Summary
Background
Alveolar echinococcosis (AE), which is caused by larval Echinococcus multilocularis, is one of the world's most dangerous neglected diseases. Currently, no fully effective treatments are available to cure this disease.
Methods
In vitro protoscolicidal assay along with in vivo murine models was applied in repurposing drugs against AE. Genome-wide identification and homology-based modeling were used for predicting drug targets. RNAi, enzyme assay, and RNA-Seq analyses were utilized for investigating the roles in parasite survival and validations for the drug target.
Findings
We identified nelfinavir as the most effective HIV protease inhibitor against larval E. multilocularis. Once-daily oral administration of nelfinavir for 28 days resulted in a remarkable reduction in parasite infection in either immune-competent or immunocompromised mice. E. multilocularis DNA damage-inducible 1 protein (EmuDdi1) is predicted as a target candidate for nelfinavir. We proved that EmuDdi1 is essential for parasite survival and protein excretion and acts as a functionally active protease for this helminth. We found nelfinavir is able to inhibit the proteolytic activity of recombinant EmuDdi1 and block the EmuDdi1-related pathways for protein export. With other evidence of drug efficacy comparison, our results suggest that inhibition of EmuDdi1 is a mechanism by which this HIV proteinase inhibitor mediates its antiparasitic action on echinococcosis.
Interpretation
This study demonstrates that nelfinavir is a promising candidate for treating echinococcosis. This drug repurposing study proves that the widely prescribed drug for AIDS treatment is potent in combating E. multilocularis infection and thus provides valuable insights into the development of single-drug therapy for highly prevalent co-infection between HIV and helminth diseases.
Funding
This work was supported by the National Natural Science Foundation of China (31802179), the Natural Science Foundation of Gansu Province, China (No. 21JR7RA027), and the State Key Laboratory of Veterinary Etiological Biology (No. SKLVEB2021YQRC01).
Keywords: Echinococcosis, HIV protease inhibitors, Nelfinavir, Co-infection, Ddi1-like protein
Research in context.
Evidence before this study
Alveolar echinococcosis is one (2nd) of the most important global, neglected, food-borne diseases. If left untreated, it invariably is fatal. However, only albendazole is currently licensed for the treatment of this disease. The current albendazole chemotherapy is far from optimal. Novel alternative drugs are urgently needed.
Added value of this study
In this study, we repurposed the HIV protease inhibitor nelfinavir as an effective candidate against the causative pathogen E. multilocularis in both immunocompetent and compromised hosts. Importantly, we proved for the first time that the Ddi1 protein in Platyhelminthes, which is conserved across parasitic helminths, is a functional aspartic protease and is essential for parasite survival. We also proved that this protein is the most likely drug target mediating the antiparasitic effect of nelfinavir.
Implications of all the available evidence
Nelfinavir and its derivatives are incorporated in first-line highly active antiretroviral therapy (HAART) for AIDS. They are also implicated in the development for treating multiple infectious diseases, even cancers. This study is the first to report nelfinavir as a promising effective drug against helminth. Helminths infect one-third of the world population and their prevalence in patients with AIDS is high. Therefore, the repurposing of the HIV protease inhibitor in this study would also provide valuable insights for the development of single-drug therapy for treating co-infections between helminth and HIV.
Alt-text: Unlabelled box
Introduction
Alveolar echinococcosis (AE) is a globally distributed zoonosis caused by the larval stage of the Echinococcus multilocularis tapeworm. As the second most important food-borne parasitic disease at the global level,1,2 it is amongst the world's most dangerous neglected diseases. Transmission of AE to humans is by consumption of the tapeworm eggs which are excreted in the feces of the definitive hosts (foxes or other canids). E. multilocularis has a wide distribution in the Northern Hemisphere, including extensive endemic regions in Asia, North America, and Europe.3,4 AE causes 18,235 new cases per annum globally, with the disease burden reaching 666,433 disability-adjusted life years.5 In many countries, AE has recently become an emerging disease with rising incidence or populations at a higher risk.5,6
AE is characterized by the slow development of a primary tumor-like lesion (also known as “parasitic cancer”), which is usually located in the liver and causes immunosuppression during chronic infection.7 Infected people will be experiencing significantly reduced quality of life and eventually death if left untreated. The case fatality of untreated or inadequately treated human AE patients is 90% within 10–15 years of diagnosis. However, treatment of this disease remains a challenge and is often expensive.8 There are very limited options for the treatment of echinococcosis. It mainly relies on surgery and drug intervention.7 A single drug, albendazole, is currently licensed for this disease. However, the current albendazole chemotherapy is far from optimal and is required continuously for many years.9 In addition, given the emerging chemoradiotherapy resistance, identification of other alternatives for drugs against AE is critical.
The repurposing of established drugs is evolving as a cost- and time-saving approach for antiparasitic drug development. HIV protease inhibitors (HIVPIs) are cornerstone agents widely prescribed for highly active antiretroviral therapy (HAART). These compounds inhibit the activity of the retroviral aspartate protease by competitively binding the active site.10 Interestingly, a marked reduction in both the incidence and prevalence of important bacterial, fungal and parasitic co-infections was observed after HIVPI treatment on AIDS, implying broad-spectrum chemotherapeutic properties for these compounds.11 Indeed, for example, the HIVPI nelfinavir has been reported to be effective in anti-cancers,12 anti-viruses (e.g., SARS-COV-2),13 and anti-parasites (e.g., Trypanosoma).14 In this sense, the repurposing of these approved drugs appears as an interesting and viable strategy for developing intervention tools against other pathogens.
We previously reported the presence of retroviral-like aspartic protease in flatworms utilizing a whole-genome screening. This indicates that these anti-HIV agents might also be inhibitors for this protease and thus might be effective against Platyhelminthes.15,16 However, their efficacies against helminths have not yet been experimentally investigated. Over one-third of people worldwide are currently infected with parasitic worms. In addition, as co-occurrence between HIV and helminth is highly frequent due to the coexisting immunosuppressive conditions caused by both pathogens, drug repurposing of HIVPIs is of high interest for developing HAART-based methods for treating such co-infection. In this study, we screened all approved HIVPIs and demonstrated that nelfinavir could effectively block the Ddi1-like enzyme in E. multilocularis that is vital for parasite survival and induces a protoscolicidal effect in either a normal or deficient immune state. Hence, this compound is a valuable candidate drug for treating alveolar echinococcosis and exerts potential for combating co-infection between helminths and HIV.
Methods
Ethics statement
All animals were handled in accordance with the guidelines specified by the Administration of Affairs Concerning Experimental Animals at Lanzhou Veterinary Research Institute. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Lanzhou Veterinary Research Institute (Reference No. LVRIAEC-2020-019).
Parasite preparation
The E. multilocularis strain,17 which was isolated from Qinghai in China, was used in this study. The protoscoleces collection was carried out as previously described.18 Briefly, parasite tissues were aseptically recovered from the peritoneal cavity of gerbils (Meriones unguiculatus) that had been intraperitoneally inoculated with protoscoleces for 3 months. The metacestode tissue was cut into thin slices and strained through a tea strainer. The collected PSCs were washed five times with sterile phosphate-buffered saline (PBS) and then cultured in DMEM medium (Gibco) at 37°C in 5% CO2. The medium was supplemented with 25% FBS (Gibco), 50 units/ml penicillin G, 50 μg/ml streptomycin, Gibco, 0.45% yeast extract (OXOID), and 0.4% glucose. The vitality and mortality of PSCs were evaluated after staining the parasite with fluorescein diacetate (FDA) and propidium iodide (PI) for 15 min.19 The initial vitality of PSCs used in this study was over 98%. In this study, animals and parasites were randomly assigned into each experimental group unless otherwise specified.
In vitro drug screening against E. multilocularis PSCs
HIV protease inhibitors (HIVPIs), including nelfinavir (NFV, CAS NO. 159989-64-7), lopinavir (LPV, CAS NO. 192725-17-0), indinavir (IDV, CAS NO.180686-37-8), darunavir (DRV, CAS NO. 206361-99-1), fosamprenavir (FAPV, CAS NO. 226700-79-4), ritonavir (RTV, CAS NO. 176655-55-3), tipranavir (TPV, CAS NO. 174484-41-4), atazanavir (ATV, CAS NO. 198904-31-3), saquinavir (SQV, CAS NO. 149845-06-7), and amprenavir (APV, CAS NO. 161814-49-9), were purchased from Macklin (Shanghai, China). Albendazole (ABZ, CAS NO. 54965-21-8) was purchased from Solarbio (Beijing, China).
To investigate the effects of HIVPIs on the viability of E. multilocularis PSCs, they were equally divided into 48-well plates (∼400 PSCs per well in triplicate) and treated with either HIVPIs, Albendazole, or DMSO at a concentration of 40 μM. The viability of PSCs was assessed every 24 h until 72 h using FDA/PI fluorescence staining method. For estimating the half-maximal effective concentration (EC50), PSCs were cultured in 48-well plates with different concentrations (ranging from 0.1 μM to 640 μM) of nelfinavir, ritonavir, and albendazole (DMSO as vehicle control). The EC50 was calculated using a nonlinear regression equation. The damage induced was quantitatively assessed by measuring the release of Phosphoglucose isomerase (PGI)20 into the supernatant medium using a Phosphoglucose Isomerase Colorimetric Assay Kit (Sigma, USA). PSCs were fixed in 3% glutaraldehyde/osmium tetroxide and embedded in epoxy resin to prepare ultra-thin tissue sections for transmission electron microscopy (TEM).
