ABSTRACT.
Nasarawa and Plateau states of north-central Nigeria have implemented programs to control schistosomiasis (SCH) and soil-transmitted helminths (STH) in children since the 1990s. Statewide mapping surveys were conducted in 2013, when 11,332 school-aged children were sampled from 226 schools. The local government areas (LGAs) then received varying combinations of mass drug administration (MDA) for the next 5 years. We revisited 196 (87%) schools in 2018 plus an additional six (202 schools in total), sampling 9,660 children. We calculated overall prevalence and intensity of infection and evaluated associations with gender; age; behaviors; water, sanitation, and hygiene (WASH); and treatment regimen. Urine heme detection dipsticks were used for Schistosoma hematobium in both surveys, with egg counts added in 2018. Stool samples were examined by Kato-Katz for Ascaris lumbricoides, Trichuris trichiura, Schistosoma mansoni, and hookworm. Schistosomiasis prevalence among sampled students dropped from 12.9% (95% confidence interval [CI]: 11.1–14.9%) to 9.0% (95% CI: 7.5–10.9%), a statistically significant change (P < 0.05). In 2018, eight LGAs still had > 1% of children with heavy-intensity schistosome infections. Prevalence of STH infection did not significantly change, with 10.8% (95% CI: 9.36–12.5%) of children positive in 2013 and 9.4% (95% CI: 8.0–10.9%) in 2018 (P = 0.182). Heavy-intensity STH infections were found in < 1% of children with hookworm, and none in children with A. lumbricoides or T. trichiura in either study. The WASH data were collected in 2018, indicating 43.6% of schools had a latrine and 14.4% had handwashing facilities. Although progress is evident, SCH remains a public health problem in Nasarawa and Plateau states.
INTRODUCTION
An estimated 1.5 billion people around the world have infections with soil-transmitted helminths (STHs) specifically Ascaris lumbricoides, Trichuris trichiura, or either hookworm: Necator americanus and Ancylostoma duodenale.1 Schistosomiasis (SCH), caused by Schistosoma mansoni or Schistosoma hematobium, has been estimated to affect more than 200 million people.2,3 School-aged children (ages 5–14 years) often carry the most burden of these infections, which contribute to malnourishment, anemia, and other morbidities.4 Nigeria is home to the largest number of people in the world in need of treatment of SCH (> 25 million),5 and the fourth-largest number of children in need of treatment of STHs (> 48 million).6
Nasarawa and Plateau states are located in north-central Nigeria and consist of 30 local government areas (LGAs, or administrative districts) across both states, 13 in Nasarawa and 17 in Plateau. Their combined population in 2018 was around 7 million. The SCH control program in these states began in 1999, focusing on urinary SCH. Initial mapping in 1999 in two LGAs found dipstick positivity (a marker for S. hematobium infections) in children ranged from 0% to 87%.7 The situation was similar 5 years later, when studies in 13 LGAs found village dipstick positivity ranging from 0% to 100%, with one LGA above 50% overall prevalence.8 These results indicated praziquantel mass drug administration (MDA) was needed.9 However, limited funds and medicines led to relatively small proportions of the population receiving treatment, reaching no more than 200,000 children per year from 1999 through 2007, a total of 1.15 million doses, either via stand-alone treatment or integrated into the MDAs of onchocerciasis and/or lymphatic filariasis (LF) programs.10 These treatments were effective at reducing hematuria.7 This changed in 2008 with a major drug donation by E-Merck through the WHO, allowing treatment to expand in frequency, reach, and to target intestinal as well as urinary SCH.11 From 2008 onward, more than 1.1 million treatments were administered each year.
Soil-transmitted helminths were addressed via the LF program’s use of albendazole and ivermectin in community-wide MDA, which took place throughout the two states. Although some LGAs began MDA in 2000 and some ended in 2009, most were treated from 2003 to 2012.12,13 At that time, both states had reached the point where they could stop MDA for LF and transitioned the STH program to focus on school-aged children using the drug mebendazole. Out of the 30 LGAs, 12 were endemic for onchocerciasis and continued annual community-wide MDA with ivermectin through 2017.14 As the LF program ended completely in 2013, it was important to establish future treatment needs; intestinal and urinary SCH were assessed at the same time in widespread school-based surveys.
Based on the prevalence results in the 2013 survey, LGAs (the administrative units for treatment decisions) were stratified to receive MDA either annually or every other year for SCH and STHs between 2013 and 2018. All 30 LGAs received mebendazole for STH in 2014, 2016, and 2018, whereas 11 (37%) received it every year. All 30 LGA received praziquantel in 2013, 2014, 2016, and 2018; 6 (20%) received it every year. Only one LGA, Karu in Nasarawa state, received both drugs each year. More detailed treatment histories are available in Supplemental Figure 1. Administratively reported coverage was generally above 90% among school-age children but dropped as low as 61% for STH in one round because of shortage of mebendazole tablets. Net school enrollment rates at the state level were between 53.2% and 62.4% in the study years,15,16 but this information was not available for the sampled schools and surrounding communities.
The 2018 follow-up survey was conducted in the same schools to measure any changes in disease burden and to determine whether the MDA frequency in the various LGAs should change. This study presents the results of the 2013 surveys along with the 2018 surveys and offers conclusions regarding the impact of MDA during those years.
MATERIALS AND METHODS
Ethics.
Ethical clearance was obtained from the Federal Ministry of Health, Nigeria, after submitting the survey proposal, which contained the study plan, consent/assent forms, and permission forms from parents/guardians and the head teacher. On arrival in the school, the consent of the school was sought after which the assents of the selected pupils were recorded.
Study design.
The design of this study was to compare samples taken from schoolchildren in 2013 and 2018 to determine changes in prevalence and intensity of A. lumbricoides, T. trichiura, hookworm and S. mansoni, and in prevalence of S. hematobium. The 2013 survey was conducted in April–June, whereas that of 2018 was June–July. The 2013 MDA was July–November, whereas that of 2018 was August–November.
In 2013, the state Ministries of Health and Education provided lists of schools in each LGA. From this list, seven to nine schools were randomly selected from each of the 30 LGAs for a total of 220 schools. Six schools were then added because they had shown high rates of positivity during heme dipstick testing between 1999 and 2007 (unpublished data). During analysis, these “high-risk” schools were placed in their own stratum as they were not randomly selected to represent the schools from the original sampling frame. During the follow-up study in 2018, security issues prevented sampling at 20 schools; we therefore surveyed 196 of the 226 schools from the 2013 sample, randomly selecting six additional schools to balance the sample. The 2018 sample included the same six high-risk schools as in 2013. Approximately, 50 children aged 6–14 years were selected per school in each time point.
