Abstract
Smart pen technology has evolved over the past decade with new features such as Bluetooth connectivity, bolus dose calculators, and integration with mobile apps and continuous glucose monitors. While similar in appearance to a traditional insulin pen, smart pens have the ability to record and store data of insulin injections. These devices have the potential to transform diabetes management for clinicians, and patients with type 1 and type 2 diabetes on insulin therapy by improving adherence, glycemic control, and addressing barriers to diabetes management. Smart pens can also highlight the relationship between insulin, food, and physical activity, and provide insight into optimizing insulin regimens. Education of clinicians and patients, and more clinical studies showing the benefits of smart pens and cost-effectiveness, are needed.
Keywords: smart pens, technology, diabetes management, type 1 diabetes, type 2 diabetes, insulin therapy
In 2019, it is estimated that 463 million adults, aged 20-79, had diabetes worldwide, and by 2045, this number is anticipated to rise to 700 million. 1 Approximately 10% of all adults with diabetes have type 1 diabetes (T1D) and 7.5% have type 2 diabetes (T2D) requiring insulin therapy.1,2 Insulin pens are the most common insulin administration method used worldwide. 3
The first insulin pen, NovoPen by Novo Nordisk (Bagsværd, Denmark), was launched in 1985 and with advances in insulin pen technology, most insulin currently available on the market offers the option for insulin pens. 4 Clinicians and patients tend to prefer insulin pens over vials and syringes since they have many advantages.3,4 These include improvement in adherence, convenience, dosing accuracy, and social acceptability.3-5
Although insulin pens have been shown to be beneficial, patients with T1D or T2D on insulin therapy may continue to struggle with diabetes management and achievement of glycemic goals. 6 Insulin therapy is challenging due to its narrow therapeutic range and risk of hypoglycemia. Patients may be on complex multiple daily injection (MDI) insulin regimens and have to decide every day the doses of insulin based on amount of carbohydrates, food choices, and physical activity. These challenges are reflected by the small percentage of patients reaching target A1c <7.0%. 7 Furthermore, patients may not be adherent to insulin therapy for reasons such as forgetting to administer insulin, injecting the incorrect type of insulin, fear of hypoglycemia, and/or administering insulin more than once.
Thus far, there has not been a way to objectively evaluate dosing practices in patients with diabetes who self-inject insulin using syringes or pens. Unreliable glucose logbooks, multiple glucometers, patients not performing self-monitoring of blood glucose, and/or having difficulties with carbohydrate counting can make assessment of insulin therapy adherence challenging and prevent patients from achieving optimal glycemic control.8-11
Clinicians have to make decisions on a patient’s insulin regimen based on the presumption that the patient is following the insulin prescription. This presumption may lead to under or over treatment, particularly if nonadherence is frequent. In a recent study using Bluetooth-enabled insulin pen cap technology, nonadherence to insulin in patients with T1D and T2D was recorded for 24% of bolus insulin administration and 36% of basal insulin administration. 12 Therefore, for patients with diabetes on insulin, missing an insulin dose is not uncommon and can negatively impact glycemic control.13-15
To address the challenges of insulin pens, “smart pens” were developed and introduced in 2007 with the ability to store data such as the date, time, and amount of previous insulin doses. 16 Smart pens are similar in appearance to insulin pens and require the patient to prime the needle, set the insulin dose, and use the depressing device for insulin delivery. Over the past decade, smart pens have evolved and recently have added connectivity features such as Bluetooth or near-field connectivity (NFC) technology to display data on applications and to download records. The InPen (Companion Medical; San Diego, CA) is the first and only FDA-approved Bluetooth-enabled wireless smart pen available in the United States, and recently, has been acquired by Medtronic (Dublin, Ireland). 11 In Europe, ESYSTA BT Pen (Emperra; Potsdam, Germany) and Pendiq 2.0 (Pendiq; Moers, Germany) are available and CE-marked.17,18 NovoPen 6 (Novo Nordisk) is not yet available, but is anticipated to launch soon in Europe. 16
All of the available connected smart pens have the ability to record and store data of the amount and timing of insulin injections, which can be reviewed by the patient in a timely manner, and provide downloadable reports to the patient and healthcare provider. 19 The InPen has additional features such as bolus dose calculator, detection of prime dose versus actual dose, reminder alerts, active insulin on board (IOB) monitoring, and integration with continuous glucose monitors. 11 A comparison of the devices is shown in Table 1.
