Abstract
The toluene-degrading strain Rhodococcus opacus PWD4 was found to hydroxylate d-limonene exclusively in the 6-position, yielding enantiomerically pure (+) trans-carveol and traces of (+) carvone. This biotransformation was studied using cells cultivated in chemostat culture with toluene as a carbon and energy source. The maximal specific activity of (+) trans-carveol formation was 14.7 U (g of cells [dry weight])−1, and the final yield was 94 to 97%. Toluene was found to be a strong competitive inhibitor of the d-limonene conversion. Glucose-grown cells did not form any trans-carveol from d-limonene. These results suggest that one of the enzymes involved in toluene degradation is responsible for this allylic monohydroxylation. Another toluene degrader (Rhodococcus globerulus PWD8) had a lower specific activity but was found to oxidize most of the formed trans-carveol to (+) carvone, allowing for the biocatalytic production of this flavor compound.
d-Limonene is the main constituent of orange and lemon peel oil (92 to 96% [26]), which is a by-product of the fruit juice industry produced in quantities of approximately 50,000 tons per year (25). Due to its low price, which varied between $0.66 and $1.45 per kg in the first half of 2000 (22), d-limonene is an attractive starting compound for industrially relevant fine chemicals and flavor compounds with identical carbon skeletons, such as carveol, carvone, and perillyl alcohol (Fig. 1). The regiospecific introduction of carbonyl or hydroxy groups by chemical catalysis, however, is difficult because the electronic properties of the allylic methylene groups (carbons 3 and 6) and the allylic methyl groups (carbons 7 and 10) are rather similar. For this reason, enzymatic oxidation was considered as early as the 1960s (7, 8), and numerous d-limonene-transforming microbial and plant cells have been described since. To date, the enzymes with the best regiospecificity have been derived from plants. The P450 enzymes limonene-3-hydroxylase and limonene-6-hydroxylase (isolated from peppermint and spearmint microsomes) convert their natural substrate l-limonene and—at lower rates—d-limonene to isopiperitenol and carveol, respectively (6, 17, 19). Another example is the oxidation of d-limonene in the 6 position to cis- and trans-carveol and carvone by Solanum aviculare and Dioscorea deltoidea (29). The specific activities of these plant enzymes, however, are insufficient for industrial applications. Microbial strains found so far to be capable of bioconversion of d-limonene generally yielded a mixture of oxidation products (reviewed in reference 23). Recent examples are the conversion of d-limonene to α-terpineol and 6-hydroxycarveol by the honey fungus Armillareira mellae (9); to isopiperitenone, limonene-1,2 trans-diol, cis-carveol, perillyl alcohol, isopiperitenol, and α-terpineol by Aspergillus cellulosae (24); to carveol, α-terpineol, perillyl alcohol, and perillyl aldehyde by Bacillus stearothermophilus BR388 (4); and the same conversions by an Escherichia coli construct containing a plasmid with chromosomal inserts from this strain (3, 5). The most regiospecific microbial biocatalysts described so far are the basidiomycete Pleurotus sapidus, which converts d-limonene to cis- and trans-carveol and carvone at a low rate (25), and the black yeast Hormonema sp. strain UOFS Y-0067, which transforms d-limonene to isopiperitenol (28).
FIG. 1.
Structures of limonene and some derivatives.
In a number of studies, strains with the capability to use d-limonene as the sole source of carbon and energy were isolated and subsequently assessed for the accumulation of degradation pathway intermediates during growth (23). Most of these studies (2, 7, 8, 18) were inconclusive, as mixtures of oxidation products were detected and it generally remained unclear whether these were genuine pathway intermediates or dead-end products resulting from an incomplete regiospecificity of the pathway enzymes. A recent, more successful example is the work of Van der Werf et al. (26), who proved that Rhodococcus erythropolis DCL14 degraded d-limonene via epoxidation at the 1,2 position and managed to clone the gene encoding the epoxidase in E. coli, resulting in a biocatalyst for the high-yield production of d-limonene-1,2-epoxide.
