Abstract
A growing suite of connected devices including Bluetooth or cellular-enabled glucose monitoring devices, smart insulin pens, pumps, fitness trackers, blood pressure, and heart rate and weight monitors present a golden opportunity to build a data-driven clinical practice model including remote monitoring capability and virtual care. This paper will discuss this approach using diabetes as a case study and smart insulin pens as a use case. As payment and practice approaches evolve, there is growing interest from both patients and their health care teams in virtual care made possible by remote monitoring capability. Here, we will define the category of smart insulin pens, describe the hallmarks of a data-driven practice model, and delineate the steps to take to incorporate remote monitoring capability with smart insulin pens into diabetes care for injection therapy patients.
Keywords: connected health, data-driven, digital health, multiple daily insulin therapy, numeracy, smart insulin pen, therapeutic inertia, value-based care
Introduction
Out of the nearly eight million individuals on insulin therapy in the United States fewer than 20%-30% of people with type 1 diabetes mellitus (T1DM) and less than 1% of people with type 2 diabetes mellitus (T2DM) use Continuous Subcutaneous Insulin Infusion (CSII). 1 The remaining rely on injection therapy, inhaled insulin, or basic patch pumps. This leaves a large population who could benefit from the availability of tools to support daily insulin dosing but who do not want the inconvenience, cost, complexity, or commitment required with insulin pump systems. A new category of insulin delivery—smart insulin pens—offer this dosing support with the convenience and lower cost of a simple, durable insulin pen paired with a diabetes management app 2 for the first time addressing challenges in optimizing insulin injection therapy: therapeutic inertia, omitted doses, miscalculated insulin doses and insulin stacking, and lack of data.
Barriers to Optimizing Insulin Therapy
Therapeutic Inertia
Defined as “the failure to initiate or intensify therapy in a timely manner according to evidence-based guidelines,” therapeutic inertia is of particular concern with insulin therapy. 3 There can be a delay of seven years or more in initiating basal insulin therapy in T2DM and then a further delay (estimated 4.3 years) to advance from basal insulin to mealtime insulin or other combinations (eg, GLP-1 receptor agonist therapy). 4 This is believed to be due to lack of time and resources to adequately educate the patient and/or lack of experience or expertise to design and implement more complex insulin therapy regimens. An example of “insulin inertia” is the tendency to set it and forget it when it comes to determining insulin therapy settings in smart insulin delivery devices for individuals with either T1DM or T2DM. If patients lack confidence in their therapy settings, they will likely find ways to work around them and/or abandon use of the technology designed to support them. Importantly, the 2020 American Diabetes Association (ADA) standards of medical care in diabetes in regard to smart insulin pens note that “provider input and education can be helpful for setting the initial dosing calculations with ongoing follow-up for adjustments as needed.” 5
Omitted Doses
Data from Multiple Daily Insulin (MDI) treated individuals using continuous glucose monitoring (CGM) devices report that one in four meals are associated with either a late or missed insulin bolus 6 and occur in both older and younger individuals with either T1DM or T2DM. Munshi et al 7 reported that insulin omission occurred in 100% of participants in a study with a Bluetooth-enabled insulin pen cap. Doses can be unintentionally omitted (forgotten) or intentionally omitted due to inconvenience, embarrassment, cost, eating pattern or disorder. Regardless, omitting just two meal-related doses per week is associated with a 0.4% increase in HbA1c levels. 8
Miscalculated Doses and Insulin Stacking
