Abstract
Background:
Hybrid closed-loop (HCL) insulin pump therapy (Medtronic 670G) is an emerging technology that is growing in use worldwide. Initial clinical trials demonstrated the effectiveness of HCL in reducing hypoglycemia and improving glucose control; however, these subjects were intensely monitored and supervised. There has been concern regarding the ability of patients to remain in auto mode. We aimed to assess HCL when used in a typical outpatient endocrine clinic.
Methods:
We initially analyzed data from 80 individuals with type 1 diabetes managed in an endocrine clinic by a single certified diabetes educator (CDE). We then included our other providers and had 230 subjects by the end of the study. Patients were either transitioned from traditional insulin pump or multiple daily insulin injection therapy (MDI) to HCL. Patients initiated to HCL pump therapy from July 2017 through February 2020 were studied. Endpoints of change in time in hypoglycemic/hyperglycemic range and time in target range were analyzed. The primary outcome was a change in percent time in the target range during manual mode compared with auto mode.
Results:
There was an 18.2% increase in average time in target range when comparing manual mode to auto mode (59.3% vs 70.1%, P < .0001). Average time in hyperglycemic range was significantly reduced by 26.7% (39.0% vs 28.6%, P < .0001) but without increasing average time in hypoglycemic range (1.7% vs 1.3%, P = 0.95).
Conclusions:
HCL was effective in reducing hyperglycemia and increasing time in the target range but did not increase hypoglycemia. These data suggest HCL will improve the metrics of glucose control.
Keywords: hybrid-closed loop insulin pump, Medtronic 670G, auto mode
Introduction
Continuous subcutaneous insulin infusion (CSII) pump therapy for the treatment of type 1 diabetes (DM1) was first introduced in the 1970s.1,2 Superiority over multiple daily injection (MDI) therapy was soon noted. Insulin pump therapy has been shown to result in the reduction of hemoglobin A1c 3 (Hgb A1c) and improved quality of life. 4 However, there have been several limitations identified as well. Traditional insulin pump therapy does not easily adapt to an individual’s changing insulin sensitivity with activity or with varying food composition intake. This reality has created an opportunity for continued improvement in insulin pump therapy. The creation of continuous glucose monitoring made the first sensor-augmented pumps possible and has now led to the hybrid closed-loop (HCL) system. The HCL system is an emerging technology for the management of DM1. It was borne out of the need for a better adaptation of an individual’s changing insulin requirements based on multiple shifting variables. Previous studies5,6 demonstrated its effectiveness in reducing hypoglycemia and improving glucose control in DM1. These subjects were intensely monitored and supervised. Further studies examining effectiveness in a typical outpatient setting have shown varying results and exposed unique challenges to using the HCL system.7-9 A randomized control trial comparing HCL therapy to sensor-augmented pump therapy showed greater Hgb A1c reduction and less hypoglycemia without changes in bodyweight or total daily insulin in the HCL group. 10
Despite the evidence suggesting advantages to the HCL, one of the most common obstacles patients encounter is staying in auto mode. Recent studies 11 have reported high rates of auto mode drop out, with the most common reason being sensor issues. Remaining in auto mode does require effort from the user. For instance, regular sensor calibration is needed to stay in auto mode. Another potential roadblock with using the HCL system is that many individuals with DM1 desire intensive blood sugar control. The default fixed 120 mg/dL target blood sugar while in auto mode may be above the individual’s preferred goal. This can lead to frustration and a desire to drop out of auto mode. In the present study, our goal was to assess the HCL system when used in a typical outpatient endocrine clinic and in a diverse patient population. The HCL system is a behavioral-driven insulin pump. We created a training program that ensured close follow-up with a consistent provider and hypothesize that this will lead to optimum results with the HCL system.
Methods
Study Population
This study was reviewed and approved by the Wake Forest University School of Medicine Institutional Review Board; IRB#00062020. The Wake Forest Baptist Health Diabetes and Endocrinology clinic initiated the implementation of the HCL system (Medtronic 670G) for the management of DM1 in July 2017. Individuals (pediatric and adult) were either transitioned from traditional insulin pump or MDI therapy to the HCL system. Sixty-three percent (63%) of individuals transitioned from the insulin pump to the HCL system, and 37% went from MDI to the HCL. Initial pump settings for HCL were taken from settings on original pump therapy. If patients were on MDI, then Pumping Protocol (Bode et al.) was used to determine new pump settings. Patients were observed in a two-week run-in period with threshold suspend and predictive alerts activated. Individuals were allowed to enter auto mode if they had demonstrated appropriate carbohydrate counting, correction of high blood glucose, calibrating the sensor at least three times daily, and uploading data regularly.
