respond to foreign antigens [88]. In addition to its ability to affect communication between cells as EV cargo, PGE2 through EP4 receptor signaling can modulate EV sorting [89]. In this regard, clinical chemoresistant breast cancer carcinoma cells express high COX2-PGE2-EP4 signaling [90] and blocking the PGE2/EP4 signaling in cancer stem cells has been reported to promote conversion of of these cells to non-cancer stem cells [89]. This effect is a consequence of increased EV release of mesenchymal markers and drug transporters from cancer stem cells, reducing the number of chemoresistant cells that continue to proliferate. Paradoxically, the COX2 inhibitor celecoxib, which reduces COX2 mRNA in several cancer cells, increases COX2 accumulation in exosomes of lung cancer cells [91]. Recipient cells of these exosomes produced higher PGE2 and vascular endothelial growth factor, thus spreading inflammatory responses.
Metabolism of arachidonic acid by 5-lipoxygenase leads to generation of leukotrienes. Among them, LTB4 has garnered much attention owing to its varied roles, including in recruitment of neutrophils and other leukocytes in autoimmune diseases [92,93]. Exosomes contain the enzyme machinery to synthesize LTB4 [94] and inhibition of the enzymes decreases exosomal LTB4 and results in loss of directionality during neutrophil migration [95]. Given the short half-life (1 min) of LTB4 in vivo, stability and longevity of LTB4 is achieved through packaging in EVs [96] and its time-dependent release from exosomes serves as a signal to recruit neutrophils [97].
Metabolism of arachidonic acid by 12-lipoxygenase (12-LOX) leads to generation of 12S-HETE [98,99], which promotes invasion and metastasis of tumors [[100], [101], [102]]. Platelets produce 12-LOX [103] and can deliver 12-LOX packaged in EVs to colon cancer cells, leading to an increased production of 12S-HETE and its esterification into cell membrane phospholipids [104]. A further example of a role for platelet EV cargo 12-LOX was demonstrated in autoimmune arthritis [105]. During inflammation, secreted PLA2-IIA is expressed in inflammatory exudates [106] and catalyzes hydrolysis of arachidonic acid, which is then metabolized to 12S-HETE by 12-LOX in the platelet EVs. When neutrophils reach the inflammatory site, signaling by 12S-HETE via BLT2 receptors on activated neutrophils triggers internalization of platelet EV cargo, which enhances inflammation [105].
4. Other signaling cargo in EVs
In recognizing the potential importance of cell-to-cell communication in T1D development, much of the work characterizing β cell EVs has focused on mRNAs, miRNAs, and protein cargo. Independent studies have identified a variety of these species in β cell EVs that can impact β cell function and survival including: miR-127 which can inhibit β cell proliferation and insulin secretion [107]; mRNAs (VEGF-A, eNOS) and miRNAs (27b, 126, 130, 296) that contribute to β cell function and islet endothelial cell angiogenesis, a key for engraftment of transplanted islets [108]; miR-21–5p, which is induced during inflammation and can increase β cell apoptosis in T1D [109,110]; 19 miRNAs and 133 mRNAs [111] and cytokines [112] that are differentially expressed by islets under proinflammatory conditions; and accumulation of the spliced form of XBP1 mRNA, which correlates with ER stress and apoptosis [113]. It has also been suggested that islet graft rejection may be a consequence of major histocompatibility complex (MHC) molecules carried by donor islet EVs that are recognized by recipient antigen presenting cells (APCs) and immune cells [114].
In other studies, EVs from various preparations have been related to consequences of islet stress in diabetes, although the precise EV cargo was not identified. For example, human bone marrow-derived mesenchymal stem cell EVs can induce tolerogenic dendritic cells to reduce inflammation and mitigate T1D progression [115]; pancreatic cancer cell-derived EVs induce ER stress genes and β cell dysfunction [116]; human islet EVs containing mRNA of differentiation factors NGN3, MAFA, and PDX1 upregulate C-peptide levels in iPSC clusters [117], suppress human islet amyloid polypeptide amyloid formation that occurs in the islets of patients with T2D [118], and trigger immune responses and activate PBMCs [119]; human pancreas-derived mesenchymal stem cells facilitate improved glucose tolerance, increases in β cell number and insulin secretion in streptozotocin-treated mice [120]; human umbilical cord mesenchymal stem cells protect β cells from hypoxia-induced apoptosis [121]; healthy and human lean adipocyte-derived EVs but not inflamed or obese adipose explant-derived EVs improve β cell function and survival [122]; and EVs from cytokine-exposed β cells promote the interaction of EV-derived CXCL10 with recipient β cell CXCR3 receptor to induce a proinflammatory islet transcriptome and β cell dysfunction [123].
5. β-cell stress, EV formation, and connection to T1D pathogenesis
Little is known regarding the intersection of β cell stress and pathways that lead to EV formation, although common players exist in both diabetes pathogenesis and EV biogenesis. As one example, cytokines such as IL-1β or TNFα have been shown to activate the sphingomyelin/ceramide pathway or increase ceramide content [124]. Proinflammatory cytokines also activate or upregulate SMases, leading to increased ceramide β cell content with associated ER stress, mitochondrial damage and caspase-3 activation [124,125]. It is conceivable that these same pathways influencing ceramide content in β cells could also affect EV biogenesis and EV properties. Ceramide-rich EVs induce apoptosis in astrocytes [30] as well as in IFNγ-primed oligodendrocytes [61], suggesting that similar mechanisms could be at play in other diseases, including diabetes. Another possible impact of stress-induced alterations in β cell lipid pathways involves dysregulation of intracellular endosome trafficking. Defects in autophagy have been associated with the development of both T1D and T2D [126]. Since significant overlap exists in the pathways leading to exosome formation and targeting of endosomes to degradation, reduced autophagy in T1D might be associated with changes in multivesicular endosome trafficking and exosome formation. Similarly, the induction of stress granule production by ER stress and the integrated stress response is thought to alter the potential content of EVs as they are shuttled through the endosome pathway. Stress granules are non-membranous cellular inclusions that contain translationally inhibited mRNAs and RNA binding proteins [127], and it has been shown that hnRNPs present in stress granules can be identified in EVs [128].
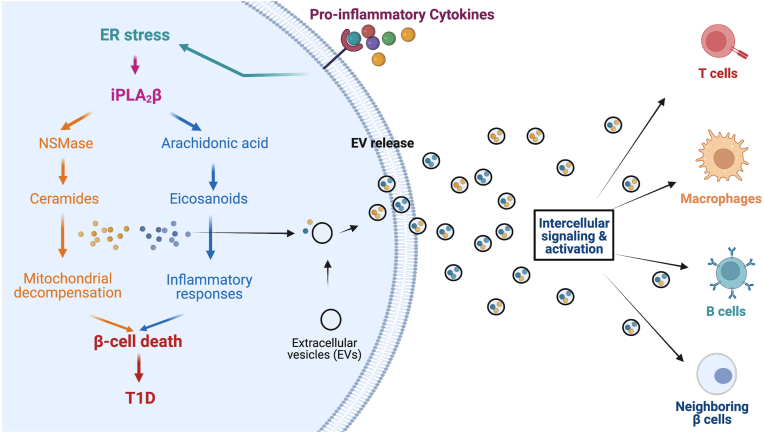
ER stress-induced β cell death is associated with increases in ceramide generation via hydrolysis of sphingomyelins by NSMase. Accumulations in ceramides cause decompensation of the mitochondria, leading to release of cytochrome C into the cytosol, inducing caspase-3-mediated β cell apoptosis [[129], [130], [131], [132]]. More recently, it was reported that the development of T1D is associated with increased production of proinflammatory eicosanoids by immune cells [133,134]. These outcomes are likely mediated by activation of the Ca2+-independent phospholipase A2β (iPLA2β). Given the observations that ER stress in β cells precedes T1D onset [135,136] and the literature available to date on EV lipid cargo, we posit that cells integral to inducing β cell death in T1D communicate with each other, in part through lipid signals delivered by EVs, as illustrated in Figure 3.
Figure 3.
Activation of iPLA2β signaling and incorporation of lipid products into exosomes. The figure depicts that stressed β cells in the setting of type 1 diabetes induce ER stress and iPLA2β activity, whose downstream products lead to both β cell death and are incorporated into exosomes for intercellular signaling to neighboring islet cells and immune cells.
The impact of the lipid load in β cell-derived EVs has received little attention, as evidence by a recent report in which a survey of EV lipid content across various donor cells did not include the β cell [137]. Notably, a study performed with RBL-2H3 mast cells revealed that, in addition to prostaglandins, the EVs from these cells contain a truncated iPLA2β protein [87]. It has also been previously reported that this isoform is generated through caspase 3-mediated cleavage of iPLA2β and that the truncated iPLA2β protein manifests greater specific activity [133,134]. Collectively, these observations provide rationale to examine the contribution of EV-derived lipid signaling shuttled between donor/recipient β cells and immune cells. As can be deduced from the reports of EV-lipid signaling in various contexts above, such investigations will undoubtedly provide insights to new therapeutic avenues and identify lipid-related biomarkers of T1D onset.
