ABSTRACT.
The worldwide resurgence of tropical bed bug Cimex hemipterus beginning in the late 1990s has led to growing concern. Molecular data on pyrethroid resistance, which is essential for the control strategies, is unknown for C. hemipterus in Iran. The current study evaluated the deltamethrin resistance status of C. hemipterus by bioassay and molecular tests. Live bed bugs were collected from sleeping quarters (dormitories) in the city of Tehran and used for insecticide bioassay tests. For bioassay evaluation, mixed-sex pools of adult bugs were exposed to deltamethrin (0.025%)-treated paper. Polymerase chain reaction assay evaluated resistance-related mutations in the voltage-gated sodium channel gene (VGSC) gene of studied populations. On the basis of the bioassay test within the 48-h exposure to deltamethrin, C. hemipterus were determined to be resistant. Knockdown time ratios (KR50) in the studied populations of C. hemipterus was 5.5-fold compared with those of the C. lectularius Teh strain. DNA sequencing of the VGSC gene revealed the presence of mutations at M918I and L1014 in C. hemipterus. According to the bioassay and molecular results of current study, C. hemipterus showed a high degree of pyrethroid resistance. The application of multiple approaches including physical, biological, and chemical tests should be regarded in future bed bug control strategies.
INTRODUCTION
Bed bugs (Cimex lectularius and Cimex hemipterus) are blood-feeding ectoparasites of significant public health concern around the world.1 In recent years, bed bugs have reemerged in many countries. Bed bugs can cause serious indoor public health issues directly by nuisance biting and indirectly by hematophagic activity.2 Their bites may cause allergic responses, including itching and erythematous nuisance biting, which can cause cutaneous and systemic reactions.3 There is no evidence related to the transmission of pathogens by bed bugs to humans; however, many studies have shown that bed bugs may be a competent vector for Bartonella quintana and Trypanosoma cruzi.4 The tropical bed bug C. hemipterus and common bed bug C. lectularius are mainly confined to tropical regions and temperate climates, respectively. Today, the tropical bed bug may be seen in subtropical or stable temperature regions due to globalization, climate change, tourism, and insecticide resistance.5,6 Chemical agents in various forms, such as fumigants, powder dust, and liquids, as well as nonchemical techniques such as heat, steam, and vacuuming, have been used to manage this pest.7,8 Pyrethroids are the most widely used chemical agent to control the bed bugs. These agents possess rapid knockdown activities and low mammalian toxicity, and they are relatively low cost.8,9 However, their overuse and misuse have led to insecticide resistance in bed bugs.9 Despite numerous studies performed on common bed bug resistance to pyrethroids,6 few studies on the resistance status of the C. hemipterus have been performed. It was documented that various mutations in the voltage-gated sodium channel gene (VGSC) that cause a “knockdown resistance” (kdr) are responsible for insecticide resistance against pyrethroids.10–13 One of the key factors to increase the bed bug infestation is poor bed bug management practices. Most residents of dormitories have no effective strategies to control bed bug infestation. This study aimed to evaluate the resistance to deltamethrin in collected tropical bed bugs by bioassay tests and molecular surveys from dormitories in Iran.
MATERIALS AND METHODS
Bed bug sampling.
Bed bugs were collected from Tehran district, Iran, during 2021–2022. Live bed bugs were collected from seven sleeping quarters, and the maximum distance between these sites was 4 km. Bed bugs were collected by forceps from crevices and cracks in the furniture and walls. For morphological identification, collected bugs were transferred to the laboratory and identified under a stereomicroscope by identification keys.15,16 Blood-fed samples were used for insecticide bioassays. Bed bugs were immediately frozen and kept at –20°C for molecular tests.
Bioassay test.
The collected adult samples from dormitories were used for insecticide bioassay tests based on previous works.12 In brief, at least three groups of 10 to 15 mixed-sex pools of adult bugs were exposed to deltamethrin-treated paper (0.025%) as a test, and an identical number of collected samples were exposed to paper impregnated with silicone oil as a control group. At continuous exposure, the knockdown and death rates were recorded. The dead, knockdown, and living samples were kept at –20°C for future analysis once the experiments were completed. Bioassay data for C. lectularius Teh strain (adult) were used as a pyrethroid-susceptible reference. WHO classification was used to determine deltamethrin resistance as fallows; mortality rate > 98% indicated susceptibility, and < 98% proposed the presence of resistance.15
Detection of kdr mutations.
