Abstract
Cryopreservation of cells and biologics underpins all biomedical research from routine sample storage to emerging cell-based therapies, as well as ensuring cell banks provide authenticated, stable and consistent cell products. This field began with the discovery and wide adoption of glycerol and dimethyl sulfoxide as cryoprotectants over 60 years ago, but these tools do not work for all cells and are not ideal for all workflows. In this Review, we highlight and critically review the approaches to discover, and apply, new chemical tools for cryopreservation. We summarize the key (and complex) damage pathways during cellular cryopreservation and how each can be addressed. Bio-inspired approaches, such as those based on extremophiles, are also discussed. We describe both small-molecule-based and macromolecular-based strategies, including ice binders, ice nucleators, ice nucleation inhibitors and emerging materials whose exact mechanism has yet to be understood. Finally, looking towards the future of the field, the application of bottom-up molecular modelling, library-based discovery approaches and materials science tools, which are set to transform cryopreservation strategies, are also included.
Subject terms: Chemical biology, Biomaterials
Cryopreservation is a platform technology that underpins the delivery of complex therapies to patients and enables fundamental cell biology by allowing the banking and recovery of viable cells. This Review summarizes the role, and opportunities, for chemistry-driven approaches to cryopreservation, beyond formulation, to the design and discovery of innovative solutions.
Introduction
Cryopreservation, the process of storing materials at sub-zero temperatures, is an essential process for fundamental research, as well as the clinical, biomedical and food sciences1. The ability to prolong the storage of biological materials, by reducing the temperature to slow the rate of degradation, has wide-reaching applications. For instance, millions of seed samples are housed frozen in the Svalbard Global Seed Vault in Norway, making cryopreservation essential to future food and crop security2. Nearly every biomedical research lab in the world keeps frozen stocks of cells to reduce phenotypic drift from continuous culture3, to serve as backups and to preserve valuable or rare cells that are hard to come by, such as specific primary and clinical samples. The ability to store oocytes, spermatozoa and embryos has revolutionized in vitro fertility treatment, allowing individuals to preserve their fertility for many years, particularly in circumstances where it could be lost (such as certain types of cancer and the associated treatments required). More recently, cryopreservation has enabled pioneering medicines for cancer treatment, such as adoptive cell therapies, by allowing patients’ cells to be successfully shipped to dedicated processing facilities and stored for future treatments. These new immunotherapies offer promising options for cancers that are difficult to treat. New techniques for tissue and organ preservation are emerging, which, if successful, could abolish long waiting lists for organ transplantation1. Finally, the COVID-19 pandemic has demonstrated that cryopreservation is imperative to afford an effective cold chain for vaccine storage and delivery, particularly for vaccines requiring ultralow-temperature storage, such as those based on mRNA technology. In all of these mentioned applications, cryopreservation is necessary to facilitate prolonged storage of the material with minimal loss in function. We should note that there is also a desire for non-cryo and hypothermic solutions for the short-term storage of cells, such as immobilizing cells in hydrogels4, which is outside of the scope of this Review.
At ultralow temperatures (below −130 °C), kinetic energy and molecular motion within a biological material decrease. Rates of chemical and biological reactions slow down and processes such metabolism, active transport, enzymatic reactions and diffusion decline. This allows the material to remain in a suspended state until the temperature is increased again. Although ultralow temperatures are not directly responsible for cryopreservation-induced damage, the freezing and thawing processes needed to achieve these temperatures inflict damage to the material in a number of ways (detailed in the next section). In order to moderate cryopreservation-induced damage, cryoprotective agents (CPAs) are employed, the most common being dimethyl sulfoxide (DMSO) and glycerol. These low-molecular-weight cryoprotectants were originally discovered over 60 years ago5,6 (not rationally selected for a particular biological/physical property), yet they have been exceptionally effective and widely used in both research and clinical applications. Stocks of red blood cells are cryopreserved using 20–40 wt% glycerol7, immortalized cell lines are routinely stored in 10% (v/v) DMSO and cells for clinical transplantation, including haematopoietic stem cells and chimeric antigen receptor (CAR)-T cells, are frequently cryopreserved in 5–10% DMSO8,9 (amongst other additives).