6. Extracellular vesicle components as potential T1D biomarkers
For clinical trial purposes, a staging scheme for T1D has been recently described [138] and includes pre-symptomatic periods of auto-antibody positivity but without overt hyperglycemia. Therefore, the development of biomarkers is deemed crucial for disease prognosis and for the identification of patients who would benefit from preventative therapies. EVs have potential as biomarkers, as they carry cellular components from the cells from which they are derived that serve as molecular “signatures” of the disease process. Because specific molecular species (miRNAs, lipids, metabolites) are enriched in EVs, interrogation of EVs for these molecules might lead to the identification of biomarkers that would be otherwise diluted and lost to detection in circulating plasma. By example, in prostate cancer, urinary EVs have been shown to carry RNAs that serve as signatures of disease outcomes [139]. Similarly, in T1D, diverse components of β cell-derived EVs, including miRNA, DNA, protein, and metabolites have been identified [140,141]. It is reported that rat and human pancreatic β cells under inflammatory stress release EVs containing GAD65, IA2, and proinsulin that can subsequently can activate dendritic cells and propagate adaptive immunity [142]. Studies have shown β cell EV production and cargo are altered upon proinflammatory cytokine exposure [111,143], reinforcing their potential to harbor biomarkers. Further, various clinical studies report differential expression of various miRNA in subjects with long-standing T1D compared to healthy controls, among which miR-21–5p is being considered as a biomarker [110,[144], [145], [146]]. With respect to diabetes, one group used lipidomic profiling of urinary exosomes to distinguish individuals with diabetic nephropathy (as compared to controls without nephropathy) and were able to identify 5 lipids as significantly different [147]. Further research is warranted to determine if a single biomarker or a group of biomarkers in these EVs reflect β cell health.
Dysregulation in lipid metabolism has been suggested to precede the onset of autoimmunity in T1D [148]. In this study, serum metabolomics was performed from samples obtained from youth who later progressed to T1D. As compared to healthy controls, youth who later progressed to T1D demonstrated increased levels of proinflammatory lysophosphotidylcholines several months before seroconversion to auto-antibody positivity. Another study demonstrated the utility of sphingolipids as biomarkers in T1D [149]. In that study, using animal models of diabetes, it was demonstrated that sphingosine-1-phosphate is elevated compared to the controls. In addition, diabetic animals demonstrated reductions in plasma levels of cytoprotective omega-9 24:1 (nervonic acid)-containing ceramide, sphingomyelin, and cerebrosides. A metabolomic study from cord blood also demonstrated that levels of phospholipids, mainly phosphatidylcholines and phosphatidylethanolamines, were decreased in T1D subjects long before diagnosis [150]. Finally, a recent study also demonstrated that the iPLA2-derived lipids are enhance in T1D subjects compared to healthy controls [151]. These different studies reveal the relevance of lipids as biomarkers of T1D progression. It is unknown if these lipids are also released as EV cargo; however, considering the aforementioned studies in which lipid EV cargo appears to contribute to disease pathogenesis, it is likely that lipid EV cargo contributes to the development of diabetes and could serve as a biomarker.
Whereas EV properties such as their relative stability and protection from circulating protease/nuclease activity are attractive for their use in biomarker development, a major challenge to their clinical utility lies with their isolation. Several biophysical approaches have been employed for the isolation of EVs including ultracentrifugation, density gradient ultracentrifugation, polymer-based precipitation, size-exclusion chromatography, and immunocapture. These techniques all have their advantages, but they also suffer from unique profiles of contaminants. For instance, while ultracentrifugation is technically simple, it results in poor recovery and large compositional variability. Polymer-based precipitation has the advantage that it is a rapid isolation technique allowing for isolation and characterization of large numbers of samples; however, polymers can alter the composition of the EVs, and the levels of polymers required to remove contaminating lipoproteins result in precipitation of a majority of the EVs of interest. Similarly, while size exclusion chromatography is gentle and has excellent recovery and repeatability/reproducibility, EVs share similar hydrodynamic diameters with large plasma lipoproteins, which results in co-fractionation of these distinct populations that can confound compositional analysis [[152], [153], [154], [155]]. This issue remains an ongoing concern that receives continually refined recommendations by the International Society for Extracellular Vesicles [8].
7. Bioengineering EVs
With the increasing body of knowledge on factors effecting EV biogenesis, secretion, contents, and (patho)-physiological roles, more research is now being focused on how EVs might be used as therapeutic tools. Indeed, EV-based therapeutics can impart many of the same effects as their cell-based counterparts while avoiding several key drawbacks associated with patient risk, scalability, and cost [156]. The distinct biological structure and function of EVs means that they possess high physiochemical stability [157], innate biocompatibility, and the ability to interact with cells through signaling, fusion, and delivery [158]. Despite these advantages, EVs must still be altered in many cases to complement or enhance their therapeutic applicability. Here, we will discuss several approaches for EV engineering and suggest how they might be applied to T1D, as well as some considerations for translational potential and commercial scalability. We will also discuss how knowledge of lipid EV cargo is crucial for therapeutic applicability.
Of the different approaches for EV functionalization, indirect engineering through changing the parent cells is perhaps the most readily adoptable method, as cell functionalization is a highly established and widely employed field. For example, it was reported that exosomes from human bone marrow mesenchymal stem cells transfected with pshFas-anti-miR-375 silence Fas and miR-375 in human islets to improve viability and function against inflammatory cytokines after transplantation [159]. Although this approach involved gene silencing, a more commonly employed method is to use tetraspanins such as CD9, C63, and CD81, which are enriched in EVs [160], as backbones for fusion proteins intended for EV loading. Tetraspanin fusion proteins are used for a variety of purposes, from investigating secretion [161] and trafficking [162] of EVs, to enabling more flexible engineering of EV surfaces [163] and efficient EV loading of therapeutic cargo [164,165]. Other groups have used Rab5a [166] or lactadherin [167], both also enriched in EVs, as backbones for fusion proteins for EV loading. Recently, it was reported that exosomes derived from HEK293 cells engineered to express an MHC-I/CD81/IL-2 fusion and a CD80/lactadherin fusion were able to selectively deliver co-stimulation and IL-2 to antigen-specific CD8+ T cells [168], highlighting the distinctive role that engineered EVs can play in antigen specific immune interactions. Engineering EVs for T1D therapeutics through cell functionalization therefore seems highly feasible. For example, β cells genetically engineered to express tetraspanin fusions containing immunomodulatory proteins or cytokines could be loaded into EVs that inherently display β cell autoantigens in native MHC. Such EVs could then function as a cell-free, antigen specific immunotherapy against diabetogenic T cells. However, cell-based EV functionalization typically results in low packaging efficiency of therapeutic cargo, which is highlighted by the large knowledge gap in the importance of post-translational modifications in conferring specific properties to proteins and sorting them into EVs [169]. There have been scattered reports of lipid post-translational modifications, such as palmitoylation, that targets a protein to EVs [170]; however, their relevance for EVs in T1D therapeutics remains to be seen.
To overcome packaging inefficiencies, many groups have focused on directly engineering EVs after purification. Direct EV engineering can be accomplished in both passive and active manners to modify the surface with membrane binding species or to encapsulate materials into the vesicle interior. Loading EV interiors with cargo is achieved through membrane permeabilization. Permeabilizing via electroporation is commonly used to load EVs with RNA cargos that then are taken up by target cells to impart therapeutic effects [[171], [172], [173]]. Active EV surface modification often includes some sort of chemical process to alter the lipids or proteins on the surface of EVs, such as through click chemistry [174,175]. Multiple groups have also devised ways to directly load peptide antigen into exosomal MHC [176,177], again highlighting the potential for EVs as antigen specific therapies. Passive surface modification relies on noncovalent interactions including hydrophobic insertions, regularly used to label EVs with hydrophobic dyes for investigating trafficking [[178], [179], [180]], and receptor-ligand binding. In one recent example, an anchor peptide that enables EV targeting of cargo through binding to CD63 was identified [181]. Coupling several of these direct engineering strategies enables a “plug-and-play” approach for internal cargo loading and external decorating to engineer “designer” EVs. For instance, EVs derived from dendritic cells (DCs) could be loaded with therapeutic RNAs like miR-375 [159] and targeted to islets through surface functionalization, or directly loaded with β cell autoantigens and functionalized with immunomodulatory proteins. Indeed, there is already precedence for using DC-derived EVs as therapies for cancer in clinical trials [182].