A sample size of 50 individuals of both sexes (about 50% male and 50% female) was intended. The students aged 6–14 years in the selected schools were assembled in two lines, one for boys and one for girls. Children were selected systematically from within these two lines, using a sampling interval derived from the target sample size and the number of children present.
Sample collection and analysis.
Each selected pupil was given two specimen bottles, one each for stool and urine. The stool samples were processed using the Kato-Katz technique. Briefly, samples were processed in the mobile laboratory by collecting approximately 40–45 mg of stool in a plastic template using a spatula over a nylon mesh. Hydrophilic cellophane soaked in glycerol malachite green solution was used as a cover slip on the stool sample placed on a glass slide. Duplicate slides were done for stool samples and read by the same microscopist for egg counts, with the average eggs per slide taken to calculate the eggs per gram (epg) of stool for each organism. Infection was defined as the presence of at least one egg of A. lumbricoides, T. trichiura, S. mansoni, N. americanus, or A. duodenale.
Urine samples were first tested with a dipstick (Combi-9), which was read and graded within 60 seconds according to the manufacturer’s guideline. Ten percent of dipstick-positive urine samples were immediately preserved in formalin for later microscopy. Urine filtration was used for egg quantification and examination by wet mount. Ten milliliters of urine were drawn from the specimen bottle into a 10-mL syringe. The gasket with a membrane filter was placed securely at the tip of the syringe. Urine was then flushed through the gasket. The membrane was retrieved with forceps and placed on a clean slide, to which one to two drops of lugols iodine solution was added, then covered with a slip. This was viewed under the microscope to count any eggs of S. hematobium.
We categorized infections according to the WHO guidance.17 Thresholds for heavy-intensity infections were ≥ 400 epg of stool for S. mansoni, ≥ 50,000 for A. lumbricoides, ≥ 4,000 for hookworm, and ≥ 10,000 for T. trichuria. As urine filtration and egg counting were not done in 2013, we can only assess intensity of infection of S. hematobium in 2018; heavy infections were set at ≥ 50 eggs per 10 mL of urine.
Dipstick tests were categorized as negative, +, ++, and +++ in 2013. “Trace” was added between negative and + in 2018. Schistosomiasis cases were defined as any of the following: dipstick result of “Trace” or higher, any S. hematobium eggs found in the urine sample, or any S. mansoni eggs in the stool sample. Schistosome infections were defined in 2013 as having the presence of hematuria or S. mansonie eggs in the stool; this definition expanded in 2018 to include the presence of S. hematobium eggs in urine. A child with a positive result by any of these tests was said to be SCH positive. Soil-transmitted helminth infection was defined as the presence of A. lumbricoides, T. trichiura, or hookworm in the stool. A child with one or more of these organisms in their stool was said to be STH positive.
Data collection and analysis.
Data were collected on Android tablets using Open Data Kit-based applications and cloud-based databases, namely, the LINKS system in 2013 and NEMO (https://getnemo.org/) in 2018.18 Standardized forms were used to gather information about the school and the individual student. Data on water, sanitation, and hygiene (WASH) facilities were collected in this study for 198 of 202 schools in 2018.
School- and student-level questionnaires were administered in 2018. Data collectors observed the water and sanitation infrastructure at the school and reviewed its treatment records. Students were asked about their behaviors regarding water, urination, and defecation. Interviewers observed whether they were wearing shoes. In addition, the survey team inspected and graded water and sanitation facilities at the surveyed schools using questionnaires provided by the Federal Ministry of Health.
Data from both surveys were consolidated in Microsoft Excel (Microsoft Corp, Redmond, WA) and then analyzed in STATA v. 15.1 (StataCorp LLC, College Station, TX). We used procedures for complex survey designs where indicated, using the school as the primary sampling unit, stratified by LGA with self-representing high-risk schools placed into their own strata. We examined the data for possible associations between SCH or STH and the presence of WASH facilities at schools, controlling for treatment regimen, age, gender, and high-risk behaviors reported by students (e.g., activities involving water for SCH, and not wearing shoes for STH). Single- and multivariate models were created with purposively chosen variables, testing for associations with logistic regression. Comparisons between proportions were made with χ2 tests, using postestimation procedures to account for the survey design.
RESULTS
Study population.
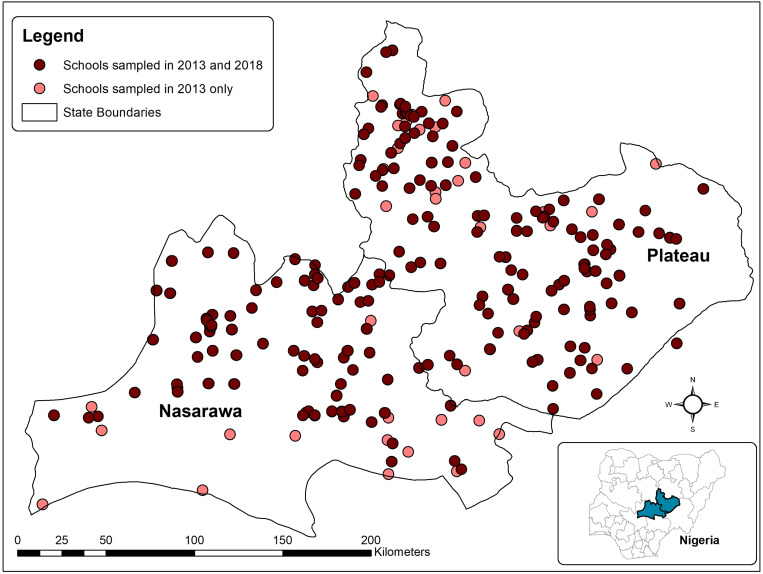
The locations of schools sampled in both years are shown in Figure 1. A total of 20,992 children were sampled across the two studies, 11,332 aged 6–15 years in 2013 (15-year-olds were sampled if they were in the same class as 14-year-olds) and 9,660 aged 6–14 years in 2018. There were more boys in both the samples (52.3% in 2013 and 51.2% in 2018), and the mean age was slightly younger in 2018 than in 2013 (Table 1). Of note, 425 (3.6%) children in 2013 and 14 (0.1%) in 2018 declined a stool test. All children received a dipstick test in 2013, and all but 6 of the 9,660 sampled children were tested by dipstick and urine filtration in 2018 (Table 2).
