Abstract
Purpose:
To report two cases of COVID-19 under treatment with a corticosteroid; in one case rhino-orbitocerebral mucormycosis and in another one rhino-orbital mucormycosis developed.
Case presentation:
A 40-year old woman and a 54-year old man with severe COVID-19 underwent corticosteroid therapy for immune-related lung injuries. The first case presented with a bilateral visual loss and complete ophthalmoplegia of the right eye. The second case presented with vision loss, proptosis, orbital inflammation, and complete ophthalmoplegia on the left side. Histopathologic, nasal endoscopic examinations, and radiologic findings confirmed mucormycosis in both patients. The patients denied orbital exenteration and were managed with systemic amphotericin B and daily endoscopic sinus debridement and irrigation with diluted amphotericin B. Because of the intracranial space involvement, the first case died. The second case was successfully managed surgically and medically.
Conclusion:
Rhino-orbital/cerebral mucormycosis may be developed in COVID-19 patients under treatment with corticosteroid, and requires prompt diagnosis and management.
Keywords: Rhino-orbitocerebral, rhino-orbital, COVID-19, corticosteroid, mucormycosis
Introduction
COVID-19 pandemic is an outbreak of coronavirus disease that was first identified in December 2019. The severity of the disease ranges from asymptomatic infection to respiratory failure and death.1 Secondary fungal or bacterial infections or coinfections are important challenges increasing the patients’ morbidity and mortality.1 Candidiasis and pulmonary aspergillosis have been common fungal infections that were reported as superinfections in COVID-19 patients.2 On the other hand, the use of corticosteroids for modulating immune-related lung injury and reducing the mortality rate in the COVID-19 patients, that need respiratory supports and supplementary oxygen,3 may predispose the patients to secondary infections that increase the risk of mortality. Herein, we report two cases of confirmed COVID-19 patients that received corticosteroid therapy; one case developed rhino-orbitocerebral mucormycosis, and another one developed rhino-orbital mucormycosis after the initiation of corticosteroid. To the best of our knowledge, rhino-orbital and/or rhino-orbitocerebral mucormycosis have rarely been reported in COVID-19 patients.4,5
Case presentation
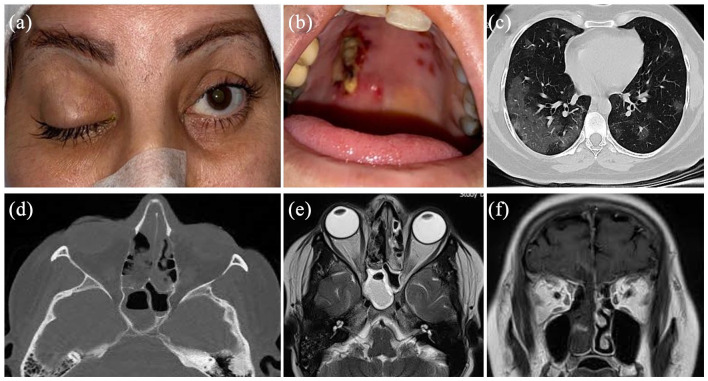
Case 1: A 40-year old woman with an unremarkable past medical history presented with respiratory symptoms of COVID-19 confirmed by PCR and chest CT scan (Figure 1). The patient had no history of immune system disorders including AIDS disease. She was admitted to the hospital and received supplementary oxygen (without invasive mechanical ventilation) in addition to intravenous (IV) remdesivir 200 mg on day 1 followed by 100 mg daily for 4 days, and IV levofloxacin (500 mg/day). On day 7, IV dexamethasone (8 mg/day) was added to mitigate immune-related lung