Abstract
In the largest sample studied to date, white matter microstructural trajectories and their relation to persistent symptoms were examined after pediatric mild traumatic brain injury (mTBI). This prospective, longitudinal cohort study recruited children aged 8–16.99 years with mTBI or mild orthopedic injury (OI) from five pediatric emergency departments. Children's pre‐injury and 1‐month post‐injury symptom ratings were used to classify mTBI with or without persistent symptoms. Children completed diffusion‐weighted imaging at post‐acute (2–33 days post‐injury) and chronic (3 or 6 months via random assignment) post‐injury assessments. Mean diffusivity (MD) and fractional anisotropy (FA) were derived for 18 white matter tracts in 560 children (362 mTBI/198 OI), 407 with longitudinal data. Superior longitudinal fasciculus FA was higher in mTBI without persistent symptoms relative to OI, d (95% confidence interval) = 0.31 to 0.37 (0.02, 0.68), across time. In younger children, MD of the anterior thalamic radiations was higher in mTBI with persistent symptoms relative to both mTBI without persistent symptoms, 1.43 (0.59, 2.27), and OI, 1.94 (1.07, 2.81). MD of the arcuate fasciculus, −0.58 (−1.04, −0.11), and superior longitudinal fasciculus, −0.49 (−0.90, −0.09) was lower in mTBI without persistent symptoms relative to OI at 6 months post‐injury. White matter microstructural changes suggesting neuroinflammation and axonal swelling occurred chronically and continued 6 months post injury in children with mTBI, especially in younger children with persistent symptoms, relative to OI. White matter microstructure appears more organized in children without persistent symptoms, consistent with their better clinical outcomes.
Keywords: concussion, diffusion tensor imaging, mild orthopedic injury, pediatric mild traumatic brain injury, pediatric traumatic injury, post‐concussive symptoms
In the largest sample studied to date, white matter microstructural trajectories and their relation to persistent symptoms were examined after pediatric mild traumatic brain injury (mTBI). White matter microstructural changes suggesting neuroinflammation and axonal swelling continued 6 months post‐injury in children with mTBI, especially in younger children when symptoms are persistent, relative to OI. White matter microstructure appears more organized in children without persistent symptoms, consistent with better clinical outcomes.
1. INTRODUCTION
Mild traumatic brain injury (mTBI) affects millions of children annually and is a major global public health concern (Nguyen et al., 2016). No evidence‐based standard of care exists for pediatric mTBI diagnosis or prognostication, largely because no objective clinical test or biomarker can accurately detect mTBI or predict outcomes to date (Ledoux et al., 2019; Mayer et al., 2018; Yeates et al., 2017).
Although mTBI often eludes detection on routine clinical neuroimaging, growing evidence points to the potential utility of diffusion tensor imaging (DTI) metrics, including fractional anisotropy (FA) and mean diffusivity (MD), for assessing mTBI (Lindsey et al., 2021; Mayer et al., 2018; Schmidt et al., 2018; Ware et al., 2020). Reduced MD has been found post‐acutely and up to 6 months post‐injury, whereas FA changes appear to be more dynamic, with cumulative evidence of higher early FA but lower FA several months after pediatric mTBI (Lindsey et al., 2021; MacDonald et al., 2019; Ware et al., 2020; Wilde et al., 2018) However, DTI metrics are not always altered (Ware et al., 2020), and both higher and lower FA have been reported early after pediatric mTBI (Lindsey et al., 2021; MacDonald et al., 2019; Mayer et al., 2018). FA measures restricted diffusion and is highest along highly organized, well‐myelinated white matter tracts (Basser & Pierpaoli, 1996). Thus, a pattern of higher followed by lower FA suggests pediatric mTBI may cause an initial neuroinflammatory response (e.g., edema), leading to persistent axonal swelling and ultimately to demyelination and degeneration (Mayer et al., 2018). However, DTI research in pediatric mTBI is still plagued by limitations (Lindsey et al., 2021; Mayer et al., 2018)
A particular need exists for longitudinal prospective studies that are well‐powered, include appropriate comparison groups, examine a broad age range, and delineate clearly defined post‐injury phases (Mayer et al., 2018). Research on early post‐injury periods is limited, and altered white matter structure is not always demonstrated post‐acutely after mTBI (Lindsey et al., 2021; MacDonald et al., 2019; Ware et al., 2020; Wilde et al., 2018). Less is known about longitudinal trajectories of change, which have only been investigated in a few studies with small samples (i.e., n <30) (Lindsey et al., 2021; Mayer et al., 2018). Accounting for the variability in white matter microstructure associated with age and biological sex also is important (Guenette et al., 2018). Age and sex may moderate outcomes in pediatric mTBI, but most published studies are characterized by small samples (i.e., are underpowered), restricted age ranges, and/or skewed male to female ratios, precluding comprehensive investigation of these moderators (Goodrich‐Hunsaker et al., 2018; Gupte et al., 2019; Manning et al., 2017; Mayer et al., 2018).
The relation of white matter microstructure to symptom recovery following pediatric mTBI is another important knowledge gap with both scientific and clinical significance. Pediatric mTBI results in highly heterogeneous outcomes, with variability observed in clinical presentation, symptom severity, and symptom duration (Ayr et al., 2009; Ledoux et al., 2019; Taylor et al., 2010). Symptom severity is typically worst during the first 2 weeks post‐injury, with full recovery occurring by 4 weeks after mTBI in approximately 75–85% of children (Ledoux et al., 2019; Mayer et al., 2020). However, no biomarker of prolonged recovery is currently available. Few studies have investigated the prognostic utility of DTI in pediatric mTBI and they have produced mixed results (Mayer et al., 2018) with associations between DTI metrics and symptom severity or duration found only in some of the studies (Lima Santos et al., 2021; Manning et al., 2017; Ware et al., 2020; Wilde et al., 2008). Because the neurophysiological effects of mTBI may persist after symptoms have resolved (Manning et al., 2017; Murdaugh et al., 2018) research addressing this knowledge gap is crucial for both understanding the neurobiology of mTBI and identifying objective neuroimaging biomarkers of prolonged recovery in affected children.
This study compared longitudinal changes in white matter microstructure in the largest cohort to date (N = 560) of children with mTBI versus orthopedic injury (OI) to better understand the time course of neurobiological recovery following pediatric mTBI (Mayer et al., 2018). To examine the association of DTI metrics with persistent symptom status in mTBI, longitudinal white matter trajectories were compared among children with mTBI with and without persistent symptoms 1‐month post‐injury and with OI. Age and sex were assessed as moderators of group differences. Based on current evidence, time post‐injury was expected to moderate differences in DTI metrics between children with mTBI and OI, with minimal group differences post‐acutely but lower FA and MD in chronic mTBI (MacDonald et al., 2019; Manning et al., 2017; Mayer et al., 2018; Ware et al., 2020; Wilde et al., 2018; Wu et al., 2018). Pediatric mTBI with persistent symptoms 1‐month post‐injury was expected to result in the largest alterations as compared with mTBI without persistent symptoms and OI.
2. MATERIALS AND METHODS
2.1. Study design and procedure
Data were drawn from the Advancing Concussion Assessment in Pediatrics (A‐CAP) study (Yeates et al., 2017). This multisite study used a prospective, concurrent cohort design to study outcomes longitudinally in pediatric mTBI versus OI. Children with OI were chosen for comparison because they are similar to those with mild TBI, both demographically and in terms of risk factors that predispose to injury, and to help control for the general effects of trauma (Ware et al., 2020; Wilde et al., 2018; Yeates et al., 2017). A‐CAP recruited children between 8–16.99 years of age who presented within 48 h of sustaining an mTBI or OI to the emergency department (ED) of five children's hospitals across Canada, of which all are members of the Pediatric Emergency Research Canada (PERC) network: Alberta Children's Hospital (Calgary), Children's Hospital of Eastern Ontario (Ottawa), Centre Hospitalier Universitaire Sainte‐Justine (Montreal), Stollery Children's Hospital (Edmonton), and British Columbia Children's Hospital (Vancouver) (Bialy et al., 2018; Yeates et al., 2017). Information about acute clinical presentation was collected during the initial ED visit, and information on mechanism of injury and a demographic questionnaire was collected at the post‐acute follow‐up (Yeates et al., 2017). Enrolled participants at each site returned for three additional follow‐up assessments: a post‐acute assessment (i.e., targeted for 10 days post‐injury; range 2–33 days) and two chronic assessments, at 3 and 6 months post‐injury. Study attrition rates did not differ between groups, and were 15, 25, and 28% for the post‐acute, 3‐, and 6‐month assessments, respectively, across groups. This is similar to other studies of pediatric mTBI (Yeates et al., 2017). All eligible participants (i.e., without MRI contraindication; see details below) completed 3 T MRI at the post‐acute assessment and were randomly assigned to complete a second MRI scan at 3 or 6 months post‐injury.
The study was conducted with the approval of the research ethics board at each study site. All participants provided written informed assent and parents/guardians provided written informed consent.