In addition to creating targeted EV cargo, knowledge of cell membrane lipid components could be used to target therapeutic EVs to specific cell types. The enrichment of phosphatidyl serine in EV membranes has also been exploited experimentally for EV targeting by fusing a phosphatidyl serine-targeting protein to a ligand for cell receptors of interest, thereby targeting the EVs to the desired cell type [183]. The extent to which the lipid composition of the EV membrane participates in specific cellular targeting is not known. However, fusion of EVs with liposomes have shown that different lipids can contribute to increased uptake. The caveat of these studies is that they were only performed in vitro, and not in vivo [184]. To add to the complexity, a recent study showed that cellular uptake does not automatically indicate integration into the cytosol; the exosomes can be re-released without apparent modification or housed in endosomes for days only to undergo degradation [185].
Any EV-based therapeutic intended for clinical use must pass regulatory rigor and conform to good manufacturing practice (GMP) grade protocols. To this end, several groups have described technologies and protocols for EV manufacturing that adhere to GMP standards [[186], [187], [188]]. Considerations for scalability and quality control are also important. Commercial bioreactors are widely available for large-scale cell expansion, with microcarriers [189] or hollow fiber bioreactors [190] now being used to reduce costs. It is possible to collect EVs from cells cultured in hollow fiber bioreactors [191], demonstrating the field's interest in commercializing EV therapeutics. Furthermore, recombinant EVs as a biological reference tool for quantifying recovery efficiencies of common techniques and normalizing and improving sensitivity of EV counting methods has been described [192]. While there are already several EV-based treatments in clinical trials for various diseases that show great promise [193], technologies such as those described above will be important for widespread, safe adoption of this class of therapeutics.
8. Conclusions
EVs represent a new horizon in the study of diabetes pathogenesis. In recent years, it has become apparent that different classes of EVs have different intracellular origins, contain sometimes distinct cargo, and have lipid content that is enriched compared to the cell of origin. Moreover, it is now appreciated that the content of EVs can vary depending on the stress state of the cell. This latter observation has significant ramifications with respect to intercellular communication, thereby allowing neighboring cells to recognize and possibly respond to states of prevailing stress by incorporation of EV cargo. Further elucidation of EV function in health and disease has the potential to leverage EVs as circulating biomarkers of prevailing cellular health and possibly engage engineered EVs for targeted therapeutics. Nevertheless, several challenges remain in leveraging EVs to better understand biology and manipulate pathologic states. These include, but are not limited to, a better understanding of how cargo (lipids, RNA, metabolites) is specifically shuttled into EVs, methods for the reproducible and reliable isolation of EVs, and methods for engineering and cargo loading of EVs for cell-directed therapies. Given the potential promise that EVs hold for therapeutics and diagnostics, recent requests for applications from major granting agencies (e.g. US National Institutes of Health) ensure that EV biology is likely to progress rapidly in the coming years to meet these and other challenges.
Acknowledgements
The authors wish to acknowledge the National Institutes of Health grants U01 DK122786 (to RGM), P30 DK020595 (to University of Chicago), and P30 DK097512 (to Indiana University) for supporting work by the authors in the area of EV biology in human islets.
Conflict of interest
None declared.
References
- 1.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Research and Clinical Practice. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Roep B.O., Thomaidou S., van Tienhoven R., Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?) Nature Reviews Endocrinology. 2020 doi: 10.1038/s41574-020-00443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson P.J., Shah A., Ntranos V., Van Gool F., Atkinson M., Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metabolism. 2019;29(5):1045–1060. doi: 10.1016/j.cmet.2019.01.021. e10. [DOI] [PubMed] [Google Scholar]
- 4.Falcone M., Fousteri G. Role of the PD-1/PD-L1 dyad in the maintenance of pancreatic immune tolerance for prevention of type 1 diabetes. Frontiers in Endocrinology. 2020;11:569. doi: 10.3389/fendo.2020.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Communication and Signaling: CCS. 2021;19(1):47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das Gupta A., Krawczynska N., Nelson E.R. Extracellular vesicles-the next frontier in endocrinology. Endocrinology. 2021;162(9):bqab133. doi: 10.1210/endocr/bqab133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pegtel D.M., Gould S.J. Exosomes. Annual Review of Biochemistry. 2019;88(1):487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 8.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salimi L., Akbari A., Jabbari N., Mojarad B., Vahhabi A., Szafert S., et al. Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells. Cell & Bioscience. 2020;10(1):64. doi: 10.1186/s13578-020-00426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanc L., Vidal M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases. 2018;9(1–2):95–106. doi: 10.1080/21541248.2016.1264352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbanelli L., Magini A., Buratta S., Brozzi A., Sagini K., Polchi A., et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes. 2013;4(2):152–170. doi: 10.3390/genes4020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricarico C., Clancy J., D'Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2016;8(4):220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Souza-Schorey C., Clancy J.W. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes & Development. 2012;26(12):1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muralidharan-Chari V., Clancy J., Plou C., Romao M., Chavrier P., Raposo G., et al. ARF6-regulated shedding of tumor-cell derived plasma membrane microvesicles. Current Biology : CB. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabhan J.F., Hu R., Oh R.S., Cohen S.N., Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(11):4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T., Gilkes D.M., Takano N., Xiang L., Luo W., Bishop C.J., et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(31):E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexy T., Rooney K., Weber M., Gray W.D., Searles C.D. TNF-α alters the release and transfer of microparticle-encapsulated miRNAs from endothelial cells. Physiological Genomics. 2014;46(22):833–840. doi: 10.1152/physiolgenomics.00079.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng F., Fussenegger M. Shedding light on extracellular vesicle biogenesis and bioengineering. Advanced Science. 2020;8(1):2003505. doi: 10.1002/advs.202003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skotland T., Hessvik N.P., Sandvig K., Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. Journal of Lipid Research. 2019;60(1):9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mir B., Goettsch C. Extracellular vesicles as delivery vehicles of specific cellular cargo. Cells. 2020;9(7):E1601. doi: 10.3390/cells9071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boal F., Mansour R., Gayral M., Saland E., Chicanne G., Xuereb J.-M., et al. TOM1 is a PI5P effector involved in the regulation of endosomal maturation. Journal of Cell Science. 2015;128(4):815–827. doi: 10.1242/jcs.166314. [DOI] [PubMed] [Google Scholar]
- 22.Record M., Silvente-Poirot S., Poirot M., Wakelam M.J.O. Extracellular vesicles: lipids as key components of their biogenesis and functions. Journal of Lipid Research. 2018;59(8):1316–1324. doi: 10.1194/jlr.E086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P., Zhang Y., Li H., Chieu H.K., Munn A.L., Yang H. AAA ATPases regulate membrane association of yeast oxysterol binding proteins and sterol metabolism. The EMBO Journal. 2005;24(17):2989–2999. doi: 10.1038/sj.emboj.7600764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skotland T., Sagini K., Sandvig K., Llorente A. An emerging focus on lipids in extracellular vesicles. Advanced Drug Delivery Reviews. 2020;159:308–321. doi: 10.1016/j.addr.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Menaldino D.S., Bushnev A., Sun A., Liotta D.C., Symolon H., Desai K., et al. Sphingoid bases and de novo ceramide synthesis: enzymes involved, pharmacology and mechanisms of action. Pharmacological Research. 2003;47(5):373–381. doi: 10.1016/s1043-6618(03)00054-9. [DOI] [PubMed] [Google Scholar]
- 26.Perry D.K. Serine palmitoyltransferase: role in apoptotic de novo ceramide synthesis and other stress responses. Biochimica et Biophysica Acta. 2002;1585(2–3):146–152. doi: 10.1016/s1388-1981(02)00335-9. [DOI] [PubMed] [Google Scholar]