Despite their utility, these common cryoprotectants present a number of issues to the biological material being frozen, and some cell types still remain challenging to cryopreserve and recover, such as human embryonic stem cells (hESCs)10, cell monolayers11–13 and multicellular systems. Red blood cells stored in glycerol require a lengthy deglycerolization process to safely remove the cryoprotectant while also avoiding osmotic shock14. DMSO has been shown to cause epigenetic changes in hepatic microtissues15, induce differentiation in embryonic stem cells16 and has potentially contributed to the leaching of plasticizers from cell storage bags17. In addition, DMSO can cause adverse effects in patients who receive cryopreserved transplanted material (noting that the clinical benefits of the transplant outweigh this)18,19. Overall, there is a clear unmet need for the design and discovery of novel cryoprotectants that can either replace or reduce the required amount of these current gold standards and address challenging sample types, such as multicellular systems and whole organ-freezing.
In this Review, we will summarize the challenges of cryopreserving biological material, describing the intricate chemical processes involved and the impact on the material being frozen. We will highlight and evaluate current and emerging chemical tools that modulate the cryopreservation process. Further, we will discuss specific methods that utilize chemical tools such as cell encapsulation and the modulation of biochemical pathways. Finally, we will introduce areas for development, outline knowledge gaps and suggest emerging approaches that would greatly benefit the field.
Challenges during cryopreservation
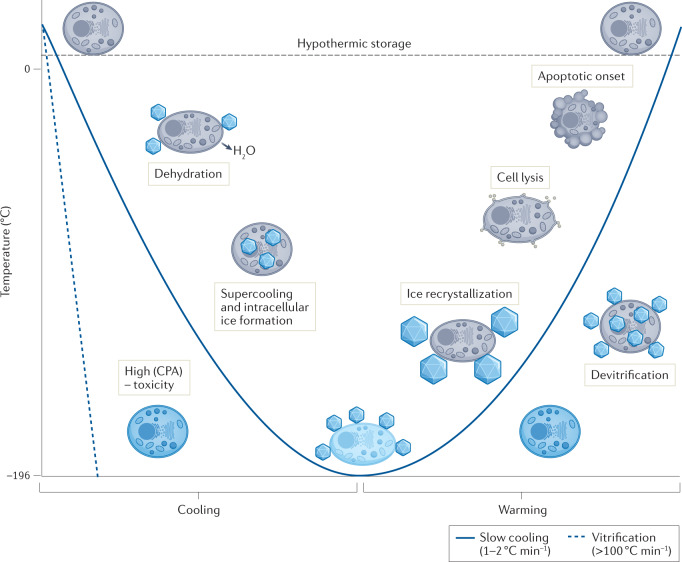
A general understanding of the challenges associated with cryopreservation is required before discussing new chemical approaches to cryopreservation. In most cases, cooling aqueous solutions below the equilibrium freezing point will inevitably result in the formation of ice crystals in the extracellular media and a decrease in the concentration of water in the sample, which has two key consequences. First, an osmotic gradient forms across the cell membrane, which causes the cell to dehydrate (Fig. 1). Some cellular dehydration is beneficial for cryopreservation outcomes, as it reduces the chance of excessive intracellular ice formation (IIF), which is often fatal — the exact underlying mechanisms are still being investigated. However, disproportionate dehydration can be irreversible and is a key cause of cryopreservation-induced damage to biological material. The second consequence is that, as ice crystals continue to grow in the extracellular media, solutes that were previously dispersed in the bulk solution become concentrated in the residual water channels between ice crystals. Cells trapped within these channels will experience a much higher concentration of solutes compared with the sample solution without ice present, ultimately leading to osmotic shock and increased toxicity.
Fig. 1. Potential mechanisms of cellular damage during cryopreservation.
