Highlights
-
•
This report reiterates the aggressive nature of uLMS and introduces the novel use of PARPi in recurrent disease.
-
•
NGS should be performed to identify functional BRCA1/2 loss in uLMS as PARPi may be a potential targeted therapy.
-
•
PARPi are effective in tumor cells that lack BRCA1/2 tumor suppressor proteins by way of synthetic lethality.
Keywords: PARP inhibitor, Leiomyosarcoma, Soft tissue sarcoma, Uterine
Abstract
Uterine leiomyosarcoma (uLMS) is an aggressive mesenchymal tumor associated with a poor prognosis. Research demonstrates that PARP inhibitors (PARPi) improve disease-stable survival in patients with somatic BRCA1/2 mutations through the process of synthetic lethality. Therefore, PARPi’s may have a role in treating gynecologic malignancies with deleterious BRCA1/2 mutations. This patient is a 50-year-old female with a history of stage IB uterine leiomyosarcoma, complicated by recurrence along the vaginal cuff and metastases to the lungs. A somatic BRCA2 mutation was identified, and the patient was started on Olaparib for treatment of recurrent disease. The patient has now been disease free for two years. We recommend next generation sequencing be performed to identify functional BRCA1/2 loss in uLMS as PARPi may be a potential targeted therapy for uLMS.
1. Introduction
Uterine leiomyosarcoma (uLMS) is a rare, aggressive mesenchymal tumor that is typically diagnosed postoperatively following hysterectomy for presumed benign uterine leiomyomas (Giuntoli et al., 2003). The surgical stage of uLMS guides treatment. Early-stage disease is generally followed with postsurgical observation, whereas patients with advanced disease are offered adjuvant chemotherapy with or without radiation (Benedet et al., 2000). Unfortunately, due to the paucity of molecular or other predictors to help identify which cases of early stage uLMS will recur, staging systems have limited utility in predicting prognosis. uLMS has high risk of recurrence and poor prognosis in all stages despite treatment. If possible, recurrences can be treated with complete gross resection (Benedet et al., 2000); however, limited treatment options exist given inadequate study attrition rates and low prevalence. Prognosis after a recurrence remains dismal, and only 50% of patients will live more than 12 months following recurrence (Arend et al., 2018); therefore, potential targeted therapies should be further investigated.
One such therapy targets poly(ADP-ribose) polymerase 1 (PARP1) by manipulating its role in DNA damage response (Mateo et al., 2019, Lee and Konstantinopoulos, 2020). Studies by Farmer et al. and Bryant et al. showed PARPi potential in the treatment of BRCA 1/2 (BReast CAncer genes 1 and 2)-defective cancers (Bryant et al., 2005, Farmer et al., 2005 Apr 14). PARPi are effective in tumor cells that lack BRCA1/2 tumor suppressor proteins by way of synthetic lethality: loss of either BRCA or PARP is not lethal, but concomitant inactivation leads to apoptosis (Mateo et al., 2019). PARP inhibition increases unrepaired SSBs, which are processed by replication to double-stranded breaks (DSBs) that, due to lack of functional BRCA1/2, cannot be repaired by homologous recombination. DSBs are further unable to be repaired by alternative error-prone non-homologous end joining, which is reliant on PARP1 (Lee and Konstantinopoulos, 2020). However, in the presence of BRCA1/2 deficiency, the impaired HR and accumulation of DSB causes synthetic lethality and cell death (Mateo et al., 2019, Bryant et al., 2005, Farmer et al., 2005 Apr 14).
The clinical significance of both germline and somatic BRCA mutations has been explored in the literature. The SOLO-3 trial was conducted in patients with germline BRCA-mutated platinum sensitive or partially platinum-sensitive relapsed ovarian cancer (Penson et al., 2020). This study demonstrated a statistically significant improvement in objective response rate (ORR) and progression-free survival (PFS) in patients who received Olaparib compared to patients that received nonplatinum chemotherapy (Penson et al., 2020). A case series found that 10% of uLMS tumors had a BRCA2 alteration (Seligson et al., 2019). Given the substantial incidence of BRCA2 alteration in uLMS tumors, patients with uLMS should be considered for germline and somatic BRCA1/2 testing (Seligson et al., 2019). Another phase II clinical trial is underway and will examine the use of Temozolomide and Olaparib for the treatment of advanced uterine leiomyosarcoma (Ingham et al., 2020). Preliminary data demonstrated an overall response rate (ORR) within six months of initiating treatment was 23% (Ingham et al., 2020). Correlative assays are being performed to evaluate whether tumors with homologous recombination deficiency or BRCA 1/2 are most sensitive to Temozolomide and Olaparib and may allow a durable response. Therefore, using next-generation sequencing (NGS), PARPi’s may have a role in treating a select group of gynecologic malignancies lacking BRCA1/2. Here we report a case of recurrent advanced stage uLMS treated with Olaparib after the identification of a somatic BRCA2 mutation by NGS. Given the current data on the treatment of uLMS with PARPi’s is scarce and insufficient, this report provides insight on an unmet need for treating uLMS tumors with somatic BRCA 1/2 mutations.