- 27.Marchesini N., Hannun Y.A. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochemistry and Cell Biology = Biochimie Et Biologie Cellulaire. 2004;82(1):27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 28.Kitatani K., Idkowiak-Baldys J., Hannun Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cellular Signalling. 2008;20(6):1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Q., Wang G., Wakade S., Dasgupta S., Dinkins M., Kong J.N., et al. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Molecular Biology of the Cell. 2014;25(11):1715–1729. doi: 10.1091/mbc.E13-12-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G., Dinkins M., He Q., Zhu G., Poirier C., Campbell A., et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4) Journal of Biological Chemistry. 2012;287(25):21384–21395. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (New York, N.Y.) 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 32.Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M.Á., et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. Journal of Biological Chemistry. 2013;288(15):10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phuyal S., Hessvik N.P., Skotland T., Sandvig K., Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS Journal. 2014;281(9):2214–2227. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 35.Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. Journal of Biological Chemistry. 2010;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsui T., Osaki F., Hiragi S., Sakamaki Y., Fukuda M. ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Reports. 2021;22(5):e51475. doi: 10.15252/embr.202051475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bianco F., Perrotta C., Novellino L., Francolini M., Riganti L., Menna E., et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO Journal. 2009;28(8):1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S., Lin Z., Jiang X., Yu X. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacologica Sinica. 2018;39(4):542–551. doi: 10.1038/aps.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skryabin G.O., Komelkov A.V., Savelyeva E.E., Tchevkina E.M. Lipid rafts in exosome biogenesis. Biochemistry. Biokhimiia. 2020;85(2):177–191. doi: 10.1134/S0006297920020054. [DOI] [PubMed] [Google Scholar]
- 40.Yáñez-Mó M., Barreiro O., Gordon-Alonso M., Sala-Valdés M., Sánchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends in Cell Biology. 2009;19(9):434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Strauss K., Goebel C., Runz H., Möbius W., Weiss S., Feussner I., et al. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. Journal of Biological Chemistry. 2010;285(34):26279–26288. doi: 10.1074/jbc.M110.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logozzi M., De Milito A., Lugini L., Borghi M., Calabrò L., Spada M., et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llorente A., Skotland T., Sylvänne T., Kauhanen D., Róg T., Orłowski A., et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochimica et Biophysica Acta. 2013;1831(7):1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Brzozowski J.S., Jankowski H., Bond D.R., McCague S.B., Munro B.R., Predebon M.J., et al. Lipidomic profiling of extracellular vesicles derived from prostate and prostate cancer cell lines. Lipids in Health and Disease. 2018;17(1):211. doi: 10.1186/s12944-018-0854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan Q., Yi K., Li X., Cui X., Yang E., Chen N., et al. Phosphatidylcholine-engineered exosomes for enhanced tumor cell uptake and intracellular antitumor drug delivery. Macromolecular Bioscience. 2021;21(8):e2100042. doi: 10.1002/mabi.202100042. [DOI] [PubMed] [Google Scholar]
- 46.Durcin M., Fleury A., Taillebois E., Hilairet G., Krupova Z., Henry C., et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. Journal of Extracellular Vesicles. 2017;6(1):1305677. doi: 10.1080/20013078.2017.1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brouwers J.F., Aalberts M., Jansen J.W.A., van Niel G., Wauben M.H., Stout T.A.E., et al. Distinct lipid compositions of two types of human prostasomes. Proteomics. 2013;13(10–11):1660–1666. doi: 10.1002/pmic.201200348. [DOI] [PubMed] [Google Scholar]
- 48.Del Boccio P., Raimondo F., Pieragostino D., Morosi L., Cozzi G., Sacchetta P., et al. A hyphenated microLC-Q-TOF-MS platform for exosomal lipidomics investigations: application to RCC urinary exosomes. Electrophoresis. 2012;33(4):689–696. doi: 10.1002/elps.201100375. [DOI] [PubMed] [Google Scholar]
- 49.Skotland T., Ekroos K., Kauhanen D., Simolin H., Seierstad T., Berge V., et al. vol. 70. European Journal of Cancer; Oxford, England: 2017. pp. 122–132. (Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers). 1990. [DOI] [PubMed] [Google Scholar]
- 50.Chen S., Datta-Chaudhuri A., Deme P., Dickens A., Dastgheyb R., Bhargava P., et al. Lipidomic characterization of extracellular vesicles in human serum. Journal of Circulating Biomarkers. 2019;8 doi: 10.1177/1849454419879848. 1849454419879848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alharbi M.G., Lee S.H., Abdelazim A.M., Saadeldin I.M., Abomughaid M.M. Role of extracellular vesicles in compromising cellular resilience to environmental stressors. BioMed Research International. 2021;2021:9912281. doi: 10.1155/2021/9912281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Zhao Y., Yin Y., Jia X., Mao L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered. 2021;12(1):8186–8201. doi: 10.1080/21655979.2021.1977767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chidester S., Livinski A.A., Fish A.F., Joseph P.V. The role of extracellular vesicles in β-cell function and viability: a scoping review. Frontiers in Endocrinology. 2020;11 doi: 10.3389/fendo.2020.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Record M., Carayon K., Poirot M., Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochimica et Biophysica Acta. 2014;1841(1):108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Zamith-Miranda D., Peres da Silva R., Couvillion S.P., Bredeweg E.L., Burnet M.C., Coelho C., et al. Omics approaches for understanding biogenesis, composition and functions of fungal extracellular vesicles. Frontiers in Genetics. 2021;12:648524. doi: 10.3389/fgene.2021.648524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Javeed N. Shedding perspective on extracellular vesicle biology in diabetes and associated metabolic syndromes. Endocrinology. 2019;160(2):399–408. doi: 10.1210/en.2018-01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Record M., Poirot M., Silvente-Poirot S. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie. 2014;96:67–74. doi: 10.1016/j.biochi.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 58.Verderio C., Gabrielli M., Giussani P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. Journal of Lipid Research. 2018;59(8):1325–1340. doi: 10.1194/jlr.R083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Blitterswijk W.J., van der Luit A.H., Veldman R.J., Verheij M., Borst J. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochemical Journal. 2003;369(Pt 2):199–211. doi: 10.1042/BJ20021528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogretmen B., Hannun Y.A. Biologically active sphingolipids in cancer pathogenesis and treatment. Nature Reviews Cancer. 2004;4(8):604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 61.Podbielska M., Szulc Z.M., Kurowska E., Hogan E.L., Bielawski J., Bielawska A., et al. Cytokine-induced release of ceramide-enriched exosomes as a mediator of cell death signaling in an oligodendroglioma cell line. Journal of Lipid Research. 2016;57(11):2028–2039. doi: 10.1194/jlr.M070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Testai F.D., Landek M.A., Dawson G. Regulation of sphingomyelinases in cells of the oligodendrocyte lineage. Journal of Neuroscience Research. 2004;75(1):66–74. doi: 10.1002/jnr.10816. [DOI] [PubMed] [Google Scholar]
- 63.Dhib-Jalbut S., Arnold D.L., Cleveland D.W., Fisher M., Friedlander R.M., Mouradian M.M., et al. Neurodegeneration and neuroprotection in multiple sclerosis and other neurodegenerative diseases. Journal of Neuroimmunology. 2006;176(1–2):198–215. doi: 10.1016/j.jneuroim.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 64.Vidaurre O.G., Haines J.D., Katz Sand I., Adula K.P., Huynh J.L., McGraw C.A., et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain. 2014;137(8):2271–2286. doi: 10.1093/brain/awu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alessenko A.V., Bugrova A.E., Dudnik L.B. Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer's disease. Biochemical Society Transactions. 2004;32(Pt 1):144–146. doi: 10.1042/bst0320144. [DOI] [PubMed] [Google Scholar]