Protoscolicidal effect of nelfinavir
To confirm the protoscolicidal effect of nelfinavir on PSCs, specific pathogen-free (SPF) BALB/c mice (female, 7-9 weeks) were infected with 2,000 PSCs that had been pre-treated with 40 μM nelfinavir or DMSO control in vitro for 48 h. The testing of viability by injection of tissue into rodents (in vivo viability test) is an established procedure.21 The mice were euthanized and necropsied 60 days post-infection. The cysts collected from each mouse were weighed and stored in 4% buffered formalin (pH = 7.2) for hematoxylin and eosin (H&E) analysis.
RNA-Seq analysis of nelfinavir treatment
PSCs were treated with 40 μM nelfinavir or DMSO as vehicle control for 12 h in the culturing medium and then collected by centrifuge (×1,000 rpm). No significant difference in mortality of PSCs was observed between the nelfinavir treatment and control. Total mRNA was extracted using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific, USA) according to the manufacturer's instructions. RNA integrity was assessed using the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). The library was constructed using NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina® according to the manufacturer's instructions and then sequenced on the Illumina Hiseq X platform. Adapters and low-quality reads were filtered from the raw reads. Hisat2 (v2.0.5)22 was used to align the clean pair-end reads onto the reference genome of E. multilocularis (ENA accession: GCA_000469725.3). DESeq2 (v1.20.0)23 was used for differential analysis between groups. Differentially expressed genes (DEGs) were defined by P < 0.05. Gene set enrichment analysis (GSEA) was done for the ranked gene list by GSEA software.24
In vitro efficacy study on metacestode vesicles
Metacestode cysts were aseptically recovered from the peritoneal cavity of gerbils (M. unguiculatus) that were intraperitoneally inoculated with protoscoleces for 3 months. The collected cysts tissues were washed five times with PBS and then cultured using the protocol described above for PSCs. After 6 h and 12 h of incubation, the fluid of metacestode cysts was collected for detection of nelfinavir. The concentrations of nelfinavir in the cyst fluid were quantitatively measured by Agilent 1290 Infinity UHPLC System under the manufacturer's instructions using a standard curve-based method. On day 3 and day 7, PSCs were collected from the cultured cysts and their viability was assessed using the method described above.
In vivo efficacy study in BALB/c mice
SPF BALB/c mice (female, 7-9 weeks) were purchased from Lanzhou veterinary research institute, CAAS and housed in a SPF facility. No criteria were used to exclude animals. Mice were intraperitoneally infected with 2,000 PSCs following a previously described method.18 After 35 days post-infection, the mice randomly assigned into each experimental group and were subject to the following treatments: (1) For oral administration study, nelfinavir, ritonavir, and albendazole were prepared as a stock solution in DMSO and diluted with 0.5% carboxymethyl cellulose (CMC) in saline solution. The mice were gavaged with 0.2 mL of corresponding drug solution (50 mg/kg body weight for each mouse) once a day for a period of 28 days. Unmedicated control mice received 0.2 mL of CMC solution (vehicle control). (2) For the intraperitoneal administration study, nelfinavir was dissolved with 10% DMSO, 40% PEG-300, 5% Tween-80, and 45% saline. The infected mice were intraperitoneally injected with 0.1 mL of corresponding drug solution (50 mg/kg body weight for each mouse) or with vehicle solution once a day for 28 days. 14 days after the treatments, all mice were euthanized, and necropsy was carried out. Blood samples were collected immediately, and sera were separated with centrifugation at 2,000 g for 15 min at 4°C. The naive mice and infected mice with a clear presence of cysts tissues in the liver were selected for the detection of total protein loss in sera. Levels of total serum protein were measured by biuret protein assay kit (Zybio) using an AU analyzer system (AU5800, Beckman Coulter) according to the manufacturer's instructions. Cysts, liver, and spleen collected from each mouse were weighed and manually inspected by microscopy.
In vivo efficacy study in immunocompromised mice
SPF Nude-BALB/c mice (female, 7–9 weeks) were purchased from Cavensbiogle (Suzhou, China) and housed in a SPF facility. No criteria were used to exclude animals. Mice were intraperitoneally infected with 2,000 PSCs 28 days before treatments. The mice were randomly divided into groups. The drugs were prepared with the protocol mentioned above. The mice were gavaged with nelfinavir (50 mg/kg body weight for each mouse), albendazole (50 mg/kg body weight for each mouse), or vehicle solution once a day for 28 days, respectively. The mice were euthanized 14 days after the treatments.
Cloning and sequence analysis of the EmuDdi1
Total RNA of E. multilocularis PSC tissues was extracted using TRIzol Reagent (Invitrogen, Thermo Fisher Scientific, USA). cDNA synthesis was done with the PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Chian). Using the above-synthesized cDNA, the full-length coding sequence of EmuDdi1 (WormBase ID: EmuJ_000898400.1) was amplified by PCR using a pair of specific primers: EmuDdi1F: 5’-ATGAGGCTTTCTTTCGTTCTTCCC-3’ and EmuDdi1R: 5’-CTAGGGGAGACTGCTCATGT-3’. The reactions were denatured at 95°C for 5 min, followed by 30 cycles of denaturation at 94°C for 40s, annealing at 58°C for 1 min and extension at 72°C for 1 min, and final extension at 72°C for 10 min. Validation of the Ddi1 in T. solium was done with RACE method (S1 File).
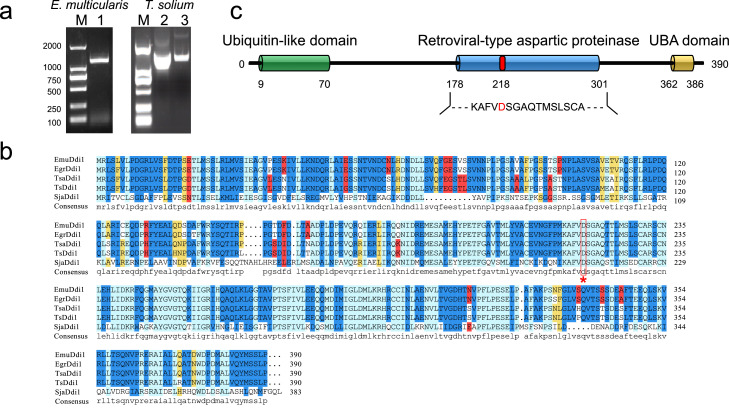
Genome-wide identification of A28 proteases was done with the Blast search server (e-value < 1e–5) in Wormbase ParaSite (https://parasite.wormbase.org/index.html). The sequence analysis of motifs was done with MOTIF Search (http://www.genome.jp/tools/motif/). The 3D structure was predicted by the SWISS-MODEL server (https://www.swissmodel.expasy.org/) based on the best hit template (PDB ID: 5ys4.1.A; identity: 52.31%; QSQE: 0.55). The predicted 3D structure for EmuDdi1 and crystal structure for the HIV protease (PDB ID: 1pro) were displayed with PyMoL (v1.20).25 Sequence alignment for the Ddi1-like orthologs was performed with Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). All these analyses were done with default settings.
Inference of the function of EmuDdi1 by RNAi
The Cy5-labeled siRNAs that specifically target the EmuDdi1 gene (siEmuDdi1: CCGACTGATGGTTTCTATT) and a Cy5-labeled negative control siRNA (NC-siRNA: Cat. No. siN0000001-1-10) were designed and synthesized by RIBOBIO (Guangzhou, China). The availability of NC-siRNA was validated by searching the E. multilocularis genome (EMULTI002). Approximately 5,000 PSCs were resuspended in 200 μL Gene Pulser Electroporation Buffer (BIO-RAD) and the siRNAs were added into the buffer at a final concentration of 2.5 μM.26,27 Transformations were done in a 4-mm electroporation cuvette at 200 V-100 μF using an exponential decay pulse (Bio-Rad). After incubation at 37°C for 30 min, 1mL DMEM medium was added to each electroporation cuvette and then transferred into 24-well culture plates. After 2 h of incubation, siRNA transfection efficiency was estimated with Laser Scanning Confocal Microscope (LSCM). At 6 h, 12 h, 24 h, 48 h, and 72 h post electroporation, the culture supernatants and PSCs were collected. The total protein concentration of the culture medium was examined with the BCA Protein Assay Kit (Beyotime, China). The release of PGI in the culture supernatant was measured with the Phosphoglucose Isomerase Colorimetric Assay Kit (Sigma, USA). The survival rate of PSCs based on the FDA/PI staining method was assessed by fluorescence microscopy (ZEISS).