Figure 1.
Locations of sampled schools in Nasarawa and Plateau states, Nigeria. This figure appears in color at www.ajtmh.org.
Table 1.
Participant demographics in 2013 and 2018 evaluations in two central Nigerian states
| Nasarawa | Plateau | Overall | ||||
|---|---|---|---|---|---|---|
| Demographic | 2013 | 2018 | 2013 | 2018 | 2013 | 2018 |
| Schools visited | 96 | 79 | 130 | 123 | 226 | 202 |
| Children sampled | 4,872 | 3,768 | 6,460 | 5,892 | 11,332 | 9,660 |
| Proportion female | 45.7% | 48.4% | 49.3% | 48.2% | 47.7% | 48.3% |
| Mean years of age | 11.4 | 10.3 | 11.3 | 10.4 | 11.4 | 10.4 |
| Primary school attendance | 71.0% | 70.2% | 63.1% | 72.6% | n/a | n/a |
| Secondary school attendance | 53.7% | 62.4% | 53.2% | 51.8% | n/a | n/a |
The participant age range was 6–15 years in 2013 and 6–14 years in 2018. School attendance is drawn from net attendance ratios in the corresponding Demographic and Health Surveys (DHS).
Table 2.
Test results for Schistosoma hematobium by diagnostic method
| Dipstick result | No egg filtration done (2013 only) | Negative by egg count (2018 only) | 1–49 eggs/10 mL urine (2018 only) | ≥ 50 eggs/10 mL urine (2018 only) | Missing egg count (2018 only) | Total |
|---|---|---|---|---|---|---|
| Negative | 9,969 | 9,174 | 15 | 0 | – | 19,158 |
| Trace | – | 88 | 31 | 4 | – | 123 |
| + | 340 | 61 | 52 | 12 | – | 465 |
| ++ | 348 | 52 | 59 | 15 | – | 474 |
| +++ | 262 | 40 | 31 | 20 | – | 353 |
| Missing | 413 | 0 | 0 | 0 | 6 | 419 |
| Total | 11,332 | 9,415 | 118 | 51 | 6 | 20,992 |
Prevalence of infection.
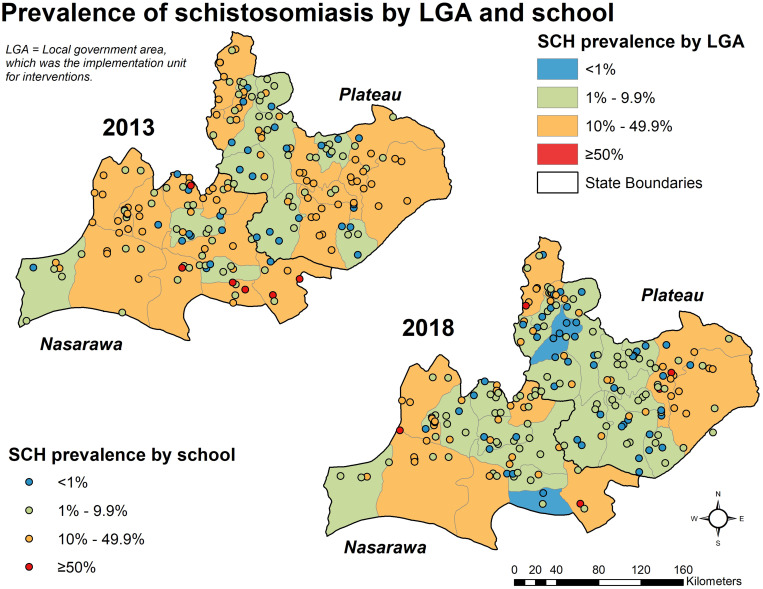
Schistosomiasis—S. hematobium or S. mansoni—prevalence among all sampled students dropped from 12.9% (95% confidence interval [CI]: 11.1–14.9%, design effect 3.0) in 2013 to 9.0% (95% CI: 7.5–10.9%, design effect 3.0) in 2018, a statistically significant change (P < 0.05) (Table 3). Nasarawa state dropped over 4% points, from 15.0% (95% CI: 11.9–18.6%) to 10.6% (95% CI: 8.1–13.8%), whereas Plateau state’s change was more modest, from 11.3% (95% CI: 9.4–13.6%) to 8.0% (95% CI: 6.1–10.4%). However, Plateau’s change in prevalence was statistically significant (P = 0.04). Both increases and decreases in SCH prevalence at the LGA and school levels were observed (Figure 2).
Table 3.
Prevalence of schistosomiasis and STH infections and distribution of causative by organisms in Nigerian children
| Organism | 2013 Prevalence estimate (95% CI) | 2018 Prevalence estimate (95% CI) |
|---|---|---|
| Among all children | ||
| Schistosomiasis | 12.9% (11.1–14.9%) | 9.0% (7.4–10.9%) |
| Only Schistosoma hematobium | 8.4% (6.8–10.2%) | 4.7% (3.7–6.0%) |
| Only Schistosoma mansoni | 4.7% (3.7–5.9%) | 4.1% (2.9–5.6%) |
| Both schistosomiasis species | 0.3% (0.2–0.6%) | 0.2% (0.1–0.4%) |
| Soil-transmitted helminths | ||
| Only Ascaris lumbricoides | 1.3% (1.0–1.6%) | 0.9% (0.7–1.3%) |
| Only hookworm | 10.0% (8.5–11.8%) | 8.5% (7.1–10.2%) |
| Only Trichuris trichiura | 0.1% (0.0–0.2%) | 0.1% (0.0–0.2%) |
| More than one organism | 0.2% (0.1–0.3%) | 0.2% (0.1–0.4%) |
| Causative organisms | ||
| Schistosomiasis infections | N = 1,461 | N = 873 |
| Only S. hematobium | 62.6% (54.9–69.8%) | 52.3% (42.3–62.2%) |
| Only S. mansoni | 35.0% (28.0–42.6%) | 45.0% (35.2–55.3%) |
| Both schistosomiasis species | 2.4% (1.4–4.2%) | 2.6% (1.6–4.2%) |
| STH infections | N = 1,225 | N = 904 |
| Only A. lumbricoides | 11.7% (9.3–14.6%) | 10.5% (7.6–14.3%) |
| Only Hookworm | 89.2% (86.2–91.6%) | 91.0% (87.8–93.5%) |
| Only T. trichiura | 0.8% (0.4–1.5%) | 1.3% (0.7–2.6%) |
| More than one organism | 1.7% (1.1–2.7%) | 2.5% (1.6–4.1%) |
CI = confidence interval.