Several challenges occur when cells are cooled for cryogenic storage, as well during rewarming. During cooling, the formation of extracellular ice crystals leads to an osmotic imbalance across the cell membrane and subsequent cell dehydration. In the absence of extracellular ice, samples might supercool below the equilibrium freezing point, leading to an increased chance of fatal intracellular ice formation. Fast cooling rates and the addition of high concentrations of cryoprotective agents (CPA) can achieve vitrification, an amorphous, ice-free state. However, high concentrations of CPA can be toxic to cells. During warming, ice recrystallization can occur, where ice crystals grow and cause mechanical damage and cell lysis. Vitrified samples may become unstable and devitrify, leading to further ice growth. Finally, cryopreservation can induce apoptosis, leading to delayed cell death post-thaw. Note that these are all extremes, do not necessarily occur simultaneously and can be partially mitigated by the addition of cryoprotective agents. Ice crystals are not to drawn to scale for illustrative purposes.
A further challenge in cryopreservation is sample supercooling, whereby the temperature of the solution cools to many degrees below the equilibrium melting point before (heterogeneous) extracellular ice nucleation occurs20. When ice does nucleate, the exothermic process causes latent heat to be released, thereby warming the solution close to the melting point, while the solution temperature simultaneously continues to decrease. Consequently, as the previously supercooled solution readjusts to the solution temperature, it experiences a greater cooling rate than solutions that nucleate at higher temperatures. Typically, inducing extracellular ice formation at temperatures close to zero drives cellular dehydration and reduces the likelihood of fatal IIF.
An additional challenge is adopting a cooling rate that leads to optimal cell survival. Slow cooling rates (<1 °C min−1) allow cells sufficient time to dehydrate and prevents IIF. However, cells become exposed to high solute concentrations as well as any (potentially toxic) cryoprotective agents that have been added for an extended period of time. In contrast though, fast cooling rates (for example, >100 °C min−1) avoid prolonged periods in high-solute solutions but can lead to IIF, as cells are not capable of dehydrating fast enough, causing irreversible damage. The optimum cooling rate for cell survival is outlined by Mazur’s two-factor hypothesis21 — the highest cell viability will be achieved by an intermediate cooling rate, which will provide a balance of these two scenarios. It is important to mention that different cell types will have different optimal cooling rates. One caveat to Mazur’s two-factor hypothesis is that, at exceptionally high cooling rates, the crystalline (ice) phase can be bypassed to achieve an ultrahigh-viscosity glass, known as vitrification (Fig. 1). It prevents catastrophic cellular damage caused by ice nucleation and intracellular ice growth during cryopreservation. However, vitrification usually requires very high concentrations of cryoprotectants that can result in osmotic stress during CPA loading and removal.
There are additional cryopreservation challenges when warming biological material back to physiological temperature. Exposure of dehydrated cells to large volumes of water or buffer solutions during the thawing process leads to an influx of water across the cell membrane and can result in swelling and cell lysis. Ice recrystallization (a type of Ostwald ripening) can occur, where ice crystals grow into larger crystals at the expense of smaller ones, leading to mechanical damage and osmotic stress22. During thawing, vitrified solutions are at risk of devitrification, where ice nucleation and subsequent ice recrystallization occur. It is important to note that the recrystallization can sometimes be used to describe nucleation from a vitrified solution, but the term is not used in this way here. More broadly, the freezing and thawing process imposes extensive stress on cells that can lead to protein denaturation23 and impacts higher-order structures, such as microtubules and the meiotic spindle in oocytes24.
Some of these cryopreservation challenges can be mitigated by the addition of permeating and non-permeating cryoprotectants (CPAs), also referred to as penetrating and non-penetrating cryoprotective agents. Permeating cryoprotectants, including DMSO and glycerol, can cross cellular membranes. By contrast, non-permeating cryoprotectants remain extracellular and some examples include polymeric materials, such as poly(vinyl pyrrolidone) and hydroxyethyl starch (HES), as well as small molecules, such as trehalose. Specific mechanisms of action for individual cryoprotectants have not been fully elucidated, with the general view that many cryoprotectants are multimodal in their protection25. Overall, cryoprotection likely arises from a combination of factors including the modulation of hydrogen bonding, effects on cell membrane properties, dilute solute effects and increases in solution viscosity at low temperatures, among others (reviewed in detail by Fuller25 and Elliott et al.26).