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from the PSCs treated with the RNAi described above. The first-strand cDNA was synthesized and the resulting cDNA was diluted 5-fold with nuclease-free ddH2O. Each reaction was assembled in a total volume of 20 μL containing 10 μL of 2 × master mix (Promega, China), 2 μL of cDNA, 2 μL of each primer (2 μM, Table S1), and 4 μL of ddH2O. The reaction mixtures were run using the 7500 Real-Time PCR System (Applied Biosystems) under the following conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. The β-actin gene of E. multilocularis (WormBase ID: EmuJ_000061200) was selected as an endogenous reference gene. The relative expression levels were calculated using the 2–ΔΔCt method.
Expression and purification of recombinant EmuDdi1 protein
To express the recombinant EmuDdi1 protein, a pair of specific primers containing Sal I and Xho I restriction sites (EmuDdi1-F: CGAGCTCATGAGGCTTTCTTT CGTTC and EmuDdi1-R: CCCTCGAGACTAGGGGAG A CTGCTCATG) were designed and synthesized. The EmuDdi1 coding sequence was amplified and cloned into the expression vector pET-28a (+). Expression was induced by 1mM IPTG at 28°C. The recombinant EmuDdi1 protein was purified by affinity chromatography on a HiTrapTM column (GE), and then dialyzed against PBS at 4°C for 12 h. The purified protein was analyzed on 12.5% SDS-polyacrylamide gels and stained with Coomassie Brilliant Blue (Solarbio, China). Non-denaturing PAGE (NativePAGE™ Gel system, ThermoFisher) was used to analyze the dimer of the recombinant EmuDdi1. The concentration of recombinant EmuDdi1 protein was determined with the BCA protein assay kit (ThermoFisher) and was stored at −80°C until used.
Aspartyl protease assays
To detect the functional enzyme activity of the purified recombinant EmuDdi1 protein, a fluorogenic substrate DABCYL-GABA-SQNYPIVQ-EDANS (Apeptide, Shanghai, China), which is a substrate for HIV protease and can also be degraded by Leishmania Ddi1,28 was synthesized. The reaction mixture contained 0.5 μM of the recombinant protein and 2 μM of substrate solution in 100 mM sodium acetate buffer (pH ranging from 5 to 9) in a volume of 200 μL and incubated at 37°C for 2 h. The optimum pH (pH = 7.2) for the reaction system was determined. The Michaelis constant (Km) and catalytic number (Kcat) of recombinant EmuDdi1 protein were determined with increasing concentrations of the fluorogenic substrate (0.03 μM to 32 μM). The initial velocity was calculated from the slope of the linear range of the fluorescence versus the time curve. The Km and Kcat values were recorded with their standard errors derived from three replicates. For the inhibition assay, the recombinant EmuDdi1 protein (0.5 μM) was pre-incubated with each HIVPI (0-160 μM) and control (DMSO) in sodium acetate buffer (pH = 7.2) for 2h at 37°C. Then 2 μM of the fluorescence substrate was added to the reaction mixture for 2h incubation at 37°C. The cleavage of the fluorogenic substrate was measured using a SYNERGY H1 microplate reader (BioTek) at an excitation wavelength of excitation of 340 nm and an emission wavelength of 490 nm.29
Statistics
Statistical analyses were performed using GraphPad Prism (v5.0). No animals or data points were excluded from analyses. Figures for RNA-seq data were plotted using R (v4.1.0). Nonlinear regression analysis was used to determine the enzyme kinetic constants (Km and Kcat). Pearson correlation coefficient (R) was calculated for the correlation analysis. The comparisons of results for in vitro or in vivo experiments were conducted with either the Student's-t-test (two-sided; confidence level of 0.95) or the Wilcoxon-rank sum test (two-sided; confidence level of 0.95). The p values for the multiple hypothesis tests were corrected by Benjamini-Hochberg (B&H) method unless otherwise noted. Values of P < 0.05 or corrected P < 0.05 were considered statistically significant.
Role of funders
The funders had no role in the conceptualization, study design, data collection, analysis, interpretation of data, in writing the paper, or in the decision to submit the paper for publication.
Results
HIV protease inhibitor nelfinavir inhibits the viability of E. multilocularis protoscoleces
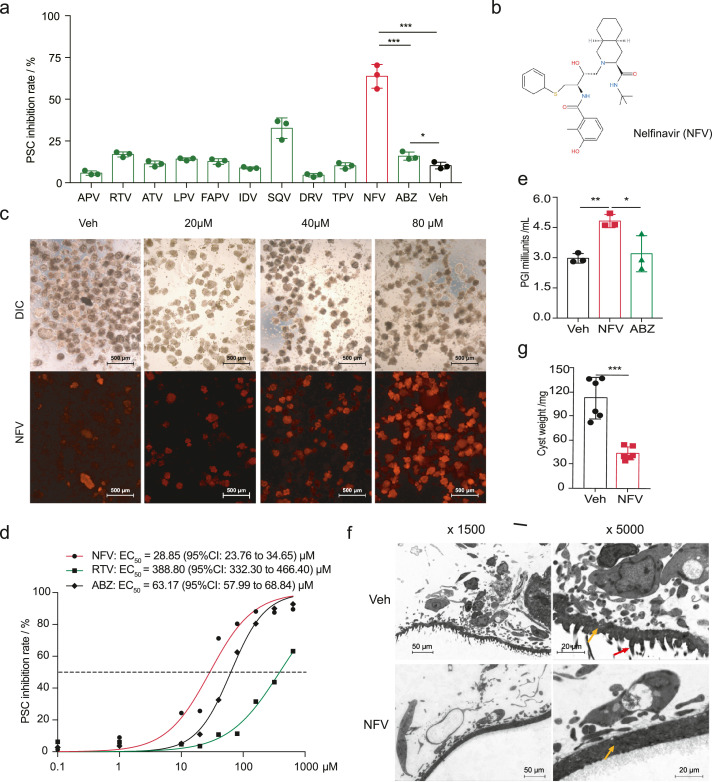
We evaluated the efficacies of 10 structurally distinct FDA-approved antiretroviral HIV protease inhibitors (HIVPIs) against E. multilocularis protoscoleces (PSCs) in vitro to test whether they have the anti-PSC activity. The screening results revealed that HIVPIs have effective and diverse anti-PSCs activities (Figure 1a). At concentrations of 40 μM, the most potent inhibitor in this screening system was the widely prescribed drug in HAART, nelfinavir (Figure 1b). A phenotypic screening revealed that nelfinavir has a protoscolicidal effect against E. multilocularis PSCs (Figure 1c). It remarkably increased mortality and reduced viability in terms of activity and motility. In comparison with the control group (DMSO treated), nelfinavir caused a dose-dependent efficacy of mortality in PSCs with a half-maximal effective concentration (EC50) of 28.84 (95% CI: 23.76 to 34.65) μM for the treatment of 72 h. This is approximately 2-fold more effective than albendazole EC50 = 63.17 (95% CI: 57.99 to 68.84) µM under such a condition (Figure 1d). This result confirmed previous findings that albendazole is not effective at a short incubation period.20 The leakage of phosphoglucose isomerase (PGI) activity into the medium supernatant was measured as an indicator for the compound efficacy in destroying metacestode tissues.20 After treatment of 48 h, the PGI activity for the nelfinavir-treated group was significantly higher than that for the control (Figure 1e). This indicates a disruption effect on the parasite body from nelfinavir.
Figure 1.
Nelfinavir effectively inhibits E. multilocularis protoscoleces (PSCs). (a) In vitro screening of HIVPIs on E. multilocularis PSCs. Albendazole (ABZ) and vehicle (Veh) were set up as positive and negative controls, respectively (n = 3). The inhibition rate represents the inhibition of viability. (b) The molecular formula of nelfinavir (NFV). (c) Staining of propidium iodide (PI) on PSCs treated with different concentrations of nelfinavir. The PSCs were stained with fluorescein diacetate (FDA) and PI for 15 min. (d) Half-maximal effective concentration (EC50) of nelfinavir and albendazole in the screening condition (n = 3). The EC50 of nelfinavir (28.84 [95% CI: 23.76 to 34.65] μM) is significantly lower than that of albendazole (63.17 [95% CI: 57.99 to 68.84] μM) or that of ritonavir (388.80 [95% CI: 332.30 to 466.40] μM). (e) Leakage of phosphoglucose isomerase (PGI) activity into the medium supernatant after nelfinavir (40μM) treatment for 48 h (n = 3). (f) Representative of transmission electron microscopy (TEM) image for the PSCs treated with nelfinavir. The PSCs were treated with 40 μM nelfinavir for 48 h. The damages of microtriches (red arrow) and surfaces were observed. The yellow arrow indicates the ‘tegument’ of the parasite. (g) Evaluation of protoscolicidal effect on PSCs for nelfinavir (n = 6). The PSCs (n=2000) treated with 40 μM nelfinavir for 48 h were injected into BALB/c mice. Cysts were weighed after two months. The results shown are representative of two or more independent experiments. Data shown are represented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 using the two-sided Student's-t test.