The genomic DNA of isolated samples was extracted using a Qiagen extraction kit (Valencia, CA). In brief, the sample cutting into small pieces with a scalpel and then homogenized in 200 µL of a lysis buffer. Subsequently, 20 µg of reconstituted proteinase K was added and then mixed immediately and incubated until the tissue was digested completely. Further steps were undertaken based on manufacturer’s instructions.
Genotyping of kdr gene.
Amplification of two VGSC regions including 513-bp and 752-bp related to kdr-type resistance to pyrethroids was performed under following conditions: 1 μL of both forward and reverse primers (10 pM), 12.5 μL of Taq DNA Polymerase 2X Master Mix RED (Ampliqon, Denmark), and 2 μL of DNA template in a total volume of 25 μL (100–150 ng/reaction). Fragment amplification was carried out using the primers BBparaF1 and BBparaR1 for the first fragment and BBparaF3 and BBparaR3 for the second regions.16 PCR for both reactions were performed under the following conditions: 95°C for 10 min for denaturation, followed by 40 cycles of 94°C for 20 seconds, 58°C for 20 seconds, and 72°C for 40 seconds, and the final extension of 70°C for 10 minutes. For detection of a point mutation at residues V410, V419, V421, and E435 at the first region and M918, L925, T929, L932, L936, and L1014 residue at the second region of VGSC gene, all the samples were sequenced (Daejeon, South Korea). The raw nucleotide sequences were aligned manually using ClustalW and analyzed by BioEdit software (version 7.0.9, Los Angeles, CA). The mutations were determined by comparing the translated sequences of samples compared with wild-type sequences (GenBank accession numbers GU123927 and GU123928) and Musca demostica (GenBank accession number AAB47604).
Statistical analysis.
The times of KT50 and KT95 in which 50% and 95% of bed bugs were paralyzed, respectively, were calculated by PriProbit version 1.63 software. Abbott’s formula was used if the control knockdown mortality was < 20%. Populations were statistically compared using chi-square test. The resistance ratio at KT50 (KR50) was calculated by comparing the rates of KT50 values of the field strains of tropical bed bugs with the corresponding knockdown time for C. lectularius Teh strain.
RESULTS
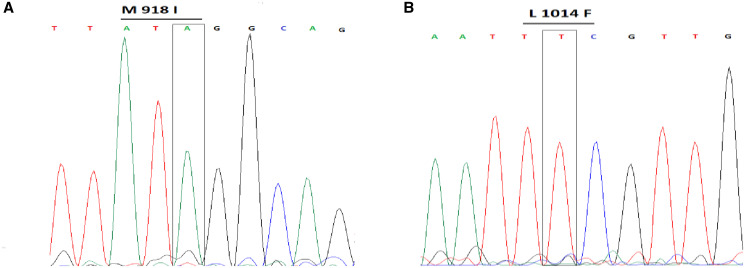
One thousand three hundred bed bugs were collected from three out of seven (42%) infested sites. On the basis of morphological identification keys, 35% and 65% of them were related to C. hemipterus and C. lectularius, respectively. From the bed bugs samples, 200 individuals of C. hemipterus were isolated randomly and used to bioassay test. On the basis of the bioassay test within the 48-h exposure to the deltamethrin, C. hemipterus were determined to be resistant. The difference between knockdown and mortality rates of C. hemipterus and C. lectularius at 24 and 48 hours were significant (P < 0.05). Knockdown and mortality rates of collected bed bugs to deltamethrin are shown in Table 1. No mortality was noted in the control bioassay experiments. KR50 indicates that resistance to deltamethrin of C. hemopterus has increased 5.5-fold compared with the common bed bug (Table 2). DNA sequencing of the domain IS6 and part of the domain I–II linker region as a first region and the domain IIS4–IIS6 as a second region of the VGSC gene revealed the presence of mutations at M918I and L1014 in C. hemipterus (Figure 1).