2.2. Participants
2.2.1. Mild TBI
Children in the mTBI group sustained a blunt head trauma resulting in at least one of the following three criteria, consistent with the World Health Organization (WHO) definition of mTBI: (i) observed loss of consciousness, (ii) glasgow coma scale score of 13–14, or (iii) at least one acute sign or symptom of concussion as noted by ED medical personnel on a standard case report form, including posttraumatic amnesia, focal neurological deficits, vomiting, headache, dizziness, or other mental status changes (Carroll et al., 2004). Children were excluded if they demonstrated prolonged neurological deterioration (e.g., glasgow coma scale <13), required neurosurgical intervention, had loss of consciousness >30 min, or posttraumatic amnesia >24 h (Yeates et al., 2017).
2.2.2. Mild OI
Children with mild OI sustained an upper or lower extremity fracture, sprain, or strain due to blunt force trauma, associated with abbreviated injury scale (AIS) score ≤4 (Committee on Injury Scaling, 1998). Children were excluded from the OI group if they had head trauma, symptoms of concussion, or any injury requiring surgical intervention or procedural sedation (Yeates et al., 2017).
2.2.3. Exclusion criteria
Both injury groups were subject to the following exclusion criteria: any other severe injury as defined by an AIS score > 4; hypoxia, hypotension, or shock during or following the injury; previous concussion within 3 months prior or any prior TBI requiring hospitalization; premorbid neurological disorder or severe neurodevelopmental disability; injury resulting from nonaccidental trauma; or severe psychiatric disorder requiring hospitalization within the past year (Yeates et al., 2017). This study also excluded any children with contraindications to MRI (e.g., metallic implants, orthodontia).
2.2.4. Symptoms
The Health and Behavior Inventory was used to assess cognitive and somatic symptoms. This measure has good internal consistency and test–retest reliability, and has been adopted as a core measure in the common data elements for pediatric TBI (Adelson et al., 2012; McCauley et al., 2012; O'Brien et al., 2021). Total premorbid (pre‐injury) symptoms were rated by parents during the post‐acute visit, and total post‐injury symptoms were rated by both parents and children weekly and also at each follow‐up assessment (Ayr et al., 2009). A reliable change index (z‐score) score comparing total 1‐month post‐injury symptom scores to premorbid scores was calculated using the following formulas based on regression analyses using data from the OI group for child ratings (O'Brien et al., 2021):
and parent ratings:
Results were used to classify children with mTBI into two groups using a critical z‐score >1.65 (one‐tailed p <.05): (i) children with mTBI and persistent symptoms (significant increase at 1‐month post‐injury relative to premorbid) and (ii) without persistent symptoms (no significant increase at 1‐month post‐injury relative to premorbid) (Ledoux et al., 2019; Mayer et al., 2020).
2.3. Diffusion MRI
Eligible participants completed at least one 3 T MRI scan without sedation. Thirty diffusion‐weighted images with different diffusion gradient encoding directions were acquired at b = 900 s/mm2, along with five images at b = 0 s/mm2, with 2.2 mm isotropic resolution at all sites (General Electric: TR/TE = 6, 12 s/70, 90 ms; Siemens: 6.3, 7.8 s/55, 90 ms) (Yeates et al., 2017).
2.3.1. Quality assurance
Visual checks of all raw images were conducted to identify and exclude scans with structural abnormalities/incidental findings, scanner artifacts (e.g., warping), incomplete acquisition, or not collected using the standardized scan parameters. Most DTI data from the CHEO site was inadvertently collected using acquisition parameters that differed from the study protocol, precluding analysis of many otherwise eligible participants. Data that passed the initial quality assessment were subsequently rated for motion by at least two trained analysts (Ashley L. Ware, Ayushi Shukla, Adrian I. Onicas). Discrepancies were resolved through a third reviewer blind to initial ratings. Diffusion‐weighted volumes with severe motion artifact were removed. Datasets with >7 volumes with severe motion artifact were excluded from subsequent analysis (Ware et al., 2021).
2.3.2. Image preprocessing
Diffusion‐weighted DICOM data were converted into NIfTI format using the dcm2niix tool in MRIcron (https://github.com/rordenlab/dcm2niix). Images were preprocessed (corrected for eddy currents and head motion, skull‐stripped, and tensor fitted) using the dtiInit preprocessing pipeline wrapper from VISTASOFT package v1.0 (https://github.com/vistalab/vistasoft) running on MATLAB v8.6.0 (R2018a; MathWorks Inc., Natick, MA).
2.3.3. Diffusion tensor imaging
Automated deterministic tractography was performed using the open‐source Automated Fiber Quantification software package v1.2 (https://github.com/yeatmanlab/AFQ) with default parameters (Yeatman et al., 2012). Nonlinear transformation was used to apply a waypoint region of interest template to individual images in native (diffusion‐weighted) space to identify the forceps major (splenium) and forceps minor (genu) of the corpus callosum, along with eight major white matter tracts in each hemisphere: corticospinal tract, inferior longitudinal fasciculus, inferior fronto‐occipital fasciculus, uncinate fasciculus, anterior thalamic radiation, cingulum cingulate gyrus (cingulum bundle), superior longitudinal fasciculus, and arcuate fasciculus. Identified tracts were visually inspected for accuracy. FA and MD were extracted along 100 segments of each identified tract and averaged to provide one value per tract per individual.
2.3.4. DTI metric harmonization
Prior to statistical analysis, the averaged DTI metrics of each tract were harmonized for scanner differences using ComBAT in RStudio v1.1.383 (R v4.0.3) (Fortin et al., 2017; R Core Team, 2017; RStudio Team, 2020). ComBAT is a validated technique for removing nonbiological variance attributable to multiple site (i.e., scanners) effects while preserving sources of variance from biological effects of interest (e.g., group, age, and sex) in DTI data (Fortin et al., 2017). As recommended by the developers, harmonization was applied to unilateral tracts and DTI metrics separately without the use of empirical Bayes (i.e., eb = FALSE) because the number of features was fewer than the sample size, and a matrix of biological covariates of interest was included to aid in preserving the effects of group, time (days) post‐injury, age at injury, and sex as fixed generalized additive mixed model (GAMM) predictors and participant as a random effect. The resulting harmonized DTI metrics were then subject to linear mixed effects (LME) modeling, as described below.
2.4. Statistical analyses
Demographic data were analyzed using t‐tests for continuous variables and techniques for categorical variables.
Multiple linear mixed effects models were computed in RStudio using the lmerTest package to investigate the relations of group (mTBI, OI), the linear and quadratic effects of time (days) post‐injury, age at injury, sex, and group by time by age, and group by time by sex interactions on harmonized FA and MD values of each examined white matter tract, controlling for the random effect of participant (Bates et al., 2015; Kuznetsova et al., 2017; R Core Team, 2017; RStudio Team, 2020). Hemisphere did not moderate group differences in preliminary analyses. Therefore, only the main effect of hemisphere was included in each model. This approach was repeated to compare DTI metrics among symptom status groups (i.e., mTBI with persistent symptoms, mTBI without persistent symptoms, and OI). The final model was:
False discovery rate (FDR) was used to correct for multiple comparisons (Benjamini & Hochberg, 1995). Standardized effect size was assessed for group differences within the context of the final model for each white matter metric using model estimates for Cohen's d, with small effect size = |0.20| d <|0.50|, medium effect size = |0.50| d <|0.80|, and large effect size = d |0.80| (Cohen, 1988). Only the effects with a 95% confidence interval range that excluded 0 were considered to be robust and are described below.
3. RESULTS
3.1. Sample
Information about the overall A‐CAP study sample and derivation of the current sample is provided in Figure 1. The final data set included 892 scans from 560 children (362 mTBI/198 OI). A total of 407 children (73%) had usable longitudinal DTI data at post‐acute and 3 months (135 mTBI/66 OI) or 6 months (129 mTBI/77 OI) assessments. A total of 241 (21%; 170 mTBI/71 OI) scans were excluded during initial quality assessment for the following reasons: incorrect acquisition parameters (e.g., <30 diffusion gradients; 104 mTBI/40 OI), severe motion artifact (33 mTBI/13 OI), incomplete acquisition (23 mTBI/6 OI), scanner artifacts (5 mTBI/4 OI), or structural abnormalities/incidental findings (2 mTBI/4 OI). Eleven scans (7 mTBI/4 OI) failed AFQ processing. A subset of the post‐acute neuroimaging data was analyzed as part of a study comparing DTI and neurite orientation dispersion and density imaging (NODDI) metrics (Shukla et al., 2021).
FIGURE 1.