Chemical tools for cryopreservation
In order to improve cryopreservation outcomes, researchers are now looking beyond chance discoveries of cryoprotectants and instead utilizing a toolbox of methodologies, from bio-inspired synthetic design to molecular modelling and emerging discovery tools. In the following sections, we will review and critically analyse some of these advancements and highlight where chemical tools are being utilized in existing and emerging areas.
Vitrification agents
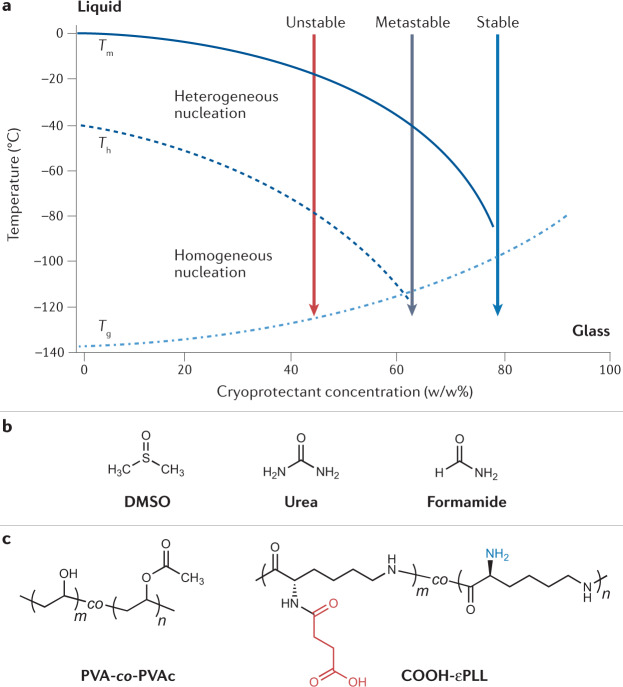
Vitrification is the clinically preferred method to cryopreserve oocytes and embryos27, with reports of superior clinical outcomes compared with slow-freezing (ice formation) protocols28. It could also be used for large-volume storage and whole-organ freezing where slow freezing is not suitable29. To achieve vitrification, high concentrations of both permeating and non-permeating CPAs are needed (for example, ethylene glycol, propylene glycol, sugars, DMSO and high-molecular-weight agents)30–32, along with rapid cooling rates, to bypass ice formation (Fig. 2a). A major challenge with this method is cryoprotectant toxicity and the need for rapid but stepwise removal of the CPA to avoid post-thaw toxicity and osmotic damage.
Fig. 2. Vitrification agents for cryopreservation.
To achieve vitrification, a sample must be cooled to obtain a glassy, amorphous state without heterogeneous (via foreign particles) or homogeneous (through random ordering of water molecules) ice nucleation. a | Schematic phase diagram of an aqueous sample with different concentrations of a cryoprotectant, adapted with permission from ref.43. Tm and the solid line denote the equilibrium melting temperature, Th and the dashed line denote the temperature of homogeneous ice nucleation, and Tg and the dashed/dotted line denote the glass transition temperature of the sample. For dilute samples (<40% cryoprotectant), vitrification is difficult to achieve due to the likelihood of nucleation occurring. b | Structures of small molecules that have been investigated for toxicity neutralization: dimethyl sulfoxide (DMSO), urea and formamide34. c | Macromolecular compounds such as poly(vinyl alcohol)-co-poly(vinyl acetate) (PVA-co-PVAc) copolymers and carboxylated ε-poly-L-lysine (COOH-εPLL) have been used to limit devitrification of samples during warming43. Panel a adapted with permission from ref.193, Elsevier.
Mixtures of permeating cryoprotectants and their effect on the viability (K+/Na+ ratios) of rabbit renal cortical slices have been analysed33. Solution toxicity was correlated to the hydrogen bonding between water and polar groups on permeating cryoprotectants. New vitrification solutions that had overall higher concentrations but lower intrinsic toxicities than standard vitrification solutions were screened for improved K+/Na+ ratios. Interestingly, the toxicity of some permeating cryoprotectants used in vitrification, such as formamide, can be reduced by the addition of compounds such as DMSO, urea and acetamide (Fig. 2b). This approach is known as cryoprotectant toxicity neutralization34. A proposed mechanism was that these small molecules might act as low-toxicity analogues of formamide, but competition experiments refuted this theory34. Instead, the neutralization is thought to be due to a general reduction in non-specific toxicity35. These initial studies corroborate that small-molecule approaches can help address cryoprotectant toxicity.