Table 1.
Comparison of Approved Connected Smart Pens.
| InPen | ESYSTA BT Pen | Pendiq 2.0 | NovoPen 6 | |
|---|---|---|---|---|
| Company | Companion Medical | Emperra | Pendiq | Novo Nordisk |
| Availability | United States | Europe | Europe | Not available |
| Approval | FDA and CE-marked | CE-marked | CE-marked | CE-marked |
| Insulins | Humalog | Any major brand including NPH and pre-mix insulin | Any major brand except NPH or pre-mix insulin | Novolog |
| Novolog | Fiasp | |||
| Fiasp | Levemir | |||
| Tresiba | ||||
| Insulin dose increments | 0.5 units | 1 unit | 0.1 units | 1 unit |
| Maximum insulin dose | 30 units | 60 units | 60 units | 60 units |
| Connects with company app on smartphone | Yes | Yes | Yes | No |
| Displays number of units last administered | Yes | Yes | Yes | Yes |
| Monitors active insulin on board | Yes | No | No | Yes |
| Collects and stores data of insulin injections | Yes | Yes | Yes | Yes |
| Insulin injection reminder | Yes | No | No | No |
| Bolus dose calculator | Yes | No | No | No |
| Integrates with CGM | Yes | No | No | Maybe |
| Downloadable reports | Yes | Yes | Yes | Yes |
| Connectivity | Bluetooth | Bluetooth | Bluetooth | NFC |
| Battery life | One year | Six months | Rechargeable | Five years |
CE, Conformité Européenne (European Conformity); CGM, continuous glucose monitoring; FDA, U.S. Food and Drug Administration; NFC, near-field connectivity.
The Benefits of Using Connected Smart Pens
Connected smart pens have many benefits and may be useful for patients of all ages with T1D or T2D on insulin therapy (Table 2).
Table 2.
Benefits and Barriers to Using Smart Pens.
| Age group | Benefits | Barriers |
|---|---|---|
| All ages | ● Assess adherence to therapy ● May improve time in range, reduce glucose variability and hypoglycemia Highlight relationship between insulin, nutrition, and physical activity ● Simplify complex regimens and reinforce important diabetes concepts (eg, timing of insulin administration, nutrition, missed boluses, etc.) ● “Pump holiday” with support of pump features such as bolus calculator and active insulin on board ● Insulin dose reminders ● Downloadable reports ● Integrate with continuous glucose monitors ● Accurate dosing and ability to dose smaller units (eg, 0.1 units) ● Cheaper, less complex and less maintenance than insulin pump |
● Patient preference ● Patient may feel embarrassed and/or forgets to use the device ● Higher cost compared to insulin pens ● Limited options of approved devices ● Requires additional training to use device ● Maintenance of smart connected insulin pen (eg, changing cartridges, utilizing adaptors or smartphone apps) ● Clinics may lack capability to download device ● Lack of educational materials and guidance for clinicians on how to interpret data and develop a plan |
| Children (≤ 18 years) | ● Help parents and/or caregivers to assess
adherence ● Accurate, timely shared information among caregivers ● Bolus dose calculator to help with carbohydrate counting ● Monitor insulin on board during activity and bedtime ● Small (0.5 unit) dosing |
● May require carrying a phone for the mobile