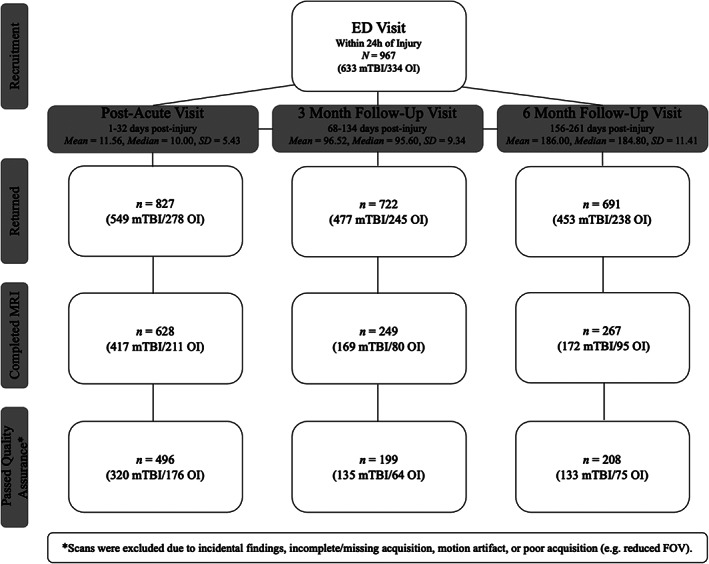
Summary data for the overall A‐CAP study sample and the derivation of the current sample. Of 3075 eligible children with mTBI or mild OI, 967 consented to participate in A‐CAP, and 846 returned for at least one assessment. Children who returned at post‐acute, 3 months, and/or 6 months did not differ from those who did not return in terms of age, sex, race, or parental education, with one exception: Children who returned at 6 months had higher parental education than those who did not return. Children completed a post‐acute MRI scan and were randomly assigned to complete a second MRI scan 3 or 6 months post‐injury. New MRI contraindications (after recruitment, e.g., orthodontia treatment) and scheduling difficulties were the most common reasons that MRI was not completed. Overall, 671 children completed at least one MRI, with a total of 1144 scans completed (758 mTBI/386 OI). Children who completed MRI were younger (M = 12.23, SD = 2.38 years; t = 5.29, p <.001) and more often male (402 male/266 female, 2 = 6.22, p = .013) than children who did not complete MRI (age M = 13.29, SD = 2.37 years; 88 male/90 female), but did not differ in race or parental education. The final sample included a total of 892 DTI scans from 560 children (see Table 1), of which 407 (73%) had longitudinal data. *Excluded 241 (21%, 170 mTBI/71 OI) scans during initial quality assessment and an additional 11 scans that failed the DTI processing pipeline. A‐CAP, Advancing Concussion Assessment in Pediatrics; mTBI, mild traumatic brain injury; OI, orthopedic injury
3.2. Demographic and injury characteristics
The mTBI and OI groups did not differ significantly in age at injury, sex, race, parental education, days post‐injury for post‐acute or chronic MRI scans, or whether the injury occurred during sport/recreation, but differed in injury mechanism (Table 1).
TABLE 1.
Demographic and injury characteristics for the sample of children
| Variable | mTBI | OI | p* |
|---|---|---|---|
| n = 362 | n = 198 | ||
| Study site [n(%)] | .002 | ||
| Calgary | 103 (28.5) | 45 (22.7) | |
| Edmonton | 87 (24.0) | 45 (22.7) | |
| Montreal | 45 (12.0) | 12 (6.0) | |
| Ottawa | 42 (11.6) | 19 (9.6) | |
| Vancouver | 85 (23.5) | 77 (38.9) | |
| Age [mean (SD) years] | 12.30 (2.45) | 12.44 (2.23) | .501 |
| Sex [n (%) male] | 224 (61.9) | 110 (55.6) | .171 |
| Parental education [n (%)] | .911 | ||
| No certificate, diploma or degree | 11 (3.2) | 4 (2.2) | |
| High school diploma or equivalent | 50 (14.7) | 24 (13.2) | |
| Trades certificate or diploma | 35 (10.3) | 16 (8.8) | |
| 2‐year college diploma | 66 (19.4) | 41 (22.5) | |
| 4‐year bachelor's degree | 125 (36.8) | 63 (34.6) | |
| Master's degree | 38 (11.2) | 24 (13.2) | |
| Doctoral degree (PhD or similar) | 10 (2.9) | 6 (3.3) | |
| Medical degree | 5 (1.5) | 4 (2.2) | |
| Race [n (%)] | .698 | ||
| White | 246 (68.0) | 132 (66.7) | |
| Asian | 30 (8.3) | 13 (6.6) | |
| Black | 15 (4.1) | 6 (3.0) | |
| Latinx | 8 (2.2) | 8 (4.0) | |
| Indigenous | 6 (1.7) | 3 (1.5) | |
| Other/mixed | 50 (13.8) | 29 (14.6) | |
| Unknown | 7 (1.9) | 7 (3.5) | |
| Mechanism of injury [n (%)] | <.001 | ||
| Bicycle related | 6 (1.9) | 10 (5.6) | |
| Fall | 138 (44.5) | 90 (50.8) | |
| Motor vehicle collision | 4 (1.3) | 0 (0.0) | |
| Struck object | 92 (29.7) | 34 (19.2) | |
| Struck person | 60 (19.4) | 20 (11.3) | |
| Other | 4 (1.3) | 12 (6.8) | |
| Unknown | 6 (1.9) | 11 (6.2) | |
| Sport‐related injury [n (%) sport/recreational play] | 260 (84.1) | 147 (83.1) | .852 |
| Symptom ratings | |||
| Premorbid (parent) | 12.36 (10.02) | 8.72 (8.17) | <.001 |
| Child 1‐month post‐injury | 13.50 (11.99) | 7.72 (7.90) | <.001 |
| Parent 1‐month post‐injury | 12.60 (10.29) | 6.61 (7.52) | <.001 |
Note: *Uncorrected p‐values reported.
Abbreviations: mTBI, mild traumatic brain injury; OI, orthopedic injury.
3.3. White matter microstructure
The group differences in DTI metrics that survived correction for multiple comparisons are summarized in Figure 2 and the model and follow‐up statistics are reported in Table 2 and Table S2. Time post‐injury moderated group differences in MD of the arcuate fasciculus and anterior thalamic radiations, across hemispheres (Figure 2b, c). Follow‐up analyses examined group differences at the average day post‐injury of each MRI scan (see Figure 1). Relative to OI, the mTBI group had higher MD in the anterior thalamic radiations at 3 months post‐injury, but lower MD in the arcuate fasciculus at 6 months post‐injury.
FIGURE 2.
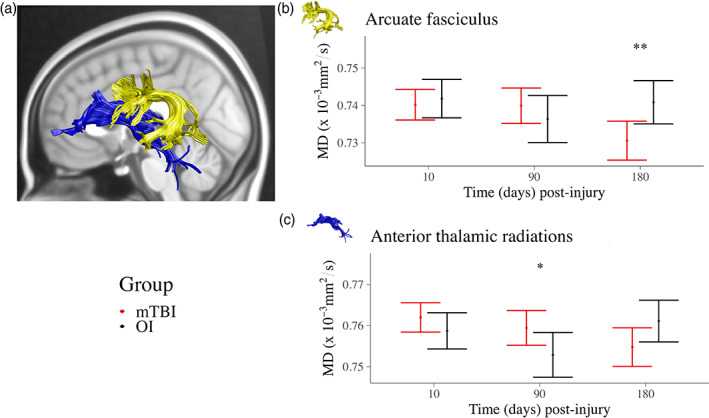
Significant group effects and interactions on DTI metrics. (a) Three of the examined tracts survived FDR correction for multiple comparisons. Box plots illustrating the group differences and summarizing effect magnitudes for (b) MD of the arcuate fasciculus (group‐by‐time interaction), and (c) MD of the anterior thalamic radiation (group‐by‐time interaction). Follow‐up analyses examined group differences within the context of the final model and for average days post‐injury at each post‐injury assessment (see Figure 1). Standardized effect size was computed for group (mTBI—OI) differences using model estimates for Cohen's d. effects sizes with 95% confidence interval range excluding zero are illustrated using * and for small and medium effect magnitudes. Complete results are provided in Table S2.
TABLE 2.