Rather than mitigating toxicity, approaches to reduce the total CPA concentration have also been explored. In small volumes, thermal diffusion distances are shorter, such that higher cooling rates can be achieved at low CPA concentrations, allowing for rapid cooling below the glass transition temperature (Tg), such that no nucleation occurs36. Droplets 70 µm in diameter of encapsulated AML-12 hepatocytes were vitrified with 1.5 M propanediol and 0.5 M trehalose, resulting in post-thaw viabilities of ~90% (ref.36). High-throughput vitrification using inkjet printing of 3T3 mouse fibroblast cells enabled cryopreservation with low CPA concentrations37. Examples of cryoprotectant-free vitrification have been reported, in which super-flash freezing was employed for picolitre sample volumes. Post-thaw viabilities of mouse myoblast (C1C12) cells and primary rat stem cells by super-flash freezing with no CPA were 70–80%, which is comparable with conventional cryopreservation protocols38. These approaches show great promise for research purposes but may not be useful for cell-therapy applications where large volumes of cells are required.
The warming cycle faces the same challenges associated with the cooling cycle; how to bypass the ice phase directly to the liquid phase. External heating can be helpful, but thermal gradients present a challenge because the outside of the sample thaws before the inside. Nanowarming exploits the local heating effect associated with magnetic nanoparticles in an alternating magnetic field, allowing homogeneous and rapid rewarming. Application of mesoporous silica-coated iron oxide nanoparticles have aided the cryopreservation of porcine arterial tissue (20 ml volume)39 and magnetite (Fe3O4) nanoparticles increased the cell viability of human induced pluripotent stem cells (hiPSCs) to 74.8% compared with 38.5% as assessed immediately post-thaw by Hoechst/SYTOX green staining40. Microporous silicon-coated iron oxide nanoparticles improved cryopreservation outcomes with samples as large as rat kidneys41. In addition, cell attachment rates of vitrified alginate-encapsulated stem cells increased from 24% to 68% with nanowarming42.
Chemical tools to prevent ice nucleation can also reduce the damage from ice formation during warming. Poly(vinyl alcohol)-co-poly(vinyl acetate) (PVA-co-PVAc) copolymers (1–3%) (Fig. 2c) were used to inhibit devitrification in solutions of 56% (w/w) ethylene glycol and DMSO43. A copolymer consisting of a 20:80 ratio of vinyl acetate to vinyl alcohol units afforded the best results, showing the importance of the molecular structure of the additive and the particularly unique ice-binding properties of PVA44. Adding 3% (w/w) PVA to solutions of aqueous 1,2-propanediol (35% (w/w)) inhibited ice nucleation as well as ice growth45. Introducing carboxylated ε-poly-L-lysine (COOH-εPLL, Fig. 2c) (discussed in greater detail in the ‘Macromolecular cryoprotectants’ section) has also shown promise as an additive in vitrification solutions. A quantity of 10% (w/v) COOH-εPLL, in a solution of 6.5 M ethylene glycol and 0.75 M sucrose, allowed successful vitrification of hiPSCs, with colony attachment rates increasing from 46.8% to 73.4% (ref.46). The mechanism of action of COOH-εPLL has yet to be fully understood but it appears to reduce osmotic stress or inhibit devitrification, as observed by differential scanning calorimetry (DSC) analysis46,47. Similar results with COOH-εPLL have been reported for the successful vitrification of hESCs48, porcine embryos49 and 3D cell constructs50.