2. Case description
A 50-year-old female initially presented to her gynecologist in May 2018 with abnormal uterine bleeding (AUB) for 3 weeks duration with interval bleeding of small clots. The endometrial biopsy performed at that time demonstrated proliferative endometrium with no evidence of atypia or malignancy. The patient’s AUB was uncontrolled with prescribed Norethindrone Acetate. She later presented to the Emergency Department for worsening vaginal bleeding, requiring intravenous conjugated estrogens and subsequent transition to Megestrol Acetate. Ultrasound at the time demonstrated multiple uterine leiomyomas with the largest measuring 5.5 cm in its greatest dimension. The patient desired definitive surgical management after failing multiple medical regimens.
She then underwent total laparoscopic hysterectomy with bilateral salpingectomy requiring morcellation in a specimen bag in August 2018. Operative report confirms there was no gross contamination of the peritoneal cavity. Final surgical pathology showed leiomyosarcoma presumed stage 1B. Computed tomography (CT) scan of the abdomen and pelvis post-operatively demonstrated no evidence of metastases.
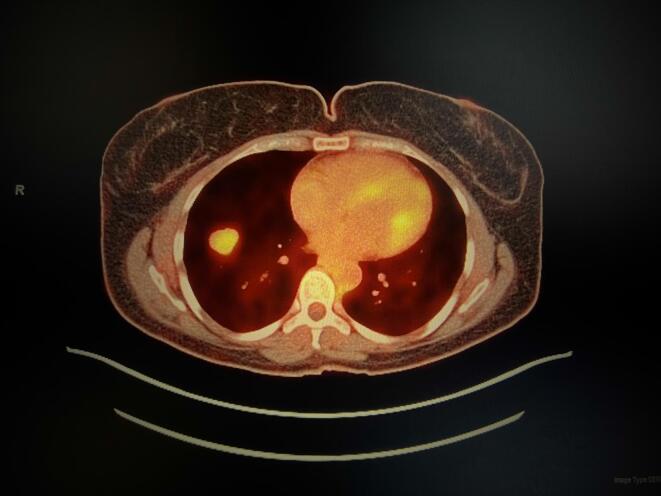
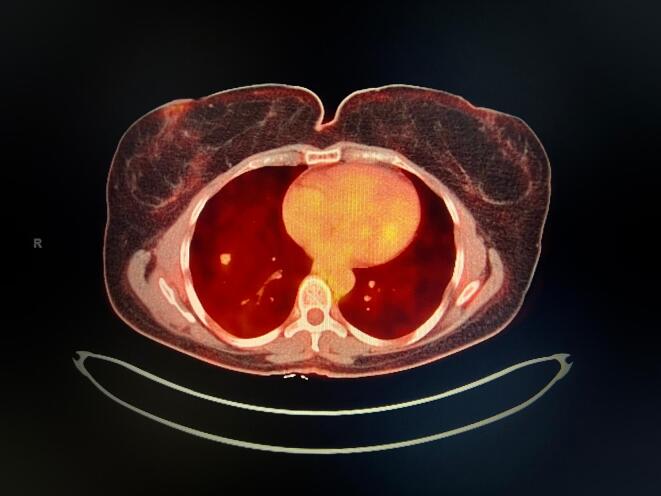
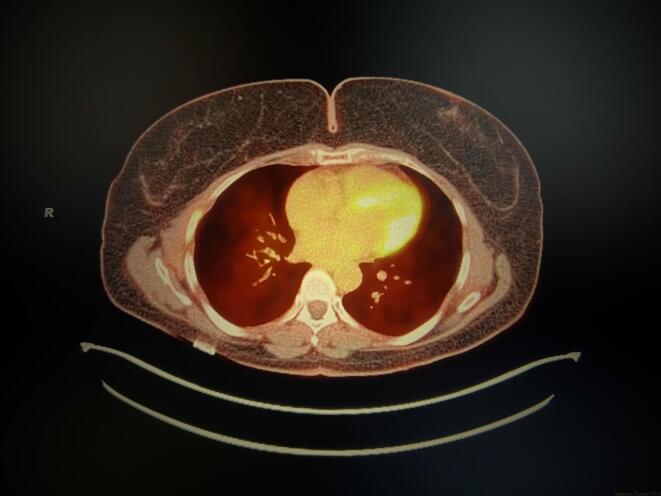
Patient was counseled on post-operative observation versus adjuvant chemotherapy. She opted to undergo chemotherapy and completed 6 cycles of Gemcitabine and Docetaxel in January 2019. CT scan of the chest, abdomen, and pelvis showed no evidence of disease in March 2019. Unfortunately, in June 2019, the patient developed a new 6 mm medial right upper lobe lung nodule discovered on CT scan which was confirmed on positron emission tomography (PET)/CT. She then underwent right upper lobe wedge resection and the pathology resulted as metastatic leiomyosarcoma with negative margins. The patient chose to undergo observation. In September 2019, surveillance CT scan demonstrated a 2.3-centimeter soft tissue nodule in the right lower lobe adjacent to the diaphragm and two soft tissue masses on the vaginal cuff. One soft tissue mass was noted in the left vaginal cuff measuring 3.4 × 3.2 cm, and the second was identified in the right vaginal cuff measuring 3.0 × 2.8 cm. PET/CT confirmed the interval appearance of the new large right lower lobe lung nodule with SUV max 5.7 (Fig. 1) and also showed hypermetabolic foci in the vaginal cuff and right inguinal nodes highly suspicious for recurrent disease with SUV max 12.2. The patient also complained of new onset vaginal bleeding and the vaginal cuff recurrence was confirmed on biopsy. The tumor was confirmed positive for a BRCA2 mutation by NGS; subsequent germline genetic testing was preformed, and the patient was negative for a BRCA1/2. In