Table 1.
Knockdown and mortality rates of bedbugs after exposure to discriminating dosages deltamethrin
| Samples | Knockdown rate (%) | Mortality rate (%) | ||
|---|---|---|---|---|
| 24 hours | 48 hours | 24 hours | 48 hours | |
| Cimex hemipterus | 54 | 72 | 49 | 65 |
| Cimex lectolarus | 100 | 100 | 100 | 100 |
Exposure time was 24 hours. Data for the C. lectularius population are presented for comparison.
Table 2.
Time (hours) taken to knockdown 50% (KT50) and 95% (KT95) of the Cimex hemipterus population when exposed to discriminating dosages of deltamethrin
| Cimex lectularius | Cimex hemipterus | ||||
|---|---|---|---|---|---|
| Insecticide | KT50 | KT95 | KT50 | KT95 | KR50 |
| Deltamethrin | 4 | 19 | 22 | 75 | 5.5 |
KR50 = knockdown time ratios. Data from C. lectularius are presented for comparison.
Figure 1.
Chromatograms showing kdr genotypes of Cimex hemipterus. A:M 918 I, transitions with change in amino acid at sites I918 (isoleucine/I: ATA) compare with sites M918 (methionine/M: ATG); B: L1014F, transitions with change in amino acid at sites F1014 (phenylalanine/F: TTC) compare with L1014 sites (leucine/L: CTG). This figure appears in color at www.ajtmh.org.
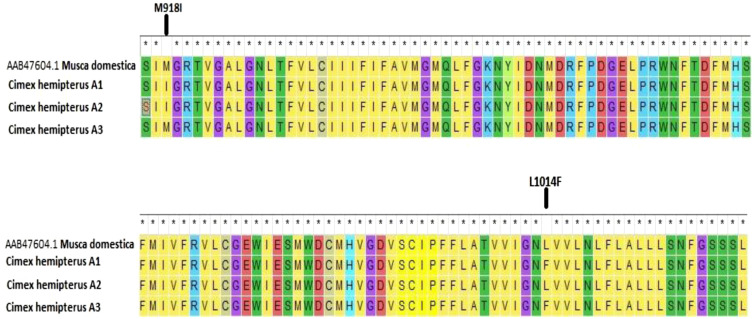
Twenty-five amplicons were randomly selected for sequencing. Kdr mutations of only M918I were observed in 12 of 25 (40.9%), M918I and L1014F in six of 25 (24%), and only L1014F in seven of 25 (28%) (Figure 2). All of these kdr mutation sites of VGSC gene seem to be associated with kdr-type resistance to pyrethroids.
Figure 2.
Gene sequences of the voltage-gated sodium channel of Cimex hemipterus aligned with that of Musca domestica (GenBank accession number: AAB47604) showing the mutations M918I and L1014F. Three haplotypes (A1, A2, and A3) were reported. This figure appears in color at www.ajtmh.org.