injuries. Eight days later, the patient complained of progressive bilateral visual loss and periorbital pain. The visual acuity was no light perception bilaterally. Ocular examinations disclosed complete blepharoptosis and ophthalmoplegia together with mild proptosis on the right side. Normal ocular motilities were observed in the left eye. Both pupils were fixed and dilated (Figure 1). Orbital CT scan disclosed opacifications of paranasal sinuses, more prominent in the posterior ethmoidal and sphenoidal sinuses, and extending to the posterior orbital space (Figure 1). MRI revealed an extension of the disease into the anterior cranial fossa (Figure 1). Systemic antibiotics including IV meropenem and vancomycin were started and the patient underwent endoscopic sinus examination where blackish necrotic tissues were observed. Histopathological examinations of the debrided necrotic tissues from paranasal sinuses disclosed areas of granulomatous inflammation with marked necrosis, prominent vasculitis, and large numbers of irregular non-septate and branched eosinophilic filaments on hematoxylin and eosin that were markedly angioinvasive (Figure 2) and confirming the diagnosis of mucormycosis. Endoscopic debridement of the involved paranasal sinuses was performed every day. IV administration of amphotericin B (4 mg/kg/day) and daily irrigation of paranasal sinuses with diluted amphotericin B were performed. The patient was monitored with regular imaging (MRI and CT scan). A multidisciplinary team composed of an otorhinolaryngologist, ophthalmologist, infectious disease specialist, and a neurosurgeon involved in the management of the patient. The patient declined orbital exenteration surgery and despite regular daily surgical debridement of the paranasal sinuses and medical therapy, she expired 3 months later because of the involvement of his central nervous system.
Figure 1.
A 40-year old COVID-19-affected woman presented with rhino-orbitocerebral mucormycosis. Complete blepharoptosis and ophthalmoplegia are seen on the right side without significant inflammatory signs (a). Note the blackish necrotic tissues in her hard palate (b). The chest CT scan shows ground-glass opacities in both lungs, particularly in peripheral regions (c). Note the presence of bilateral opacifications of the ethmoidal and sphenoidal sinuses (d and e) that were propagated into the intracranial space through the cribriform plate (f).
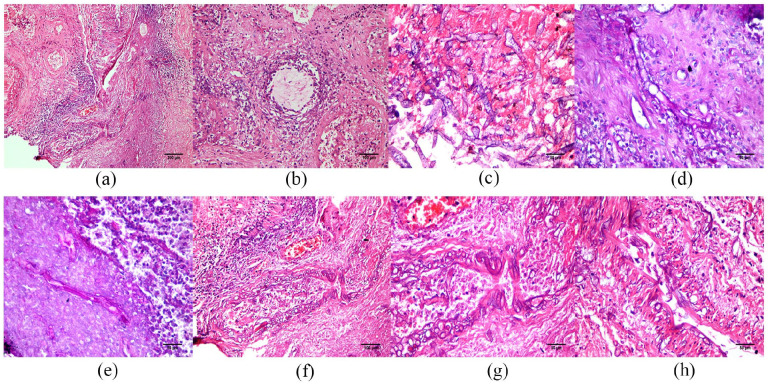
Figure 2.
Representative histopathological microphotographs of debrided tissues from the patients’ paranasal sinuses. Note the presence of granulomatous inflammation (a) with marked necrosis (asterisk), prominent vasculitis (a and b), and large numbers of irregular non-septate branching mucor hyphae (c) (hematoxylin and eosin) (a–c: case 1). The mucor hyphae show reactivity on Periodic acid-Schiff (d and e, case 2) and the eosinophilic irregular filaments are markedly angioinvasive (f–h, case 1) (hematoxylin and eosin).