Ice recrystallization inhibitors
Ice recrystallization, a type of Ostwald ripening, describes the increase in ice crystal size over time in an already frozen material (Fig. 3A). This phenomenon is a contributor to cell death during the thawing phase of cryopreservation and leads to osmotic stress21. A diverse range of ice-binding proteins that inhibit ice recrystallization activity are known51, with antifreeze glycoproteins inhibiting ice growth at 0.022 µg ml−1 (ref.52), whereas even the most active synthetic ice recrystallization inhibitors (IRIs) are orders of magnitude less effective53. In 1992, Carpenter and Hansen reported that the antifreeze protein from Pseudopleuronectes americanus reduced post-thaw haemolysis of red blood cells, providing the first demonstration that ice recrystallization inhibition benefits cryopreservation54. When discussing ice recrystallization inhibition for cryopreservation, it is crucial to note that ‘how much inhibition is needed for cryopreservation benefit?’ is a deceptively challenging question, as any agent can have multiple modes of action, which may vary across different cell lines, cryoprotectant type, freeze/thaw cycle and other variables in any cryopreservation procedure. Adding complexity, ice recrystallization inhibition is a continuum, not an on/off property, and any material can slow ice growth at sufficiently high concentrations. Therefore, cryopreservation cannot simply be attributed to ice recrystallization inhibition, as opposed to other effects (especially when high concentrations are used). To highlight this, poly(D/L-serine) and poly(ethylene glycol) (PEG), which have limited ice recrystallization inhibitory activity, can aid red blood cell cryopreservation at high concentrations (100 mg ml−1)55. By contrast, oligoproline, which is a weak IRI, is a potent cryopreservation enhancer for monolayers of A549 cells56 (at ~10 mg ml−1) and oocytes57. Proving a causative link between materials that inhibit ice recrystallization and improved cryopreservation can also be challenging, and some structural features, such as amphipathy58,59 (Fig. 3Bc), can be associated with both. It is also crucial to highlight that additional ice-binding effects can be seen, including thermal hysteresis and dynamic ice shaping (which can be detrimental to the sample)54. The mechanism of ice binding is beyond the scope of this Review, but it is clear that multiple molecular-level mechanisms can give rise to this macroscopic effect53,60–64.
Fig. 3. Ice recrystallization inhibitors.
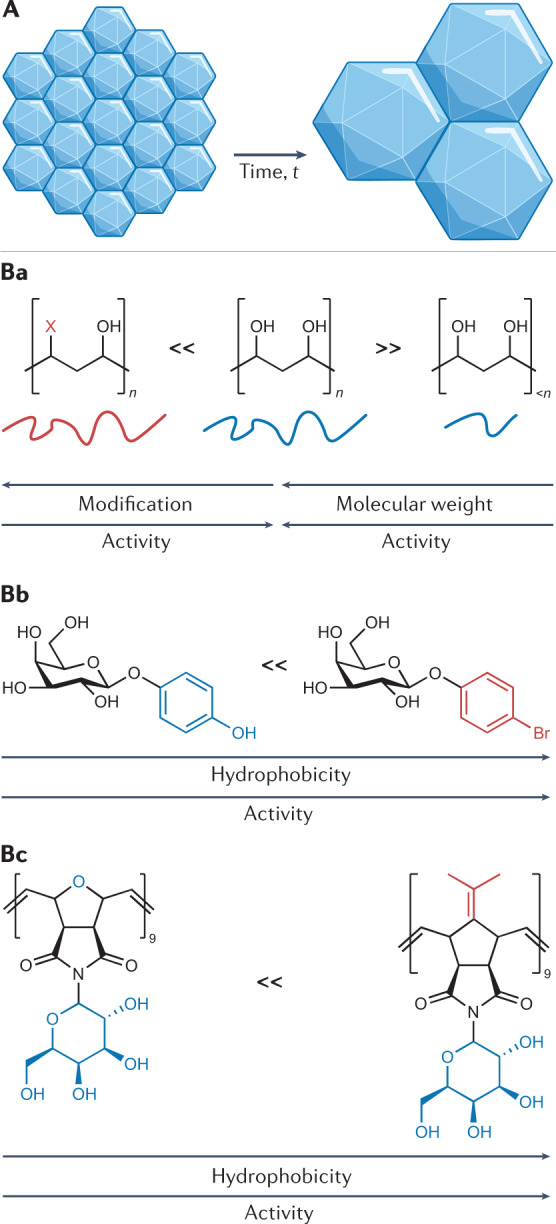
Ice recrystallization describes the growth of larger ice crystals at the expense of smaller crystals and is a form of Ostwald ripening, as depicted in A. Ice recrystallization inhibitors (IRIs) are molecules/materials that slow the growth of ice crystals, for instance, poly(vinyl alcohol) is a highly active macromolecular IRI (B). The chemical structures of IRIs are crucial to their function and the design rules can vary between classes. Removing the hydroxyl groups of PVA (for example, to add more hydrophobic groups) or decreasing the molecular weight reduces ice recrystallization inhibitory activity (Ba)69. O-aryl-glycosides (Bb) are an example of small-molecular-weight IRIs that have been shown to improve red blood cell recovery194. For this class of IRI, an increase in hydrophobicity leads to an increase in their ice recrystallization inhibitory activity. Facial amphiphilicity has been found to be crucial for a range of materials to have ice recrystallization inhibitory activity and is a feature of many IRI-proteins, but also synthetic polymers that can slow ice growth, such as those shown in Bc (ref.59).