Incubation of PSCs in the presence of nelfinavir resulted in dramatic morphological changes. After a 48 h incubation period, PSCs treated with nelfinavir showed a complete disruption with reduced turgidity, diminished microthrix border, and irregular surfaces (Figure 1f). To further confirm the protoscolicidal effect of nelfinavir on PSCs, we infected BALB/c mice with the PSCs that had been pre-treated with or without nelfinavir in vitro. The mice were then euthanized 60 days after the infection for measuring the parasite burden. Weights of cysts were remarkably reduced in the mice infected with nelfinavir-treated PSCs (Figure 1g), indicating potent killing activity for nelfinavir on PSCs. Collectively, these results indicate that the HIVPI nelfinavir is effective in inhibiting the viability of E. multilocularis PSCs in vitro.
Nelfinavir inhibits the viability of E. multilocularis metacestode vesicles
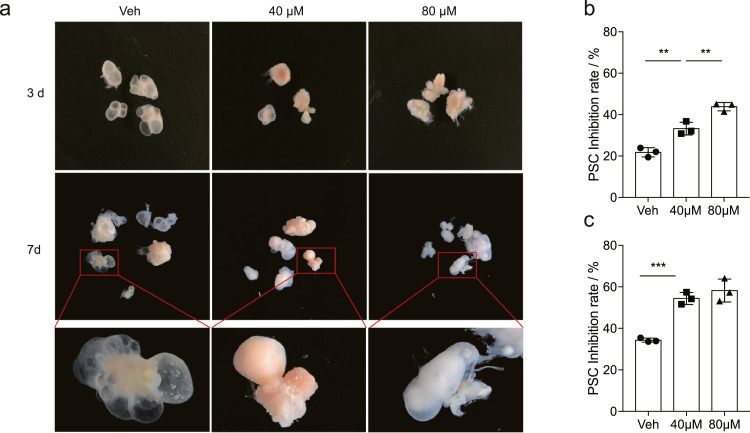
In order to test the efficacy of nelfinavir on metacestode vesicles, we collected cysts isolated from the gerbils that had been infected with E. multilocularis for 3 months and in vitro cultured them with or without the presence of nelfinavir. The liquid chromatography analysis revealed that a time-dependent accumulation of nelfinavir occurred in the vesicle fluid of the cysts after treatment (6 h and 12 h) (Figure S1), indicating that nelfinavir is able to effectively penetrate the metacestode vesicles. Furthermore, incubation of metacestode vesicles in the presence of nelfinavir for 3 days or 7 days resulted in dramatic morphological changes, in which shrank surfaces and reduced fluid were observed (Figure 2a). In particular, the “budding” of vesicles from metacestode tissues was obviously blocked after the nelfinavir exposure, whereas the E. multilocularis metacestode tissue cultures exposed to the DMSO solvent control budded off vesicles apparently (Figure 2a). More importantly, nelfinavir treatment resulted in a significantly increased mortality of the PSCs within the cysts in comparison with the control at either day 3 or day 7 (Figure 2b and c). Together, these data suggest that nelfinavir is effective in inhibiting the growth of metacestode vesicles.
Figure 2.
Nelfinavir effectively inhibits E. multilocularis metacestode vesicles. The metacestode cysts, which were isolated from gerbils that had been infected with PSCs for 3 months, were treated with nelfinavir (at 40 μM and 80 μM) DMSO control (Veh). (a) The nelfinavir treatment reduced fluid within cysts, shrunk laminated layer surfaces, and blocked the ‘budding’ of the cysts. On day 3 (b) or day 7 (c), the viability of PSCs within the cysts was significantly inhibited by the treatment of nelfinavir. The results shown are representative of two independent experiments. Data shown are represented as mean ± SD (n = 3). **P < 0.01, ***P < 0.001 using the two-sided Student's t-test.
Nelfinavir blocks the growth of parasite cysts in E. multilocularis infected mice
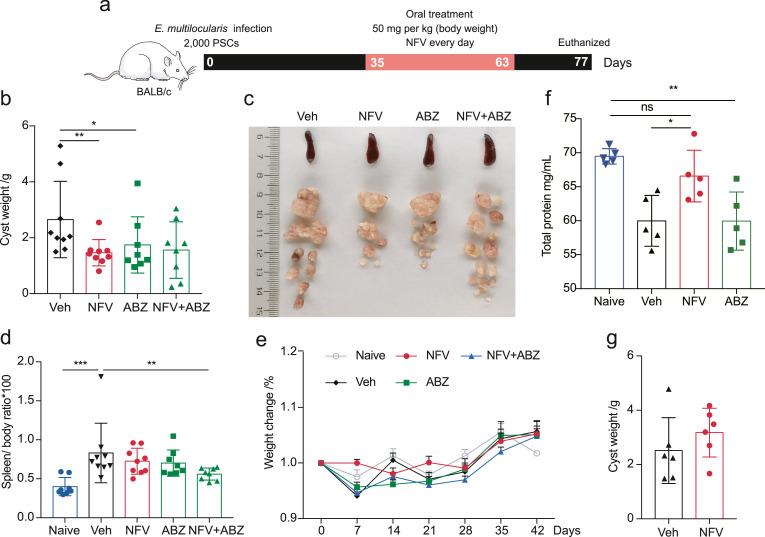
The in vivo impact of treatment on parasite load was investigated in a mouse model (E. multilocularis PSC-infected BALB/c mice) (Figure 3a). After a period of 28 days of daily oral application (50 mg/kg/day), nelfinavir greatly reduced parasite burden (weight of cysts) in comparison with mice in a vehicle-treated control group. Albendazole showed slightly lower efficacy (Figure 3b and c). After necropsy, the liver, spleen, heart, and kidneys of each mouse were weighed and carefully examined. Splenomegaly is a hallmark of the inflammation induced by helminth infections30 (Figure 3d). Neither nelfinavir nor albendazole treatment resulted in a significant reduction of spleen weights (Figure 3d). All the mice tolerated nelfinavir treatment without compound-related abnormalities in terms of body weights (Figure 3e), liver and heart (data not shown), and physical activity. Furthermore, mice with oral nelfinavir treatment suffered less loss of total serum protein (indicated by albumin and globulin) (Figure 3f). This indicates that nelfinavir probably protects the liver function, which was typically damaged in liver echinococcosis. A combination of nelfinavir (50 mg/kg/day) and albendazole (50 mg/kg/day) did not show a synergistic effect against cysts in terms of cyst weight. However, it showed an apparent reduction of cyst sizes was observed (Figure 3b and c). Of note, the combination of the two drugs resulted in reduced spleen weights compared to vehicle-treated mice, indicative of the potential in controlling the development of splenomegaly (Figure 3d). We also tested the administration of nelfinavir via an intraperitoneal injection route, but no significant reduction of parasite load was observed (Figure 3g). These data clearly show that oral administration of nelfinavir is effective against echinococcosis in vivo.
Figure 3.
Nelfinavir has potent activity against larval E. multilocularis in immunocompetent mice. BALB/c mice were infected with 2000 E. multilocularis PSCs (a). A once-daily dose of nelfinavir (NFV) or albendazole (ABZ) (50 mg/kg/day) was gavaged for 28 days (n = 8–9). The mice were euthanized after 14 days at the end of treatment. The nelfinavir treatment markedly reduced the weights of cysts (b-c). Spleen size (d), weight change (e) and total protein (f) (n = 5) in serum were also evaluated. Treatment of nelfinavir administered via intraperitoneal injection was accessed and no apparent efficacy was observed (g) (n = 6). The results shown are representative of two or more independent experiments. Data shown are represented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 using the two-sided Student's-t test.
Nelfinavir is effective in immunocompromised mice
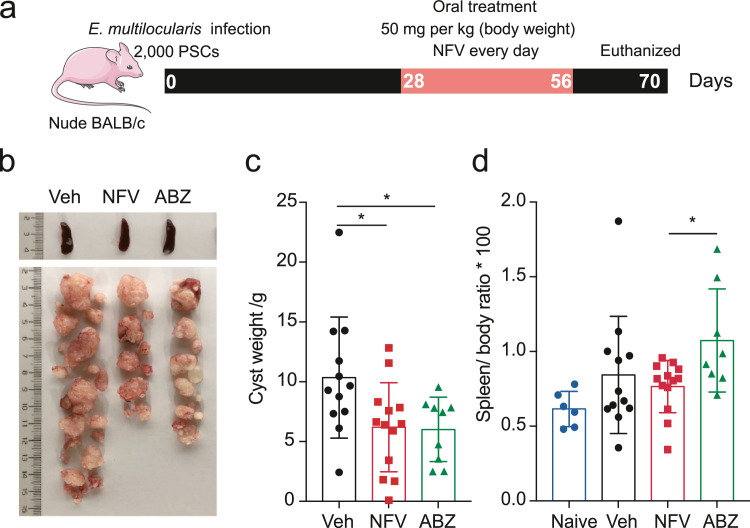
Co-incidence between helminth infections and immunosuppression conditions (e.g., AIDS.) occurs frequently worldwide.31 Recent studies have reported that host immunity is associated with the degree of efficacy of anti-AE treatment using Albendazole.32 Patients with echinococcosis and low CD4 counts showed poor response to Albendazole treatment.33 In order to assess whether nelfinavir could serve as an alternative medicine against echinococcosis in immunocompromised status, we tested the effectiveness of nelfinavir in immunodeficient mice (Figure 4a). For athymic nude mice infected with E. multilocularis PSCs, nelfinavir treatment (50 mg/kg/day) showed a significant reduction of parasite mass compared to the vehicle-treated group (Figure 4b and c). Consistent with the situation in wild-type mice, E. multilocularis infection in immunocompromised mice typically enlarged the spleen (Figure 4d). The group treated with nelfinavir exerted reduced spleen sizes, in comparison with the albendazole-treated group (Figure 4d). These results suggest that nelfinavir still keeps effective during the immunodeficiency state and thus is a candidate for treating co-infection between HIV and echinococcosis.