app ● Easy to misplace compared to an insulin pump ● Lack of educational materials and guidance for clinicians on how to interpret data and develop a plan |
| Pregnancy | ● May improve adherence and achieve target
glycemic goals ● Bolus dose calculator to help with carbohydrate counting ● Educate patients on the relationship between insulin, nutrition, and physical activity throughout pregnancy |
● Lack of educational materials and guidance for clinicians on how to interpret data and develop a plan |
| Elderly (≥ 65 years) |
● Assist patients who have difficulties
problem-solving (eg, cannot calculate
doses) ● Caregiver is able to accurately monitor insulin administration and assist patient if needed ● May help clinicians identify challenges (eg, missed boluses, stacking boluses, etc.) and simplify insulin regimens to lower risk of hypoglycemia and treatment burden |
● No Medicare coverage ● Need for dexterity ● Visual impairment ● Moderate to severe cognitive dysfunction ● Lack of educational materials and guidance for clinicians on how to interpret data and develop a plan |
Improve Adherence and Glycemic Control
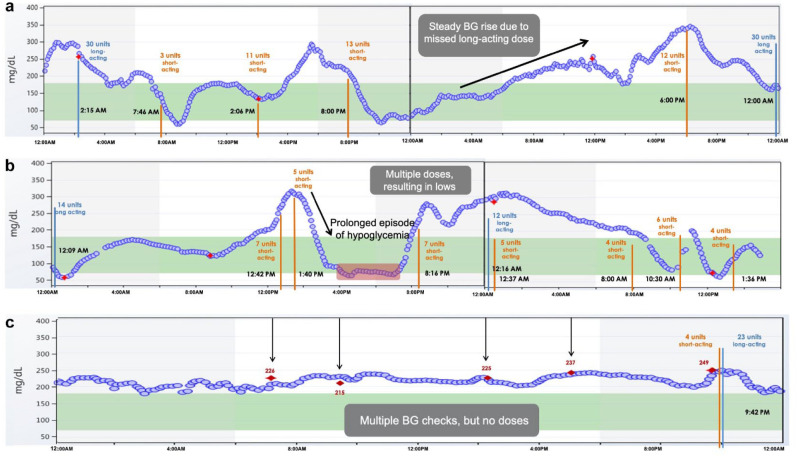
With the ability to record insulin injections, smart pens may help patients monitor their adherence to insulin therapy and assist clinicians in clinical decision making. Smart pens when combined with continuous glucose monitoring (CGM) can show patients, in real-time, the consequences of behaviors such as missing insulin doses or insulin stacking, and this provides an opportunity for targeted diabetes education (Figure 1). A recent proof-of-concept study using NovoPen6 in patients with T1D on MDI with CGM showed that smart pens can help improve adherence to insulin therapy. 20 Participants had a 43% reduction in missed bolus doses at follow-up (≥180 days) compared to baseline using the smart pen. This study also found a significant increase of 1.9 hours/day for time in range (TIR) with a reduction in time spent in hyperglycemia (>180 mg/dL) and hypoglycemia (<54 mg/dL). Improving adherence to insulin therapy not only has a positive effect on glycemic control but may also reduce healthcare costs and utilization.21,22
Figure 1.
Smart pens plus CGM can reveal nonadherence to insulin regimens and may help healthcare providers create individualized treatment plans and provide targeted education.
(a) A patient with T1D has inconsistent timing of bedtime long-acting (basal) insulin administration. The patient misses a dose of basal insulin in a 24-hour period and consequently experiences hyperglycemia (black rising arrow). (b) A patient with T1D administers multiple short-acting insulin doses within two hours in response to hyperglycemia. The patient subsequently experiences a prolonged episode of hypoglycemia (highlighted in red) due to insulin stacking. (c) A patient with T1D and history of fear of hypoglycemia skips mealtime short-acting insulin boluses despite steady glucose levels in the hyperglycemic range. The red diamond symbol indicates fingerstick glucose checks. The orange vertical lines indicate short-acting insulin administration and blue vertical lines indicate long-acting insulin administration. CGM, continuous glucose monitoring; T1D, type 1 diabetes.