October 2019, the patient then underwent radiation therapy and had a total of 5000 cGy for the lung metastasis and 3500 cGy in 10 fractions at 350 cGy for the vaginal cuff recurrence. The patient was thoroughly counseled and declined chemotherapy. She was started on Olaparib 300 mg (mg) twice daily in December 2019 following the identification of BRCA2 deep deletion. The only adverse effects noted by the patient was grade one nausea. No dose modifications were needed. PET/CT in February 2020 showed interval decrease in size of the right lower lobe nodule (Fig. 2) now measuring 1.6 cm, SUV max 2.3, previously 5.7 and minimal-to-mild residual flurorine-18-fluorodeoxyglucose uptake (FDG) activity at the vaginal cuff, SUV max of 2.7, previously 12.2. The most recent imaging in March 2022 continues to show no FDG activity in the lung parenchyma (Fig. 3) and vaginal cuff consistent with a complete response of the target lesions.
Fig. 1.
PET/CT demonstrating the interval appearance of a 2.3 cm right lower lobe lung nodule with SUV max 5.7.
Fig. 2.
PET/CT showed interval decrease in size of the right lower lobe nodule now measuring 1.6 cm with SUV max 2.3.
Fig. 3.
PET/CT reveals no FDG activity in the lung parenchyma.
3. Discussion
This patient’s disease course reiterates the aggressive nature of uLMS and introduces the novel use of PARPi in recurrent uLMS. After initiation of Olaparib, this patient demonstrated prolonged response and is now disease-free for almost two years. Various preclinical studies have demonstrated the use of PARPi to treat uLMS (Seligson et al., 2019 Jul, Ingham et al., 2020, Hensley et al., 2020 Jul 15, Pan et al., 2021 May, Oza et al., 2020 Dec). To the best of our knowledge, only three other studies have been published corroborating results of ongoing stable disease after PARPi in uLMS (Ingham et al., 2020, Pan et al., 2021 May, Oza et al., 2020 Dec).
There are no randomized phase III trials that reveal any impact of adjuvant radiation therapy on progression free or overall survival in patients with uterine sarcomas. Reed et al., examined the role of adjuvant radiation in uterine sarcomas (Reed et al., 2008). This study demonstrated no difference in local or distant progression rates for uLMS depending on radiation treatment (Reed et al., 2008). This supports the thought that uLMS likely undergoes early hematogenous dissemination, causing lung metastases (Reed et al., 2008, Tirumani et al., 2014). This mechanism of metastasis likely explains why radiation therapy has not demonstrated a benefit in the treatment of uLMS. Our patient received radiation treatment for her second recurrence of uLMS however, based on current literature and molecular studies there is little reason to attribute her complete remission to radiation alone.