It is estimated that two out of every three adults in the United States are unable to perform rudimentary math due to limited numeracy skills. 9 In individuals with diabetes, 25% could not determine what glucose values were within a specified target range, 56% could not correctly count the carbohydrates in a prepackaged snack, and 59% could not calculate an insulin dose based on a blood glucose reading and a specified carbohydrate intake. 10 Limited diabetes-related numeracy skills have the potential to impact an individual’s ability to achieve glycemic targets. 11 Insulin stacking (overlap of bolus doses) is estimated to occur in 60% of insulin bolus doses putting the individual at risk for hypoglycemia. 12
Lack of Data
Unlike insulin pump users, people who use traditional insulin delivery devices such as syringes or insulin pens must make daily dosing decision without access to the amount or timing of previous insulin doses given or residual active insulin. In addition, it is necessary to also consider other confounding variables such as stress, sickness, schedule changes, travel, and so forth. Clinicians too are left to make therapy decisions in a data vacuum making it difficult to optimize the insulin regimen or adjust therapy settings in an informed manner. 13
A number of devices (caps, clips, pens) and apps have been developed or are being developed to address these barriers in order to support safe and effective MDI therapy including features such as digital log books, dose reminders, and bolus calculators. The use of automated bolus calculators has been shown to decrease HbA1c by up to one percentage point without an increase in the total daily dose, supporting the contention that individuals need help with optimizing their insulin. 14 Individuals with T1DM on MDI therapy and using automated bolus calculators report reduced fear of hypoglycemia, improved confidence in their dose accuracy, and increased treatment satisfaction.15,16
The Roadmap to Smart Insulin Pens
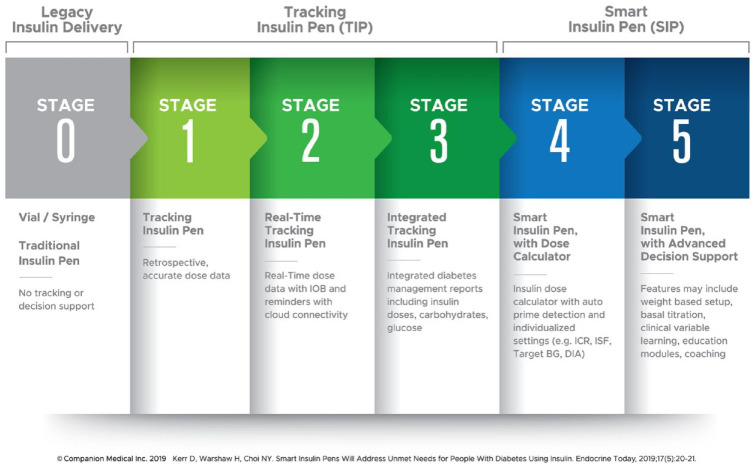
A number of years ago, Kowalski proposed a roadmap for the development of artificial pancreas systems. 17 Subsequently, Kerr and Warshaw 18 proposed a conceptually similar roadmap for the development of smart insulin pen (SIP) systems (Figure 1). This is a particularly useful concept as it helps define where products currently are in the development process as well as future development potential.
Figure 1.
Roadmap to smart insulin pens.
The roadmap starts with legacy insulin delivery at Stage 0 including traditional pens, vials, and syringes offering no dosing support. Next, tracking insulin pens (TIP) are introduced encompassing three stages:
Stage 1: The pen, cap, or clip is capable of tracking the last dose administered by the device allowing retrospective dose data review. An example of a commercially available Stage 1 TIP in the United States is the NovoPen Echo® by Novo Nordisk®, Plainsboro, NJ. Another is Clipsilun by Diabnext, Paris, France, released commercially through Amazon in 2020.
Stage 2: Incorporates real-time dose tracking with cloud connectivity enabling active insulin (insulin-on-board or IOB) tracking, ability to trigger missed dose notifications, and introduces remote monitoring capability.
Stage 3: Integrates related data from other connected devices or apps such as glucose and carbohydrate, and provides integrated data reports for the patient to share with their care team when needed.
Next the roadmap progresses to smart insulin pens (SIPs) encompassing two stages:
Stage 4: In addition to the real-time tracking capability and integrated data reports from TIPs, the Stage 4 SIP adds clinical decision support through an automated insulin dose calculator based on individualized insulin therapy settings with auto prime-detection. The only commercially available Food and Drug Administration (FDA)-cleared Stage 4 SIP in the United States is the InPen™ by Companion Medical, San Diego, CA. It is noted that the ADA 2020 Standards of Medical Care in Diabetes indicate that smart pens may be useful for some patients to help with dose capture and dosing recommendations as well as titrating insulin doses. The recommendations further state that individuals choosing to use bolus calculators use those that are FDA approved. 5
Stage 5 SIP: A stage 5 SIP automates weight-based insulin therapy setup, provides continuous dose titration, and delivers data-driven education modules and coaching. Stage 5 SIPs are currently in various stages of development.