Highlight Challenges Between Insulin and Food
The timing of mealtime insulin boluses is key to achieving optimal post-prandial glycemic control. Clinical studies have shown that injecting rapid-acting insulin 15-20 minutes pre-meal reduced hyperglycemia by 30% and hypoglycemia compared to injection immediately before the meal.23,24 With smart pen features such as dose reminder alerts and the ability to view active IOB, patients may be more successful in timing the dose of insulin boluses in relation to meals and avoid insulin stacking.
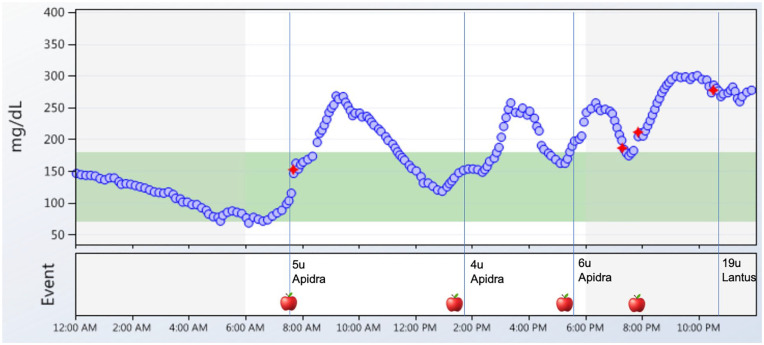
Meal composition (fat, protein, and carbohydrates) can also significantly affect post-prandial glucose. Previous studies have shown that dietary fat can lead to sustained post-prandial hyperglycemia up to five hours. 25 Using Bluetooth-enabled insulin pen cap technology and CGM in patients with T1D on MDI, 37% of mealtime boluses resulted in elevated three-hour post-prandial glucose levels >180 mg/dL despite insulin being injected at the appropriate time. 26 This study also showed that late or missed mealtime boluses occurred frequently and resulted in post-prandial glucose excursions. Therefore, smart pens combined with CGM may be used as an education tool to teach patients and give immediate feedback on dosing and timing of insulin and meal choices (Figure 2). It can also inform healthcare providers whether insulin to carbohydrate ratios (ICR) and insulin sensitivity factors (ISF) are appropriate.
Figure 2.
Smart pens plus CGM can highlight the relationship between insulin and food. A daily glucose report shows that a patient consumed four meals per day (apple symbol) and injected rapid acting insulin (Apidra) to cover meals and long-acting insulin at bedtime (Lantus). Data from the pen revealed that the patient was injecting rapid-acting insulin immediately before or shortly after eating, and consequently the patient experienced post-prandial hyperglycemia. There was one episode of a missed mealtime insulin dose at 8:00 PM (presence of apple symbol without Apidra administration). The patient had a consistent pattern of post-breakfast hyperglycemia and a review of dietary intake uncovered that the patient was eating a donut regularly at breakfast time. The red diamond symbol indicates fingerstick calibrations for the CGM and blue vertical lines indicate insulin administration.
Address Barriers to Diabetes Management in Different Diabetes Populations
For patients with T1D, smart pens offer similar features as insulin pumps such as bolus calculator and IOB but are less expensive, complex, and are easier to maintain. Smart pens can be helpful for children and their parents, and young adults to assist with carbohydrate counting, teach the impact of exercise on glucose control, and educate them on important diabetes concepts (eg, timing of insulin, relationship between insulin and food, etc.). Moreover, in children with more than one caregiver involved, smart pens offer the ability to share data on insulin injections. In addition, in young adults with T1D on MDI who are at increased risk of diabetic ketoacidosis and severe hypoglycemia due to insulin omission or over stacking, smart pens can assist with bolus reminders and active IOB. The downloadable data can also identify these challenges and help clinicians and patients devise a personalized plan to mitigate hypoglycemia and hyperglycemia (Figure 1). 27
For those patients who want to transition from MDI to an insulin pump, smart pens may help to optimize insulin doses, ICR, and ISF. Conversely, the InPen can be considered for patients who are looking for a “pump holiday” but want to maintain the features of a pump such as IOB, and bolus and correction dose calculator. In addition, smart pens when combined with CGM may help patients adjust insulin when physically active as shown in a recent study of professional cyclists with T1D. 28 Smart pen and CGM data were used during a five-day race to adjust bolus and basal doses for physical activity and macronutrient intake.