- 66.Mielke M.M., Bandaru V.V.R., Haughey N.J., Xia J., Fried L.P., Yasar S., et al. Serum ceramides increase the risk of Alzheimer disease. Neurology. 2012;79(7):633–641. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mielke M.M., Haughey N.J., Bandaru V.V.R., Schech S., Carrick R., Carlson M.C., et al. Plasma ceramides are altered in MCI and predict cognitive decline and hippocampal volume loss. Alzheimer's and Dementia : The Journal of the Alzheimer’s Association. 2010;6(5):378–385. doi: 10.1016/j.jalz.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dinkins M.B., Enasko J., Hernandez C., Wang G., Kong J., Helwa I., et al. Neutral sphingomyelinase-2 deficiency ameliorates Alzheimer's disease pathology and improves cognition in the 5XFAD mouse. Journal of Neuroscience. 2016;36(33):8653–8667. doi: 10.1523/JNEUROSCI.1429-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinkins M.B., Wang G., Bieberich E. Sphingolipid-enriched extracellular vesicles and Alzheimer's disease: a decade of research. Journal of Alzheimer's Disease : JAD. 2017;60(3):757–768. doi: 10.3233/JAD-160567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bengoa-Vergniory N., Roberts R.F., Wade-Martins R., Alegre-Abarrategui J. Alpha-synuclein oligomers: a new hope. Acta Neuropathologica. 2017;134(6):819–838. doi: 10.1007/s00401-017-1755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sackmann V., Sinha M.S., Sackmann C., Civitelli L., Bergström J., Ansell-Schultz A., et al. Inhibition of nSMase2 reduces the transfer of oligomeric α-synuclein irrespective of hypoxia. Frontiers in Molecular Neuroscience. 2019;12:200. doi: 10.3389/fnmol.2019.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shamseddine A.A., Airola M.V., Hannun Y.A. Roles and regulation of Neutral Sphingomyelinase-2 in cellular and pathological processes. Advances in Biological Regulation. 2015;57:24–41. doi: 10.1016/j.jbior.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pieragostino D., Cicalini I., Lanuti P., Ercolino E., di Ioia M., Zucchelli M., et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Scientific Reports. 2018;8:3071. doi: 10.1038/s41598-018-21497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elsherbini A., Bieberich E. Ceramide and exosomes: a novel target in cancer biology and therapy. Advances in Cancer Research. 2018;140:121–154. doi: 10.1016/bs.acr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng Q., Li X., Wang Y., Dong M., Zhan F., Liu J. The ceramide pathway is involved in the survival, apoptosis and exosome functions of human multiple myeloma cells in vitro. Acta Pharmacologica Sinica. 2018;39(4):561–568. doi: 10.1038/aps.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin M., Liao W., Dong M., Zhu R., Xiao J., Sun T., et al. Exosomal neutral sphingomyelinase 1 suppresses hepatocellular carcinoma via decreasing the ratio of sphingomyelin/ceramide. FEBS Journal. 2018;285(20):3835–3848. doi: 10.1111/febs.14635. [DOI] [PubMed] [Google Scholar]
- 77.Deng Z., Mu J., Tseng M., Wattenberg B., Zhuang X., Egilmez N.K., et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nature Communications. 2015;6:6956. doi: 10.1038/ncomms7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shin K.-O., Ha D.H., Kim J.O., Crumrine D.A., Meyer J.M., Wakefield J.S., et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de Novo synthesis of ceramides in atopic dermatitis. Cells. 2020;9(3):680. doi: 10.3390/cells9030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scesa G., Moyano A.L., Bongarzone E.R., Givogri M.I. PORT-TO-PORT delivery: mobilization of toxic sphingolipids via extracellular vesicles. Journal of Neuroscience Research. 2016;94(11):1333–1340. doi: 10.1002/jnr.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. Journal of Lipid Research. 2018;59(11):2037–2046. doi: 10.1194/jlr.R084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nature Reviews Immunology. 2015;15(8):511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilroy D.W., Newson J., Sawmynaden P., Willoughby D.A., Croxtall J.D. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. Federation of American Societies for Experimental Biology Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2004;18(3):489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 83.Thurnher M. Lipids in dendritic cell biology: messengers, effectors, and antigens. Journal of Leukocyte Biology. 2007;81(1):154–160. doi: 10.1189/jlb.0706438. [DOI] [PubMed] [Google Scholar]
- 84.Perrin-Cocon L., Agaugué S., Coutant F., Masurel A., Bezzine S., Lambeau G., et al. Secretory phospholipase A2 induces dendritic cell maturation. European Journal of Immunology. 2004;34(8):2293–2302. doi: 10.1002/eji.200324797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laulagnier K., Grand D., Dujardin A., Hamdi S., Vincent-Schneider H., Lankar D., et al. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Letters. 2004;572(1–3):11–14. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 86.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J.-F., et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochemical Journal. 2004;380(Pt 1):161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Subra C., Grand D., Laulagnier K., Stella A., Lambeau G., Paillasse M., et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. Journal of Lipid Research. 2010;51(8):2105–2120. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng Z.-B., Ju S., Zhuang X., Xiang X., Mu J., Liu Y., et al. Exosomes-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver natural killer T cells. Journal of Immunology (Baltimore, Md. : 1950) 2013;190(7):3579–3589. doi: 10.4049/jimmunol.1203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin M.-C., Chen S.-Y., He P.-L., Herschman H., Li H.-J. PGE2/EP4 antagonism enhances tumor chemosensitivity by inducing extracellular vesicle-mediated clearance of cancer stem cells. International Journal of Cancer. 2018;143(6):1440–1455. doi: 10.1002/ijc.31523. [DOI] [PubMed] [Google Scholar]
- 90.Lin M.-C., Chen S.-Y., Tsai H.-M., He P.-L., Lin Y.-C., Herschman H., et al. PGE2/EP4 signaling controls the transfer of the mammary stem cell state by lipid rafts in extracellular vesicles. Stem Cells (Dayton, Ohio) 2017;35(2):425–444. doi: 10.1002/stem.2476. [DOI] [PubMed] [Google Scholar]
- 91.Kim J., Hong S.-W., Kim S., Kim D., Hur D.Y., Jin D.-H., et al. Cyclooxygenase-2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes. International Journal of Oncology. 2018;52(2):613–620. doi: 10.3892/ijo.2017.4227. [DOI] [PubMed] [Google Scholar]
- 92.Filgueiras L.R., Brandt S.L., Wang S., Wang Z., Morris D.L., Evans-Molina C., et al. Leukotriene B4-mediated sterile inflammation favors susceptibility to sepsis in murine type 1 diabetes. Science Signaling. 2015;8(361):ra10. doi: 10.1126/scisignal.2005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kihara Y., Yokomizo T., Kunita A., Morishita Y., Fukayama M., Ishii S., et al. The leukotriene B4 receptor, BLT1, is required for the induction of experimental autoimmune encephalomyelitis. Biochemical and Biophysical Research Communications. 2010;394(3):673–678. doi: 10.1016/j.bbrc.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 94.Esser J., Gehrmann U., D'Alexandri F.L., Hidalgo-Estévez A.M., Wheelock C.E., Scheynius A., et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. The Journal of Allergy and Clinical Immunology. 2010;126(5):1032–1040. doi: 10.1016/j.jaci.2010.06.039. 1040.e1-1040. [DOI] [PubMed] [Google Scholar]
- 95.Majumdar R., Tavakoli Tameh A., Parent C.A. Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biology. 2016;14(1):e1002336. doi: 10.1371/journal.pbio.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Marleau S., Dallaire N., Poubelle P.E., Borgeat P. Metabolic disposition of leukotriene B4 (LTB4) and oxidation-resistant analogues of LTB4 in conscious rabbits. British Journal of Pharmacology. 1994;112(2):654–658. doi: 10.1111/j.1476-5381.1994.tb13125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Subramanian B.C., Majumdar R., Parent C.A. The role of LTB4-BLT1 AXIS in chemotactic gradient sensing and directed leukocyte migration. Seminars in Immunology. 2017;33:16–29. doi: 10.1016/j.smim.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kulkarni A., Nadler J.L., Mirmira R.G., Casimiro I. Regulation of tissue inflammation by 12-lipoxygenases. Biomolecules. 2021;11(5):717. doi: 10.3390/biom11050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hernandez-Perez M., Kulkarni A., Samala N., Sorrell C., El K., Haider I., et al. A 12-lipoxygenase-Gpr31 signaling axis is required for pancreatic organogenesis in the zebrafish. Federation of American Societies for Experimental Biology Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2020;34(11):14850–14862. doi: 10.1096/fj.201902308RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dilly A., Ekambaram P., Guo Y., Cai Y., Tucker S.C., Fridman R., et al. Platelet-type 12-lipoxygenase induces MMP9 expression and cellular invasion via activation of PI3K/Akt/NF-κB. International Journal of Cancer. Journal International du Cancer. 2013;133(8):1784–1791. doi: 10.1002/ijc.28165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nie D., Tang K., Diglio C., Honn K.V. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 2000;95(7):2304–2311. [PubMed] [Google Scholar]