During a screening of a collection of toluene-degrading and naphthalene-degrading bacteria, various strains were found to be capable of transforming d-limonene to a compound with the same high-pressure liquid chromatography (HPLC) retention time and mass spectrum as carveol (unpublished data). We describe here the kinetics of biotransformation of d-limonene by chemostat-grown cells of one of the positive strains, Rhodococcus opacus PWD4, and identified the isolated product as (+) trans-carveol by 1H nuclear magnetic resonance (NMR), gas chromatography (GC), and optical rotation studies.
Bacterial strains.
R. opacus PWD4 (DSMZ 44313) was isolated by W. A. Duetz and coworkers (unpublished results) from a topsoil sample from a petrol station in Hilversum (The Netherlands) in April 1992 by plating a bacterial soil extract on a mineral agar medium supplied with toluene as a C source via the gas phase. The taxonomy was based on morphological studies and partial 16S RNA sequencing by the Deutsche Sammlung von Microorganismen and Zellculturen (Braunschweig, Germany), which showed a 100% sequence identity with the type strain R. opacus DSMZ 43205. Rhodococcus globerulus PWD8, isolated from a topsoil sample from a meadow in Scherpenzeel (The Netherlands) in February 1992 as described above, showed 100% sequence identity with the type strain R. globerulus DSMZ 43954.
Growth substrate range of R. opacus PWD4.
R. opacus PWD4 was inoculated onto seven agar-based mineral medium plates without any added C source (12) and incubated in desiccators together with solutions of benzene (5%, vol/vol), ethylbenzene (10%, vol/vol), toluene (10%, vol/vol), o-xylene (10%, vol/vol), m-xylene (10%, vol/vol), or p-xylene (10%, vol/vol) in hexadecane or with pure hexadecane (control). After 1 week of growth at ambient temperature, all strains were scored for growth in comparison to the control plate. Benzene, toluene, and ethylbenzene resulted in significantly larger colonies of R. opacus PWD4 than of the control and so apparently could serve as sole C sources. No significant growth was observed with either o-xylene, m-xylene, or p-xylene as the sole C source. This growth substrate range is compatible with the presence of a tod pathway (15).
Biotransformation kinetics of d-limonene by chemostat-grown R. opacus PWD4.
R. opacus PWD4 was grown on a mineral medium (13) supplied with 5 mM glucose in a 100-ml Erlenmeyer-shaped chemostat (11) at a dilution rate of 0.03 h−1. Toluene was supplied as an additional C source via the gas phase as described previously (10), allowing the cells to reach a density of 1 to 1.3 g (vol/vol) liter−1. The cell dry weight was determined as described previously (12). Aliquots of 5 ml of cells were sampled from the chemostat, centrifuged, washed with a K2HPO4-KH2PO4 buffer (50 mM, pH 7.0), and resuspended in 5 ml of the same buffer supplied with 100 mM glucose. This cell suspension was transferred to a headspace vial with a total volume of 43 ml (National Scientific Company, Lawrenceville, Ga.); 1.0 μl of d-limonene was added as a pure compound via a microsyringe, followed by magnetic stirring at 1,000 rpm and 30°C. The headspace vial was closed using a screw cap with a Teflon-coated insert. At regular time intervals, samples of 100 μl were taken using a syringe with a needle (without opening the vial in order to prevent the loss of gaseous d-limonene from the headspace), centrifuged in Eppendorf tubes at 15,000 rpm for 3 min, and analyzed by liquid chromatography (LC)-mass spectrometry (MS) Agilent 1100 series) equipped with (i) a diode array detector and a mass detector in series and (ii) a cooled autosampler from CTC (Zwingen, Switzerland). Solvent 1 was acetonitrile-methanol (1:1 [vol/vol])–0.1% formic acid. Solvent 2 was water–0.1% formic acid. Solvents 1 and 2 were used in a 30:70 (vol/vol) ratio for 10 min followed by a steep gradient to 90% solvent 1, at a total run time of 13.2 min, using a Zorbax SB-C18 column (30 by 2 mm). The MS settings were as follows: atmospheric pressure chemical ionization mode; positive ionization; fragmentor voltage, 50 V; gas temperature, 350°C; vaporizer temperature, 375°C; drying gas (N2) flow rate, 4 liter min−1; nebulizer pressure, 0.023 N/m2; capillary voltage, 2,000 V; corona current, 6 μA. The MS was run in the selective ion mode set to detect the following masses (relative dwell times in parentheses): 135 (40%), 137 (5%), 151 (10%), 153 (7%), and 176 (15%). trans-carveol and cis-carveol gave retention times of 7.3 and 8.1 min, respectively, and a mass signal of 135, while carvone gave a mass signal of 151 and a retention time of 7.4 min.