Statistical results for linear mixed effects models with significant effects of group that survived FDR correction on DTI metrics of examined white matter tracts
| Predictors | Arcuate fasciculus MD | Anterior thalamic radiation MD | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimates | 95% CI | Statistic | p* | Estimates | 95% CI | Statistic | p* | |
| Intercept | 0.74 | 0.73 to 0.74 | 371.90 | <.001 | 0.76 | 0.76 to 0.76 | 443.88 | <.001 |
| Group (OI—mTBI) | 2.46e−3 | ‐3.71e−2 to 0.01 | 0.79 | .432 | ‐1.74e−3 | −0.01 to 3.53e−3 | −0.65 | .517 |
| Time (days) post‐injury | −0.15 | −0.22 to −0.08 | −4.38 | <.001 | −0.12 | −0.19 to −0.06 | −3.58 | <.001 |
| Time2 | −0.08 | −0.15 to −0.01 | −2.16 | .030 | −0.02 | −0.09 to 0.05 | −0.48 | .629 |
| Age at injury (centered) | −0.01 | −0.01 to −0.01 | −9.83 | <.001 | −3.92e−3 | −0.01 to −1.92e−3 | −3.83 | <.001 |
| Sex (female—male) | 0.01 | 0.01 to 0.02 | 4.42 | <.001 | 0.01 | 4.19e−3 to 0.01 | 3.92 | <.001 |
| Hemisphere (left—right) | 3.41e−3 | 2.15e−3 to 7.83e−3 | 5.31 | <.001 | 0.02 | 0.01 to 0.02 | 25.59 | <.001 |
| Group × time | 0.12 | 0.03 to 0.22 | 2.56 | .011 | 0.15 | 0.06 to 0.25 | 3.15 | .002 |
| Group × time2 | 0.16 | 0.05 to 0.28 | 2.86 | .004 | 0.15 | 0.04 to 0.26 | 2.67 | .008 |
| Group × age | 3.59e−3 | −6.50e−4 to 0.01 | 1.66 | .097 | 1.70e−3 | −1.91e−3 to 0.01 | 0.93 | .354 |
| Group × sex | −5.40e−4 | −0.01 to 0.01 | −0.13 | .898 | −4.10e−4 | −0.01 to 0.01 | −0.12 | .908 |
| Time × age | 3.92e−3 | −0.03 to 0.04 | 0.21 | .833 | −0.04 | −0.07 to −5.10e−4 | −1.99 | .047 |
| Time2 × age | −1.76e−3 | −0.04 to 0.04 | −0.08 | .933 | −0.02 | −0.06 to 0.03 | −0.76 | .447 |
| Time × sex | 0.12 | 0.04 to 0.20 | 2.88 | .004 | 0.12 | 0.04 to 0.20 | 2.84 | .005 |
| Time2 × sex | 0.07 | −0.02 to 0.15 | 1.51 | .130 | 0.01 | −0.08 to 0.10 | 0.28 | .783 |
| Group × time × age | 0.03 | −0.04 to 0.09 | 0.86 | .390 | 0.03 | −0.03 to 0.09 | 0.97 | .333 |
| Group × time2 × age | −0.01 | −0.08 to 0.07 | −0.13 | .895 | −0.06 | −0.14 to 0.01 | −1.71 | .087 |
| Group × time × sex | −0.08 | −0.21 to 0.05 | −1.22 | .224 | −0.12 | −0.25 to 0.01 | −1.88 | .061 |
| Group × time2 × sex | −0.20 | −0.35 to −0.05 | −2.55 | .011 | −0.12 | −0.27 to 0.02 | −1.66 | .096 |
| Random effects | ||||||||
| ICC | 0.76 | 0.69 | ||||||
| Observations | 1594 | 1784 | ||||||
| Marginal R 2/conditional R 2 | 0.193/0.803 | 0.157/0.735 | ||||||
Note: Effect sizes, that is, Cohen's d (95% CI), for the pairwise comparisons between the mTBI and OI groups are reported in Table S2. *Uncorrected p‐values reported. Bolded = uncorrected p <.05; bolded/italic = FDR corrected p <.05.
Abbreviations: CI, confidence interval; mTBI, mild traumatic brain injury; OI, orthopedic injury.
3.4. Symptom status
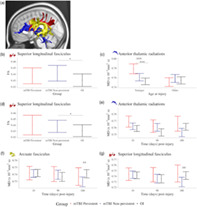
Persistent symptom status based on child and parent report was available for 275 (76%) and 286 (79%) children with mTBI (see Table S1). Children with persistent symptoms (child n = 68; parent n = 54) did not differ from those without persistent symptoms in terms of study site, age, maternal education, race, mechanism of injury, or whether the injury was sport related. Sex did not differ by symptom status based on parent ratings, but there were less males (41%) with persistent symptoms than without persistent symptoms (72%) based on child ratings (p < .001).
DTI metrics differed among symptom groups derived based on child and parent report (see Figure 3). The model results are reported in Tables 3 and 4 respectively for analyses based on child and parent report, and follow‐up statistics are provided in Table S3. In groups based on child report, superior longitudinal fasciculus FA was consistently higher in mTBI without persistent symptoms relative to OI across time (Figure 3b). Age at injury also moderated differences between groups based on child report in anterior thalamic radiations MD (Figure 3c). In younger children (i.e., 10th percentile of age at injury; ~9.40 years), MD of the anterior thalamic radiation was higher in mTBI with persistent symptoms relative to mTBI without persistent symptoms and relative to OI, across time post‐injury.
FIGURE 3.
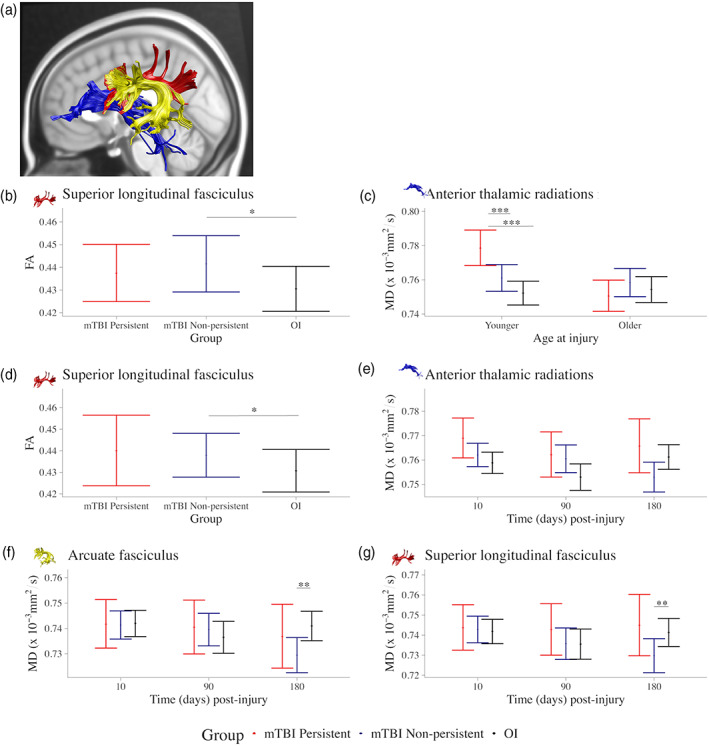
Significant effects involving symptom status group based on child repot on DTI metrics. Graphs illustrating differences and their effect magnitudes in (a) regional tract metrics among symptoms status groups based on child report for (b) FA of the superior longitudinal fasciculus (main effect of group) and (c) MD of the anterior thalamic radiation (group by age at injury interaction), and among symptoms status groups based on parent report for (d) FA of the superior longitudinal fasciculus (main effect of group) and MD of the (e) anterior thalamic radiations, (f) arcuate fasciculus, and (g) superior longitudinal fasciculus (group by time interaction). Follow‐up analyses examined group differences within the context of the final model and for the 10th and 90th percentile of age at injury. Standardized effect size was computed for group (mTBI—OI) differences using model estimates for Cohen's d effects sizes with 95% confidence interval range excluding zero are illustrated using *, **, and *** for small, medium, and large effect magnitudes. Complete results are provided in Table S3.
TABLE 3.
Statistical results for linear mixed effects models with significant effects of symptom groups based on child report that survived FDR correction on DTI metrics of examined white matter tracts
| Predictors | Superior longitudinal fasciculus FA | Anterior thalamic radiations MD | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimates | CI | Statistic | p* | Estimates | CI | Statistic | p* | |
| (Intercept) | 0.44 | 0.43 to 0.45 | 85.61 | <.001 | 0.77 | 0.76 to 0.77 | 238.81 | < .001 |
| Group a | ‐ | ‐ | 6.43 | .002 | ‐ | ‐ | 3.05 | .293 |
| Time | 0.04 | −0.29 to 0.38 | 0.25 | .805 | −0.13 | −0.27 to 0.02 | −1.74 | .081 |
| Time2 | 0.03 | −0.25 to 0.31 | 0.20 | .845 | 0.02 | −0.11 to 0.14 | 0.25 | .805 |
| Age at injury (centered) | 0.01 | 1.00e‐3 to 0.02 | 2.77 | .006 | −0.01 | −0.01 to −0.01 | −4.84 | <.001 |
| Sex (female–male) | 3.02e−3 | −0.01 to 0.02 | 0.40 | .688 | 4.96e−3 | −4.56e−3 to 0.01 | 1.02 | .307 |
| Hemisphere (left–right) | 0.03 | 0.02 to 0.03 | 16.81 | <.001 | 0.01 | 0.01 to 0.02 | 23.51 | <.001 |
| Group × (time + time2) b | ‐ | ‐ | 0.42 | .794 | ‐ | ‐ | 2.81 | .024 |
| Group × age a | ‐ | ‐ | 2.12 | .122 | ‐ | ‐ | 6.75 | .001 |
| Group × sex a | ‐ | ‐ | 0.41 | .661 | ‐ | ‐ | 0.34 | .714 |
| Time × age | 0.06 | −0.12 to 0.23 | 0.61 | .543 | 0.01 | −0.06 to 0.09 | 0.38 | .704 |
| Time2 × age | −0.07 | −0.25 to 0.10 | −0.80 | .425 | −4.50e−3 | −0.08 to 0.07 | −0.12 | .907 |
| Time × sex | −0.06 | −0.47 to 0.36 | −0.26 | .793 | 0.20 | 0.03 to 0.38 | 2.32 | .020 |
| Time2 × sex | 3.00e−3 | −0.39 to 0.40 | 0.01 | .988 | −0.07 | −0.24 to 0.10 | −0.78 | .433 |
| Group × (time + time2) × age b | ‐ | ‐ | 0.61 | .659 | ‐ | ‐ | 1.72 | .142 |
| Group × (time + time2) × sex b | ‐ | ‐ | 0.68 | .609 | ‐ | ‐ | 2.42 | .047 |
| Random effects | ||||||||
| ICC | 0.36 | 0.68 | ||||||
| Observations | 1534 | 1538 | ||||||
| Marginal R 2/conditional R 2 | 0.176/0.468 | 0.177/0.734 | ||||||
Note: Effect sizes, that is, Cohen's d (95% CI), for the pairwise comparisons between the symptom's groups are reported in Table S3. *Uncorrected p‐values reported. Bolded = uncorrected p <.05; bolded/italic = FDR corrected p <.05.
df = 2.
df = 4.