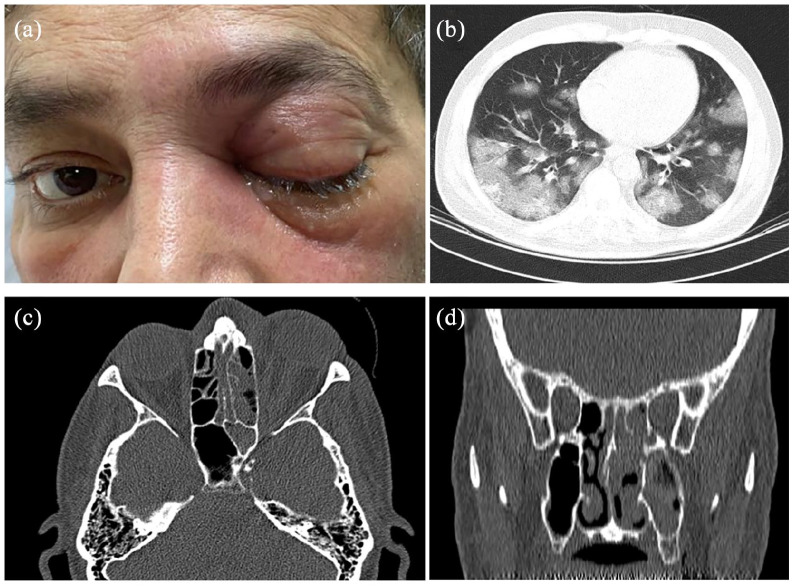
Case 2: A 54-year old man with a history of well-controlled non-insulin-dependent diabetes mellitus (DM) was affected by COVID-19, confirmed by polymerase chain reaction (PCR) and chest CT scan (Figure 3). He was managed by intravenous (IV) remdesivir 200 mg on day 1 followed by 100 mg daily for 4 days, IV levofloxacin (500 mg/day), and non-invasive respiratory supports to control the respiratory symptoms of COVID-19. Six days later, IV dexamethasone (8 mg/day) was started result in high blood sugar that was controlled by insulin injections. On the 7th day, the patient complained of left orbital pain and periorbital swelling together with progressive vision loss. The visual acuity was 20/20 on the right side and light perception on the left. The ocular signs on the left side included blepharoptosis, deficient ocular motilities, proptosis, mild conjunctival injection, and chemosis (Figure 3). CT scan revealed unilateral opacifications of the left orbit and paranasal sinuses (Figure 3). With the clinical suspicion of bacterial superinfection, dexamethasone was discontinued and IV vancomycin and Piperacillin-Tazobactam were started. On the next day, the left side vision changed to no light perception, and the left globe and pupil were fixed. The patient underwent endoscopic sinus surgery and removal of the blackish necrotic tissues from paranasal sinuses. Histopathological examinations disclosed areas of necrotizing granulomatous inflammation with eosinophilic non-septate and branched irregular hyphae on hematoxylin and eosin, which were reactive on Periodic acid-Schiff stain (Figure 2). The cerebral extension of the infection was excluded based on unremarkable brain MRI. With the diagnosis of a rhino-orbital mucormycosis, debridement of the blackish necrotic tissues was attempted from the left maxillary, ethmoidal, and sphenoidal sinuses. In addition to IV infusion of amphotericin B (3 mg/kg/day), the patient underwent daily endoscopic sinus debridement and paranasal sinus irrigation with diluted amphotericin B. The patient declined orbital exenteration and was successfully managed by a multidisciplinary in-hospital team composed of an otolaryngologist, a neurosurgeon, an infectious diseases specialist, an endocrinologist, and an ophthalmologist. Two months later, when the patient’s condition became stable, that is, no necrotic tissues in sinus endoscopy, negative histopathology for mucormycosis, and normal range of the serum inflammatory markers for three consecutive days, the patient was discharged with oral posaconazole (800 mg/day). Based on paranasal endoscopic and imaging results, the patient’s condition was stable during a 7-month follow-up with no evidence of active infection.
Figure 3.
A 54-year old COVID-19 affected man with diabetes mellitus and presented with rhino-orbital mucormycosis. Periorbital inflammation, complete blepharoptosis, and ophthalmoplegia are seen on the left side (a). The chest CT scan discloses multiple mostly peripheral ground-glass opacities with the crazy paving appearance in the lungs and compatible with COVID-19 (b). CT scan shows opacifications in the left maxillary, sphenoidal, and ethmoidal sinuses together with orbital tissue involvement (c and d).