Hallmarks of a Data-Driven Practice Model
According to a 2017 systematic review of studies evaluating technology-enabled Diabetes Self-Management Education and Support, there were improvements in HbA1c ranging from 0.1% to 0.8% in 18 of the 25 studies. This review identified four key elements that were incorporated into the most effective interventions. They included two-way communication; analyses of patient-generated health data (PGHD); tailored education; and individualized feedback. The authors referred to this as a technology-enabled self-management feedback loop, which connects people with their health care team. 19 Health care team members’ review of PGHD can lead to more immediate adaptions to the care plan by providing insights for shared decision-making and proactive patient-team communication that engages patients in their care with members of the health care team. However, to be of value, technology tools and the resulting PGHD must be integrated into clinical practice, becoming a critical part of what is done versus merely what’s being added to it.
Recently, one of the authors (JM) published the three hallmarks of a data-driven practice and they are reviewed in Table 1, including steps to take to incorporate SIPs and the resulting data into clinical practice. 20
Table 1.
Hallmarks of a Data-Driven Practice.
| Hallmark 1: Identification | Hallmark 2: Configuration | Hallmark 3: Ongoing collaborative use of data |
|---|---|---|
| Identify the right technology tools for each person with diabetes (PwD) as a
standard of care. This includes helping the patient identify the best route of insulin delivery for them. SIP indications for use: ❑ T1D or T2D ❑ On mealtime insulin ❑ Counting or estimating carbohydrates or taking fixed dose amounts Tech assessment: ❑ Has smart phone/uses apps ❑ Is monitoring blood glucose on regular basis |
Configure the technology tool to match the routine, lifestyle, and therapy plan
of the individual. For SIP this includes: 1. Provide personalized insulin therapy settings (for how to calculate see resource below) 2. Assure that the meal schedule in the app is adjusted to the person’s daily routine 3. Connect to available glucose monitoring devices 4. Agree on a plan for sharing the data with the care team. 5. Assure the patient has up to date prescriptions for basal insulin, rapid-acting insulin cartridges, and connected glucose monitoring supplies. 6. Be sure to check insulin injection technique, injection sites, understanding of priming, and proper insulin storage. 7. Discuss ability of PwDs to afford their insulin and supplies 8. Set clear expectations regarding use of SIP as part of daily self-management plan: ❑ Advise the patient to check the app home screen for the last dose amount and time, last glucose, and any active insulin when unsure if they took a dose or not. ❑ Encourage the patient to check their blood glucose and use the bolus calculator each time they dose. ❑ While fine-tuning settings and learning carbohydrate counting, ask the patient to check their glucose two hours after their meal time doses to determine if a correction dose is needed. ❑ Remind patients to log additional insulin doses such as long-acting insulin and short-acting doses taken without SIP in the Logbook. ❑ Suggest patients review their Logbook as needed for daily history and adjust reminders for doses and glucose checks as needed ❑ Ask patients to share data reports and engage with the care team between health care visits per plan ❑ Update Therapy Settings as care plan evolves. |
Use the data on an ongoing basis in collaboration with the PwD to optimize
care. For SIPs this includes determining data report workflow: The Receive, Review, Respond Model. Receive: ❑ Who receives the reports and assures the right team member reviews the report to take action? ❑ When and how will reports be received and on what cadence? Review ❑ Who will review and determine if care plan changes are warranted and if so what member of the care team will be responsible to address (prescribing clinician, diabetes care and education specialist, care coordinator, or health coach)? Respond: ❑ How does the designated care team member use the behavior-focused data to have a focused conversation with the patient about their self-management plan? ❑ How does the designated care team member use the clinical data to facilitate therapy changes? ❑ How are care plan changes communicated to the patient and to the rest of the care team? |
| Resource: Stanford University has designed a website, https://www.diabeteswise.org/ to help PwDs identify the right technology tools for their particular situation. Clinicians can use this website as a shared decision-making tool in partnership with their patients. |
Resource: Consensus Statement by AACE/ACE Insulin Pump Management Task Force. Endo Practice, 2014. 21 See Table 4 in Consensus Statement for instructions in calculating individualized insulin therapy settings. (Open access reference) |
Resource: Figure 2 Guideline on Reviewing Integrated SIP Data Report |
While there can be a technology learning curve for many persons with diabetes (PwDs), with perseverance and help from the care team in configuring the tool to match their circumstances, PwDs can ultimately benefit as more empowered and active participants in their care. Having data for the first time to guide daily dosing decisions and to inform clinician decisions regarding insulin regimen optimization has revealed significant gaps in care for injection therapy patients and the need for establishing practice protocols for incorporating SIP in a data-driven practice model. Ultimately, much of this work can be automated in the Stage 5 SIPs which are being developed. These practice protocols include:
Checking insulin injection technique, site rotation, timing of insulin dose relative to meal, priming, storing insulin
Optimizing the basal insulin therapy regimen including identifying when to add mealtime coverage in T2DM
Calculating rapid-acting insulin therapy settings: target glucose, duration of insulin action (DIA), insulin to carbohydrate ratio (ICR) or meal doses, and insulin sensitivity factor (ISF)
SIP data review including refining the insulin therapy settings on an ongoing basis; may include procedures for remote monitoring and providing virtual care
Prescribing and training on the use of connected glucose monitoring devices including optimal blood glucose check schedules to support the prescribed insulin regimen and for setting clear expectations for the patient on the optimal use of SIPs.