- 102.Schneider M., Wortmann M., Mandal P.K., Arpornchayanon W., Jannasch K., Alves F., et al. Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia. 2010;12(3):254–263. doi: 10.1593/neo.91782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Funk C.D., Furci L., FitzGerald G.A. Molecular cloning, primary structure, and expression of the human platelet/erythroleukemia cell 12-lipoxygenase. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(15):5638–5642. doi: 10.1073/pnas.87.15.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Contursi A., Schiavone S., Dovizio M., Hinz C., Fullone R., Tacconelli S., et al. Platelets induce free and phospholipid-esterified 12-hydroxyeicosatetraenoic acid generation in colon cancer cells by delivering 12-lipoxygenase. Journal of Lipid Research. 2021;62:100109. doi: 10.1016/j.jlr.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duchez A.-C., Boudreau L.H., Naika G.S., Bollinger J., Belleannée C., Cloutier N., et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(27):E3564–E3573. doi: 10.1073/pnas.1507905112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lambeau G., Gelb M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annual Review of Biochemistry. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 107.Shen Z., Jiang H., Hsu H.-T., Qian L., Fu Q., Shen M., et al. MicroRNA-127 inhibits cell proliferation via targeting Kif3b in pancreatic β cells. Aging (Albany NY) 2019;11(5):1342–1355. doi: 10.18632/aging.101835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Figliolini F., Cantaluppi V., De Lena M., Beltramo S., Romagnoli R., Salizzoni M., et al. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS One. 2014;9(7):e102521. doi: 10.1371/journal.pone.0102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sims E.K., Lakhter A., Anderson-Baucum E., Kono T., Tong X., Evans-Molina C. MicroRNA 21 targets BCL2 mRNA to increase cell apoptosis in rat and human beta cells. Diabetologia. 2017;60(6):1057–1065. doi: 10.1007/s00125-017-4237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lakhter A.J., Pratt R.E., Moore R.E., Doucette K.K., Maier B.F., DiMeglio L.A., et al. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018;61(5):1124–1134. doi: 10.1007/s00125-018-4559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krishnan P., Syed F., Jiyun Kang N., Mirmira R.G., Evans-Molina C. Profiling of RNAs from human islet-derived exosomes in a model of type 1 diabetes. International Journal of Molecular Sciences. 2019;20(23):E5903. doi: 10.3390/ijms20235903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumano K., Kanak M.A., Saravanan P.B., Blanck J.P., Liu Y., Vasu S., et al. Withaferin A inhibits lymphocyte proliferation, dendritic cell maturation in vitro and prolongs islet allograft survival. Scientific Reports. 2021;11:10661. doi: 10.1038/s41598-021-90181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hosoi T., Nakashima M., Ozawa K. Incorporation of the endoplasmic reticulum stress-induced spliced form of XBP1 mRNA in the exosomes. Frontiers in Physiology. 2018;9:1357. doi: 10.3389/fphys.2018.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mattke J., Vasu S., Darden C.M., Kumano K., Lawrence M.C., Naziruddin B. Role of exosomes in islet transplantation. Frontiers in Endocrinology. 2021;12:681600. doi: 10.3389/fendo.2021.681600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Favaro E., Carpanetto A., Caorsi C., Giovarelli M., Angelini C., Cavallo-Perin P., et al. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59(2):325–333. doi: 10.1007/s00125-015-3808-0. [DOI] [PubMed] [Google Scholar]
- 116.Javeed N., Sagar G., Dutta S.K., Smyrk T.C., Lau J.S., Bhattacharya S., et al. Pancreatic cancer-derived exosomes causes paraneoplastic β-cell dysfunction. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2015;21(7):1722–1733. doi: 10.1158/1078-0432.CCR-14-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ribeiro D., Andersson E.-M., Heath N., Persson-kry A., Collins R., Hicks R., et al. Human pancreatic islet-derived extracellular vesicles modulate insulin expression in 3D-differentiating iPSC clusters. PLoS One. 2017;12(11):e0187665. doi: 10.1371/journal.pone.0187665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ribeiro D., Horvath I., Heath N., Hicks R., Forslöw A., Wittung-Stafshede P. Extracellular vesicles from human pancreatic islets suppress human islet amyloid polypeptide amyloid formation. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(42):11127–11132. doi: 10.1073/pnas.1711389114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rutman A.K., Negi S., Gasparrini M., Hasilo C.P., Tchervenkov J., Paraskevas S. Immune response to extracellular vesicles from human islets of langerhans in patients with type 1 diabetes. Endocrinology. 2018;159(11):3834–3847. doi: 10.1210/en.2018-00649. [DOI] [PubMed] [Google Scholar]
- 120.Cooper T.T., Sherman S.E., Bell G.I., Dayarathna T., McRae D.M., Ma J., et al. Ultrafiltration and injection of islet regenerative stimuli secreted by pancreatic mesenchymal stromal cells. Stem Cells and Development. 2021;30(5):247–264. doi: 10.1089/scd.2020.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen J., Chen J., Cheng Y., Fu Y., Zhao H., Tang M., et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Research & Therapy. 2020;11:97. doi: 10.1186/s13287-020-01610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gesmundo, I., Pardini, B., Gargantini, E., Gamba, G., Birolo, G., Fanciulli, A., et al., n.d. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic β cells. JCI Insight 6(5): e141962, Doi: 10.1172/jci.insight.141962. [DOI] [PMC free article] [PubMed]
- 123.Javeed N., Her T.K., Brown M.R., Vanderboom P., Rakshit K., Egan A.M., et al. Pro-inflammatory β cell small extracellular vesicles induce β cell failure through activation of the CXCL10/CXCR3 axis in diabetes. Cell Reports. 2021;36(8):109613. doi: 10.1016/j.celrep.2021.109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gurgul-Convey E. Sphingolipids in type 1 diabetes: focus on beta-cells. Cells. 2020;9(8):E1835. doi: 10.3390/cells9081835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lei X., Bone R.N., Ali T., Zhang S., Bohrer A., Tse H.M., et al. Evidence of contribution of iPLA2β-mediated events during islet β-cell apoptosis due to proinflammatory cytokines suggests a role for iPLA2β in T1D development. Endocrinology. 2014;155(9):3352–3364. doi: 10.1210/en.2013-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muralidharan C., Linnemann A.K. β-Cell autophagy in the pathogenesis of type 1 diabetes. American Journal of Physiology. Endocrinology and Metabolism. 2021;321(3):E410–E416. doi: 10.1152/ajpendo.00151.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Riggs C.L., Kedersha N., Ivanov P., Anderson P. Mammalian stress granules and P bodies at a glance. Journal of Cell Science. 2020;133(16):jcs242487. doi: 10.1242/jcs.242487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jimenez L., Yu H., McKenzie A.J., Franklin J.L., Patton J.G., Liu Q., et al. Quantitative proteomic analysis of small and large extracellular vesicles (EVs) reveals enrichment of adhesion proteins in small EVs. Journal of Proteome Research. 2019;18(3):947–959. doi: 10.1021/acs.jproteome.8b00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ali T., Lei X., Barbour S.E., Koizumi A., Chalfant C.E., Ramanadham S. Alterations in β-cell sphingolipid profile Associated with ER stress and iPLA2β: another contributor to β-cell apoptosis in type 1 diabetes. Molecules. 2021;26(21):6361. doi: 10.3390/molecules26216361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lei X., Zhang S., Barbour S.E., Bohrer A., Ford E.L., Koizumi A., et al. Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression. Journal of Biological Chemistry. 2010;285(9):6693–6705. doi: 10.1074/jbc.M109.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lei X., Zhang S., Bohrer A., Barbour S.E., Ramanadham S. Role of calcium-independent phospholipase A2β in human pancreatic islet β-cell apoptosis. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(11):E1386–E1395. doi: 10.1152/ajpendo.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lei X., Zhang S., Bohrer A., Ramanadham S. Calcium-independent phospholipase A2 (iPLA2 beta)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. Journal of Biological Chemistry. 2008;283(50):34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Atsumi G., Murakami M., Kojima K., Hadano A., Tajima M., Kudo I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2alpha inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. Journal of Biological Chemistry. 2000;275(24):18248–18258. doi: 10.1074/jbc.M000271200. [DOI] [PubMed] [Google Scholar]
- 134.Ramanadham S., Hsu F.-F., Zhang S., Jin C., Bohrer A., Song H., et al. Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2β) and suppressed by inhibition of iPLA2β. Biochemistry. 2004;43(4):918–930. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marhfour I., Lopez X.M., Lefkaditis D., Salmon I., Allagnat F., Richardson S.J., et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55(9):2417–2420. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- 136.Tersey S.A., Nishiki Y., Templin A.T., Cabrera S.M., Stull N.D., Colvin S.C., et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61(4):818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skotland T., Sandvig K., Llorente A. Lipids in exosomes: current knowledge and the way forward. Progress in Lipid Research. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 138.Insel R.A., Dunne J.L., Atkinson M.A., Chiang J.L., Dabelea D., Gottlieb P.A., et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the endocrine society, and the American diabetes association. Diabetes Care. 2015;38(10):1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pang B., Zhu Y., Ni J., Thompson J., Malouf D., Bucci J., et al. Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics. 2020;10(5):2309–2326. doi: 10.7150/thno.39486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia-Contreras M., Brooks R.W., Boccuzzi L., Robbins P.D., Ricordi C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. European Review for Medical and Pharmacological Sciences. 2017;21(12):2940–2956. [PubMed] [Google Scholar]
- 141.Grieco G.E., Fignani D., Formichi C., Nigi L., Licata G., Maccora C., et al. Extracellular vesicles in immune system regulation and type 1 diabetes: cell-to-cell communication mediators, disease biomarkers, and promising therapeutic tools. Frontiers in Immunology. 2021;12:682948. doi: 10.3389/fimmu.2021.682948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cianciaruso C., Phelps E.A., Pasquier M., Hamelin R., Demurtas D., Alibashe Ahmed M., et al. Primary human and rat β-cells release the intracellular autoantigens GAD65, IA-2, and proinsulin in exosomes together with cytokine-induced enhancers of immunity. Diabetes. 2017;66(2):460–473. doi: 10.2337/db16-0671. [DOI] [PubMed] [Google Scholar]