Simplified glycopeptide-based IRIs, as opposed to total synthesis of glycoproteins, were reported from the first chemistry-driven attempt to discover new IRIs65,66. Following this in a specific protocol, aryl glycosides IRIs were shown to mitigate damage to red blood cells, increasing post-thaw yields from ~40% to >80% (ref.67). A hydrophobic face has been characterized as crucial for the activity associated with these small-molecule IRIs61,68 (Fig. 3Bb), and this element has also been seen in macromolecular IRIs59. The placement of the hydrophobic groups is essential, as more hydrophobic does not always lead to significant increases in activity, as seen for hydrophobic modified PVA69 (Fig. 3Ba) and fluorinated glycopeptides70.
Intracellular ice is often fatal, and conventional CPAs effectively mitigate this. Hence, whether IRIs are required inside cells remains a question. The permeable IRI p-bromophenyl-β-D-glucoside (Fig. 3Bb) diminished intracellular ice growth in human umbilical vein endothelial cells71. By contrast, a recombinant type III α-fetoprotein protected A549 cell monolayers during cryopreservation (increasing recovery from 10 to >40% at 5% DMSO), but only when applied extracellularly72. Interestingly, cryopreserving the cells in suspension gave only very small gains. This could be because the suspension system was already optimized or that the lack of cell–cell and cell–substrate contacts for cells frozen in suspension meant that intracellular ice and ice recrystallization was not a primary mechanism of damage73. Other IRIs, which are non-permeable, have demonstrated that they can mitigate (but not remove) cellular damage, including PVA for red blood cell cryopreservation22 and graphene oxide for horse sperm74. The effect of ice recrystallization inhibition on the post-thaw cell viability of three human cell lines (WRL-68, HepG2 and HEK 293) with different sugars was explored75. The relative uptake (and, hence, intracellular/extracellular location compared with the non-permeable trehalose) could not be factored in despite there being a link to the IRI’s location. Taking this into account, IRIs clearly have potential as extracellular supplemental additives, alongside traditional CPAs, rather than as a replacement. Further, the cryopreservation of rat lung was improved by an IRI, as there were about fourfold fewer damaged membranes compared with the control76, showing application beyond cell cultures, where the IRI must penetrate a complex matrix.
Inspired by these positive results in cryopreservation, there is an increasing number of new molecules and polymers being developed that can inhibit ice recrystallization activity, but have yet to be evaluated for cell cryopreservation. These include nanomaterials60,77, organometallic self-assemblies78, self-assembled organic dyes79, peptides80 and nylon-3-based polymers81, all of which have been previously reviewed44,62,82. Finally, ice recrystallization inhibition has been associated with aiding protein cryopreservation by reducing irreversible aggregation during the freeze/thaw transition, but has so far only been demonstrated with PVA and a self-assembled peptide80,83.