Figure 2.
Prevalence of schistosomiasis by LGA and school. This figure appears in color at www.ajtmh.org.
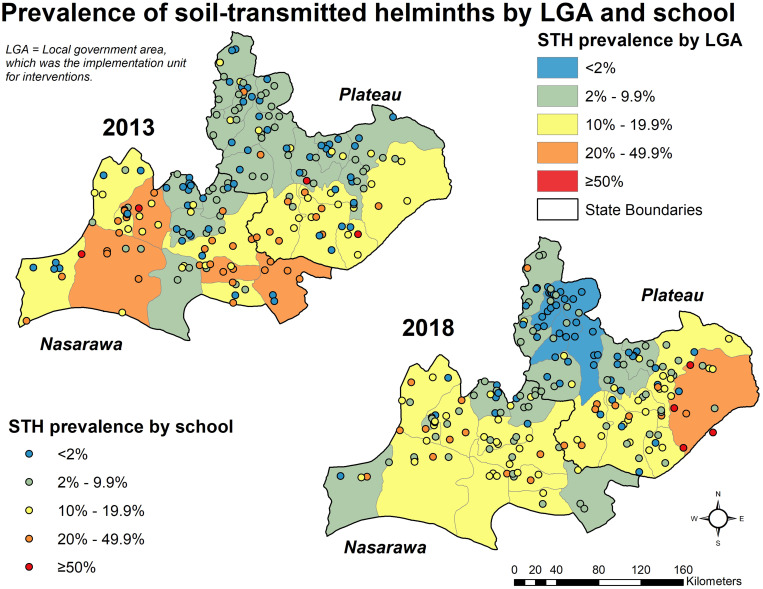
Prevalence of STH infection (any of the three main organisms) did not show statistically significant reductions, with 10.8% (95% CI: 9.36–12.5%, design effect 2.9) of children positive in 2013 and 9.4% (95% CI: 8.0–10.9%, design effect 2.6) positive in 2018 (P = 0.182). By state, Nasarawa saw a slight drop from 14.5% (95% CI: 11.7–17.9%) to 11.2% (95% CI: 9.1–13.6%), whereas Plateau state remained steady from 8.0% (95% CI: 6.5–9.8%) in 2013 to 8.2% (95% CI: 6.3–10.5%) in 2018. Neither of these changes were statistically significant (P > 0.05). A mix of increases and decreases in STH prevalence were seen (Figure 3).
Figure 3.
Prevalence of soil-transmitted helminths by LGA and school. This figure appears in color at www.ajtmh.org.
In 2013, the “high-risk schools” had significantly higher rates of any schistosome infection than the randomly selected schools, 31.3% (95% CI: 17.7–52.9%) versus 12.3% (95% CI: 10.6–14.2%, P = 0.01). In 2018, these high-risk schools saw a 21%-point reduction in SCH infection from 31.3% to 10.2% (P = 0.03), and the difference with randomly selected schools disappeared; 10.2% (95% CI: 4.4–21.9%) in the high-risk group versus 9.0% (95% CI: 7.4–10.9%, P = 0.79). Schistosoma hematobium accounted for 78.1% and 87.1% of infections detected in high-risk schools at each timepoint.
Two LGAs saw statistically significant decreases in any schistosome infection. Keana LGA in Nasarawa and Barkin Ladi in Plateau experienced drops from 32.6% to 0.9%, and 7.4% to 0.4%, respectively. For STH, only one LGA, Langtang North in Plateau state, saw a statistically significant increase in its overall STH prevalence, increasing from 4.7% (95% CI: 2.3–9.2%) in 2013 to 15.9% (95% CI: 11.7–21.1%, P = 0.00) in 2018. This was driven by an increase in hookworm infection in the LGA from 3.9% (95% CI: 2.2–7.0%) to 14.8% (95% CI: 10.0–2.1%). This change occurred predominantly in boys, who saw 15% point increase, as opposed to only 7% for girls. One LGA, Mangu in Plateau state, saw a significant drop in STH infections from 3.9% (95% CI: 1.6–6.2%) to 0.4% (upper confidence limit [UCL] = 1.2%).
The predominant SCH infection in positive children was S. hematobium (65.0% in 2013 to 55.0% in 2018), whereas S. mansoni was found in 37.8% versus 47.6% of the samples, respectively. These findings would have been substantially different, had we only used dipsticks or egg counts in 2018 to find S. hematobium rather than both (Table 2). Hookworm remained the most common STH among infected children in both surveys (89.2% in 2013 and 91.0% in 2018), followed by ascaris (11.7% and 10.5%), whereas Trichuris was the least common (0.8% and 1.3%). Multiple infections were uncommon; only three children were positive for all three STHs, all in 2018. A small proportion of children had both SCH and a STH infection, 2.1% in 2013 and 1.3% in 2018. More detailed results for each organism, both individually and combined, are shown in Table 3.
Schistosomiasis was more common in boys than in girls in both surveys. Overall, 12.2% of boys were positive, compared with 9.9% of girls (P < 0.05). This correlation held in both 2013 (14.0% versus 11.6%, P < 0.05) and 2018 (10.1% versus 7.9%, P < 0.05). Similar associations were found for STH infections; 11.6% of boys and 8.6% of girls were positive for one or more STH (P < 0.05). This was true in both 2013 (12.5% versus 8.9%, P < 0.05) and 2018 (10.4% versus 8.2%, P < 0.05). By organism, the statistically significant associations with gender were for S. mansoni only in 2018 (4.8% for boys, 3.8% for girls, P = 0.03); S. hematobium and hookworm also showed gender-specific associations (P < 0.05) but at both timepoints. There were no statistically significant gender-specific correlations with A. lumbricoides or T. Trichuris.
Children aged 6–10 years were less frequently infected with SCH than their older peers in both 2013 (11.9% versus 13.5%, P < 0.05) and 2018 (7.2% versus 11.0%, P < 0.05). As with gender, associations with age group for S. mansoni were not statistically significant in 2013 (P = 0.61) but were in 2018 (P < 0.05). Although there was a statistically significant association between the older age group and S. hematobium in the combined analysis, with prevalence of 6.8% in older children and 5.2% in younger (P < 0.05), this relationship was not significant when either survey year was analyzed individually. There were no statistically significant associations between age group and STH infection.