We initially examined the charts of 80 subjects. We were then able to pull aggregate data from 230 users of the HCL system by the end of the study. For the initial 80 subjects, pre-auto mode time points were chosen to maximize time in manual mode. Auto mode time points were chosen to optimize time in auto mode. Comparison of time in target range, hypoglycemia range, and hyperglycemia range was done for most subjects at one month on auto mode. Pre-Hgb A1c was the most recent value prior to going into auto mode. This was compared with the Hgb A1c at least 3 months after entering auto mode. We chose a follow-up endpoint of August 1, 2020, for all participants to determine if they were still using the HCL system.
End Points
The primary outcome was percent time in the target range (BG 70-180 mg/dL) in auto mode compared with percent time in the target range when the auto mode was off. We also assessed percent time in hypoglycemic range (BG less than 70 mg/dL), percent time in hyperglycemic range (BG 181 to greater than 250 mg/dL), and change in Hgb A1c. The initial 80 participants worked with the same certified diabetes educator (CDE).
Statistical Methods
Pre-auto and post-auto mode outcomes were described as means and were compared using Wilcoxon tests. A linear mixed model was used to assess the relationship between time adhering to the system, time in the target range, and time in the hypoglycemic range. P-values less than .05 were considered significant. All analyses were conducted using SAS software version 9.4.
Results
Of the initial 80 participants, the mean age was 42.5 (SD 16.2). Half of the participants were male, and half were female. The majority of participants were white (n=73, 91%), whereas five participants identified as black and two participants identified as other.
For the initial 80 users, there was an 18.2% increase in average percent of time in the target range when comparing manual mode to auto mode (59.3% vs 70.1%, P < .0001, Figure 1). There was also a 26.7% reduction in average percent of time in hyperglycemic range (39.0% vs 28.6%, P < 0.0001, Figure 1) but without an increase in average percent of time in hypoglycemic range (1.7% vs 1.3%, P = 0.95, Figure 1). Increase in time adhering to the system was significantly associated with improvement in time in the target range (P < .0001) but was not significantly associated with hypoglycemia (P = 0.93). There was a decrease in average Hgb A1c after transitioning to HCL (8.06% vs 7.37%, P < .0006). We further expanded our data collection to include the patients of 24 providers trained on the HCL system by multiple CDEs. In the 230 users included, we saw an increase in aggregate percent time in range when in auto mode (70.33% vs 55.44%, Figure 2). Aggregate percent time above range was lower in auto mode (27.92% vs 42.05%, Figure 2), and aggregate percent time below range was lower while in auto mode (1.74% vs 2.51%, Figure 2).
Figure 1.
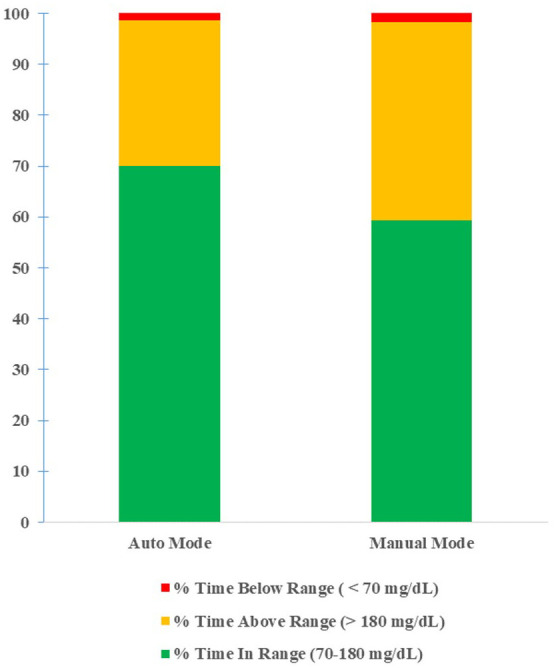
Sensor glucose distribution of auto mode and manual mode (80 users).
Figure 2.
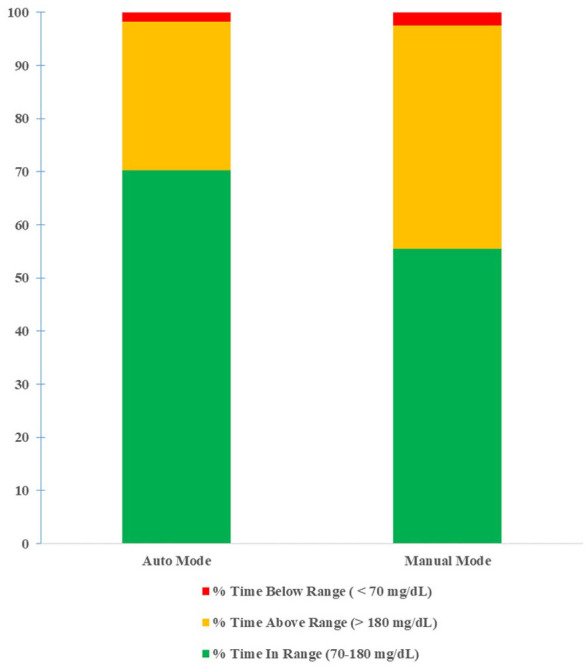
Sensor glucose distribution of auto mode and manual mode (230 users).