While early predictive biomarkers focused on germline BRCA1/2 mutations, next generation sequencing (NGS) assays now test for somatic BRCA1/2 mutations in DNA (Mateo et al., 2019). These deleterious mutations in BRCA1/2 result in decreased double-stranded DNA-break repair capability via the homologous recombination (HR) pathway which enables PARPi treatment (Seligson et al., 2019). Multiple studies demonstrate an increased prevalence of somatic BRCA2 mutations in patients with uLMS. A study by Seligson et al., examined frequency of gene alterations in the HR pathway among 1,236 patients with soft tissue sarcomas (STS) (Seligson et al., 2019). This study found that the overall frequency of gene alterations via HR pathway was 11%, and loss of BRCA2 was most common within the uLMS subtype (Seligson et al., 2019). Furthermore, a prospective study by Hensley et al. examined the utility of NGS in an attempt to understand survival correlations and therapeutic targets (Hensley et al., 2020). This study sequenced tumors from 80 patients with uLMS and found that loss-of-function mutations were most common (Hensley et al., 2020). Specifically, loss-of-function mutations were identified in TP53 (56%), RB1 (51%) and BRCA2 (5%) (Hensley et al., 2020). Among the patients with BRCA2 mutations, both patients had a sustained partial response to PARPi (Hensley et al., 2020). Homologous recombination deficiency (HRD) impairs normal DNA damage repair which results in loss or duplication in chromosomal regions. While HRD is common practice for determining genomic instability and presence of BRCA1/2 mutations in ovarian cancer, for the treatment of leiomyosarcoma NGS screens allows for determination of tumor mutational burden and understanding of mutations not just within BRCA1/2 but also RB1, ATRX, MAP2K4, MED12. Lastly, a case report by Pan et al., discussed a patient with metastatic uLMS who progressed on Gemcitabine-Docetaxel, Doxorubicin and Temozolomide. This patient’s tumor had a somatic BRCA2 deep deletion and subsequently rapidly responded to Olaparib (Pan et al., 2021). Drawing on the efficacy of PARPi in SOLO-3 (Penson et al., 2020) and the phase II clinical study mentioned above, Olaparib was chosen in the setting of resistant disease, highlighting the evolution of precision medicine.
We recommend that NGS be performed to identify functional BRCA1/2 loss in uLMS. PARPi may be a potential targeted therapy for uLMS. Currently, a single-arm, open-label, multi-center phase 2 clinical trial is underway to evaluate combined Olaparib and Temozolomide as treatment for advanced uterine leiomyosarcoma (Ingham et al., 2020).
Informed consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Synopsis: This article is a case report describing a rare case of recurrent, metastatic uterine leiomyosarcoma stabilized using a PARP inhibitor, Olaparib.
Credit authorship contribution statement
Natalie Shammas: Conceptualization, Writing – original draft, Writing – review & editing. Tiffany Yang: Conceptualization, Writing – original draft, Writing – review & editing. Alireza Abidi: Supervision, Conceptualization, Writing – original draft, Writing – review & editing. Malaika Amneus: Supervision, Conceptualization, Writing – original draft, Writing – review & editing. Melissa Hodeib: Supervision, Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Arend R.C., Toboni M.D., Montgomery A.M., Burger R.A., Olawaiye A.B., Monk B.J., Herzog T.J. Systemic Treatment of Metastatic/Recurrent Uterine Leiomyosarcoma: A Changing Paradigm. Oncologist. 2018 Dec;23(12):1533–1545. doi: 10.1634/theoncologist.2018-0095. Epub 2018 Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedet J.L., Bender H., Jones H., 3rd, Ngan H.Y., Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int. J. Gynaecol. Obstet. 2000 Aug;70(2):209–262. PMID: 11041682. [PubMed] [Google Scholar]
- Bryant H.E., Schultz N., Thomas H.D., Parker K.M., Flower D., Lopez E., Kyle S., Meuth M., Curtin N.J., Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005 Apr 14;434(7035):913–917. doi: 10.1038/nature03443. Erratum. In: Nature. 2007 May 17;447(7142):346. [DOI] [PubMed] [Google Scholar]
- Farmer H., McCabe N., Lord C.J., Tutt A.N., Johnson D.A., Richardson T.B., Santarosa M., Dillon K.J., Hickson I., Knights C., Martin N.M., Jackson S.P., Smith G.C., Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005 Apr 14;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Giuntoli R.L., 2nd, Metzinger D.S., DiMarco C.S., Cha S.S., Sloan J.A., Keeney G.L., Gostout B.S. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol. Oncol. 2003 Jun;89(3):460–469. doi: 10.1016/s0090-8258(03)00137-9. [DOI] [PubMed] [Google Scholar]
- Hensley M.L., Chavan S.S., Solit D.B., Murali R., Soslow R., Chiang S., Jungbluth A.A., Bandlamudi C., Srinivasan P., Tap W.D., Rosenbaum E., Taylor B.S., Donoghue M.T.A., Hyman D.M. Genomic Landscape of Uterine Sarcomas Defined Through Prospective Clinical Sequencing. Clin. Cancer Res. 2020 Jul 15;26(14):3881–3888. doi: 10.1158/1078-0432.CCR-19-3959. Epub 2020 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham, M., Allred, J.B., Gano, K., et al., 2020. NCI protocol 10250: A phase II study of temozolomide and olaparib for the treatment of advanced uterine leiomyosarcoma. Presented at: ASCO20 Virtual Scientific Program. J. Clin. Oncol.