Figure 4.
Nelfinavir is effective against larval E. multilocularis in immunocompromised mice. The nude-BALB/c mice were infected with 2000 E. multilocularis PSCs (a) and treated with nelfinavir (NFV) or albendazole (ABZ) (50 mg/kg/day) for 28 days. Weight of cysts (b-c) and spleen size (splenomegaly) were measured. The results shown are representative of two or more independent experiments. Data shown are represented as mean ± SD (n = 6 for Naïve, n = 12 for Veh, n = 13 for NFV, and n = 9 for ABZ). *P < 0.05 using the two-sided Student's-t test.
E. multilocularis Ddi1-like protein is a retroviral-type target candidate for nelfinavir
Next, we attempted to identify the potential target for nelfinavir. As an inhibitor for HIV protease, nelfinavir is speculated to interact with the homologs from the retroviral-like aspartyl protease family (Peptidase A28). Utilizing a genome-wide search, our previous study has proved the presence of the single-copy Ddi1-like homologue (Ddi1) from this family in parasitic flatworms.16 Herein, we further verified the open reading frame of the Ddi1-like gene in the tapeworms E. multilocularis (EmuDdi1) and T. solium by genome-wide search of retroviral-like protease, PCR, and rapid amplification of cDNA ends (RACE) (Figure 5a). RNA-seq data mining revealed that EmuDdi1 is constitutively expressed in all the studied survival conditions or developmental stages for E. multilocularis (Figure S2). Sequence alignment indicates that Ddi1-like proteins are conserved among the cestodes and trematodes (Figure 5b) in having an N-terminal ubiquitin-like domain (UBL, PF00240), the central retroviral-type aspartic proteinase (RVP, PF09668) domain and a UBA-like domain at C-terminal (UBA, PF14555) (Figure 5c). HIVPIs can block protein function by competitively fitting the active site of HIV aspartic protease. The protein homology-modeling analysis revealed that the predicted tertiary structure of EmuDdi1 highly resembled that of HIV protease at the active site cavity in having the flexible flaps, and the dimer interface (Figure S3). These data suggest that EmuDdi1 might be a candidate target as the HIV protease inhibitor.
Figure 5.
Identification of DNA damage-inducible 1 protein (Ddi1) gene in Platyhelminthes. The Ddi1 genes in E. multilocularis and Taenia solium were validated by PCR (left) or RACE (right; lanes 2–3 indicate the product of 5’-RACE and 3’-RACE, respectively) amplification (a). The alignment of protein sequences of Ddi1 in flatworms indicates that Ddi1 is highly conserved in these species (b). Ddi1 of E. multilocularis (EmuDdi1) consists of an Ubiquitin-like domain at N-terminal, a central retrovirus-like aspartic protease, and a UBA-like domain at C-terminal (c).
Ddi1 gene is essential for parasite survival and inhibits protein excretion
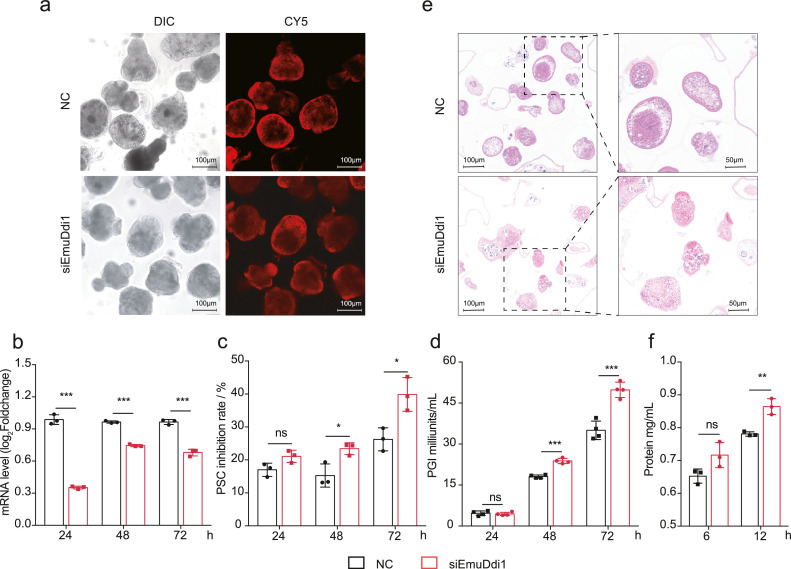
To determine whether EmuDdi1 might serve as a target for intervention against echinococcosis, we investigated the role of this protein in parasite survival. To this end, we suppress the gene expression of EmuDdi1 in PSCs via RNA interference (RNAi) (Figure 6a: transfection of siRNA into PSCs). After siRNA introduction by electroporation, the mRNA level was significantly reduced as compared to irrelevant negative siRNA (NC) (Figure 6b). The survival rate of dsRNA-treated PSCs after 48 h and 72 h was decreased to 77% and 60%. This is significantly lower than that of the NC control group (85% and 74%) (Figure 6c). The PGI activity in the supernatant medium was significantly increased after RNAi for 48 h and 72 h. This indicates that the suppression of EmuDdi1 was effective to lead to the disruption of metacestode tissues (Figure 6d). Accordingly, histological analysis showed RNAi treatment resulting in a significant cytocidal effect in the PSC, featured by apparent morphological alterations and disruption with irregular and fissured surfaces (Figure 6e). RNAi of EmuDdi1 for 12 h significantly promoted protein secretion of PSCs into the culture medium (Figure 6f). This is in accordance with the reported roles of its orthologs in the inhibition of protein export, involving protein degradation in the proteasome for non-helminth species.34, 35, 36 These results suggest that EmuDdi1 is essential for parasite survival and could potentially provide effective therapeutic treatments.
Figure 6.
RNAi on E. multilocularis Ddi1 (EmuDdi1) gene reduces the parasite's survival. Transformations of siEmuDdi1 or negative control were performed on PSCs with high efficiency (a), as indicated by the mRNA level of EmuDdi1 after 2 h measured by qRT-PCR (b). The inhibition rate of PSC viability (c) and PGI into the culture medium supernatant (d) were gradually increased across the duration of RNAi (n = 3). The morphology and integrity of the parasite body were changed (e). Total protein in the serum-free medium was increased at 12 h post-treatment (f), at which the parasite body had not been disrupted by RNAi. The results shown are representative of two or more independent experiments. Data shown are represented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 using the two-sided Student's t-test.
Nelfinavir inhibits the actively functional enzyme EmuDdi1
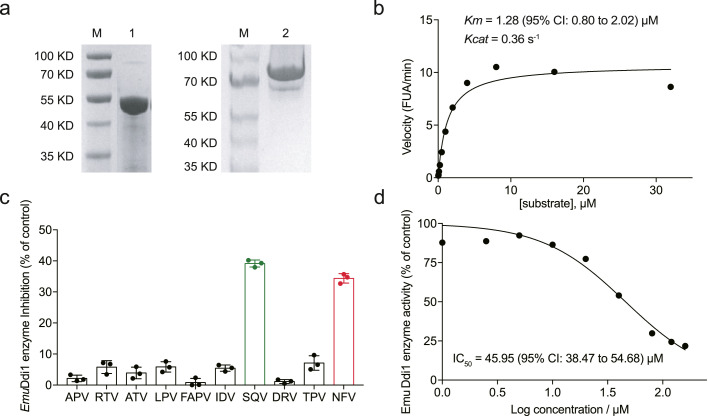
Next, we investigated whether EmuDdi1 could be inhibited by retroviral aspartyl protease inhibitors. As the roles of Ddi1 in flatworms are almost unknown, we wondered whether EmuDdi1 is an actively functional enzyme. Recombinant EmuDdi1 protein was expressed in a prokaryotic expression system. Electrophoretic analysis revealed that this protein was assembled into a form of homodimer (Figure 7a). The proteolytic activity of the E. multilocularis recombinant Ddi1-like enzyme was analyzed against a synthetic substrate for HIV proteinase. In accordance with the functions of its orthologs in several protozoan parasites, we observed substrate hydrolysis capability on the retropepsin substrate for the recombinant EmuDdi1 in vitro. In contrast to the other protozoan Ddi1-like enzymes with the optimal pH at an acidic condition, EmuDdi1 has its optimum at pH 7-7.5 (Figure S4). EmuDdi1 hydrolyzed the peptide with a Km of 1.28 (95% CI: 0.80 to 2.02) μM and a Kcat of 0.36 s-1 (Figure 7b). This is the first direct evidence that the flatworm Ddi1-like protein is capable of proteolytic activity.