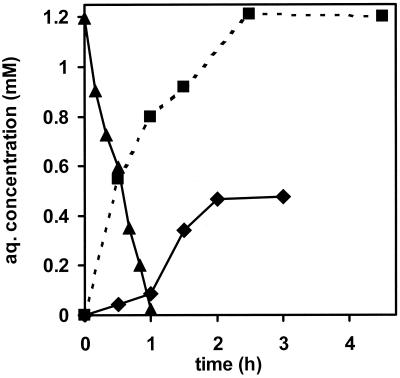
During the incubation with d-limonene, a compound with the same retention time and mass signal as trans-carveol was formed (Fig. 2). The maximal concentration was measured after 2.5 h (1.21 mM). The initial specific activity was 14.7 U (g of cells [dry weight])−1. The only other oxidation product detected was carvone (15 μM, or 1.3% of the trans-carveol formed). The molar yield of trans-carveol (determined by LC-MS) was approximately 97% (mol/mol) if the standard of carveol is assumed to be 100% pure, or 94% (mol/mol) if the standard of carveol is only 97% (indicated as the minimum purity by the supplier). The following isomers of carveol and carvone had distinctly different retention times and/or mass spectra and were not detected as products: perillyl alcohol (135, 176, 10.0 min), perillylaldehyde (151, 11.1 min), limonene oxide (135, 11.0. min), and limonene diol (153, 7.0 min). The latter compound was prepared by acid hydrolysis (1 h, 0.1 M HCl, 100°C) of limonene oxide.
FIG. 2.
Kinetics of formation of trans-carveol from d-limonene by washed, chemostat-grown cells of R. opacus PWD4 (5 ml, 1.1 g [dry weight] liter−1) in a closed headspace vial in the absence (■) and presence (⧫) of toluene. The experiment was done in a closed headspace vial with a total volume of 43 ml, implying that 68% of the toluene was in the gaseous phase and that the toluene consumption rate is actually 3.1-fold higher than suggested by the curve. aq., aqueous.
Further product identification by GC, 1H NMR, and specific optical rotation.
Products formed were extracted in dichloromethane or chloroform and analyzed by GC using a 30-m capillary column (DB 1701; J&W Scientific, Folsom, Calif.) on a Varian model 3400 GC (Varian Associates, Walnut Creek, Calif.) equipped with a flame ionization detector, using N2 as the carrier gas at a flow rate of 1.0 ml min−1. The detector and injector temperatures were 250 and 200°C, respectively. The temperature was programmed to increase from 50 to 90°C at a rate of 3°C min−1, from 90 to 210°C at 5°C min−1, and from 210 to 250°C at 12°C min−1. These conditions resulted in retention times of 33.38 and 33.88 min for trans-carveol and cis-carveol, respectively, using a commercially available mixture of 55% (−) trans-carveol and 45% (−) cis-carveol (Aldrich). A gas chromatogram of the chloroform extract showed only one significant peak, corresponding exactly to the retention time of trans-carveol.