- 143.Giri K.R., de Beaurepaire L., Jegou D., Lavy M., Mosser M., Dupont A., et al. Molecular and functional diversity of distinct subpopulations of the stressed insulin-secreting cell's vesiculome. Frontiers in Immunology. 2020;11:1814. doi: 10.3389/fimmu.2020.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tesovnik T., Kovač J., Pohar K., Hudoklin S., Dovč K., Bratina N., et al. Extracellular vesicles derived human-miRNAs modulate the immune system in type 1 diabetes. Frontiers in Cell and Developmental Biology. 2020;8:202. doi: 10.3389/fcell.2020.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sabatier F., Darmon P., Hugel B., Combes V., Sanmarco M., Velut J.-G., et al. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51(9):2840–2845. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 146.Assmann T.S., Recamonde-Mendoza M., De Souza B.M., Crispim D. MicroRNA expression profiles and type 1 diabetes mellitus: systematic review and bioinformatic analysis. Endocrine Connections. 2017;6(8):773–790. doi: 10.1530/EC-17-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donoso-Quezada J., Ayala-Mar S., González-Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics and applications. Traffic (Copenhagen, Denmark) 2021;22(7):204–220. doi: 10.1111/tra.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Orešič M., Simell S., Sysi-Aho M., Näntö-Salonen K., Seppänen-Laakso T., Parikka V., et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. Journal of Experimental Medicine. 2008;205(13):2975–2984. doi: 10.1084/jem.20081800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fox T.E., Bewley M.C., Unrath K.A., Pedersen M.M., Anderson R.E., Jung D.Y., et al. Circulating sphingolipid biomarkers in models of type 1 diabetes. Journal of Lipid Research. 2011;52(3):509–517. doi: 10.1194/jlr.M010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.La Torre, D., Seppänen-Laakso, T., Larsson, H.E., Hyötyläinen, T., Ivarsson, S.A., Lernmark, A., et al., 2013. Decreased cord-blood phospholipids in young age-at-onset type 1 diabetes. Diabetes 62(11): 3951–3956, Doi: 10.2337/db13-0215. [DOI] [PMC free article] [PubMed]
- 151.Nelson A.J., Stephenson D.J., Bone R.N., Cardona C.L., Park M.A., Tusing Y.G., et al. Lipid mediators and biomarkers associated with type 1 diabetes development. JCI Insight. 2020;5(16):138034. doi: 10.1172/jci.insight.138034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Burton J.B., Carruthers N.J., Stemmer P.M. Enriching extracellular vesicles for mass spectrometry. Mass Spectrometry Reviews. 2021 doi: 10.1002/mas.21738. [DOI] [PubMed] [Google Scholar]
- 153.Coumans F.A.W., Brisson A.R., Buzas E.I., Dignat-George F., Drees E.E.E., El-Andaloussi S., et al. Methodological guidelines to study extracellular vesicles. Circulation Research. 2017;120(10):1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 154.Salomon C., Das S., Erdbrügger U., Kalluri R., Kiang Lim S., Olefsky J.M., et al. Extracellular vesicles and their emerging roles as cellular messengers in endocrinology: an endocrine society scientific statement. Endocrine Reviews: Bnac009. 2022 doi: 10.1210/endrev/bnac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Simonsen J.B. What are we looking at? Extracellular vesicles, lipoproteins, or both? Circulation Research. 2017;121(8):920–922. doi: 10.1161/CIRCRESAHA.117.311767. [DOI] [PubMed] [Google Scholar]
- 156.Théry C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nature Reviews Immunology. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 157.Clayton A., Harris C.L., Court J., Mason M.D., Morgan B.P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. European Journal of Immunology. 2003;33(2):522–531. doi: 10.1002/immu.200310028. [DOI] [PubMed] [Google Scholar]
- 158.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biology. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 159.Wen D., Peng Y., Liu D., Weizmann Y., Mahato R.I. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2016;238:166–175. doi: 10.1016/j.jconrel.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 160.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nature Reviews Immunology. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 161.Mathieu M., Névo N., Jouve M., Valenzuela J.I., Maurin M., Verweij F.J., et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nature Communications. 2021;12(1):4389. doi: 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luo W., Dai Y., Chen Z., Yue X., Andrade-Powell K.C., Chang J. Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-CD63 fusion protein. Communications Biology. 2020;3(1):114. doi: 10.1038/s42003-020-0830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Curley N., Levy D., Do M.A., Brown A., Stickney Z., Marriott G., et al. Sequential deletion of CD63 identifies topologically distinct scaffolds for surface engineering of exosomes in living human cells. Nanoscale. 2020;12(22):12014–12026. doi: 10.1039/d0nr00362j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yim N., Ryu S.-W., Choi K., Lee K.R., Lee S., Choi H., et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nature Communications. 2016;7:12277. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Duong N., Curley K., Brown A., Campanelli A., Do M.A., Levy D., et al. Decoy exosomes as a novel biologic reagent to antagonize inflammation. International Journal of Nanomedicine. 2019;14:3413–3425. doi: 10.2147/IJN.S196975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stickney Z., Losacco J., McDevitt S., Zhang Z., Lu B. Development of exosome surface display technology in living human cells. Biochemical and Biophysical Research Communications. 2016;472(1):53–59. doi: 10.1016/j.bbrc.2016.02.058. [DOI] [PubMed] [Google Scholar]
- 167.Takahashi Y., Nishikawa M., Shinotsuka H., Matsui Y., Ohara S., Imai T., et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. Journal of Biotechnology. 2013;165(2):77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 168.Yamano T., Lyu X., Hanayama R. 714 Selective expansion of antigen-specific CD8 T cells with engineered antigen presenting exosome. Journal for ImmunoTherapy of Cancer. 2021;9(Suppl 2) doi: 10.1136/jitc-2021-SITC2021.714. [DOI] [Google Scholar]
- 169.Gurunathan S., Kang M.-H., Qasim M., Khan K., Kim J.-H. Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of extracellular vesicles. International Journal of Nanomedicine. 2021;16:3357–3383. doi: 10.2147/IJN.S310357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Carnino J.M., Ni K., Jin Y. Post-translational modification regulates formation and cargo-loading of extracellular vesicles. Frontiers in Immunology. 2020;11:948. doi: 10.3389/fimmu.2020.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.El-Andaloussi S., Lee Y., Lakhal-Littleton S., Li J., Seow Y., Gardiner C., et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nature Protocols. 2012;7(12):2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 172.Wahlgren J., De L Karlson T., Brisslert M., Vaziri Sani F., Telemo E., Sunnerhagen P., et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Research. 2012;40(17):e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B., et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nature Communications. 2018;9(1):2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smyth T., Petrova K., Payton N.M., Persaud I., Redzic J.S., Graner M.W., et al. Surface functionalization of exosomes using click chemistry. Bioconjugate Chemistry. 2014;25(10):1777–1784. doi: 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang P., Dong B., Zeng E., Wang F., Jiang Y., Li D., et al. In vivo tracking of multiple tumor exosomes labeled by phospholipid-based bioorthogonal conjugation. Analytical Chemistry. 2018;90(19):11273–11279. doi: 10.1021/acs.analchem.8b01506. [DOI] [PubMed] [Google Scholar]
- 176.Hsu D.-H., Paz P., Villaflor G., Rivas A., Mehta-Damani A., Angevin E., et al. Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. Journal of Immunotherapy (Hagerstown, Md.: 1997) 2003;26(5):440–450. doi: 10.1097/00002371-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 177.Zhao Z., McGill J., Gamero-Kubota P., He M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab on a Chip. 2019;19(10):1877–1886. doi: 10.1039/c8lc01279b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Topping L.M., Thomas B.L., Rhys H.I., Tremoleda J.L., Foster M., Seed M., et al. Targeting extracellular vesicles to the arthritic joint using a damaged cartilage-specific antibody. Frontiers in Immunology. 2020;11:10. doi: 10.3389/fimmu.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Whitham M., Parker B.L., Friedrichsen M., Hingst J.R., Hjorth M., Hughes W.E., et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metabolism. 2018;27(1):237–251. doi: 10.1016/j.cmet.2017.12.001. e4. [DOI] [PubMed] [Google Scholar]
- 180.Gerwing M., Kocman V., Stölting M., Helfen A., Masthoff M., Roth J., et al. Tracking of tumor cell-derived extracellular vesicles in vivo reveals a specific distribution pattern with consecutive biological effects on target sites of metastasis. Molecular Imaging and Biology. 2020;22(6):1501–1510. doi: 10.1007/s11307-020-01521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gao X., Ran N., Dong X., Zuo B., Yang R., Zhou Q., et al. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Science Translational Medicine. 2018;10(444):eaat0195. doi: 10.1126/scitranslmed.aat0195. [DOI] [PubMed] [Google Scholar]