- Lee, E.K., Konstantinopoulos, P.A., 2020. PARP inhibition and immune modulation: scientific rationale and perspectives for the treatment of gynecologic cancers. Ther. Adv. Med. Oncol. Jul 24;12:1758835920944116. doi: 10.1177/1758835920944116. [DOI] [PMC free article] [PubMed]
- Mateo J., Lord C.J., Serra V., Tutt A., Balmaña J., Castroviejo-Bermejo M., Cruz C., Oaknin A., Kaye S.B., de Bono J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019 Sep 1;30(9):1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza J., Doshi S.D., Hao L., Musi E., Schwartz G.K., Ingham M. Homologous recombination repair deficiency as a therapeutic target in sarcoma. Semin. Oncol. 2020 Dec;47(6):380–389. doi: 10.1053/j.seminoncol.2020.10.002. Epub 2020 Oct 24. [DOI] [PubMed] [Google Scholar]
- Pan M., Ganjoo K., Karam A. Rapid Response of a BRCA2/TP53/PTEN-Deleted Metastatic Uterine Leiomyosarcoma to Olaparib: A Case Report. Perm J. 2021 May;25 doi: 10.7812/TPP/20.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penson, R.T., Valencia, R.V., Cibula, D., Colombo, N., Leath, C.A. 3rd, Bidziński, M., Kim, J.W., Nam, J.H., Madry, R., Hernández, C., Mora, P.A.R., Ryu, S.Y., Milenkova, T., Lowe, E.S., Barker, L., Scambia, G., 2020. Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J. Clin. Oncol. Apr 10;38(11):1164-1174. doi: 10.1200/JCO.19.02745. Epub 2020 Feb 19. PMID: 32073956; PMCID: PMC7145583. [DOI] [PMC free article] [PubMed]
- Reed, N.S., Mangioni, C., Malmström, H., Scarfone, G., Poveda, A., Pecorelli, S., Tateo, S., Franchi, M., Jobsen, J.J., Coens, C., Teodorovic, I., Vergote, I., Vermorken, J.B.; European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer. 2008 Apr;44(6):808-18. doi: 10.1016/j.ejca.2008.01.019. Epub 2008 Apr 2. Erratum in: Eur. J. Cancer. 2008 Jul;44(11):1612. PMID: 18378136. [DOI] [PubMed]
- Seligson N.D., Kautto E.A., Passen E.N., Stets C., Toland A.E., Millis S.Z., Meyer C.F., Hays J.L., Chen J.L. BRCA1/2 Functional Loss Defines a Targetable Subset in Leiomyosarcoma. Oncologist. 2019 Jul;24(7):973–979. doi: 10.1634/theoncologist.2018-0448. Epub 2018 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirumani, S.H., Deaver, P., Shinagare, A.B., Tirumani, H., Hornick, J.L., George, S., Ramaiya, N.H., 2014. Metastatic pattern of uterine leiomyosarcoma: retrospective analysis of the predictors and outcome in 113 patients. J. Gynecol. Oncol. Oct;25(4):306-12. doi: 10.3802/jgo.2014.25.4.306. Epub 2014 Aug 5. PMID: 25142630; PMCID: PMC419530. [DOI] [PMC free article] [PubMed]