For the purpose of 1H NMR analysis, the overflow from the chemostat described above was collected on ice for 2 days, yielding 120 ml of a cell suspension of R. opacus PWD4 of 1.25 g (dry weight) liter−1. The cells were washed with a K2HPO4-KH2PO4 buffer (50 mM, pH 7.0) and resuspended in 100 ml of the same buffer supplied with 100 mM glucose. This cell suspension was transferred to a 2-liter bottle. The suspension was stirred at 1,000 rpm using a 80- by 9-mm stirring bar at 30°C, and 20 μl of d-limonene was added. After 4 h of incubation, the bottle was opened and stirred for another 30 min (to allow for evaporation of residual d-limonene) before the cells were separated from the supernatant by centrifugation. The supernatant was extracted at 0°C with 3 volumes of deuterated chloroform (Aldrich). The three fractions of deuterated chloroform (5 ml) were pooled and concentrated at room temperature and atmospheric pressure, yielding 2 ml of a 26 mM solution (4 mg ml−1) of trans-carveol (as determined by LC-MS). 1H NMR spectra were recorded at 400 MHz on a Varian system. The resulting 1H NMR spectrum was indistinguishable from the previously reported spectrum for trans-carveol prepared chemically [by reduction of (−) carvone] by Yasui et al. (31). Chemical shifts in parts per million relative to trimethylsilane were 5.59 (s, 1H), 4.74 (s, 1H), 4.73 (s, 1H), 4.02 (s, 1H), 2.32 (m, 1H), 2.14 (m, 1H), 1.94 (m, 1H), 1.89 (m, 1H), 1.80 (s, 3H), 1.74 (s, 3H), 1.62 (m, 1H), and 1.59 (s, 1H).
The same procedure was followed with nondeuterated chloroform for the purpose of measurement of the specific optical rotation using a Perkin-Elmer (Applied Biosystems, Foster City, Calif.) model 241 polarimeter. The specific optical rotation (1% in chloroform) was measured to be +244°, which is comparable to the value (+210°) reported previously (16).
The regiospecific and stereospecific bioconversion of d-limonene to trans-carveol (10a) has not been reported previously for microorganisms but has been described to occur naturally in the fruit of caraway (Carum carvi) by (+) limonene-6-hydroxylase (1). As far as we know, the observed specific trans-carveol synthesis rates are 1 or more orders of magnitude higher than any previously described monohydroxylations of either d- or l-limonene. For example, the only other microbial biocatalyst capable of regiospecific oxidation of d-limonene at the 6 position (the basidiomycete P. sapidus) reached carveol and carvone concentrations of approximately 0.5 mM only after 12 days (25).
Reversible inhibition of trans-carveol production by toluene.
Chemostat-grown cells of R. opacus PWD4 (transferred to headspace vials as described above) were supplied with 2.0 μl of toluene (corresponding to 0.019 mmol) in addition to 1.0 μl of d-limonene (Fig. 2). The toluene concentration was monitored over time by taking 200-μl samples from the headspace via a gastight syringe and subsequently transferring the gas samples into a 2-ml HPLC vial filled with 500 μl of acetonitrile. After shaking (to allow all toluene to dissolve in the acetonitrile), the toluene was quantified with an HPLC diode array detector (DAD) using the same column and HPLC configuration at a ratio of solvent 1 to solvent 2 of 1:1 and was detected and quantified by the DAD signal at 210 nm. The initial specific activity for trans-carveol formation in the presence of toluene was 0.8 U (g of cells [dry weight])−1. The average aqueous toluene depletion rate in the first hour of incubation was 22 μM min−1, corresponding to a specific toluene consumption rate of 57 U (g of cells [dry weight])−1 after correction for the toluene present in the gaseous form in the headspace (68% of total amount, assuming a Henry coefficient of 0.30 [14]). In this phase, the trans-carveol production rate was 0.9 U (g of cells [dry weight])−1. After all toluene was depleted, the specific trans-carveol formation rate increased 9-fold to 7.8 U (g of cells [dry weight])−1. Comparison of the maximal toluene degradation rates with the maximal trans-carveol formation rates measured in the experiment without toluene (14.7 U [g of cells {dry weight}]−1) indicates a relative activity on d-limonene of 26%.