Discussion
Mucormycosis is an acute and potentially fatal fungal infection caused by fungi related to the mucoraceae family.6 These fungi are opportunist organisms and can be found in fruit, soil, feces, and may be cultured from the nasal and oral mucosa of healthy humans.7 The pathogen as an asexual spore-forming fungus can infect the oral and nasal cavities through inhalation. In the presence of a normal immune system, the spores are removed by phagocytic leukocytes.7 The pathogen can transform into hyphae form in individuals with predisposing factors such as uncontrolled DM (particularly in the presence of ketoacidosis), malignancy (such as lymphoma and leukemia), renal failure, organ transplantation, advanced rheumatologic disorders using immunosuppressive agents (such as prolonged use of corticosteroids), AIDS, extensive burn, and chronic sinusitis.8,9 In these conditions, leukocytes have less efficacy on the hyphae forms of fungi and the pathogen may proliferate more easily.9 The organism proliferates and invades the vessel walls of the infected region and results in thrombosis, ischemia, and necrosis.7 The infection can directly spread into the paranasal sinuses and then invade into orbital and intracranial spaces by direct spread or via the bloodstream.9 This form is rhino-orbito-cerebral mucormycosis and is the most common type of human mucormycosis.7 The cutaneous, pulmonary, gastrointestinal, and disseminated form of mucormycosis are uncommon presentations of this infection.10
Some studies reported fungal superimposition or coinfection in COVID-19 patients.11 The studies disclosed that T lymphocytes (CD4 and CD8) are lower in severe COVID-19, and levels of IL-2 R, IL-6, IL-10, and TNF-α are markedly higher in these cases.12 COVID-19 has never been reported as a predisposing factor for rhino-orbital and/or rhino-orbitocerebral mucormycosis. In our study, in case #1, COVID-19 and the related short term corticosteroid therapy were the only predisposing factors conducting the patient to rhino-orbitocerebral mucormycosis. This may indicate that in the presence of COVID-19 even short-term corticosteroid therapy can predispose the patient to mucormycosis. In the case #2, COVID-19, and the corresponding short-term corticosteroid therapy resulted in high blood sugar followed by rhino-orbital mucormycosis.
The symptoms presenting in rhino-orbitocerebral mucormycosis are facial pain and paresthesia, headache, periorbital and nasal swelling, inflammation, eyelid drooping, proptosis, external and internal ophthalmoplegia, visual loss, and blackish necrosis of palate and nasal mucosa.6 The disease usually initiates on the nasal and oral mucosa and spreads to paranasal sinuses.9 It propagates into the orbital space through the lamina papyracea. Vision loss is due to the involvement of optic nerve or retinal supplying vessels. intracranial space can be involved directly through the orbital orifices and sinus walls, or through the bloodstream. Cavernous sinus thrombosis as another complication results in damage to the cranial nerves III, IV, V1, V2, and VI.13 Regular examination and imaging (CT and MRI) are crucial to detect the propagation of the mucormycosis.6 Based on the infected region, the imaging findings may include opacifications of involved paranasal sinuses, bone destruction of sinus walls, alterations of intraorbital tissue signal with or without focal mass, cavernous sinus filling defect, intracranial focal mass, and/or alteration of the meningeal signal.6,9 Mucormycosis is confirmed by the detection of blackish necrotic tissues in the involved region and histopathology. The histological stains that identify the mucor structures include hematoxylin and eosin, Periodic acid-Schiff (PAS), and Gomori methenamine silver (GMS).7 Histopathologic examinations disclose relatively broad non-septate hyphae with right angle branches, necrotizing granulomatous inflammation, and vasculitis together with the presence of mucor hyphae within the vascular wall and lumen.10 In the current study, both cases had unilateral complete internal and external ophthalmoplegia. Case 1 presented with the bilateral visual loss but no significant proptosis or periorbital inflammation. In this patient, imagings disclosed that the infection was concentrated more posteriorly particularly in the posterior air cells of the ethmoidal and sphenoidal sinuses, and near the orbital apex. Brain MRI also showed propagation of the infection into the anterior cranial fossa. Case 2 had a significant unilateral proptosis, orbital inflammation, and vision loss. Imaging revealed the involvement of left ethmoidal, sphenoidal, and maxillary sinuses. Endoscopic sinus surgery and histopathological analyses confirmed mucormycosis in both cases.