Figure 7.
Nelfinavir inhibits the proteolysis activity of recombinant EmuDdi1. EmuDdi1 was expressed in an E. coli system. Based on the amino acid compositions, this molecular weight of the recombinant protein was predicted to be appropriately 45 KD in the monomer (left) and 90 KD in the dimer (right) (a). At an optimum pH of 7.2, the recombinant EmuDdi1 was able to hydrolyze the retro-pepsin substrate with a Km = 1.28 (95% CI: 0.80 to 2.02) μM and a Kcat = 0.36 s–1 (b). The HIVPIs, including lopinavir (LPV), indinavir (IDV), darunavir (DRV), fosamprenavir (FAPV), ritonavir (RTV), tipranavir (TPV), atazanavir (ATV), amprenavir (APV), nelfinavir (NFV) and saquinavir (SQV) were tested (n = 3). The enzyme activity of this recombinant protein (at 0.5 μM) could be apparently blocked by nelfinavir (NFV) and saquinavir (SQV) (40 μM) (c). The half-maximal inhibitory concentration (IC50) of nelfinavir on the recombinant EmuDdi1 was 45.95 μM (d). The results shown are representative of two or more independent experiments. Data shown are represented as mean ± SD.
To test whether HIVPIs inhibit the activity of EmuDdi1, we tested all the 10 HIVPIs against the recombinant Ddi1-like aspartyl proteinase activity. Thereto, we detected the hydrolysis of aspartyl proteinase substrates in triple replicates (Figure 7c). Similar to the screening of HIVPIs against PSCs (Figure 1a), nelfinavir and saquinavir outcompeted others with the highest efficacy against EmuDdi1. The observed inhibition rate was nearly 40% against the Ddi1 ortholog in E. multilocularis (Figure 7c). The half-maximal inhibitory concentration (IC50) of nelfinavir on the recombinant EmuDdi1 was 45.95 μM (95% CI: 38.47-54.68) (Figure 7d). These data show that nelfinavir is an inhibitor of the actively functional enzyme EmuDdi1.
Antiparasitic action of nelfinavir is tightly linked with blocking EmuDdi1 enzymatic activity
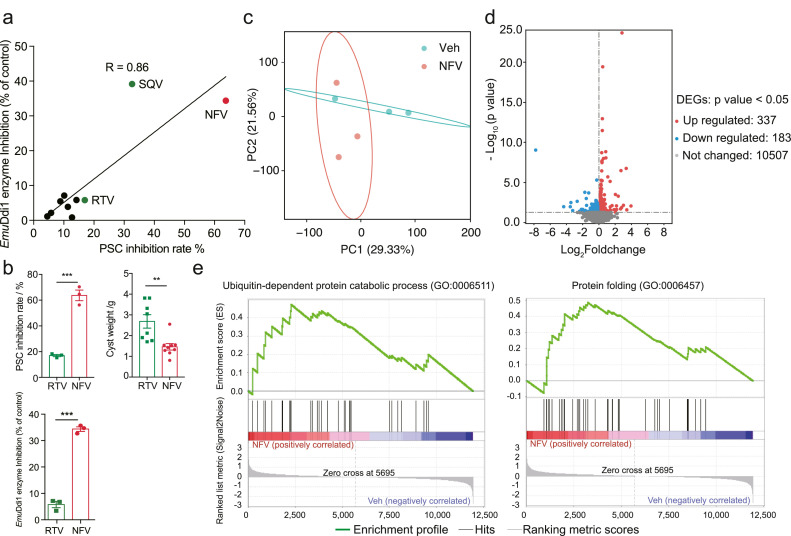
The above evidence suggests that the protoscolicidal activity of nelfinavir might be mediated by inhibiting the Ddi1 function. When we measured diverse HIVPI analogs against EmuDdi1 enzymatic activity and PSCs growth in vitro, we found a tight correlation (R = 0.86, P < 0.05) (Figure 8a), which indicates that the anti-Emu activity is directly mediated by EmuDdi1 inhibition. Further genetic and structural insights are needed to unambiguously establish EmuDdi1 as the target. However, we noted that the HIV protease inhibitor ritonavir was inactive against E. multilocularis PSCs in vitro and cysts in mice, and did not inhibit EmuDdi1 enzymatic activity (Figure 8b).
Figure 8.
Antiparasitic action of nelfinavir is associated with blocking EmuDdi1 enzymatic activity. The inhibition efficacy of HIVPIs on PSCs in vitro is significantly correlated with the inhibition efficacy of HIVPIs (40 μM) on the recombinant EmuDdi1 (Pearson R = 0.86, P < 0.05) (a). In contrast to nelfinavir (NFV), ritonavir (RTV) is ineffective in enzyme activity and exerts no efficacy on inhibiting PSCs in vitro (n = 3) and cysts in vivo (n = 8 for RTV; n = 9 for NFV) (b) (40 μM), further supporting the antiparasitic action of HIVPIs is mediated by blocking the functions of EmuDdi1. RNA-Seq analysis (n = 3 for biological replicates) reveals that nelfinavir treatment could change the gene expression patterns, as indicated by the Principle component analysis (PCA) (c). Differentially expressed genes (DEGs) for both up-regulated (n = 337) and down-regulated (n = 183) were identified. Gene Set Enrichment Analysis (GSEA) showed that biological processes pertaining to protein export and proteolysis are up-regulated after nelfinavir treatment (|NES| > 1 and NOM P < 0.01). The results shown are representative of two or more independent experiments for panels a and b. Data shown are represented as mean ± SD. **P < 0.01, ***P < 0.001 using the two-sided Student's t-test.
Although the roles of the Ddi1-like gene in helminths remain to be determined, it has previously been reported that functions of the gene orthologs in several species are conserved. It usually serves as a shuttling factor in the ubiquitin-proteasome system and inhibits protein export in yeast and mammals.36,37 To clarify the links between nelfinavir and Ddi1, we profiled the gene expressions of PSCs after 12 h of treatment with nelfinavir using RNA-Seq. As indicated by the principal coordinate analysis (PCA) (Figure 8c), the nelfinavir treatment markedly altered the gene expression patterns with 337 genes up-regulated and 183 genes down-regulated (Figure 8d). The genes involved in protein export were significantly up-regulated (Table S2). Gene set enrichment analysis (GSEA) revealed that processes of protein excretion, such as ‘UBIQUITIN-DEPENDENT_PROTEIN_ CATABOLIC_PROCESS’, ‘GOLGI_VESICLE_TRAN SPORT’, and ‘PROTEIN_FOLDING’ were significantly enriched in the group with nelfinavir treatment (Figure 8e) (Table S3). This suggests that nelfinavir treatment is probably involved in damaging the function of Ddi1. It also supports the inhibition of Emu-Ddi1 as the mechanism of action for nelfinavir. Taken together, our data suggest that nelfinavir is a drug candidate for echinococcosis by directly inhibiting EmuDdi1 enzymatic activity.
Discussion
Echinococcosis is one of the most important food-borne diseases worldwide. Nevertheless, effective treatment for this important infectious disease is lacking. This study reports a repurposing effort for the HIV protease inhibitor nelfinavir, with well-known antimicrobial mechanisms, to treat this severe zoonotic disease. Among all HIV protease inhibitors, nelfinavir stood out as a potent E. multilocularis inhibitor. This drug candidate exerts high efficacy in either immune normal and immunocompromised mice, in line with the efficacy of the only clinically available drug-albendazole in reducing the parasite mass. As indicated by spleen enlargement in nude mice, nelfinavir might be more effective than albendazole in controlling the inflammation caused by the chronic infection. Consistent with this notion, previous studies already reported the anti-inflammation effects of nelfinavir.38 For example, this compound is capable of activating PP2 and inhibiting MAPK signaling in macrophages to reduce inflammatory responses.38 If this can be confirmed, nelfinavir may offer additional beneficial effects independent of antiparasitic activity by reducing the severity of chronic immune activation in helminth infection. Such an effect is not known for albendazole and was not observed in this study. Nelfinavir is originally designed as a competitive inhibitor targeting the activity core of HIV aspartic protease. However, nelfinavir and its derivatives are now implicated in development for treating multiple infectious or non-infectious diseases, like SARS-COV-2,13 malaria,11 and even cancers.12 Herein, we, for the first time, report that this drug has the potential to combat a helminth infection. As immunosuppression can be caused by both HIV infection and chronic helminth infections, a high prevalence of co-infection between these two diseases is observed.31,39 Therefore, screening and treatment of helminths have been considered as part of the management of HIV and AIDS in primary health care.40 In this regard, the repurposing of nelfinavir would be of great value for treating co-infections. However, the efficacy of this drug in treating other helminthiasis remains determined.