Biotransformation by glucose-grown cells of R. opacus PWD4.
Biotransformation experiments performed with cells of R. opacus PWD4 that were grown in an overnight batch culture of a mineral medium with glucose as the sole C source did not result in the formation of trans-carveol or any other oxidation products from d-limonene. This observation (in addition to the reversible inhibition by toluene described above) suggests that one of the enzymes from a toluene degradation pathway is responsible for the biotransformation. The observed growth subtrate range is compatible with the presence of a tod pathway (15), which involves two oxygenases (toluene 2,3-dioxygenase and catechol 2,3-dioxygenase). Taking into account that toluene 2,3-dioxygenase has been previously found to be capable of monooxygenations (e.g., indene to 1-indenol [30]), this enzyme seems to be a more likely candidate. Additional research, however, is required to confirm this hypothesis.
Coproduction of trans-carveol and carvone by R. globerulus PWD8.
R. globerulus PWD8 was grown on a mineral medium agar plate with toluene as the sole C source as described above. After 4 days of growth, cells were scraped off the agar surface and suspended in a K2HPO4-KH2PO4 buffer (50 mM, pH 7.0) to a cell concentration of 3 g (dry weight) liter−1. The cell suspension (2.5 ml) was incubated with 0.5 μl of d-limonene in a headspace vial as described above. Samples were taken at 0, 2, 4, 6, 8, and 24 h and centrifuged; then the supernatant was analyzed by LC-MS for the formation of trans-carveol, carvone, and other possible products. The initial trans-carveol formation rate (average of the first hour) was 0.73 mM h−1, corresponding to a specific activity of 3 U (g of cells [dry weight])−1. After 2 h, more than 90% of d-limonene added was transformed to trans-carveol (1.1 mM), and a carvone concentration of 0.08 mM was formed. The concentration of carvone gradually increased to maximally 0.29 mM after 27 h, while the trans-carveol concentration decreased accordingly, indicating that the trans-carveol formed was converted slowly to carvone.
Although the specific activity of R. globerulus PWD8 was lower than that of R. opacus PWD4, the partial through conversion to (+) carvone illustrates the potential applicability of such strains for the production of (+) carvone, which is used as a flavor compound. For this purpose, the produced (+) trans-carveol would need to be completely further oxidized, by the action of either a dehydrogenase or an oxidase. Possibly, mutants of R. globerulus PWD8 that convert most or all trans-carveol to carvone could be generated. Another possibility would be the coexpression of a gene encoding a suitable (+) trans-carveol dehydrogenase. A carveol dehydrogenase active on all four stereoisomers of carveol has been described elsewhere (27). A specific (+) trans-carveol dehydrogenase was found in the fruit of caraway (1). The equilibrium constant of the conversion of trans-carveol to carvone by a NAD+-dependent dehydrogenase cannot be exactly calculated since the Gibbs free energies of formation for trans-carveol and carvone are unknown. Using the group contribution theory of Mavrovouniotis (20, 21), it can be estimated that the Gibbs free energy change of an oxidation of a cyclic alcohol to the corresponding cyclic ketone at physiological NADH/NAD+ ratios is approximately 8 kJ mol−1 (thus slightly favoring the alcohol). The energetically favorable conjugation of the carbonyl group with the C⩵C double bond in carvone, however, is likely to cause a negative Gibbs free energy change and may so result in an equilibrium toward carvone.
Acknowledgments
We thank Dongliang Chang for help with generation of the proton NMR data.