Addressing cost concerns regarding the individual’s ability to afford insulin and related supplies.
The high cost of insulin has been a significant barrier to patients being able to afford their insulin and is receiving considerable attention by policy makers and national organizations. 22 Technology tools such as smart insulin pump systems and the considerably lower-cost smart insulin pen systems are irrelevant if the patient cannot afford the insulin they need. It is noted that the insulin cartridges required for smart insulin pens are typically available at a similar cost to insulin vials and traditional insulin pens.
Remote Monitoring Capability and Virtual Care
Diabetes requires hundreds of daily decisions, and episodic (every three-six months) medical visits are insufficient for a majority of individuals living with diabetes, particularly those using insulin. Connected devices offer the possibility of remote patient monitoring and more frequent, brief but timely touchpoints as needed with the care team. The ability to communicate regularly with PwDs and monitor their clinical and lifestyle data offers clinicians an opportunity to improve quality of care and health outcomes, creating a new remote, continuous care model. Data-driven conversations allow for more objective, focused, collaborative, and less interrogative discussion fostering shared decision-making (see Figure 2).
Figure 2.
(a) Guide to using integrated insights by InPen data report and (b) Guide to using the insights by InPen integrated data report.
Increasingly, virtually delivered diabetes services may be covered by private payers within fee-for-service or value-based models of payment. The Medicare Remote Monitoring codes are listed in Table 2.
Table 2.
Medicare Remote Patient Monitoring Codes.
|
CPT Code 99453
PRODUCT TRAINING Remote monitoring of physiologic parameters initial set-up and patient education on use of equipment |
|
CPT Code 99454
WIRELESS DATA TRANSMISSION DATA COLLECTION ALERTS & MESSAGING Device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days, when at least 16 days have data. |
|
CPT Codes 99457 & 99458
PATIENT MANAGEMENT RPM treatment management services. Twenty minutes or more of clinical staff/physician/other qualified healthcare professional time in a calendar month requiring interactive communication with the patient/caregiver during the month. Code 99458 allows for an additional 20 minutes above the initial 20 minutes. |
Abbreviation: RPM, remote patient monitoring.
Source: Centers for Medicare and Medicaid Services. Physician Fee Schedule CY 2020 Final Rule. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched. Accessed March 21, 2020.
The 2020 guidance notes that remote patient monitoring services involve “establishing, implementing, revising, and monitoring a specific treatment plan for a patient,” and that these services can be provided by clinical staff and billed “incident to” a billing practitioner’s services under “general supervision” of a practitioner—meaning that the clinical staff need not be present in the same physical location as the billing practitioner. With this change, staff members can perform these services outside the clinic, allowing for even better scale as costs (such as office space, travel, and staffing) can be significantly reduced. As payment models evolve to match the changing health care environment, health care teams will be able to expand their service options.
Conclusion
Our connected future enabling data-driven continuous care models is here. Enterprising health care teams who embrace these capabilities along with evolving payment models will endeavor to develop person-centric approaches to address the unmet needs of their diabetes population including those on insulin injection therapy, improving access, reach, and effectiveness of their services.
Footnotes
Abbreviations: ADA, American Diabetes Association; CGM, Continuous Glucose Monitoring; FDA, Food and Drug Administration; MDI, Multiple Daily Insulin Therapy; PGHD, Patient-Generated Health Data; PwD, Person with Diabetes; SIP, Smart Insulin Pen; TIP, Tracking Insulin Pen.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Janice MacLeod is an employee of Companion Medical.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Janice MacLeod  https://orcid.org/0000-0001-8689-4938
https://orcid.org/0000-0001-8689-4938
References
- 1. Sikes KA, Weyman K. Diabetes and the use of insulin pumps. Endocrinology advisor. https://www.endocrinologyadvisor.com/home/decision-support-in-medicine/endocrinology-metabolism/diabetes-and-the-use-of-insulin-pumps/. Accessed July 17, 2020.