Ivermectin treatment of onchocerciasis appeared protective against STH infection (odds ratio 0.6, P < 0.05), with significantly lower STH prevalence among those LGAs at both timepoints (8.6% versus 12.4% in 2013 [P = 0.02] and 7.5% versus 10.8% in 2018 [P = 0.03]). However, when a difference-in-difference analysis was done, it showed that the changes in STH prevalence was not statistically significant between those from LGAs receiving annual ivermectin and those not (P = 0.53, Supplemental Figure 2).
There were no significant associations between STH infection and the number of years since the last community-wide LF MDA nor the cumulative years of LF MDA. The prevalence of STH among children living in LGAs that stopped LF MDA in 2012 versus 2009 was 10.5% versus 11.3% (P = 0.71) in 2013, and 9.8% versus 8.5% (P = 0.50) in 2018. However, the drop in STH infection seen from 2013 to 2018 in the LGAs that stopped LF MDA in 2009 was statistically significant (P = 0.03).
LGAs that had higher baseline prevalence of SCH received praziquantel annually instead of every other year. In 2013, those who would receive praziquantel every year had a prevalence of 16.0% compared with 12.1% in those who would receive it every other year; by 2018, the prevalence dropped to 13.0% versus 7.9%, respectively. The decreases in each group were statistically significant and although the prevalence decreased more in the group receiving MDA every other year, the interaction between treatment schedule and time was not statistically significant (P = 0.45).
WASH data and associations with infection.
Questionnaires on student behavior and the school environment were collected in 2018. The WASH data were collected from 198/202 schools. Of these, 88 (44.4%) had latrines, 29 (14.7%) had facilities for handwashing, and 66 (33.3%) had a source of water. Of the schools with latrines, 67 (76.1%) had separate facilities for boys and girls. Most latrines were in poor condition, with 65.9% of latrines described as dirty and poorly maintained by data collectors. No schools had provisions for students to dry their hands after washing, and only one school provided soap.
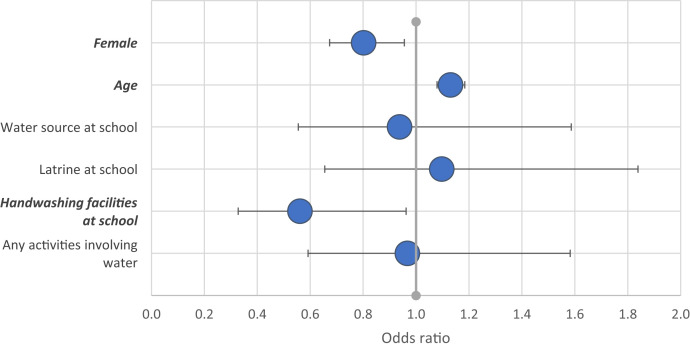
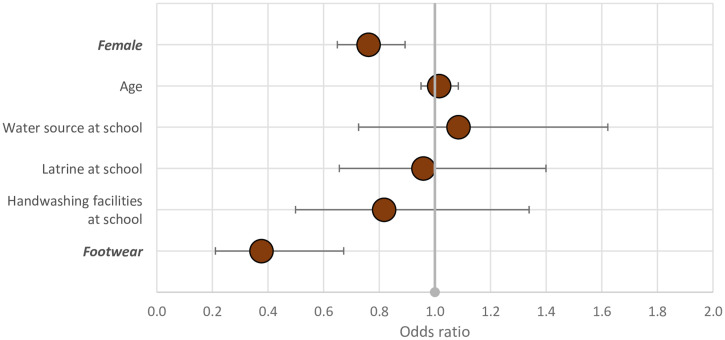
We found that having SCH was less likely in girls and younger children aged 6–10 years, and there was an association with handwashing facilities (Figure 4). Girls were similarly less likely to have an STH infection, but there was no association with age or WASH variables. Wearing shoes appeared protective in this sample (Figure 5); 97% of children wore shoes, and 8% of those who did had hookworm, compared with 20% of those who did not (P = 0.03).
Figure 4.
Odds ratio of a multivariable model exploring associations with schistosomiasis infection. Statistically significant variables are shown in bold. This figure appears in color at www.ajtmh.org.
Figure 5.
Possible associations with STH infection in 9,454 Nigerian children, 2018. Statistically significant variables are shown in bold italics. This figure appears in color at www.ajtmh.org.
Intensity of infection and dipstick sensitivity.
Most infections were of low intensity (Table 4). Heavy-intensity infections were uncommon in both samples (Table 5). Out of 30 LGAs surveyed, six were above the threshold of 1% prevalence of heavy infections for elimination of S. hematobium as a public health problem. Likewise, two of the 30 LGAs exceeded this 1% threshold for S. mansoni (Supplemental Table 1). Among children infected with S. mansoni (N = 962), heavy infections were found among 17.0% (95% CI: 9.7–28.2%) in 2013 and 7.5% (95% CI: 3.5–15.1%) in 2018. Across both studies, 1,430 children were positive for S. hematobium, either by dipstick or by urine filtration. Of children with S. hematobium identified by egg count (N = 239 or 2.5% [95% CI: 1.8–3.5%] of children in 2018), 21.3% (95% CI: 13.7–31.7%) had heavy infections. Heavy-intensity infections were found in < 1% of children with hookworm, and none were found in children with A. lumbricoides or T. trichiura in either timepoint in any LGA. When analysis was limited to children with hookworm, 0.5% (95% CI: 0.1–2.3%) of them had heavy infections in 2013, compared with 0.1% (95% CI: 0.0–0.8%) in 2018 (P = 0.3).
Table 4.
Average parasite intensity among infected children from stool samples
| State | Parasite | Mean* eggs per gram of stool (maximum)† | |
|---|---|---|---|
| 2013 | 2018 | ||
| Nasarawa | Ascaris lumbricoides | 782.4 (8,640) | 459.7 (7,332) |
| Hookworm | 162.0 (3,720) | 154.6 (6,240) | |
| Schistosoma mansoni | 96.4 (1,440) | 157.9 (1,200) | |
| Trichuris trichiura | 88.0 (216) | 32.0 (96) | |
| Plateau | A. lumbricoides | 202.0 (3,240) | 172.7 (1,200) |
| Hookworm | 450.3 (24,480) | 154.8 (2,796) | |
| S. mansoni | 454.1 (11,520) | 134.9 (2,316) | |
| T. trichiura | 174.9 (576) | 114.0 (192) | |
NB = thresholds for heavy-intensity infections were ≥ 400 eggs per gram (epg) of stool for S. mansoni, ≥ 50,000 for A. lumbricoides, ≥ 4,000 for hookworm, and ≥ 10,000 for T. trichiura.