We were able to follow-up with 60 of the initial 80 participants at the end of the study. Forty-two participants were still utilizing auto mode. The auto mode dropout rate was 30%. Out of the 18 participants who were no longer utilizing auto mode, one was still wearing the sensor. Of the subjects who stopped using the auto mode, 44.4% came from the MDI group, and 55.6% came from the prior insulin pump group. If analyzed by group, 26% of the pump users dropped out of auto mode, and 36% of the MDI patients dropped out of auto mode. The most reported reason for auto mode drop out was sensor problems and sensor error (eight participants). Four subjects reported that the reason for dropout was too much work to stay in auto mode. Three subjects stated that sensor cost was the main reason for dropout, and three participants reported too many sensor alerts as the cause for auto mode dropout.
Discussion
The HCL system was effective in reducing hyperglycemia while increasing time in the glucose target range. There was no associated increase in hypoglycemia. We further examined those patients who did not show improved control and found that these individuals often used the HCL system suboptimally. The HCL system is a behavior-driven pump, and improper practices can lead to reduced blood sugar control. Some of these poor behaviors include putting in “fake carbs,” bolusing after eating, and inaccurate carb counting. When determining if changes to pump settings need to be made, it is important to discuss with the patient their habits related to insulin pump use. The pre-pump assessment and continued follow-up with a certified diabetes educator is crucial for success. Our patients regularly met before and after the initiation of the HCL system. We consider the uniform approach obtained by using a single educator contributed to our success in most patients. They were also encouraged to routinely upload pump data for review which prompted quick changes if needed. The present study had a 30% auto mode dropout rate which is similar to what has been reported. 11 Most common reasons for dropout were sensor issues and the perceived high amount of work it took to remain in auto mode.
Conclusions
These data suggest the adoption of the HCL system can be successfully adopted in a general endocrine outpatient clinic. Optimal management requires a team approach with the inclusion of a certified diabetes educator in addition to close follow-up with patients. Our aggregate data of 230 patients among multiple providers and educators suggest that the technology is adaptable to a variety of practice sites from community-based practices to an academic medical center. The consistent improvements in glucose control metrics support increased use of this technology.
Footnotes
Abbreviations: CGM, continuous glucose monitor; DM, diabetes mellitus; MDI, multiple daily injection; HCL, hybrid-closed loop; Hgb A1c, hemoglobin A1c; CDE, certified diabetes educator; CSII, continuous subcutaneous insulin infusion.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Joseph Aloi has received research and consulting fees from Medtronic Diabetes.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chinenye O. Usoh  https://orcid.org/0000-0002-0697-9753.
https://orcid.org/0000-0002-0697-9753.
References
- 1. Tamborlane WV, Sherwin RS, Genel M, et al. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300:573-578. [DOI] [PubMed] [Google Scholar]
- 2. Pickup JC, Keen H, Parsons JA, et al. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. Br Med J. 1978;1:204-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeVries JH, Snoek FJ, Kostense PJ, et al. A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control. Diabetes Care. 2002;25:2074-2080. [DOI] [PubMed] [Google Scholar]
- 4. EQuality1 Study Group–Evaluation of QUALITY of Life and Costs in Diabetes Type 1; Nicolucci A, et al. Quality of life and treatment satisfaction in adults with Type 1 diabetes: a comparison between continuous subcutaneous insulin infusion and multiple daily injections. Diabet Med. 2008;25:213-220. [DOI] [PubMed] [Google Scholar]
- 5. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407-1408. [DOI] [PubMed] [Google Scholar]
- 6. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aleppo G, Webb KM. Integrated insulin pump and continuous glucose monitoring technology in diabetes care today: a perspective of real-life experience with the MinimedTM 670G hybrid closed-loop system. Endocr Pract. 2018;24:684-692. [DOI] [PubMed] [Google Scholar]
- 8. Akturk HK, Giordano D, Champakanath A, et al. Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22:583-589. [DOI] [PubMed] [Google Scholar]
- 9. Lepore G, Scaranna C, Corsi A, et al. Switching from suspend-before-low insulin pump technology to a hybrid closed-loop system improves glucose control and reduces glucose variability: a retrospective observational case-control study. Diabetes Technol Ther. 2020;22:321-325. [DOI] [PubMed] [Google Scholar]
- 10. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392:1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lal RA, Basina M, Maahs DM, et al. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42:2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]