- 2. Bailey TS, Stone JY. A novel pen-based Bluetooth-enabled insulin delivery system with insulin dose tracking and advice. Expert Opin Drug Deliv. 2017;14(5):697-703. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Overcoming therapeutic inertia, 2019. https://professional.diabetes.org/meeting/other/overcoming-therapeutic-inertia.
- 4. Bergenstal RM, Peyrot M, Dreon DM, et al. Implementation of basal-bolus therapy in type 2 diabetes: a randomized controlled trial comparing bolus insulin delivery using an insulin patch with an insulin pen. Diabetes Technol Ther. 2019;21(5):273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Diabetes technology: standards of medical care in diabetes – 2020. Diabetes Care. 2019;43(S1):S77-S88. [DOI] [PubMed] [Google Scholar]
- 6. Norlander LM, Anderson S, Levy CJ, et al. Late and missed meal boluses with multiple daily insulin injections. Diabetes, 2018;67(suppl 1):992-P. [Google Scholar]
- 7. Munshi MN, Slyne C, Greenberg JM, et al. Nonadherence to insulin therapy detected by Bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care. 2019; 42(6):1129-1131. [DOI] [PubMed] [Google Scholar]
- 8. Randlov J, Poulsen JU. How much do forgotten insulin injections matter to hemoglobin A1c in people with diabetes? A simulation study. J Diabetes Sci Technol. 2008;2(2):229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaugg SD, Dogbey G, Collins K, et al. Diabetes numeracy and blood glucose control: association with type of diabetes and source of care. Clin Diabetes. 2014;32(4): 152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148(10):737-746. [DOI] [PubMed] [Google Scholar]
- 11. Marden S, Thomas PW, Sheppard ZA, Knott J, Lueddeke J, Kerr D. Poor numeracy skills are associated with glycaemic control in type 1 diabetes. Diabet Med. 2012;29(5):662-669. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt S, Norgaard K. Bolus calculators. J Diabetes Sci and Technol. 2014;8(5):1035-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klonnof DC, Kerr D. Smart pens will improve insulin therapy. J Dia Sci Tech. 2018;12(3):551-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ziegler R, Cavan DA, Cranston I, et al. Use of an insulin bolus advisor improves glycemic control in multiple daily insulin injection (MDI) therapy patients with suboptimal glycemic control: first results from the ABACUS trial. Diabetes Care. 2013; 36(11):3613-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnard KD, Parkin CG, Young A, et al. Use of an automated bolus calculator reduces fear of hypoglycemia and improves confidence in dosage accuracy in patients with type 1 diabetes mellitus treated with multiple daily insulin injections. J Diab Sci Tech. 2012;6(1):144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vallejo Mora MDR, Carreira M, Anarte MT, Linares F, Olveira G, González Romero S. Bolus calculator reduces hypoglycemia in the short term and fear of hypoglycemia in the long term in subjects with type 1 diabetes (CBMDI study). Diabetes Technol Ther. 2017. 19(7):402-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kowalski A. Pathway to artificial pancreas systems revisited going downstream. Diabetes Care. 2015;38(6):1036-1043. [DOI] [PubMed] [Google Scholar]
- 18. Kerr D, Warshaw H. Smart insulin pens will address critical unmet needs for people with diabetes using insulin. Endocrine Today. 2019;17(5):21-22. [Google Scholar]
- 19. Greenwood DA, Gee PM, Fatkin KJ, Peeples M. A systematic review of reviews evaluating technology-enabled diabetes self-management education and support. J Diabetes Sci Technol. 2017;11(5):1015-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MacLeod J. Presenting the case for smart insulin pens. ADCES in Practice. 2020;8(4):48-52. [Google Scholar]
- 21. Grunberger G, et al. Consensus statement by AACE/ACE insulin pump management task force. Endo Practice. 2014;20(5):463-489. [DOI] [PubMed] [Google Scholar]
- 22. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care. 2018;41(6):1299-1311. [DOI] [PubMed] [Google Scholar]