TABLE 4.
Statistical results for linear mixed effects models with significant effects of symptom groups based on parent report that survived FDR correction on DTI metrics of examined white matter tracts
| Superior longitudinal fasciculus FA | Arcuate fasciculus MD | Superior longitudinal fasciculus MD | Anterior thalamic radiations MD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | Statistic | p | Estimates | CI | Statistic | p | Estimates | CI | Statistic | p | Estimates | CI | Statistic | p |
| (Intercept) | 0.44 | 0.43 to 0.45 | 71.62 | <.001 | 0.74 | 0.73 to 0.75 | 158.12 | <.001 | 0.74 | 0.73 to 0.76 | 135.52 | <.001 | 0.77 | 0.76 to 0.77 | 193.10 | < .001 |
| Group a | ‐ | ‐ | 5.73 | .003 | ‐ | ‐ | 0.32 | .730 | ‐ | ‐ | 0.21 | .813 | ‐ | ‐ | 1.33 | .265 |
| Time (days) post‐injury | 0.11 | −0.25 to 0.46 | 0.59 | .557 | −0.07 | −0.22 to 0.08 | −0.96 | .337 | 0.02 | −0.18 to 0.22 | 0.18 | .854 | −0.06 | −0.21 to 0.09 | −0.80 | .423 |
| Time2 | 0.04 | −0.29 to 0.38 | 0.25 | .799 | −0.02 | −0.16 to 0.13 | −0.25 | .806 | 0.03 | −0.17 to 0.23 | 0.27 | .790 | 0.09 | −0.05 to 0.24 | 1.25 | .210 |
| Age (centered) | 0. 02 | 0.01 to 0.03 | 4.95 | < .001 | −0.01 | −0.02 to −0.01 | −4.54 | <.001 | −0.02 | −0.02 to −0.01 | −4.58 | <.001 | −0.01 | −0.01 to −2.73e−3 | −3.01 | .003 |
| Sex (female—male) | −2.670e−3 | −0.02 to 0.01 | −0.32 | .746 | 0.01 | −3.92e−3 to 0.02 | 1.34 | .180 | 3.06e−3 | −0.01 to 0.02 | 0.41 | .681 | −2.60e−4 | −0.01 to 0.01 | −0.05 | .961 |
| Hemisphere (left—right) | 0.03 | 0.02 to 0.03 | 16.70 | < .001 | 3.40e−03 | 2.06e−3 to 4.75e−3 | 4.95 | <.001 | 0.01 | 4.58e−3 to 0.01 | 7.32 | <.001 | 0.01 | 0.01 to 0.02 | 23.64 | < .001 |
| Group × (time + time2) b | ‐ | ‐ | 0.34 | .851 | ‐ | ‐ | 3.12 | .014 | ‐ | ‐ | 3.63 | .006 | ‐ | ‐ | 3.83 | .004 |
| Group × age a | ‐ | ‐ | 3.92 | .020 | ‐ | ‐ | 1.52 | .221 | ‐ | ‐ | 3.96 | .020 | ‐ | ‐ | 1.85 | .159 |
| Group × sex a | ‐ | ‐ | 0.64 | .529 | ‐ | ‐ | 1.11 | .895 | ‐ | ‐ | 0.53 | .592 | ‐ | ‐ | 1.24 | .290 |
| Time × age | 0.13 | −0.11 to 0.37 | 1.06 | .287 | −0.05 | −0.16 to 0.05 | −0.95 | .343 | −0.09 | −0.23 to 0.05 | −1.26 | .206 | −0.09 | −0.19 to 0.01 | −1.85 | .064 |
| Time2 × age | 0.09 | −0.14 to 0.32 | 0.79 | .427 | 4.07e−03 | −0.10 to 0.10 | 0.08 | .936 | −0.01 | −0.14 to 0.13 | −0.10 | .921 | −0.06 | −0.16 to 0.04 | −1.16 | .248 |
| Time × sex | −0.14 | −0.59 to 0.32 | −0.59 | .554 | 0.08 | −0.11 to 0.27 | 0.84 | .402 | 0.02 | −0.23 to 0.28 | 0.18 | .857 | 0.18 | −3.65e−3 to 0.37 | 1.92 | .055 |
| Time2 × sex | 0.01 | −0.45 to 0.47 | 0.06 | .952 | −0.12 | −0.32 to 0.07 | −1.24 | .215 | −0.16 | −0.43 to 0.12 | −1.12 | .262 | −0.13 | −0.33 to 0.06 | −1.33 | .185 |
| Group × (time + time2) × age b | ‐ | ‐ | 0.33 | .861 | ‐ | ‐ | 0.65 | .627 | ‐ | ‐ | 0.55 | .702 | ‐ | ‐ | 1.70 | .148 |
| Group × (time + time2) × sex b | ‐ | ‐ | 0.65 | .630 | ‐ | ‐ | 1.88 | .112 | ‐ | ‐ | 0.49 | .743 | ‐ | ‐ | 2.90 | .021 |
| Random effects | ||||||||||||||||
| ICC | 0.35 | 0.76 | 0.68 | 0.68 | ||||||||||||
| Observations | 1566 | 1397 | 1566 | 1570 | ||||||||||||
| Marginal R 2/conditional R 2 | 0.179/0.468 | 0.179/0.801 | 0.149/0.728 | 0.158/0.732 | ||||||||||||
Note: Effect sizes, that is, Cohen's d (95% CI), for the pairwise comparisons between the symptoms groups are reported in Table S3. *Uncorrected p‐values reported. Bolded = uncorrected p <.05; bolded/italic = FDR corrected p < .05.
df = 2.
df = 4.
Superior longitudinal fasciculus FA also differed among groups derived based on parent report (Figure 3d), whereby FA was higher in children with mTBI without persistent symptoms relative to OI, across time post‐injury. Time post‐injury moderated differences in MD of the anterior thalamic radiations, arcuate fasciculus, and superior longitudinal fasciculus among groups derived based on parent report (Figure 3e–g). MD was lowest in children with mTBI without persistent symptoms relative to OI; this effect was more robust for the arcuate and superior longitudinal fasciculi then for the anterior thalamic radiations.
4. DISCUSSION
These results from the largest longitudinal DTI study of pediatric mTBI to date suggest that mTBI can cause subtle, long‐term changes to white matter microstructure as compared to extracranial traumatic injury, and that both time post‐injury and symptom status are important moderators of those effects in children. Importantly, altered white matter microstructure was not apparent globally, but appeared only in three of the examined tracts, consistent with the subtle nature of pediatric mTBI compared to more severe injuries (Lindsey et al., 2021; Mayer et al., 2018).
Relative to OI, children with mTBI demonstrated no post‐acute alterations in white matter microstructure, although changes were apparent across time, including modest elevations in MD of the anterior thalamic radiations 3 months post‐injury with robust reductions in MD of the arcuate fasciculus at 6 months post‐injury. However, further examination of DTI metrics revealed different white matter microstructural trajectories among children with mTBI with versus without persistent symptoms (1‐month post‐injury) compared to those with OI. FA was higher across time and MD was lower at 6 months post‐injury in mTBI without persistent symptoms relative to OI. Further, MD was generally higher in mTBI with persistent symptoms compared with mTBI without persistent symptoms and OI in younger children, across time post‐injury. Thus, the neurobiological heterogeneity associated with discrete clinical outcomes was initially blurred by collapsing across symptom status. This pattern of results may explain the inconsistencies across published studies of mostly smaller samples that had variable post‐injury assessment times and restricted age ranges and did not always take symptom status into account (Guenette et al., 2018; Manning et al., 2017; Mayer et al., 2018; Ware et al., 2020). In particular, previous inconsistencies in DTI metrics in pediatric mTBI could reflect differences in the timing of acquisition or clinical outcomes of children with mTBI (Mayer et al., 2018; Ware et al., 2020).
Higher anterior thalamic radiation MD chronically in children with mTBI, and across time in younger children with persistent symptoms, relative to OI could reflect vasogenic edema, accumulation of extracellular liquid, or demyelination and axonal loss following the breakdown of the blood–brain barrier and degradation of the axonal membrane (Mayer et al., 2018; Parekh et al., 2015). Other common pathologies that can alter water diffusion include vascular changes, the disintegration of axonal microfilaments of neurons, and demyelination (Mayer et al., 2018; Parekh et al., 2015). Whether early and persistently high MD promotes remyelination and protection against degeneration or, in contrast, acts as a precursor to damaged microstructure (e.g., demyelination or axonal injury associated with Wallerian‐type degeneration) is unclear (Mayer et al., 2018; Parekh et al., 2015; Yano et al., 2018). Differentiating these effects in pediatric mTBI is a critical research goal because identifying a feasible neuroprotective strategy to limit axonal damage and neurodegeneration could lead to clinical interventions that prevent demyelination or promote remyelination. This goal is perhaps particularly important for the clinical management of children who have prolonged symptom recovery. The lack of FA differences between children with persistent symptoms and OI was surprising and could reflect pre‐existing differences between the groups with and without persistent symptoms, the severity of the systemic injury, or potentially transient brain effects such as edema.