Nelfinavir exerts high efficiency in inhibiting both PSCs and metacestode vesicles in a dose-dependent manner. Previous pharmacokinetics and pharmacodynamics studies have shown that lower plasma concentrations and high variations were found in some patients medicated with this drug at different doses and regimens.41 In this study, the EC50 (∼29 uM) results on protoscoleces in vitro and IC50 (∼45 uM) on the recombinant enzyme indicate that the inhibiting efficiency of nelfinavir on this parasite is dose-dependent and thus the lower concentration of the drug might cause reduced efficacy. Accordingly, the dose and pharmacodynamics are important factors that need to be taken into account if nelfinavir would be used in treating echinococcosis. In addition, this study utilized a secondary infection model with PSCs in BALB/c mice for evaluating the effects of nelfinavir on metacestode vesicles in vivo, for which the developments of cysts might not be synchronous.42 As a limitation in this study, the in vivo efficacy of nelfinavir treatment at different developmental stages of the parasite needs further investigation.
Our results suggest that nelfinavir is effective in the immunodeficient host. As co-infection between HIV and helminth is common,43 this finding would provide insights for future development of single-drug therapy for the co-infection. A previous study reported that ABZ is less efficacious in athymic nude mice than in wild-type mice.32 In contrast, we observed a significant reduction of cyst mass after ABZ treatment in athymic nude mice. The differences in the methods of secondary intraperitoneal infection and in the ways for drug administration might account for this inconsistency of the observed results between the studies. In addition, it is noteworthy that the nelfinavir treatment in this study was unable to reduce parasite growth to the same absolute level found in immunocompetent mice. Relatedly, we also note that the absolute value of parasite mass in vehicle-treated nude mice is also higher than that in vehicle-treated immunocompetent mice. This phenomenon of increased parasite growth might be reasonably interpreted by the fact that nude mice partially lose the ability to kill parasites, whereas only a small proportion of PSCs injected could survive in immunocompetent mice.44,45 In this study, nelfinavir treatment could cause a similar fold-change (∼0.5) of cyst weights comparable in either immunocompetent or immunodeficient mice.
The presence of a single copy gene encoding aspartic protease in the flatworm genomes provided us an opportunity to validate the likely drug target for nelfinavir. Our results revealed that the DNA damage-inducible (Ddi1) protein exists in all the flatworms and is essential for parasite survival, although its roles in these species are still unknown. Ddi1-like proteins in helminths are conserved and have an N-terminal ubiquitin-like domain and a central retroviral-type aspartic proteinase domain. The ubiquitin-like domain of Ddi1 in yeast and mammals is involved in binding proteasomes and in protein export.35,37,46 Our analyses suggest that this function is still conserved in E. multilocularis, as knockdown of this gene resulted in a significant increase of protein excretion in PSCs. Further, the recombinant EmuDdi1 protein is capable of proteolytic activity in detectable enzyme activity from the prokaryotic expression system. This activity is probably mediated by the retroviral-type proteinase domain. This is the first direct evidence for a Ddi1-like protein from flatworms acting as an active enzyme that requires a still unknown activation mechanism. In contrast to the orthologs in protozoan parasites,29,35 which have an optimum acidic pH, EmuDdi1 is more effective at a neutral to near-alkaline pH (7-7.5), indicative of unique characteristics for this worm ortholog. Notably, Saccharomyces cerevisiae Ddi1 is also active at physiological (neutral pH) conditions (pH 7.4), although it is only capable of hydrolyzing polyubiquitinated substrates.37 In this study, we did not test whether EmuDdi1 had the ability to degrade polyubiquitinated proteins/substrates. According to a recent study,29 the recombinant Plasmodium Ddi1 is able to hydrolyze both ubiquitinated proteasome substrates and the retropepsin substrate at pH 5.0. Thus, it is possible that EmuDdi1 also has the capability to hydrolyze polyubiquitinated substrates. We also provide comprehensive target validations for nelfinavir as an echinococcosis drug. The inhibition of nelfinavir on Ddi1 protein has also been proved in multiple protozoan parasites, including Leishmania and Plasmodium.29,35 Therefore, the antiparasitic activity of HIVPIs might be conserved in these parasites. RNA-Seq analysis of the parasites treated with nelfinavir provides more evidence for its inhibition action of nelfinavir being directly (at least partially) mediated by blocking the protein export and the proteasome-related functions of EmuDdi1.37 In accordance with this hypothesis, significant reductions in the prevalence of ascariasis, trichuriasis, hookworm infection, and strongyloidiasis have also been reported after starting a highly active antiretroviral therapy (HAART),47 although the association can be alternatively interpreted by the reconstitution of cellular immunity after HAART. As Ddi1 is conserved among species, nelfinavir possibly also blocks the activity of the homologs in hosts. Indeed, studies have revealed that nelfinavir is able to inhibit the function of Ddi1 proteins in mammals.48,49 However, the binding capabilities of nelfinavir to Ddi1 presumably differ between parasites and hosts, since many differences in sequences still exist. In accordance with this notion, a previous study found that the Ddi1 protein in Leishmania is more sensitive to nelfinavir than in humans.29 Moreover, it is unclear whether nelfinavir could be metabolized by parasites but it has been definitely determined that nelfinavir can be metabolized by systems, e.g., the cytochrome P450, in mammals.50 This difference of metabolism may also potentially account for the observed high tolerability for the host in treating echinococcosis using nelfinavir in this study. However, further genetic and structural insights are needed to unambiguously validate Ddi1 as the target for nelfinavir against echinococcosis. In addition, since the HIV-protease inhibitor has shown broad activity in treating various infectious or non-infectious diseases, even cancers, it possibly involves multiple drug targets against the helminth.
In conclusion, our study presents the HIV protease inhibitor nelfinavir as a promising drug candidate against the helminth disease echinococcosis. Nelfinavir demonstrated in vivo efficacy in either normal or immunocompromised mice and thus has the potential to be a dual-therapeutic drug for co-infections between HIV and Echinococcus. The EmuDdi1 gene is essential in parasite survival and participates in the process of protein excretion for the parasite. Inhibition of the EmuDdi1 ortholog might be the mechanism by which the HIV proteinase inhibitor therapy mediates its antiparasitic effect on echinococcosis. Further pharmacological preclinical evaluations are needed to support the initiation of animal or human clinical trials.
Contributors
SW designed the study and conceived analyses. SW, ZLL, and XLG wrote the initial manuscript, and all authors provided invaluable feedback and insights into analyses and the manuscript. ZLL, XLG, YGW, AJG, YZ, SYZ, XLL, SHZ, WH, LXP, QYZ, and XPC prepared samples and performed mouse work. ZLL and XLG verified the data. SW was blinded for the outcome assessment and data analysis. All authors approved the final version of the manuscript.
Data sharing statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgements
We thank Professor Guan Zhu from Jilin University for support and consultation in study design and execution. This work was supported by the National Natural Science Foundation of China (31802179), the Natural Science Foundation of Gansu Province, China (No. 21JR7RA027), and the State Key Laboratory of Veterinary Etiological Biology (No. SKLVEB2021YQRC01).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104177.