Means are calculated on positive children only.
Average egg count per gram of stool was gathered through Kato-Katz technique and calculated by taking the average of two slide readings and multiplying it by 24.
Table 5.
Frequency and prevalence of infection by intensity and organism in 2013 and 2018
| Organism | Intensity of infection | 2013 (%) | 2018 (%) | Total |
|---|---|---|---|---|
| Schistosoma hematobium | Any | 950 (8.4) | 480 (4.2) | 1,430 |
| Light | 0 (0) | 423 (3.7) | 423 | |
| Heavy | 0 (0) | 57 (0.5) | 57 | |
| Schistosoma mansoni | Any | 546 (4.8) | 416 (3.7) | 962 |
| Light | 340 (3) | 265 (2.3) | 605 | |
| Moderate | 113 (1) | 120 (1.1) | 233 | |
| Heavy | 93 (0.8) | 31 (0.3) | 124 | |
| Hookworm | Any | 1,093 (9.6) | 823 (7.3) | 1,916 |
| Light | 1,074 (9.5) | 816 (7.2) | 1,890 | |
| Moderate | 13 (0.1) | 6 (0.1) | 19 | |
| Heavy | 6 (0.1) | 1 (0) | 7 | |
| Ascaris lumbricoides | Any | 143 (1.3) | 95 (0.8) | 238 |
| Light | 140 (1.2) | 94 (0.8) | 234 | |
| Moderate | 3 (0) | 1 (0) | 4 | |
| Heavy | 0 (0) | 0 (0) | 0 | |
| Trichuris trichiura | Any | 10 (0.1) | 12 (0.1) | 22 |
| Light | 10 (0.1) | 12 (0.1) | 22 | |
| Moderate | 0 (0) | 0 (0) | 0 | |
| Heavy | 0 (0) | 0 (0) | 0 | |
| Total | 20,992 | |||
DISCUSSION
We observed a significant reduction in the prevalence and intensity of SCH in school-aged children after 3–5 years of interventions in Nasarawa and Plateau states. There was also a modest reduction in the prevalence of STH infections over that time, with variable results across LGAs and schools. Prevalence of high-intensity infections was below 1% for all organisms and timepoints at the state level and in the majority of LGAs. The study draws on a large sample that allowed us to observe the effect of LGA-wide interventions on the same communities.
It is important to view the results of our 2013 and 2018 surveys in the context of other MDA programs. Praziquantel MDA was launched in two LGAs (Pankshin in Plateau state and Akwanga in Nasarawa state) in 1999 after heme dipstick mapping for S. hematobium.7 This scope of SCH control expanded modestly through additional dipstick mapping and praziquantel treatment until the E-Merck/WHO donation of praziquantel in 2008. This donation allowed higher frequency of treatment of more groups, not just those communities found through dipstick testing.18 Lymphatic filariasis MDA using ivermectin and albendazole was ubiquitous in all LGAs of the two-state area for the bulk of the 2000s, ending completely by 2013.12,13,19 Ivermectin was administered in many LGAs from the 1990s to 2017,20 and its ability to suppress STH infection is suggested here.21 Unfortunately, true baseline surveys for STH were not obtained to evaluate the impact of the LF program undoubtedly had on STH prevalence and intensity, but the end of LF treatment was a major impetus for performing the 2013 survey reported in this paper.22
As described elsewhere in Nigeria, we saw more SCH and STH infections in boys and older children.23–28 In contrast to other studies, comorbid infections were relatively uncommon, with only 2.1% of infected children having more than one STH.29,30 Hookworm was the most common STH and T. trichiura the least common in this sample, which is similar to other studies in Nigeria,27,31–33 and predictions from spatial models.34 Ascaris lumbricoides, the second-most frequent STH in these samples, has been the most common STH in other Nigerian contexts as well.20,35,36
Using two diagnostic methods for S. hematobium in 2018 affected our conclusions. Dipsticks more than doubled the number of positive children, with 241 children found negative through urine filtration but positive by dipstick test. When combined and using filtration-derived egg counts as the gold standard, dipsticks had a sensitivity of 93.7% and a specificity of 97.4%, but a positive predictive value of only 48.2%. Had they not been included, we would have seen a switch from SH to SM as the predominant organism, leading us to consider environmental changes that favored Biomphalaria snails over Bulinus species or differential effects of praziquantel on the two parasites. We also would have seen more dramatic drops in prevalence, had we only used one or the other test.
We sorted the LGAs into WHO’s treatment categories according the point estimates of prevalence. In 2013 (Figure 2), all 30 LGAs had SCH prevalence rates between 1% and 50%. By 2018, two LGAs had dropped into the lowest category (indicated in blue on the map). None had gone toward a “higher risk” category, indicated in orange or red on the map.
The picture for STH is more complicated. Given that there are more categories and organisms in play, it is not surprising that there is more shifting among them, especially as some LGAs’ prevalence increased between the two studies. In 2013, no LGAs were in the lowest risk category, and 16 were in the second lowest risk group (Figure 3). Five dropped to the lowest category and 10 in the second in 2018, and most LGAs were in the middle category of 10–20% prevalence.
Although the reduction in mean intensity of infection for most organisms was not statistically significant, we are encouraged that the range of intensities was much smaller in 2018. Comparing 2013–2018, the maximum egg count was 11,520 versus 2,316 for S. mansoni, 24,480 versus 6,240 for hookworm, and 576 versus 192 for T. trichiura in the two study years, respectively. The maximum count of A. lumbricoides showed less-impressive reductions, from 8,640 to 7,332, which is surprising given its sensitivity to mebendazole.37 Nonetheless, these results suggest an impact of the program on overall parasite intensity.
Most schools did not have adequate water and sanitation facilities, with 66% lacking latrines, 85% lacking facilities for handwashing, and 76% lacking a source of drinking water available to students. We do not know what sources of water were available to students outside of school. The majority of the population of these states did not have access to improved sanitation in 2013, but the proportions having access nearly doubled by 2018 to from 35.8% to 65.7% in Nasarawa and 18.8% to 35.6% in Plateau.15,16 Other studies have reported poor hygiene conditions in this area, with higher rates of infection than observed here.38 Provision of clean drinking water and sanitation infrastructure has been shown to reduce infection elsewhere in Nigeria,31,39 although the investment required for significant, sustained impacts on morbidity would be substantial.31,39 Shoes, which were worn by 97.1% of students in 2018, were protective here as they have been elsewhere, which is unsurprising given that hookworm was the most common STH.40 Our results show that MDA is helpful in reducing infection, but that it is insufficient on its own to completely eliminate these diseases as public health problems or to interrupt transmission. Investment in WASH infrastructure and behavior change will be needed to achieve these goals.