- 182.Fernández-Delgado I., Calzada-Fraile D., Sánchez-Madrid F. Immune regulation by dendritic cell extracellular vesicles in cancer immunotherapy and vaccines. Cancers. 2020;12(12):E3558. doi: 10.3390/cancers12123558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ramasubramanian L., Kumar P., Wang A. Engineering extracellular vesicles as nanotherapeutics for regenerative medicine. Biomolecules. 2019;10(1):E48. doi: 10.3390/biom10010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jhan Y.-Y., Prasca-Chamorro D., Palou Zuniga G., Moore D.M., Arun Kumar S., Gaharwar A.K., et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. International Journal of Pharmaceutics. 2020;573:118802. doi: 10.1016/j.ijpharm.2019.118802. [DOI] [PubMed] [Google Scholar]
- 185.O'Brien K., Ughetto S., Mahjoum S., Nair A.V., Breakefield X.O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Reports. 2022;39(2):110651. doi: 10.1016/j.celrep.2022.110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pachler K., Lener T., Streif D., Dunai Z.A., Desgeorges A., Feichtner M., et al. A Good Manufacturing Practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy. 2017;19(4):458–472. doi: 10.1016/j.jcyt.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 187.Andriolo G., Provasi E., Lo Cicero V., Brambilla A., Soncin S., Torre T., et al. Exosomes from human cardiac progenitor cells for therapeutic applications: development of a GMP-grade manufacturing method. Frontiers in Physiology. 2018;9:1169. doi: 10.3389/fphys.2018.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen Y.-S., Lin E.-Y., Chiou T.-W., Harn H.-J. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Ci Ji Yi Xue Za Zhi = Tzu-Chi Medical Journal. 2020;32(2):113–120. doi: 10.4103/tcmj.tcmj_182_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Petry F., Weidner T., Czermak P., Salzig D. Three-dimensional bioreactor technologies for the cocultivation of human mesenchymal stem/stromal cells and beta cells. Stem Cells International. 2018;2018:2547098. doi: 10.1155/2018/2547098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hanley P.J., Mei Z., Durett A.G., Cabreira-Hansen M. da G., Cabreira-Harrison M. da G., Klis M., et al. Efficient manufacturing of therapeutic mesenchymal stromal cells with the use of the Quantum Cell Expansion System. Cytotherapy. 2014;16(8):1048–1058. doi: 10.1016/j.jcyt.2014.01.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yan I.K., Shukla N., Borrelli D.A., Patel T. Use of a hollow fiber bioreactor to collect extracellular vesicles from cells in culture. Methods in Molecular Biology. 2018:35–41. doi: 10.1007/978-1-4939-7652-2_4. [DOI] [PubMed] [Google Scholar]
- 192.Geeurickx E., Tulkens J., Dhondt B., Van Deun J., Lippens L., Vergauwen G., et al. The generation and use of recombinant extracellular vesicles as biological reference material. Nature Communications. 2019;10(1):3288. doi: 10.1038/s41467-019-11182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cully M. Exosome-based candidates move into the clinic. Nature Reviews Drug Discovery. 2021;20(1):6–7. doi: 10.1038/d41573-020-00220-y. [DOI] [PubMed] [Google Scholar]
- 194.Henne W.M., Buchkovich N.J., Emr S.D. The ESCRT pathway. Developmental Cell. 2011;21(1):77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 195.Wollert T., Hurley J.H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wei J., Lv L., Wan Y., Cao Y., Li G., Lin H., et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology (Baltimore, Md. 2015;61(4):1284–1294. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Odorizzi G. The multiple personalities of Alix. Journal of Cell Science. 2006;119(Pt 15):3025–3032. doi: 10.1242/jcs.03072. [DOI] [PubMed] [Google Scholar]
- 198.Anand S., Foot N., Ang C.-S., Gembus K.M., Keerthikumar S., Adda C.G., et al. Arrestin-domain containing protein 1 (Arrdc1) regulates the protein cargo and release of extracellular vesicles. Proteomics. 2018;18(17):e1800266. doi: 10.1002/pmic.201800266. [DOI] [PubMed] [Google Scholar]
- 199.Savina A., Fader C.M., Damiani M.T., Colombo M.I. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic (Copenhagen, Denmark) 2005;6(2):131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 200.Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature Cell Biology. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 1-13. [DOI] [PubMed] [Google Scholar]
- 201.Hsu C., Morohashi Y., Yoshimura S.-I., Manrique-Hoyos N., Jung S., Lauterbach M.A., et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. The Journal of Cell Biology. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Fauré J., et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science (New York, N.Y.) 2004;303(5657):531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 203.Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nature Cell Biology. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 204.Yuan X., Bhat O.M., Lohner H., Zhang Y., Li P.-L. Endothelial acid ceramidase in exosome-mediated release of NLRP3 inflammasome products during hyperglycemia: evidence from endothelium-specific deletion of Asah1 gene. Biochimica et Biophysica Acta, Molecular and Cell Biology of Lipids. 2019;1864(12):158532. doi: 10.1016/j.bbalip.2019.158532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ghossoub R., Lembo F., Rubio A., Gaillard C.B., Bouchet J., Vitale N., et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nature Communications. 2014;5(1):3477. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 206.Aung T., Chapuy B., Vogel D., Wenzel D., Oppermann M., Lahmann M., et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Matsumura Y., Ban N., Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2008;295(4):L698–L707. doi: 10.1152/ajplung.90352.2008. [DOI] [PubMed] [Google Scholar]
- 208.Alonso R., Rodríguez M.C., Pindado J., Merino E., Mérida I., Izquierdo M. Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Journal of Biological Chemistry. 2005;280(31):28439–28450. doi: 10.1074/jbc.M501112200. [DOI] [PubMed] [Google Scholar]
- 209.Funakoshi Y., Hasegawa H., Kanaho Y. Regulation of PIP5K activity by Arf6 and its physiological significance. Journal of Cellular Physiology. 2011;226(4):888–895. doi: 10.1002/jcp.22482. [DOI] [PubMed] [Google Scholar]
- 210.Ghim J., Chelakkot C., Bae Y.-S., Suh P.-G., Ryu S.H. Accumulating insights into the role of phospholipase D2 in human diseases. Advances in Biological Regulation. 2016;61:42–46. doi: 10.1016/j.jbior.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 211.Egea-Jimenez A.L., Zimmermann P. Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles. Journal of Lipid Research. 2018;59(9):1554–1560. doi: 10.1194/jlr.R083964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Piper R.C., Katzmann D.J. Biogenesis and function of multivesicular bodies. Annual Review of Cell and Developmental Biology. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kooijman E.E., Chupin V., Fuller N.L., Kozlov M.M., de Kruijff B., Burger K.N.J., et al. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry. 2005;44(6):2097–2102. doi: 10.1021/bi0478502. [DOI] [PubMed] [Google Scholar]
- 214.Kooijman E.E., Chupin V., de Kruijff B., Burger K.N.J. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic (Copenhagen, Denmark) 2003;4(3):162–174. doi: 10.1034/j.1600-0854.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 215.Bissig C., Gruenberg J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harbor Perspectives in Biology. 2013;5(10):a016816. doi: 10.1101/cshperspect.a016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Morel E., Chamoun Z., Lasiecka Z.M., Chan R.B., Williamson R.L., Vetanovetz C., et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nature Communications. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rocha N., Kuijl C., van der Kant R., Janssen L., Houben D., Janssen H., et al. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. The Journal of Cell Biology. 2009;185(7):1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Phuyal S., Skotland T., Hessvik N.P., Simolin H., Øverbye A., Brech A., et al. The ether lipid precursor hexadecylglycerol stimulates the release and changes the composition of exosomes derived from PC-3 cells. Journal of Biological Chemistry. 2015;290(7):4225–4237. doi: 10.1074/jbc.M114.593962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hirsova P., Ibrahim S.H., Krishnan A., Verma V.K., Bronk S.F., Werneburg N.W., et al. Lipid-induced signaling causes release of inflammatory extracellular vesicles from hepatocytes. Gastroenterology. 2016;150(4):956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kajimoto T., Okada T., Miya S., Zhang L., Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nature Communications. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 221.Pfrieger F.W., Vitale N. Cholesterol and the journey of extracellular vesicles. Journal of Lipid Research. 2018;59(12):2255–2261. doi: 10.1194/jlr.R084210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hessvik N.P., Øverbye A., Brech A., Torgersen M.L., Jakobsen I.S., Sandvig K., et al. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cellular and Molecular Life Sciences: CM. 2016;73(24):4717–4737. doi: 10.1007/s00018-016-2309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]