Appendix. Supplementary materials
References
- 1.Gomez-Marin J. World Health Organization; 2014. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites: FAO. [Google Scholar]
- 2.Casulli A. Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob Health. 2020;8(4):e470–e481. doi: 10.1016/S2214-109X(20)30066-8. [DOI] [PubMed] [Google Scholar]
- 3.Kotwa JD, Isaksson M, Jardine CM, et al. Echinococcus multilocularis infection, Southern Ontario, Canada. Emerg Infect Dis. 2019;25(2):265–272. doi: 10.3201/eid2502.180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paternoster G, Boo G, Wang C, et al. Epidemic cystic and alveolar echinococcosis in Kyrgyzstan: an analysis of national surveillance data. Lancet Glob Health. 2020;8(4):e603–e611. doi: 10.1016/S2214-109X(20)30038-3. [DOI] [PubMed] [Google Scholar]
- 5.Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4(6):e722. doi: 10.1371/journal.pntd.0000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottstein B, Stojkovic M, Vuitton DA, Millon L, Marcinkute A, Deplazes P. Threat of alveolar echinococcosis to public health–a challenge for Europe. Trends Parasitol. 2015;31(9):407–412. doi: 10.1016/j.pt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Wen H, Vuitton L, Tuxun T, et al. Echinococcosis: advances in the 21st century. Clin Microbiol Rev. 2019;32(2) doi: 10.1128/CMR.00075-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torgerson PR, Schweiger A, Deplazes P, et al. Alveolar echinococcosis: from a deadly disease to a well-controlled infection. Relative survival and economic analysis in Switzerland over the last 35 years. J Hepatol. 2008;49(1):72–77. doi: 10.1016/j.jhep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Vuitton DA, Bresson-Hadni S. Alveolar echinococcosis: evaluation of therapeutic strategies. Expert Opin Orphan Drugs. 2014;2(1):67–86. [Google Scholar]
- 10.Roberts NA, Martin JA, Kinchington D, et al. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- 11.Mastrolorenzo A, Rusconi S, Scozzafava A, Barbaro G, Supuran CT. Inhibitors of HIV-1 protease: current state of the art 10 years after their introduction. From antiretroviral drugs to antifungal, antibacterial and antitumor agents based on aspartic protease inhibitors. Curr Med Chem. 2007;14(26):2734–2748. doi: 10.2174/092986707782360141. [DOI] [PubMed] [Google Scholar]
- 12.Subeha MR, Telleria CM. The anti-cancer properties of the HIV protease inhibitor nelfinavir. Cancers. 2020;12(11) doi: 10.3390/cancers12113437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohashi H, Watashi K, Saso W, et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 2021;24(4) doi: 10.1016/j.isci.2021.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfonso Y, Monzote L. HIV protease inhibitors: effect on the opportunistic protozoan parasites. Open Med Chem J. 2011;5:40–50. doi: 10.2174/1874104501105010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Wang S, Luo Y, et al. Comparative genomics reveals adaptive evolution of Asian tapeworm in switching to a new intermediate host. Nat Commun. 2016;7:12845. doi: 10.1038/ncomms12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Wei W, Luo X, Wang S, Hu S, Cai X. Comparative genomic analysis of aspartic proteases in eight parasitic platyhelminths: insights into functions and evolution. Gene. 2015;559(1):52–61. doi: 10.1016/j.gene.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Guo X, Zhang X, Yang J, et al. Suppression of nemo-like kinase by miR-71 in Echinococcus multilocularis. Exp Parasitol. 2017;183:1–5. doi: 10.1016/j.exppara.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Spiliotis M, Brehm K. Axenic in vitro cultivation of Echinococcus multilocularis metacestode vesicles and the generation of primary cell cultures. Methods Mol Biol. 2009;470:245–262. doi: 10.1007/978-1-59745-204-5_17. [DOI] [PubMed] [Google Scholar]
- 19.Fabbri J, Elissondo MC. Comparison of different staining methods for determination of viability on Mesocestoides vogae tetrathyridia. Parasitol Res. 2019;118(2):687–692. doi: 10.1007/s00436-018-6143-9. [DOI] [PubMed] [Google Scholar]
- 20.Stadelmann B, Scholl S, Muller J, Hemphill A. Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure-activity relationship of thiazolides against Echinococcus multilocularis metacestodes. J Antimicrob Chemother. 2010;65(3):512–519. doi: 10.1093/jac/dkp490. [DOI] [PubMed] [Google Scholar]
- 21.Reuter S, Manfras B, Merkle M, Harter G, Kern P. In vitro activities of itraconazole, methiazole, and nitazoxanide versus Echinococcus multilocularis larvae. Antimicrob Agents Chemother. 2006;50(9):2966–2970. doi: 10.1128/AAC.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delano WL. PyMOL: an open-source molecular graphics tool, CCP4 Newsl. Protein Crystallogr. 2002;40(1):82–92.
- 26.Mizukami C, Spiliotis M, Gottstein B, Yagi K, Katakura K, Oku Y. Gene silencing in Echinococcus multilocularis protoscoleces using RNA interference. Parasitol Int. 2010;59(4):647–652. doi: 10.1016/j.parint.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Mousavi SM, Afgar A, Mohammadi MA, et al. Biological and morphological consequences of dsRNA-induced suppression of tetraspanin mRNA in developmental stages of Echinococcus granulosus. Parasit Vectors. 2020;13(1):190. doi: 10.1186/s13071-020-04052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perteguer MJ, Gomez-Puertas P, Canavate C, Dagger F, Garate T, Valdivieso E. Ddi1-like protein from Leishmania major is an active aspartyl proteinase. Cell Stress Chaperones. 2013;18(2):171–181. doi: 10.1007/s12192-012-0368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onchieku NM, Kumari S, Pandey R, et al. Artemisinin binds and inhibits the activity of plasmodium falciparum Ddi1, a retroviral aspartyl protease. Pathogens. 2021;10(11) doi: 10.3390/pathogens10111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosenza-Contreras M, de Oliveira ECRA, Mattei B, et al. The Schistosomiasis SpleenOME: unveiling the proteomic landscape of splenomegaly using label-free mass spectrometry. Front Immunol. 2018;9:3137. doi: 10.3389/fimmu.2018.03137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Braun A, Trawinski H, Wendt S, Lubbert C. Schistosoma and other relevant helminth infections in HIV-positive individuals-an overview. Trop Med Infect Dis. 2019;4(2) doi: 10.3390/tropicalmed4020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Marreros N, Rufener R, Hemphill A, Gottstein B, Lundstrom-Stadelmann B. Short communication: efficacy of albendazole in Echinococcus multilocularis-infected mice depends on the functional immunity of the host. Exp Parasitol. 2020;219 doi: 10.1016/j.exppara.2020.108013. [DOI] [PubMed] [Google Scholar]
- 33.Dumitru I, Cernat R, Dumitru E, Rugina S. The XXVIth World Congress on Echinococcosis. 2015. Cystic Echinococcosis in HIV infected patients. [Google Scholar]
- 34.Lehrbach NJ, Ruvkun G. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. Elife. 2016;5 doi: 10.7554/eLife.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White RE, Powell DJ, Berry C. HIV proteinase inhibitors target the Ddi1-like protein of Leishmania parasites. FASEB J. 2011;25(5):1729–1736. doi: 10.1096/fj.10-178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabriely G, Kama R, Gelin-Licht R, Gerst JE. Different domains of the UBL-UBA ubiquitin receptor, Ddi1/Vsm1, are involved in its multiple cellular roles. Mol Biol Cell. 2008;19(9):3625–3637. doi: 10.1091/mbc.E07-05-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yip MCJ, Bodnar NO, Rapoport TA. Ddi1 is a ubiquitin-dependent protease. Proc Natl Acad Sci USA. 2020;117(14):7776–7781. doi: 10.1073/pnas.1902298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallet MA, Reist CM, Williams JC, et al. The HIV-1 protease inhibitor nelfinavir activates PP2 and inhibits MAPK signaling in macrophages: a pathway to reduce inflammation. J Leukoc Biol. 2012;92(4):795–805. doi: 10.1189/jlb.0911447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans EE, Siedner MJ. Tropical parasitic infections in individuals infected with HIV. Curr Trop Med Rep. 2017;4(4):268–280. doi: 10.1007/s40475-017-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeleke OA, Yogeswaran P, Wright G. Intestinal helminth infections amongst HIV-infected adults in Mthatha General Hospital, South Africa. Afr J Prim Health Care Fam Med. 2015;7(1):910. doi: 10.4102/phcfm.v7i1.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gatti G, Castelli-Gattinara G, Cruciani M, et al. Pharmacokinetics and pharmacodynamics of nelfinavir administered twice or thrice daily to human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2003;36(11):1476–1482. doi: 10.1086/375062. [DOI] [PubMed] [Google Scholar]
- 42.al Nahhas S, Gabrion C, Walbaum S, Petavy AF. In vivo cultivation of Echinococcus multilocularis protoscoleces in micropore chambers. Int J Parasitol. 1991;21(3):383–386. doi: 10.1016/0020-7519(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 43.Borkow G, Bentwich Z. HIV and helminth co-infection: is deworming necessary? Parasite Immunol. 2006;28(11):605–612. doi: 10.1111/j.1365-3024.2006.00918.x. [DOI] [PubMed] [Google Scholar]
- 44.Rogan MT. T-cell activity associated with secondary infections and implanted cysts of Echinococcus granulosus in BALB/c mice. Parasite Immunol. 1998;20(11):527–533. doi: 10.1046/j.1365-3024.1998.00180.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang WB, Jones MK, Li J, McManus DP. Echinococcus granulosus: pre-culture of protoscoleces in vitro significantly increases development and viability of secondary hydatid cysts in mice. Exp Parasitol. 2005;110(1):88–90. doi: 10.1016/j.exppara.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Sha Z, Goldberg AL. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr Biol. 2014;24(14):1573–1583. doi: 10.1016/j.cub.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachur TP, Vale JM, Coelho IC, Queiroz TR, Chaves Cde S. Enteric parasitic infections in HIV/AIDS patients before and after the highly active antiretroviral therapy. Braz J Infect Dis. 2008;12(2):115–122. doi: 10.1590/s1413-86702008000200004. [DOI] [PubMed] [Google Scholar]
- 48.Fassmannova D, Sedlak F, Sedlacek J, Spicka I, Grantz Saskova K. Nelfinavir inhibits the TCF11/Nrf1-mediated proteasome recovery pathway in multiple myeloma. Cancers. 2020;12(5) doi: 10.3390/cancers12051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu Y, Wang X, Wang Y, Wang Y, Li J, Yu FX. Nelfinavir inhibits human DDI2 and potentiates cytotoxicity of proteasome inhibitors. Cell Signal. 2020;75 doi: 10.1016/j.cellsig.2020.109775. [DOI] [PubMed] [Google Scholar]
- 50.Jarvis B, Faulds D. Nelfinavir. A review of its therapeutic efficacy in HIV infection. Drugs. 1998;56(1):147–167. doi: 10.2165/00003495-199856010-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.