Smart pens may also be advantageous in older adults with T1D or T2D on MDI to assess adherence.12,29 Older adults with diabetes are at increased risk of developing cognitive dysfunction and this can interfere with their ability to follow an insulin plan. 30 Smart pens can help provide reminders for insulin administration and assist patients who have difficulties with problem-solving (eg, cannot calculate insulin doses). Moreover, using smart pens with CGM may help clinicians simplify insulin regimens to reduce the risk of hypoglycemia, lower the treatment burden, and ensure that the patient and/or caregiver can follow the insulin plan consistently.
Patients with diabetes who have challenges with numeracy tend to have worse glycemic control. 31 Smart pens could help address this barrier with the use of a bolus dose calculator. The InPen syncs with a built-in dose calculator on the mobile app that can advise how much rapid-acting insulin and/or correction dose to administer based on either number of carbohydrates, meal size estimation (small, medium, or large), or fixed dosing. 32 Another advantage of smart pens is that insulin can be accurately delivered in small doses such as 0.1-0.5 unit increments.
In addition, smart pens may play a role in diabetes management of pregnant patients with T1D, T2D, or gestational diabetes. A multicenter randomized trial demonstrated that in pregnant patients with T1D, MDI plus CGM users compared to pump plus CGM users were more likely to achieve target A1c and have increase TIR by 24 weeks gestation.33,34 Therefore, smart pens may have the potential to improve adherence and achieve glycemic goals, which are important for maternal and fetal outcomes. Clinicians can utilize the smart pen downloadable reports to educate patients on the relationships between insulin, nutrition, and physical activity throughout their pregnancy.
Furthermore, smart pens may be beneficial for patients with T1D or T2D who are hospitalized or admitted to nursing homes to provide accurate dosing and reduce the risk of errors. Insulin administration errors are common in hospitals and nursing homes, and can lead to serious consequences.35-37 Smart pens could help nursing staff record insulin delivery and administer the appropriate insulin at the correct dose and time. If used along with CGM, clinicians may also be able to better address glycemic excursions that occur with frequent changes in health status.
Challenges of Adopting Connected Smart Pens Into Clinical Practice
While smart pens have been available for the past decade, patients and the healthcare community have been slow to adopt and embrace this new technology. Currently, there are few clinical studies with published data showing benefits in adherence, improvement in A1c, and TIR. 20 Other barriers to using smart pens include limited availability of the devices worldwide, higher cost compared to traditional insulin pens, additional training costs, and technology inertia. 3 In addition, clinicians need to be able to download the smart pen data, have the necessary skills to interpret the data, and formulate an appropriate insulin plan. Furthermore, clinicians and patients need to take into account the user experience of smart pens and their respective apps. There are also patient-specific barriers that can restrict the use of smart pens (Table 2).
However, the use of smart pens is gaining recognition. The American Diabetes Association recently published guidelines recommending the use of smart pens in diabetes management. 38 In order to disseminate the use of smart pens, there need to be education and guidelines for patients and healthcare providers on how to use the technology, interpret the data, and implement appropriate management plans as well as the benefits of smart pens. Healthcare providers also need to recognize the advantage of combining smart pens with CGM for real-time data to create individualized treatment plans for their patients. In addition, smart pens would be more readily adopted if costs were substantially reduced or subsidized and covered by Medicare. Finally, more clinical studies are needed to investigate the benefits of smart pens and their impact on diabetes outcomes, cost-effectiveness, and quality of life.