In contrast, children who had more favorable clinical outcomes (i.e., with mTBI without persistent symptoms) demonstrated higher superior longitudinal fasciculus FA and lower MD in the thalamic radiations and superior longitudinal fasciculus. This is indicative of more restricted water diffusion. Recent research similarly demonstrated higher FA in long association fibers in adolescents and young adults with short time to recovery and improved cognition following mTBI (Fakhran et al., 2014; Lima Santos et al., 2021; Mustafi et al., 2018). Higher FA is associated with greater resiliency during adolescence in typical development (Galinowski et al., 2015). Current findings therefore could reflect resilience of more organized white matter microstructure to the effects of pediatric mTBI, whereby better brain reserve leads to better outcomes in children. However, neuroimaging research in resiliency is scarce and superior longitudinal fasciculus FA was not investigated in current studies (Feder et al., 2019).
The current findings are clinically relevant in signaling persistent neurophysiological changes after pediatric mTBI. Some emerging evidence has indicated that DTI parameters could ultimately be used to enhance clinical management in affected children with mTBI (Taylor et al., 2015). Inclusion of post‐acute DTI metrics can increase the sensitivity and specificity of clinical prediction model for pediatric mTBI (Lima Santos et al., 2021) and FA values, specifically, were more predictive of recovery time than initial symptom burden in children and young adult (i.e., age 10–38 years) with mTBI in other studies (Fakhran et al., 2014). Previous findings also show that post‐acute DTI is related to symptom burden in chronic periods after pediatric mTBI, but not differentially for children with mTBI as compared with mild OI (Ware et al., 2020). Here, DTI metrics showed little evidence of providing a biomarker for prolonged brain injury or recovery given the findings regarding persistent symptom status, although less robust findings suggest that higher FA (across time) with lower MD post‐acutely may signal short recovery time post‐injury. The prognostic utility of the findings remains to be fully established. However, the minor and regionally specific post‐acute differences between mTBI and OI detected here indicate that using DTI metrics in individual patients early post‐injury is not likely to aid clinical diagnosis or detection of mTBI in children.
White matter regions appeared to be differentially vulnerable to the biomechanical forces that disrupt brain tissue in mTBI (e.g., through straining and deformation) (Mayer et al., 2018; Okamoto et al., 2019; Schmidt et al., 2018). Significant group differences were limited to only the superior longitudinal fasciculus, arcuate fasciculus, and anterior thalamic radiation. Prior evidence suggests that pediatric mTBI alters the microstructure of these regions (Manning et al., 2017; Mayer et al., 2018; Wu et al., 2018). The superior longitudinal fasciculus connects parietal, prefrontal, and temporal cortex, while the arcuate is an adjacent frontal‐temporal connection. The anterior thalamic radiations connect the prefrontal cortex, regions of the limbic system, and thalamus (Grodd et al., 2020). Alterations in these pathways could have implications for specific symptoms of pediatric mTBI, such as problems with attention, executive functioning, memory, mood, and motivation (Grodd et al., 2020; MacDonald et al., 2019).
The expected age‐related increases of FA and decreases of MD were found across groups (Lebel et al., 2019). In typical development, frontal‐temporal white matter pathways and the superior longitudinal fasciculus continue to develop into the second decade of life (peak FA occurs at age 24–25 years), a more protracted development than other regions (Lebel et al., 2019). This may increase the susceptibility of these regions to the effects of pediatric mTBI. Sex was not a robust moderator of group differences, but was associated with MD, which was higher in females as compared with males in the anterior thalamic radiations. Studies of typically developing children have not consistently reported sex differences, although some evidence suggests differences in DTI metrics (i.e., FA and MD) (Lebel et al., 2019). In pediatric mTBI, sex differences are not well understood and warrant further study (Gupte et al., 2019; Mayer et al., 2018).
This study has limitations. The sample was recruited from the ED of several children's hospitals and may not represent children with injuries who receive clinical care in other settings or who do not seek medical attention. The sample mostly comprised children who were White and of higher socioeconomic status, and the results may not be generalizable to the broader population. We cannot be certain whether white matter microstructural differences existed pre‐injury between children with mTBI versus OI, although the emergence of group differences over time suggests this is unlikely. White matter microstructure was measured using deterministic tractography, which offers better sensitivity than voxel‐wise approaches to alterations in pediatric mTBI (Goodrich‐Hunsaker et al., 2018) but could limit reproducibility given the additional processing steps required to generate tract‐specific measures (Schilling et al., 2021). More advanced water diffusion models (e.g., multiple diffusion‐weighted b‐values; increased angular resolution) could potentially increase understanding of the specific neuropathology (e.g., cytotoxic vs. vasogenic edema, as well as axonal and myelin deformation) that follows pediatric mTBI (Mayer et al., 2018). Moreover, advanced computational approaches to investigate brain networks (e.g., structural connectome, graph theory) may be more sensitive than region‐based analyses to the nature of pediatric mTBI. Parents completed the premorbid symptoms questionnaire retrospectively at the post‐acute assessment, which may introduce recall bias (Brooks et al., 2014). Finally, we examined whether MRI metrics were related to symptom persistence at 1‐month post‐injury in the children with mTBI relative to premorbid symptoms (Ledoux et al., 2019). Future research should investigate the relationship of MRI metrics to more specific symptoms and to symptom status trajectories across time post‐injury.
4.1. Conclusions and future directions
This is the largest prospective, longitudinal study of DTI in pediatric mTBI to date. We found differences in white matter microstructure that emerged chronically after mTBI, with different trajectories as a function of symptom status at 1‐month post‐injury. Overall, the results highlight the importance of a framework that considers both time post‐injury and clinical outcomes (i.e., symptom status) when examining the neurobiological effects of pediatric mTBI. The next step in our research is to link these microstructural changes to other clinical and functional outcomes, to continue to help identify biomarkers for pediatric mTBI.
CONFLICT OF INTEREST
None of the authors report any financial or personal conflict of interests.
AUTHOR CONTRIBUTIONS
Ashley L. Ware: Designed analyses, processed images, analyzed data, drafted manuscript. Keith O. Yeates: Designed parent study, provided data, designed statistical analyses, edited manuscript: Ayushi Shukla: Processed imaging data, edited manuscript. Adrian Onicas: Processed imaging data, edited manuscript. Sunny Guo: Edited manuscript. Naomi Goodrich‐Hunsaker: Processed imaging data; edited manuscript. Nishard Abdeen: Edited manuscript. Miriam H. Beauchamp: Edited manuscript. Christian Beaulieu: Edited manuscript. Bruce Bjornson: Edited manuscript. William Craig: Edited manuscript. Mathieu Dehaes: Edited manuscript. Sylvain Deschenes: Edited manuscript. Quynh Doan: Edited manuscript. Stephen B. Freedman: Edited manuscript. Bradley G. Goodyear: Edited manuscript. Jocelyn Gravel: Edited manuscript. Andrée‐Anne Ledoux: Edited manuscript. Roger Zemek: Edited manuscript. Catherine Lebel: Designed parent study of concussion and orthopedic injury, designed image analyses, edited manuscript. Data was verified by Ashley L. Ware, Catherine Lebel, and Keith O. Yeates.
Supporting information
Table S1 Demographic and injury characteristics for mTBI with and without persistent symptoms
Table S2. Follow‐up pairwise comparisons for significant group differences in DTI metrics after FDR correction. Pairwise comparisons conducted within the context of the final fitted model. Bolded/italicized = 95% confidence interval range excludes 0
Table S3. Follow‐up pairwise comparisons for significant differences in DTI metrics among symptom groups based on child and parent report. Pairwise comparisons conducted within the context of the final fitted model. Bolded/italicized = 95% confidence interval range excludes 0
ACKNOWLEDGMENTS
Canadian Institutes of Health Research (FDN143304), Ronald and Irene Ward Chair in Pediatric Brain Injury (KOY); Canada Research Chair (CL); Harley N. Hotchkiss‐Samuel Weiss and Killam Postdoctoral Fellowship (ALW); Alberta Children's Hospital Foundation Professorship in Child Health and Wellness (SBF).
APPENDIX A.
A.1. PEDIATRIC EMERGENCY RESEARCH CANADA A‐CAP STUDY GROUP CO‐INVESTIGATORS
Carolyn Emery, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Lianne Tomfohr, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Tyler Williamson, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Karen Barlow, PhD: university of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Francois Bernier, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Brian Brooks, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Ashley Harris, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Ryan Lamont, MD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Kathryn Schneider, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Kelly Mrklas, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study. Angelo Mikrogianakis, PhD: University of Calgary, Calgary, AB, Canada; Site co‐investigator, Assisted in design of parent study, coordinated recruitment at site.