DISCUSSION
Many arthropods, such as mosquitoes, ticks, scabies, and fleas are major pests and threats to human health.17 So far, several insecticides have been used to control these pest species in Iran. Repeated use of these agents to control pests has led to the development of resistant.18 For many years, bed bugs were controlled in Iran through various methods, such as boiling, using minerals, biological control with fungi, and cleaning of equipment. Then, the insecticidal properties of DDT were discovered and introduced to control the pests. DDT was widely used for control of malaria vector in Iran. With the emergence of insect resistant to DDT, other insecticidal agents were introduced.19 Pyrethroids were introduced in 1994 and became the most important group of synthetic insecticides against medical pests. In the past decade, the widespread use of these compounds against malarial vectors in Iran has led to resistance in other insects, including bed bugs.20 The current research is the first attempt to investigate pyrethroid resistance in C. hemipterus isolated from several dormitory locations in Iran. Knockdown time ratios (KR50) in the studied populations of C. hemipterus was 5.5-fold compared with those of the C. lectularius Teh strain. The results showed that the resistance to deltamethrin in the studied populations has increased. The bioassay results in this study were similar to previous studies in different parts of the world.6,21,22 Thus, reconsideration of new strategy to control C. hemipterus is important in the studied areas. The previous study by Punchihewa et al. to evaluate the insecticide resistant of C. hemipterus to deltamethrin in Sri Lanka showed a high percentage of resistance among 2016 isolated strain compared with susceptible strains of 2002, which were the same as our results.24
The C. lectularius Teh strain was used as an insecticide-susceptible control sample because no susceptible strain of C. hemipterus could be sourced. Similar use of this strain as a reference has been made in other investigations.12,18
The main point mutations related to insecticidal resistant in a range of important agricultural pests and disease vector to pyrethroids found in domain IS6 and part of the domain I–II linker region as a first region (V410,V419,V421, and E435) and the domain IIS4–IIS6 as a second region of the VGSC gene (M918, L925, T929, L932, and L1014).23,24 Another recent study has introduced novel mutations of C. hemipterus, including L899V and D953G.9 The first characterization of the VGSC gene of both resistant and susceptible strains of C. lectularius was established in United States,25 and then many similar studies were accomplished in different parts of the world.10,11,20,24 On the basis of the current study, three haplotypes, including haplotype A (only M918I; 40.9% [12/25]), B (M918I and L1014F; 24% [6/25]), and C (L1014F; 28% [7/25]) were observed. L1014F mutation was observed for the first time from housefly Musca domestica, and then it was determined as super-kdr mutation from other insect pest including bed bugs.16 The appearance of super-kdr mutation including M918I and L1014F from this study emphasizes the possibility of expansion of pyrethroid resistance isolate in Iran. Similar to our study, M918I and L1014F mutations were observed in another public health pest.9,16,26,27
CONCLUSION
The application of effective insecticides, as well as consideration of biological and socioeconomic risk factors and the side effects of current public pest control measures, need to be considered in the establishment of revised control strategies. On the basis of the bioassay and molecular results of the current study, C. hemipterus has shown a high degree of resistance to pyrethroid. The application of multiple approaches, including physical, biological, and chemical tests, should be regarded in future bed bug control.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1. Doggett SL Dwyer DE Peñas PF Russell RC , 2012. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 25: 164–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doggett SL Geary MJ Russell RC , 2004. The resurgence of bed bugs in Australia: with notes on their ecology and control. Environ Health 4: 30–38. [Google Scholar]
- 3. Zorrilla-Vaca A Silva-Medina MM Escandón-Vargas K , 2015. Bedbugs, Cimex spp.: their current world resurgence and healthcare impact. Asian Pac J Trop Dis 5: 342–352. [Google Scholar]
- 4. Leulmi H Bitam I Berenger JM Lepidi H Rolain JM Almeras L Raoult D Parola P , 2015. Competence of Cimex lectularius bed bugs for the transmission of Bartonella quintana, the agent of trench fever. PLoS Negl Trop Dis 9: e0003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suwannayod S Chanbang Y Buranapanichpan S , 2010. The life cycle and effectiveness of insecticides against the bed bugs of Thailand. Southeast Asian J Trop Med Public Health 41: 548. [PubMed] [Google Scholar]