REFERENCES
- 1.Bouwmeester H, Gershenzon J J, Konings M C J M, Croteau R. Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway. I. Demonstration of enzyme activities and their changes with development. Plant Physiol. 1998;117:901–912. doi: 10.1104/pp.117.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadwallader K R, Braddock R J, Parish M E, Higgins D P. Bioconversion of dextro limonene by Pseudomonas gladioli. J Food Sci. 1989;54:1241–1245. [Google Scholar]
- 3.Chang H C, Gage D A, Oriel P J. Cloning and expression of a limonene degradation pathway from Bacillus stearothermophilus in Escherichia coli. J Food Sci. 1995;60:551–553. [Google Scholar]
- 4.Chang H C, Oriel P J. Bioproduction of perillyl alcohol and related monoterpenes by isolates of Bacillus stearothermophilus. J Food Sci. 1994;59:660–662. [Google Scholar]
- 5.Cheong T K, Oriel P J. Cloning and expression of the limonene hydroxylase of Bacillus stearothermophilus BR388 and utilization in two-phase limonene conversions. Appl Biochem Biotechnol. 2000;84:903–915. doi: 10.1385/abab:84-86:1-9:903. [DOI] [PubMed] [Google Scholar]
- 6.Croteau, R. B., S. L. Lupien, and F. Karp. December 1998. Recombinant materials and methods for the production of limonene hydroxylases. Patent WO9859042A1.
- 7.Dhavalikar R S, Bhattacharyya P K. Microbiological transformations of terpenes. Part VIII. Fermentation of limonene in a soil pseudomonad. Indian J Biochem. 1966;3:144–157. [PubMed] [Google Scholar]
- 8.Dhavalikar R S, Rangachari P N, Bhattacharyya P K. Microbiological transformations of terpenes. Part IX. Pathways of degradation of limonene in a soil pseudomonad. Indian J Biochem. 1966;3:158–164. [PubMed] [Google Scholar]
- 9.Draczynska L B. Oxidation of selected p-menthane derivatives by means of Armilariella mellea (honey fungus), a parasite of woodlands. J Basic Microbiol. 1987;27:191–196. [Google Scholar]
- 10.Duetz W A, Marques S, De Jong C, Ramos J L, Van Andel J G. Inducibility of the TOL catabolic pathway in Pseudomonas putida(pWWO) growing on succinate in continuous culture: evidence of carbon catabolite repression control. J Bacteriol. 1994;176:2354–2361. doi: 10.1128/jb.176.8.2354-2361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Duetz, W. A., C. Jourdat, and B. Witholt. November 2000. PCT patent.
- 11.Duetz W A, Marques S, Wind B, Ramos J L, Van Andel J G. Catabolite repression of the toluene degradation pathway in Pseudomonas putida harboring pWWO under various conditions of nutrient limitation in chemostat culture. Appl Environ Microbiol. 1996;62:601–606. doi: 10.1128/aem.62.2.601-606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duetz W A, Ruedi L, Hermann R, O' Connor K, Buchs J, Witholt B. Methods for intense aeration, growth, storage, and replication of bacterial strains in microtiter plates. Appl Environ Microbiol. 2000;66:2641–2646. doi: 10.1128/aem.66.6.2641-2646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duetz W A, Van Andel J G. Stability of TOL plasmid pWWO in Pseudomonas putida mt-2 under non-selective conditions in continuous culture. J Gen Microbiol. 1991;137:1369–1374. doi: 10.1099/00221287-137-6-1369. [DOI] [PubMed] [Google Scholar]
- 14.Eastcott L, Shiu W Y, Mackay D. Environmentally relevant physicochemical properties of hydrocarbons: a review of data and development of simple correlations. Oil Chem Pollut. 1988;4:191–216. [Google Scholar]