Ware, A. L. , Yeates, K. O. , Tang, K. , Shukla, A. , Onicas, A. I. , Guo, S. , Goodrich‐Hunsaker, N. , Abdeen, N. , Beauchamp, M. H. , Beaulieu, C. , Bjornson, B. , Craig, W. , Dehaes, M. , Doan, Q. , Deschenes, S. , Freedman, S. B. , Goodyear, B. G. , Gravel, J. , Ledoux, A.‐A. , Zemek, R. , Lebel, C. , & Pediatric Emergency Research Canada A‐CAP Study Team (2022). Longitudinal white matter microstructural changes in pediatric mild traumatic brain injury: An A‐CAP study. Human Brain Mapping, 43(12), 3809–3823. 10.1002/hbm.25885
[Correction added on 04 May 2022, after first online publication: Quynh Doan's affiliation is updated and subsequent affiliations were re‐numbered.]
Funding information Canadian Institutes of Health Research, Grant/Award Number: FDN143304
DATA AVAILABILITY STATEMENT
A dataset with deidentified participant data and a data dictionary will be made available upon reasonable request from any qualified investigator, subject to a signed data access agreement.
REFERENCES
- Adelson, P. D. , Pineda, J. , Bell, M. J. , Abend, N. S. , Berger, R. P. , Giza, C. C. , Hotz, G. , & Wainwright, M. S. (2012). Common data elements for pediatric traumatic brain injury: Recommendations from the working group on demographics and clinical assessment. Journal of Neurotrauma, 29(4), 639–653. 10.1089/neu.2011.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayr, L. K. , Yeates, K. O. , Taylor, H. G. , & Browne, M. (2009). Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. Journal of the International Neuropsychological Society, 15(1), 19–30. 10.1017/S1355617708090188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser, P. J. , & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. Journal of Magnetic Resonance. Series B, 111(3), 209–219. 10.1006/jmrb.1996.0086 [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme 4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57(1), 289–300. [Google Scholar]
- Bialy, L. , Plint, A. , Zemek, R. , Johnson, D. , Klassen, T. , Osmond, M. , & Freedman, S. B. (2018). Pediatric emergency research Canada: Origins and evolution. Pediatric Emergency Care, 34(2), 138–144. 10.1097/PEC.0000000000001360 [DOI] [PubMed] [Google Scholar]
- Brooks, B. L. , Kadoura, B. , Turley, B. , Crawford, S. , Mikrogianakis, A. , & Barlow, K. M. (2014). Perception of recovery after pediatric mild traumatic brain injury is influenced by the “good old days” bias: Tangible implications for clinical practice and outcomes research. Archives of Clinical Neuropsychology, 29(2), 186–193. 10.1093/arclin/act083 [DOI] [PubMed] [Google Scholar]
- Carroll, L. , Cassidy, J. D. , Holm, L. , Kraus, J. , & Coronado, V. (2004). Methodological issues and research recommendations for mild traumatic brain injury: The who collaborating centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine, 36(0), 113–125. 10.1080/16501960410023877 [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). L. Erlbaum Associates. 10.4324/9780203771587 [DOI] [Google Scholar]
- Committee on Injury Scaling . (1998). Abbreviated Injury Scale. Association for the Advancement of Automotive Medicine. [Google Scholar]
- Fakhran, S. , Yaeger, K. , Collins, M. , & Alhilali, L. (2014). Sex differences in white matter abnormalities after mild traumatic brain injury: Localization and correlation with outcome. Radiology, 272(3), 815–823. 10.1148/radiol.14132512 [DOI] [PubMed] [Google Scholar]
- Feder, A. , Fred‐Torres, S. , Southwick, S. M. , & Charney, D. S. (2019). The biology of human resilience: Opportunities for enhancing resilience across the life span. Biological Psychiatry, 86(6), 443–453. 10.1016/j.biopsych.2019.07.012 [DOI] [PubMed] [Google Scholar]
- Fortin, J. P. , Parker, D. , Tunç, B. , Watanabe, T. , Elliott, M. A. , Ruparel, K. , Roalf, D. R. , Satterthwaite, T. D. , Gur, R. C. , Gur, R. E. , Schultz, R. T. , Verma, R. , & Shinohara, R. T. (2017). Harmonization of multi‐site diffusion tensor imaging data. Neuroimage, 161, 149–170. 10.1016/j.neuroimage.2017.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinowski, A. , Miranda, R. , Lemaitre, H. , Paillère Martinot, M. L. , Artiges, E. , Vulser, H. , Goodman, R. , Penttilä, J. , Struve, M. , Barbot, A. , Fadai, T. , Poustka, L. , Conrod, P. , Banaschewski, T. , Barker, G. J. , Bokde, A. , Bromberg, U. , Büchel, C. , Flor, H. , … the IMAGEN Consortium . (2015). Resilience and corpus callosum microstructure in adolescence. Psychological Medicine, 45(11), 2285–2294. 10.1017/S0033291715000239 [DOI] [PubMed] [Google Scholar]
- Goodrich‐Hunsaker, N. J. , Abildskov, T. J. , Black, G. , Bigler, E. D. , Cohen, D. M. , Mihalov, L. K. , Bangert, B. A. , Taylor, H. G. , & Yeates, K. O. (2018). Age‐ and sex‐related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: A comparison of voxelwise and tractography methods. Journal of Neuroscience Research, 96(4), 626–641. 10.1002/jnr.24142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodd, W. , Kumar, V. J. , Schüz, A. , Lindig, T. , & Scheffler, K. (2020). The anterior and medial thalamic nuclei and the human limbic system: Tracing the structural connectivity using diffusion‐weighted imaging. Scientific Reports, 10(1), 10957. 10.1038/s41598-020-67770-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette, J. P. , Shenton, M. E. , & Koerte, I. K. (2018). Imaging of concussion in young athletes. Neuroimaging Clinics of North America, 28(1), 43–53. 10.1016/j.nic.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte, R. , Brooks, W. , Vukas, R. , Pierce, J. , & Harris, J. (2019). Sex differences in traumatic brain injury: What we know and what we should know. Journal of Neurotrauma, 36(22), 3063–3091. 10.1089/neu.2018.6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Christensen, R. H. B. (2017). Lmer test package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Lebel, C. , Treit, S. , & Beaulieu, C. (2019). A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in Biomedicine, 32(4), e3778. 10.1002/nbm.3778 [DOI] [PubMed] [Google Scholar]
- Ledoux, A. A. , Tang, K. , Yeates, K. O. , Pusic, M. V. , Boutis, K. , Craig, W. R. , Gravel, J. , Freedman, S. B. , Gagnon, I. , Gioia, G. A. , Osmond, M. H. , Zemek, R. L. , & for the Pediatric Emergency Research Canada (PERC) Concussion Team . (2019). Natural progression of symptom change and recovery from concussion in a pediatric population. JAMA Pediatrics, 173(1), e183820. 10.1001/jamapediatrics.2018.3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Santos, J. P. , Kontos, A. P. , Mailliard, S. , Eagle, S. R. , Holland, C. L. , Suss, S. J., Jr. , Abdul‐waalee, H. , Stiffler, R. S. , Bitzer, H. B. , Blaney, N. A. , Colorito, A. T. , Santucci, C. G. , Brown, A. , Kim, T. , Iyengar, S. , Skeba, A. , Diler, R. S. , Ladouceur, C. D. , Phillips, M. L. , … Versace, A. (2021). White matter abnormalities associated with prolonged recovery in adolescents following concussion. Frontiers in Neurology, 12, 681467. 10.3389/fneur.2021.681467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey, H. M. , Hodges, C. B. , Greer, K. M. , Wilde, E. A. , & Merkley, T. L. (2021). Diffusion‐weighted imaging in mild traumatic brain injury: A systematic review of the literature. Neuropsychology Review. Advance online publication. 10.1007/s11065-021-09485-5 [DOI] [PubMed] [Google Scholar]
- MacDonald, C. L. , Barber, J. , Wright, J. , Coppel, D. , de Lacy, N. , Ottinger, S. , Peck, S. , Panks, C. , Sun, S. , Zalewski, K. , & Temkin, N. (2019). Longitudinal clinical and neuroimaging evaluation of symptomatic concussion in 10‐ to 14‐year‐old youth athletes. Journal of Neurotrauma, 36(2), 264–274. 10.1089/neu.2018.5629 [DOI] [PubMed] [Google Scholar]