Conclusion
Connected smart pens have the potential to help patients with T1D or T2D on insulin therapy improve adherence, glycemic control, and address challenges related to diabetes management. Further education of healthcare providers and patients, cost reduction, integration with CGM, and large clinical studies are needed to advance this technology for widespread use. The technology of smart pens is still in the early development phase with untapped potential that can enhance diabetes care and change the way how MDI therapy is used in the future.
Footnotes
Abbreviations: CE, Conformité Européenne; CGM, continuous glucose monitoring; FDA, U.S. Food and Drug Administration; ICR, Insulin to carbohydrate ratio; IOB, Insulin on board; ISF, Insulin sensitivity factor; MDI, multiple daily injections; NFC, Near-field communication; TIR, Time in range; T1D, Type 1 Diabetes; T2D, Type 2 Diabetes.
Author(s) contributions: All authors accept responsibility for all aspects of the work.
Conception of the work: SS and ET
Drafting and revising: SS, MM and ET
Final approval of the version: SS, MM and ET
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ET is a consultant for Medtronic. MM is a consultant for Sanofi and Lilly. SS declares that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ET and MM’s time is covered by NIH DP3 Grant (1DP3DK112214-01). SS does not receive financial support for research, authorship, and/or publication of this article.
ORCID iD: Sarah L. Sy  https://orcid.org/0000-0003-2237-2537
https://orcid.org/0000-0003-2237-2537
References
- 1. Saeedi P, Petersohn I, Paraskevi S, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 2. Basu S, Yudkin JS, Kehlenbrink S, et al. Estimation of global insulin use for type 2 diabetes, 2018-30: a microsimulation analysis. Lancet Diabetes Endocrinol. 2019;7:25-33. [DOI] [PubMed] [Google Scholar]
- 3. Klonoff DC, Kerr D. Smart pens will improve insulin therapy. Diabetes Technol Ther. 2018;12:551-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selam JL. Evaluation of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lasalvia P, Barahona-Correa JE, Romero-Alvernia DM, et al. Pen devices for insulin self-administration compared with needle and vial: systematic review of the literature and meta-analysis. J Diabetes Sci Technol. 2016;10:959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weinger K, Beverly EA. Barriers to achieving glycemic targets: who omits insulin and why? Diabetes Care. 2010;33:450-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blackwell M, Wheeler BJ. Clinical review: the misreporting of log-book, download, and verbal self-measured blood glucose in adults and children with type 1 diabetes. Acta Diabetol. 2016;54:1-8. [DOI] [PubMed] [Google Scholar]
- 9. Mazze RS, Shamoon H, Pasmantier R, et al. Reliability of blood glucose monitoring by patients with diabetes mellitus. Am J Med. 1984;77:211-217. [DOI] [PubMed] [Google Scholar]
- 10. Tenderich A. Use of blood glucose meters among people with type 2 diabetes: patient perspectives. Diabetes Spectr. 2013;26:67-70. [Google Scholar]
- 11. Bailey TS, Stone JY. A novel pen-based Bluetooth-enabled insulin delivery system with dose tracking and advice. Expert Opin Drug Deliv. 2017;14:697-703. [DOI] [PubMed] [Google Scholar]
- 12. Munshi MN, Slyne C, Greenberg JM, et al. Nonadherence to insulin therapy detected by bluetooth-enabled pen cap is associated with poor glycemic control. Diabetes Care. 2019;42:1129-1131. [DOI] [PubMed] [Google Scholar]
- 13. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218-1224. [DOI] [PubMed] [Google Scholar]
- 14. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burdick J, Chase HP, Slover RH, Knievel K, Scrimgeour L, Maniatas AK. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113:e221-4. [DOI] [PubMed] [Google Scholar]
- 16. Kesavadev J, Saboo B, Krishna M, Krishnan G. Evolution of insulin delivery devices: from syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. 2020;11:1251-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ESYSTA BT. Pen [Internet]. Germany: Emperra GmbH E-Health Technologies; 2020. [cited June 11 2020]. https://www.emperra.com/de/esysta/bt-pen/