- 15.Gibson D T, Hensley M, Yoshioka H, Mabry T J. Formation of (+)-cis 2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 16.Hamada H. Enantioselectivity in the biotransformation of monocyclic and bicyclic monoterpenoids with the cultured cells of Nicotiana tabacum. Bull Chem Soc Jpn. 1988;61:869–878. [Google Scholar]
- 17.Haudenschild C, Schalk M, Karp F, Croteau R. Functional expression of regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha spp.) in Escherichia coli and Saccharomyces cerevisiae. Arch Biochem Biophys. 2000;379:127–136. doi: 10.1006/abbi.2000.1864. [DOI] [PubMed] [Google Scholar]
- 18.Kieslich K, Abraham W R, Stumpf B, Thede B, Washausen P. Progress in essential oil research. Berlin, Germany: Walter de Gruyter & Co.; 1986. pp. 367–394. [Google Scholar]
- 19.Lupien S, Karp F, Wildung M, Croteau R. Regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha) species: cDNA isolation, characterization, and functional expression of (−)-4s-limonene-3-hydroxylase and (−)-4s-limonene-6-hydroxylase. Arch Biochem Biophys. 1999;368:181–192. doi: 10.1006/abbi.1999.1298. [DOI] [PubMed] [Google Scholar]
- 20.Mavrovouniotis M L. Estimation of standard Gibbs energy changes of biotransformations. J Biol Chem. 1991;266:14440–14445. [PubMed] [Google Scholar]
- 21.Mavrovouniotis M L. Group contributions for estimating standard Gibbs free energies of formation of biochemical compounds in aqueous solutions. Biotechnol Bioeng. 1990;36:1070–1082. doi: 10.1002/bit.260361013. [DOI] [PubMed] [Google Scholar]
- 22.Mazzaro D. Orange oil, d-limonene market usettled due to Brazilian delays. Chem Marketing Rep. 2000;258(4):18. [Google Scholar]
- 23.Mikami Y. Microbial conversion of terpenoids. Biotechnol Gen Eng Rev. 1988;6:271–320. [Google Scholar]
- 24.Noma Y, Yamasaki S, Asakawa Y. Biotransformation of limonene and related compounds by Aspergillus cellulosae. Phytochemistry. 1992;31:2725–2727. [Google Scholar]
- 25.Onken J, Berger R G. Effects of R-(+)-limonene on submerged cultures of the terpene transforming basidiomycete Pleurotus sapidus. J Biotechnol. 1999;69:163–168. doi: 10.1016/s0168-1656(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 26.Van der Werf M J, Swarts H J, De Bont J A M. Rhodococcus erythropolis DCL14 contains a novel degradation pathway for limonene. Appl Environ Microbiol. 1999;65:2092–2102. doi: 10.1128/aem.65.5.2092-2102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Werf M J, Van der Ven C, Barbirato F, Eppink Michel H M, De Bont J A M, Van Berkel W J H. Stereoselective carveol dehydrogenase from Rhodococcus erythropolis DCL14. A novel nicotinoprotein belonging to the short chain dehydrogenase/reductase superfamily. J Biol Chem. 1999;274:26296–26304. doi: 10.1074/jbc.274.37.26296. [DOI] [PubMed] [Google Scholar]
- 28.Van Dyk M S, Van Rensburg E, Moleki N. Hydroxylation of (+) limonene, (−) α-pinene and (−) β-pinene by a Hormonema sp. Biotechnol Lett. 1998;20:431–436. [Google Scholar]
- 29.Vanek T, Valterova I, Vankova R, Vaisar T. Biotransformation of (−)-limonene using Solanum aviculare and Dioscorea deltoidea immobilized plant cells. Biotechnol Lett. 1999;21:625–628. [Google Scholar]
- 30.Wacket L P, Kwart L D, Gibson D T. Benzylic monooxygenation catalyzed by toluene dioxygenase from Pseudomonas putida F1. Biochemistry. 1988;27:1360–1367. doi: 10.1021/bi00404a041. [DOI] [PubMed] [Google Scholar]
- 31.Yasui K, Fugami K, Tanaka S, Tamaru Y. Unsymmetrical ketone synthesis via a three-component connection reaction of organozines, allylating agents and carbon monoxide. J Org Chem. 1995;60:1365–1380. [Google Scholar]