This study is limited by its focus on children attending school in an area with sub-optimal school enrollment. The prevalence of these ailments in the broader population could be quite different, especially given that low enrollment of children is associated with less adequate sanitation and higher rates of these infections.41 It is likely that, because MDA is based in schools, some children are systematically not reached, especially girls, who can be enrolled at lower rates than boys, such as in 2013 in Nasarawa state (69.3% versus 72.6% in 2013 and 67.2% versus 72.7% in 2018).15,16 However, this could be mitigated by the longstanding use of community-based treatment structures (i.e., volunteer community drug distributors) to reach children not in school, which occurs in some LGAs in these two states.7,10 We calculated prevalence using statistical procedures appropriate for cluster sampling, which widened our CIs. We were missing the original sample frames and thus could not incorporate weights. The study is subject to nonresponse bias because of insecurity that prevented revisiting some schools, as well as a lack of community- or individual-level treatment data, which could further explain the results.
These results could have implications for future MDA schedules per the WHO recommendations.17 As of 2018, 9 of the 30 LGAs could consider reducing the frequency of MDA based on the results of this study. However, since high-intensity schistosome infections remain, such decisions should be made with caution. The treatment algorithm is more complex for STH: based on 2018 results, nine LGAs could reduce the frequency of mebendazole treatment, whereas four could increase it. The other 17 could continue as before. Of note, three LGAs were conducting MDA for STH every year between 2013 and 2018, when only every other year was required. Given the odds or reinfection,42 particularly in areas with poor WASH infrastructure, decisions to lessen the intensity of MDA should not be made lightly.
CONCLUSION
Schistosomiasis and STH in Nasarawa and Plateau states of central Nigeria remain public health problems in this context despite progress achieved through MDA over the past decade. Research into MDA coverage, targeting, and frequency, as well as efforts to improve water safety and sanitation, are needed to further control these diseases and thereby improve the health of these children and their communities.
Supplemental Material
ACKNOWLEDGMENTS
We would like to thank a number of people for their assistance with this program and study: from Nasarawa State University, Professor Gideon Amuga; from the University of Jos, Professor D. Dakul; from the Nasarawa State Ministry of Health, Simon Damishi, Abari Ogah, Gladys Ogah, and Abubakar Umar; from the Plateau State Ministry of Health, Ibrahim Gontor and Henry Fildan; and finally, from The Carter Center-Nigeria, the late Chuwang Gwomkudu, Sunday Pam, George Jar, Bitrus Gofor, Feyi Fadipe, David Alheri, and the late John Umaru. We would like to thank Dr. Karen Hamre and Dr. Jenna Coalson of The Carter Center for their review of the manuscript.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1. Pullan RL Smith JL Jasrasaria R Brooker SJ , 2014. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chitsulo L Loverde P Engels D , 2004. Schistosomiasis. Nat Rev Microbiol 2: 12–13. [DOI] [PubMed] [Google Scholar]
- 3. Vos T et al. 2012. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Committee WE , 2002. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser 912: i–vi, 1–57, back cover. [PubMed] [Google Scholar]
- 5. WHO , 2018. Schistosomiasis: Estimated Number of Individuals in the Country Requiring Preventive Chemotherapy for Schistosomiasis Annually: 2018. Available at: https://apps.who.int/neglected_diseases/ntddata/sch/sch.html. Accessed October 12, 2020.
- 6. WHO , 2018. Soil-Transmitted Helminthiases: Number of Children (Pre-SAC and SAC) Requiring Preventive Chemotherapy for Soil-Transmitted Helminthiases: 2018. Available at: https://apps.who.int/neglected_diseases/ntddata/sth/sth.html. Accessed October 12, 2020.
- 7. Hopkins DR et al. 2002. Lymphatic filariasis elimination and schistosomiasis control in combination with onchocerciasis control in Nigeria. Am J Trop Med Hyg 67: 266–272. [DOI] [PubMed] [Google Scholar]
- 8. Center TC , 2005. Summary: 2004 Program Review for The Lions-Carter Center SightFirst River Blindness Programs. Cameroon, Ethiopia, Nigeria, OEPA, Sudan, and Uganda. 3–5 March 2005. Atlanta, GA: The Carter Center, 128. [Google Scholar]
- 9. Njepuome NA et al. 2009. Nigeria’s war on terror: fighting dracunculiasis, onchocerciasis, lymphatic filariasis, and schistosomiasis at the grassroots. Am J Trop Med Hyg 80: 691–698. [PubMed] [Google Scholar]
- 10. Evans D McFarland D Adamani W Eigege A Miri E Schulz J Pede E Umbugadu C Ogbu-Pearse P Richards FO , 2011. Cost-effectiveness of triple drug administration (TDA) with praziquantel, ivermectin and albendazole for the prevention of neglected tropical diseases in Nigeria. Ann Trop Med Parasitol 105: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gutman J Fagbemi A Alphonsus K Eigege A Miri ES Richards FO Jr , 2008. Missed treatment opportunities for Schistosomiasis mansoni, in an active programme for the treatment of urinary schistosomiasis in Plateau and Nasarawa states, Nigeria. Ann Trop Med Parasitol 102: 335–346. [DOI] [PubMed] [Google Scholar]
- 12. Eigege A et al. 2017. Criteria to stop mass drug administration for lymphatic filariasis have been achieved throughout Plateau and Nasarawa States, Nigeria. Am J Trop Med Hyg 97: 677–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eigege A et al. 2020. Post-treatment surveillance for lymphatic filariasis in Plateau and Nasarawa States, Nigeria: results of transmission assessment surveys. Am J Trop Med Hyg 102: 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richards FO et al. 2020. The interruption of transmission of human onchocerciasis by an annual mass drug administration program in Plateau and Nasarawa States, Nigeria. Am J Trop Med Hyg 102: 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. (Nigeria) NPC , ICF, 2019. Nigeria Demographic and Health Survey 2018. The DHS Program, Abuja, Nigeria and Rockville, MD, 748.