- Manning, K. Y. , Schranz, A. , Bartha, R. , Dekaban, G. A. , Barreira, C. , Brown, A. , Fischer, L. , Asem, K. , Doherty, T. J. , Fraser, D. D. , Holmes, J. , & Menon, R. S. (2017). Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology, 89(21), 2157–2166. 10.1212/WNL.0000000000004669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A. R. , Kaushal, M. , Dodd, A. B. , Hanlon, F. M. , Shaff, N. A. , Mannix, R. , Master, C. L. , Leddy, J. J. , Stephenson, D. , Wertz, C. J. , Suelzer, E. M. , Arbogast, K. B. , & Meier, T. B. (2018). Advanced biomarkers of pediatric mild traumatic brain injury: Progress and perils. Neuroscience and Biobehavioral Reviews, 94, 149–165. 10.1016/j.neubiorev.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A. R. , Stephenson, D. D. , Dodd, A. B. , Robertson‐Benta, C. R. , Pabbathi Reddy, S. , Shaff, N. A. , Yeates, K. O. , van der Horn, H. J. , Wertz, C. J. , Park, G. , Oglesbee, S. J. , Bedrick, E. J. , Campbell, R. A. , Phillips, J. P. , & Quinn, D. K. (2020). Comparison of methods for classifying persistent post‐concussive symptoms in children. Journal of Neurotrauma, 37(13), 1504–1511. 10.1089/neu.2019.6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley, S. R. , Wilde, E. A. , Anderson, V. A. , Bedell, G. , Beers, S. R. , Campbell, T. F. , Chapman, S. B. , Ewing‐Cobbs, L. , Gerring, J. P. , Gioia, G. A. , Levin, H. S. , Michaud, L. J. , Prasad, M. R. , Swaine, B. R. , Turkstra, L. S. , Wade, S. L. , Yeates, K. O. , & Pediatric TBI Outcomes Workgroup . (2012). Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. Journal of Neurotrauma, 29(4), 678–705. 10.1089/neu.2011.1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh, D. L. , King, T. Z. , Sun, B. , Jones, R. A. , Ono, K. E. , Reisner, A. , & Burns, T. G. (2018). Longitudinal changes in resting state connectivity and white matter integrity in adolescents with sports‐related concussion. Journal of the International Neuropsychological Society, 24(08), 781–792. 10.1017/S1355617718000413 [DOI] [PubMed] [Google Scholar]
- Mustafi, S. M. , Harezlak, J. , Koch, K. M. , et al. (2018). Acute white‐matter abnormalities in sports‐related concussion: A diffusion tensor imaging study from the NCAA‐DoD CARE consortium. Journal of Neurotrauma, 35, 2653–2664. 10.1089/neu.2017.5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, R. , Fiest, K. M. , McChesney, J. , Kwon, C. S. , Jette, N. , Frolkis, A. D. , Atta, C. , Mah, S. , Dhaliwal, H. , Reid, A. , Pringsheim, T. , Dykeman, J. , & Gallagher, C. (2016). The international incidence of traumatic brain injury: A systematic review and meta‐analysis. Canadian Journal of Neurological Sciences, 43(6), 774–785. 10.1017/cjn.2016.290 [DOI] [PubMed] [Google Scholar]
- O'Brien, H. , Minich, N. M. , Langevin, L. M. , et al. (2021). Normative and psychometric characteristics of the health and behavior inventory among children with mild orthopedic injury presenting to the emergency department: Implications for assessing postconcussive symptoms using the child sport concussion assessment tool 5th edition (child SCAT5). Clinical Journal of Sport Medicine, 31(5), e221–e228. 10.1097/JSM.0000000000000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, R. J. , Romano, A. J. , Johnson, C. L. , & Bayly, P. V. (2019). Insights into traumatic brain injury from MRI of harmonic brain motion. Journal of Experimental Neuroscienc., 13, 117906951984044. 10.1177/1179069519840444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh, M. B. , Gurjarpadhye, A. A. , Manoukian, M. A. C. , Dubnika, A. , Rajadas, J. , & Inayathullah, M. (2015). Recent developments in diffusion tensor imaging of brain. Radiology‐Open Journal, 1(1), 1–12. 10.17140/roj-1-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2017). R: A language and environment for statistical Computing. R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/ [Google Scholar]
- RStudio Team . (2020). RStudio: Integrated Development for R. RStudio, PBC. Retrieved from http://www.rstudio.com/ [Google Scholar]
- Schilling, K. G. , Rheault, F. , Petit, L. , Hansen, C. B. , Nath, V. , Yeh, F. C. , Girard, G. , Barakovic, M. , Rafael‐Patino, J. , Yu, T. , Fischi‐Gomez, E. , Pizzolato, M. , Ocampo‐Pineda, M. , Schiavi, S. , Canales‐Rodríguez, E. J. , Daducci, A. , Granziera, C. , Innocenti, G. , Thiran, J. P. , … Descoteaux, M. (2021). Tractography dissection variability: What happens when 42 groups dissect 14 white matter bundles on the same dataset? Neuroimage, 243, 118502. 10.1016/j.neuroimage.2021.118502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, J. , Hayward, K. S. , Brown, K. E. , Zwicker, J. G. , Ponsford, J. , van Donkelaar, P. , Babul, S. , & Boyd, L. A. (2018). Imaging in pediatric concussion: A systematic review. Pediatrics, 141(5), e20173406. 10.1542/peds.2017-3406 [DOI] [PubMed] [Google Scholar]
- Shukla, A. , Ware, A. L. , Guo, S. , Goodyear, B. , Beauchamp, M. H. , Zemek, R. , Craig, W. , Doan, Q. , Beaulieu, C. , Yeates, K. O. , Lebel, C. , & Pediatric Emergency Research Canada A‐CAP study team . (2021). Examining brain white matter after pediatric mild traumatic brain injury using neurite orientation dispersion and density imaging: An A‐CAP study. NeuroImage: Clinical, 32, 102887. 10.1016/j.nicl.2021.102887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, H. G. , Dietrich, A. , Nuss, K. , Wright, M. , Rusin, J. , Bangert, B. , Minich, N. , & Yeates, K. O. (2010). Post‐concussive symptoms in children with mild traumatic brain injury. Neuropsychology, 24(2), 148–159. 10.1037/a0018112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, H. G. , Orchinik, L. J. , Minich, N. , Dietrich, A. , Nuss, K. , Wright, M. , Bangert, B. , Rusin, J. , & Yeates, K. O. (2015). Symptoms of persistent behavior problems in children with mild traumatic brain injury. The Journal of Head Trauma Rehabilitation, 30(5), 302–310. 10.1097/HTR.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, A. L. , Shukla, A. , Goodrich‐Hunsaker, N. J. , Lebel, C. , Wilde, E. A. , Abildskov, T. J. , Bigler, E. D. , Cohen, D. M. , Mihalov, L. K. , Bacevice, A. , Bangert, B. A. , Taylor, H. G. , & Yeates, K. O. (2020). Post‐acute white matter microstructure predicts post‐acute and chronic post‐concussive symptom severity following mild traumatic brain injury in children. NeuroImage: Clinical, 25, 102106. 10.1016/j.nicl.2019.102106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, A. L. , Shukla, A. , Guo, S. , et al. (2021). Participant factors that contribute to magnetic resonance imaging motion artifacts in children with mild traumatic brain injury or orthopedic injury. Brain Imaging and Behavior. Advance online publication. 10.1007/s11682-021-00582-w [DOI] [PubMed] [Google Scholar]
- Wilde, E. A. , McCauley, S. R. , Hunter, J. V. , et al. (2008). Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology, 70(12), 948–955. 10.1212/01.wnl.0000305961.68029.54 [DOI] [PubMed] [Google Scholar]
- Wilde, E. A. , Ware, A. L. , Li, X. , et al. (2018). Orthopedic injured versus uninjured comparison groups for neuroimaging research in mild traumatic brain injury. Journal of Neurotrauma, 36, 239–249. 10.1089/neu.2017.5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T. , Merkley, T. L. , Wilde, E. A. , Barnes, A. , Li, X. , Chu, Z. D. , McCauley, S. R. , Hunter, J. V. , & Levin, H. S. (2018). A preliminary report of cerebral white matter microstructural changes associated with adolescent sports concussion acutely and subacutely using diffusion tensor imaging. Brain Imaging and Behavior, 12(4), 962–973. 10.1007/s11682-017-9752-5 [DOI] [PubMed] [Google Scholar]
- Yano, R. , Hata, J. , Abe, Y. , Seki, F. , Yoshida, K. , Komaki, Y. , Okano, H. , & Tanaka, K. F. (2018). Quantitative temporal changes in DTI values coupled with histological properties in cuprizone‐induced demyelination and remyelination. Neurochemistry International, 119, 151–158. 10.1016/j.neuint.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Yeates, K. O. , Beauchamp, M. , Craig, W. , Doan, Q. , Zemek, R. , Bjornson, B. , Gravel, J. , Mikrogianakis, A. , Goodyear, B. , Abdeen, N. , Beaulieu, C. , Dehaes, M. , Deschenes, S. , Harris, A. , Lebel, C. , Lamont, R. , Williamson, T. , Barlow, K. M. , Bernier, F. , … Pediatric Emergency Research Canada (PERC) . (2017). Advancing concussion assessment in pediatrics (A‐CAP): A prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: Study protocol. BMJ Open, 7(7), e017012. 10.1136/bmjopen-2017-017012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Myall, N. J. , Wandell, B. A. , & Feldman, H. M. (2012). Tract profiles of white matter properties: Automating fiber‐tract quantification. PLoS One, 7(11), e49790. 10.1371/journal.pone.0049790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographic and injury characteristics for mTBI with and without persistent symptoms
Table S2. Follow‐up pairwise comparisons for significant group differences in DTI metrics after FDR correction. Pairwise comparisons conducted within the context of the final fitted model. Bolded/italicized = 95% confidence interval range excludes 0
Table S3. Follow‐up pairwise comparisons for significant differences in DTI metrics among symptom groups based on child and parent report. Pairwise comparisons conducted within the context of the final fitted model. Bolded/italicized = 95% confidence interval range excludes 0
Data Availability Statement
A dataset with deidentified participant data and a data dictionary will be made available upon reasonable request from any qualified investigator, subject to a signed data access agreement.