- 6. Tawatsin A Thavara U Chompoosri J Phusup Y Jonjang N Khumsawads C Bhakdeenuan P Sawanpanyalert P Asavadachanukorn P Mulla MS , 2011. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol 48: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 7. Davies T Field L Williamson M , 2012. The re‐emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 26: 241–254. [DOI] [PubMed] [Google Scholar]
- 8. Elliott M Janes N , 1978. Synthetic pyrethroids—a new class of insecticide. Chem Soc Rev 7: 473–505. [Google Scholar]
- 9. Dang K Doggett SL Singham GV Lee C-Y , 2017. Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp. (Hemiptera: Cimicidae). Parasit Vectors 10: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akhoundi M Chebbah D Sereno D Marteau A Jan J Bruel C Elissa N Izri A , 2021. Widespread mutations in voltage-gated sodium channel gene of Cimex lectularius (Hemiptera: Cimicidae) populations in Paris. Int J Environ Res Public Health 18: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dang K Toi CS Lilly DG Bu W Doggett SL , 2015. Detection of knockdown resistance mutations in the common bed bug, Cimex lectularius (Hemiptera: Cimicidae), in Australia. Pest Manag Sci 71: 914–922. [DOI] [PubMed] [Google Scholar]
- 12. Ghavami MB, Ghahremani Z, Raeisi N, Taghiloo B, 2021. High levels of pyrethroid resistance and super-kdr mutations in the populations of tropical bed bug, Cimex hemipterus, in Iran. Parasites Vectors 14: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Myamba J Maxwell C Asidi A Curtis C , 2002. Pyrethroid resistance in tropical bedbugs, Cimex hemipterus, associated with use of treated bednets. Med Vet Entomol 16: 448–451. [DOI] [PubMed] [Google Scholar]
- 14. Bennett GW Gondhalekar AD Wang C Buczkowski G Gibb TJ , 2016. Using research and education to implement practical bed bug control programs in multifamily housing. Pest Manag Sci 72: 8–14. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization , 2016. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. Geneva, Switzerland: WHO. [Google Scholar]
- 16. Dang K Toi CS Lilly DG Lee CY Naylor R Tawatsin A Thavara U Bu W Doggett SL , 2015. Identification of putative kdr mutations in the tropical bed bug, Cimex hemipterus (Hemiptera: Cimicidae). Pest Manag Sci 71: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 17. Vazirianzadeh B Dehghani R Mehdinejad M Sharififard M Nasirabadi N , 2014. The first report of drug resistant bacteria isolated from the brown-banded cockroach, Supella longipalpa, in Ahvaz, south-western Iran. J Arthropod-Borne Dis 8: 53. [PMC free article] [PubMed] [Google Scholar]
- 18. Kassiri H Dehghani R Doostifar K Rabbani D Limoee M Chaharbaghi N , 2020. Insecticide resistance in urban pests with emphasis on urban pests resistance in Iran: a review. Entomol Appl Sci Lett 7: 32–54. [Google Scholar]
- 19. Vatandoost H Raeisi A Saghafipour A Nikpour F Nejati J , 2019. Malaria situation in Iran: 2002–2017. Malar J 18: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samiei A Tavassoli M Mardani K , 2020. Molecular analysis of pyrethroid resistance in Cimex hemipterus (Hemiptera: Cimicidae) collected from different parts of Iran. Veterinary Research Forum: Faculty of Veterinary Medicine, Urmia University, Urmia, Iran, 243. [DOI] [PMC free article] [PubMed]
- 21. Lilly DG Dang K Webb CE Doggett SL , 2018. Are Australian field‐collected strains of Cimex lectularius and Cimex hemipterus (Hemiptera: Cimicidae) resistant to deltamethrin and imidacloprid as revealed by topical assay? Austral Entomol 57: 77–84. [Google Scholar]
- 22. Punchihewa R de Silva WPP Weeraratne TC Karunaratne SP , 2019. Insecticide resistance mechanisms with novel ‘kdr’ type gene mutations in the tropical bed bug Cimex hemipterus . Parasit Vectors 12: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rinkevich FD Du Y Dong K , 2013. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol 106: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vander Pan A, Kuhn C, Schmolz E, von Samson-Himmelstjerna G, Krücken J, 2020. Detection of target-site and metabolic resistance to pyrethroids in the bed bug Cimex lectularius in Berlin, Germany11Note: Supplementary data associated with this article. International Journal for Parasitology: Drugs and Drug Resistance 14: 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon KS Kwon DH Strycharz JP Hollingsworth CS Lee SH Clark JM , 2008. Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae). J Med Entomol 45: 1092–1101. [DOI] [PubMed] [Google Scholar]
- 26. Biduda S, Lin C, Saleh F, Konradsen F, Hansson H, Schiøler K L, Alifrangis M (2019). Temporal Pattern of Mutations in the Knockdown Resistance (kdr) Gene of Aedes aegypti Mosquitoes Sampled from Southern Taiwan. The American Journal of Tropical Medicine and Hygiene 101 (5): 973–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soderlund DM , 2012. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol 86: 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]