- 16. NPC/Nigeria NPC, International I , 2014. Nigeria Demographic and Health Survey 2013. Abuja, Nigeria and Rockville, MD: National Population Commission, Federal Republic of Nigeria and ICF International. [Google Scholar]
- 17. WHO , 2011. Helminth Control in School-Age Children: A Guide for Managers of Control Programmes. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 18. Pavluck A Chu B Mann Flueckiger R Ottesen E , 2014. Electronic data capture tools for global health programs: evolution of LINKS, an android-, web-based system. PLoS Negl Trop Dis 8: e2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. King JD Eigege A Umaru J Jip N Miri E Jiya J Alphonsus KM Sambo Y Graves P Richards F Jr , 2012. Evidence for stopping mass drug administration for lymphatic filariasis in some, but not all local government areas of Plateau and Nasarawa States, Nigeria. Am J Trop Med Hyg 87: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gyang VP et al. 2019. Intestinal parasitic infections: current status and associated risk factors among school aged children in an archetypal African urban slum in Nigeria. J Microbiol Immunol Infect 52: 106–113. [DOI] [PubMed] [Google Scholar]
- 21. Turner HC Truscott JE Bettis AA Hollingsworth TD Brooker SJ Anderson RM , 2016. Analysis of the population-level impact of co-administering ivermectin with albendazole or mebendazole for the control and elimination of Trichuris trichiura. Parasite Epidemiol Control 1: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mupfasoni D Montresor A Mikhailov A King J , 2016. The impact of lymphatic filariasis mass drug administration scaling down on soil-transmitted helminth control in school-age children. Present situation and expected impact from 2016 to 2020. PLoS Negl Trop Dis 10: e0005202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afiukwa FN Nwele DE Uguru OE Ibiam GA Onwe CS Ikpo AU Agumah NB Odoemena OF , 2019. Transmission dynamics of urogenital schistosomiasis in the rural community of Ebonyi State, south eastern Nigeria. J Parasitol Res 2019: 7596069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ben SA Useh MF , 2017. A comparative study on the efficacy of praziquantel and albendazole in the treatment of urinary schistosomiasis in Adim, Cross River State, Nigeria. Int Health 9: 288–293. [DOI] [PubMed] [Google Scholar]
- 25. Morenikeji O Quazim J Omoregie C Hassan A Nwuba R Anumudu C Adejuwon S Salawu O Jegede A Odaibo A , 2014. A cross-sectional study on urogenital schistosomiasis in children; haematuria and proteinuria as diagnostic indicators in an endemic rural area of Nigeria. Afr Health Sci 14: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dahal AS, Francis EO, Francis JE, Wamtas FI, 2019. Soil-transmitted helminths and associated risk factors among elementary school pupils in Dadin Kowa, Jos. Niger Med J 60: 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Odinaka KK Nwolisa EC Mbanefo F Iheakaram AC Okolo S , 2015. Prevalence and pattern of soil-transmitted helminthic infection among primary school children in a rural community in Imo State, Nigeria. J Trop Med 2015: 349439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sowemimo OA Asaolu SO , 2011. Current status of soil-transmitted helminthiases among pre-school and school-aged children from Ile-Ife, Osun State, Nigeria. J Helminthol 85: 234–238. [DOI] [PubMed] [Google Scholar]
- 29. Aribodor DN Bassey SA Yoonuan T Sam-Wobo SO Aribodor OB Ugwuanyi IK , 2019. Analysis of schistosomiasis and soil-transmitted helminths mixed infections among pupils in Enugu State, Nigeria: implications for control. Infect Dis Health 24: 98–106. [DOI] [PubMed] [Google Scholar]
- 30. Babamale OA Ugbomoiko US Heukelbach J , 2018. High prevalence of Plasmodium falciparum and soil-transmitted helminth co-infections in a periurban community in Kwara State, Nigeria. J Infect Public Health 11: 48–53. [DOI] [PubMed] [Google Scholar]
- 31. Anunobi JT Okoye IC Aguzie IO Ndukwe YE Okpasuo OJ , 2019. Risk of soil-transmitted helminthiasis among Agrarian communities of Kogi State, Nigeria. Ann Glob Health 85: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Douglas KE Amadi CU , 2016. Soil transmitted helminth infection among farmers in Ukwa West Local Government Area, Abia State, south-east, Nigeria. Niger J Med 25: 24–32. [PubMed] [Google Scholar]
- 33. Oyebamiji DA Ebisike AN Egede JO Hassan AA , 2018. Knowledge, attitude and practice with respect to soil contamination by soil-transmitted helminths in Ibadan, southwestern Nigeria. Parasite Epidemiol Control 3: e00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karagiannis-Voules DA et al. 2015. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: a systematic review and geostatistical meta-analysis. Lancet Infect Dis 15: 74–84. [DOI] [PubMed] [Google Scholar]
- 35. Caroline Okeke O Obiageli Ubachukwu P , 2015. A cross-sectional study of Ascaris lumbricoides infection in a rural community in Ebonyi State, Nigeria: prevalence and risk factors. Iran J Public Health 44: 1430–1432. [PMC free article] [PubMed] [Google Scholar]
- 36. Olopade BO Idowu CO Oyelese AO Aboderin AO , 2018. Intestinal parasites, nutritional status and cognitive function among primary school pupils in Ile-Ife, Osun State, Nigeria. Afr J Infect Dis 12: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mrus J Baeten B Engelen M Silber SA , 2018. Efficacy of single-dose 500 mg mebendazole in soil-transmitted helminth infections: a review. J Helminthol 92: 269–278. [DOI] [PubMed] [Google Scholar]
- 38. Abe EM Echeta OC Ombugadu A Ajah L Aimankhu PO Oluwole AS , 2019. Helminthiasis among School-age children and hygiene conditions of selected schools in Lafia, Nasarawa State, Nigeria. Trop Med Infect Dis 4: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ekanem EE Akapan FM Eyong ME , 2017. Urinary schistosomiasis in school children of a southern Nigerian community 8 years after the provision of potable water. Niger Postgrad Med J 24: 201–204. [DOI] [PubMed] [Google Scholar]
- 40. Okoyo C Campbell SJ Williams K Simiyu E Owaga C Mwandawiro C , 2020. Prevalence, intensity and associated risk factors of soil-transmitted helminth and schistosome infections in Kenya: impact assessment after five rounds of mass drug administration in Kenya. PLoS Negl Trop Dis 14: e0008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hotez PJ et al. 2006. Helminth infections: soil-transmitted helminth infections and schistosomiasis. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, eds. Disease Control Priorities in Developing Countries. Washington, DC: The World Bank, Oxford University Press, 467–482. [PubMed] [Google Scholar]
- 42. Jia TW Melville S Utzinger J King CH Zhou XN , 2012. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis 6: e1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.