- 18. Pendiq GmbH. Pendiq 2.0 User Manual. Germany: Pendiq; 2017. [Google Scholar]
- 19. Sangave NA, Aungst TD, Patel DK. Smart connected insulin pens, caps, and attachments: a review of the future of diabetes technology. Diabetes Spectr. 2019;32:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adolfsson P, Vaever Hartvig N, Kaas A, Bech Moller J, Hellman J. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen [published online ahead of print March 11, 2020]. Diabetes Technol Ther. doi: 10.1089/dia.2019.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33:74-109. [DOI] [PubMed] [Google Scholar]
- 22. Sarbacker GB, Urteaga EM. Adherence to insulin therapy. Diabetes Spectr. 2016;29:166-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cobry E, McFann K, Messer L, et al. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with Type 1 diabetes. Diabetes Technol Ther. 2010;12:173-177 [DOI] [PubMed] [Google Scholar]
- 24. Slattery D, Amiel SA, Choudhary P. Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet Med. 2018;35: 306-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079-1094. [DOI] [PubMed] [Google Scholar]
- 26. Toschi E, Slyne C, Greenberg JM, et al. Examining the relationship between pre- and postprandial glucose levels and insulin bolus timing using bluetooth-enabled insulin pen cap technology and continuous glucose monitoring. Diabetes Technol Ther. 2020;22:19-24. [DOI] [PubMed] [Google Scholar]
- 27. Karges B, Schwandt A, Heidtmann B, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318:1358-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moser O, Dietrich M, McCarthy O, Bracken RM, Eckstein ML. Bolus insulin dose depends on previous-day race intensity during 5 days of professional road-cycle racing in athletes with type 1 diabetes: a prospective observational study [published online ahead of print May 8, 2020]. Diabetes Obes Metab. doi: 10.1111/dom.14083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toschi E, Munshi MN. Benefits and challenges of diabetes technology use in older adults. Endocrinol Metab Clin N Am. 2020;49:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munshi MN. Cognitive dysfunction in older adults with diabetes: what a clinician needs to know. Diabetes Care. 2017;40:461-467. [DOI] [PubMed] [Google Scholar]
- 31. Marden S, Thomas PW, Sheppard ZA, Knott J, Lueddeke J, Kerr D. Poor numeracy skills are associated with glycaemic control in type 1 diabetes. Diabet Med. 2012;29:662-669. [DOI] [PubMed] [Google Scholar]
- 32. Companion Medical. InPen System: Instructions for Use. San Diego: Companion Medical; 2020. [Google Scholar]
- 33. Feig DS, Corcoy R, Donovan LE, et al. Pumps or multiple daily injections in pregnancy involving type 1 diabetes: a prespecified analysis of the CONCEPTT randomized trial. Diabetes Care. 2018;41:2471-2479. [DOI] [PubMed] [Google Scholar]
- 34. Feig DS, Corcoy R, Donovan LE, et al. Response to comment on Feig et al. pumps or multiple daily injections in pregnancy involving type 1 diabetes: a prespecified analysis of the CONCEPTT randomized trial. Diabetes Care. 2019;42:e98-e99. [DOI] [PubMed] [Google Scholar]
- 35. Cornish W. Safe and appropriate use of insulin and other antihyperglycemic agents in hospital. Can J Diabetes. 2014;38:94-100. [DOI] [PubMed] [Google Scholar]
- 36. Greene SB, Williams CE, Pierson S, Hansen RA, Carey TS. Medication error reporting in nursing homes: identifying targets for patient safety improvement. Qual Saf Health Care. 2010;19:218-222. [DOI] [PubMed] [Google Scholar]
- 37. Hellman R. Patient safety and inpatient glycemic control: translating concepts into action. Endocr Pract. 2006;12(suppl 3):49-55. [DOI] [PubMed] [Google Scholar]
- 38. American Diabetes Association. Diabetes technology: standards of medical care in diabetes – 2020. Diabetes Care. 2020;43(suppl 1):s77-s88. [DOI] [PubMed] [Google Scholar]