Rhinoorbitocerebral mucormycosis is a relatively fatal infection and in cases of brain involvement, mortality rises to 50%–85%.14 Survival in mucormycosis depends on early diagnosis, alleviation of basic predisposing factors, aggressive debridement of necrotic tissues, and appropriate systemic antifungal agents.7 Predisposing factors such as corticosteroid therapy should be discontinued, and blood sugar should be controlled restrictively. Systemic amphotericin B and its liposomal formulation are the first drug of choice for the treatment of mucormycosis and significantly improves the survival rate.13 Posaconazole is an oral antifungal agent that has been used as step-down therapy after initial control of the mucormycosis by amphotericin B.15 Regular daily debridement of necrotic tissues from paranasal sinuses is necessary to prevent the propagation of mucormycosis.6 Also, irrigation of the sinuses and the involved regions with diluted amphotericin B is recommended.6 Orbital exenteration in the presence of focal mass or extensive necrotic tissues has been suggested in most studies.6 In our cases, predisposing factors were controlled, IV amphotericin B was prescribed, daily paranasal sinus debridement and irrigation with diluted amphotericin B were performed. Debridement of necrotic tissues was continued until the necrotic tissues were not detectable in three consecutive days. In our patients, although there was no intraorbital focal mass, due to the extension of the necrotic tissues, orbital exenteration was suggested that was declined by the patients. Unfortunately, case #1 died because of the propagation of the mucormycosis into intracranial space. Case #2 survived by proper medical therapy and regular debridement and irrigation of the paranasal sinuses.
Conclusions
COVID-19 patients undergoing corticosteroid therapy have a risk of rhino-orbital and/or rhino-orbitocerebral mucormycosis, particularly when another risk factor such as DM is present. In these patients, vision changes, orbital pain, and orbital inflammation should be promptly evaluated, otherwise, the propagation of the infection into the intracranial space may be fatal.
Footnotes
Authors’ note: This paper has not been presented as an oral or poster presentation anywhere.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: A written informed consent form was obtained from the patient to publish these data and images.
ORCID iDs: Amirreza Veisi https://orcid.org/0000-0001-8304-3804
Abbas Bagheri https://orcid.org/0000-0002-6736-7435
Mohamad Hasan Rikhtehgar https://orcid.org/0000-0002-5957-8785
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229): 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes S, Troise O, Donaldson H, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting [published online ahead of print, 2020 Jun 27]. Clin Microbiol Infect 2020; 26(10): 1395–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19-preliminary report. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werthman-Ehrenreich A.Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med 2020; 42: 264.e5-264.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus 2020; 12(9): e10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohindra S, Mohindra S, Gupta R, et al. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses 2007; 50: 290–296. [DOI] [PubMed] [Google Scholar]
- 7.Goel S, Palaskar S, Shetty VP, et al. Rhinomaxillary mucormycosis with cerebral extension. J Oral Maxillofac Pathol 2009; 13(1): 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viterbo S, Fasolis M, Garzino-Demo P, et al. Management and outcomes of three cases of rhinocerebral mucormycosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 112: e69–e74. [DOI] [PubMed] [Google Scholar]
- 9.Afroze SN, Korlepara R, Rao GV, et al. Mucormycosis in a diabetic patient: a case report with an insight into its pathophysiology. Contemp Clin Dent 2017; 8: 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mignogna MD, Fortuna G, Leuci S, et al. Mucormycosis in immunocompetent patients: a case-series of patients with maxillary sinus involvement and a critical review of the literature. Int J Infect Dis 2011; 15: e533–e540. [DOI] [PubMed] [Google Scholar]
- 11.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy M, Rosengart A, Schuetz AN, et al. Mold infections of the central nervous system. N Engl J Med 2014; 371: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhu S, Alqahtani M, Al Shehabi M.A fatal case of rhinocerebral mucormycosis of the jaw after dental extractions and review of literature. J Infect Public Health 2018; 11: 301–303. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg RN, Mullane K, Van Burik JA, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother 2006; 50: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]