Macromolecular cryoprotectants
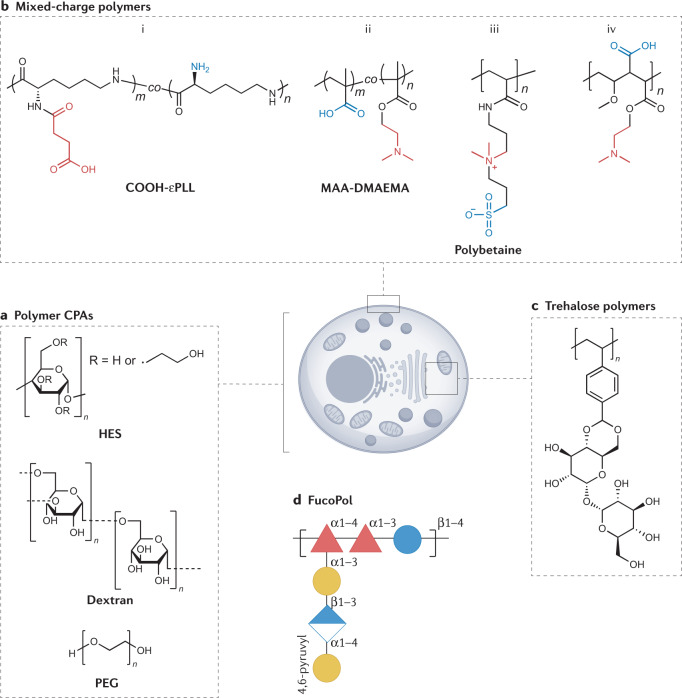
The inclusion of macromolecules, such as polymers, proteins and/or polysaccharides, in cryopreservation solutions is widespread. For example, hydroxyethyl starch is used as a red blood cell protectant84, and serum proteins85, dextran86 or PEG87 (amongst others) are added into cryoprotectant solutions (Fig. 4a). However, interest has peaked in discovering new synthetic and natural polymers that can replace or reduce the amount of organic solvents and increase post-thaw yields, including those that do not have specific ice binding or ice recrystallization inhibitory activity (see previous sections). Carboxylation of ε-poly-L-lysine (Fig. 4bi) introduced remarkable cryoprotective functionality that enabled the recovery of viable mouse fibroblast cells in the absence of DMSO88. Follow-up studies have shown that the polyampholyte motif — polymers with mixed cationic and anionic side chains — was essential for function, rather than the specific polymer used89,90. It was thought that polyampholytes act through controlling ice recrystallization inhibition, but the magnitude of this activity is very low compared with the potent IRI PVA88,91. Despite this low activity, polyampholytes outperform ice recrystallization inhibitory materials and can work in vitrification as well as slow-freezing protocols92. Liposomal dye leakage assays suggest that polyampholytes can protect by lowering the lipid Tg, supported by observations that increasingly hydrophobic polymers lead to more cryoprotectant activity93. In contrast though, a photochemical high-throughput screen of ampholyte copolymers (Fig. 4bii) for red blood cell and human lung (A549) cell cryopreservation suggested that a small increase in hydrophilicity, rather than hydrophobicity, improved post-thaw cell recovery, highlighting the complex associated trends94. Solid-state NMR suggests that, upon cooling, polyampholytes can increase viscosity and, thus, ‘trap’ ions from solution and prevent osmotic shock47. Finally, the precise chemical nature of the charged side chains is also key, as ‘any type of charge’ does not necessarily work; polybetaines show significantly lower cryoprotectant activity95 (Fig. 4biii). Reference96 provides a review on polyampholyte cell recoveries.
Fig. 4. Macromolecular cryoprotectants for cryopreservation.
There is a huge interest in using macromolecular cryoprotectants to mimic the action of antifreeze proteins, as well as investigate new methods of protection during cold temperature exposure. This started with the use of polymers such as hydroxyethyl starch (HES), dextran and poly(ethylene glycol) (PEG) (a). More recently, polyampholytes (polymers with alternating positive and negative charges) have been shown to be beneficial for cryopreservation of mammalian cells, such as bi (ref.88), bii (ref.91) and biv (ref.98). Their modes of action are still being elucidated, but may involve membrane stabilization and ion trapping. Polybetaines (biii) with mixed charges on the same polymer branch have, so far, not shown the same benefit95. Alternative macromolecular structures that are of interest for cryopreservation include those with trehalose side chains (c)102,103 and FucoPol (d)106. CPAs, cryoprotective agents.
A crucial consideration of polyampholytes is that they are not replacements for DMSO. Post-thaw viabilities of cells with polyampholytes can be high when cryopreserved with both polyampholytes and DMSO together, but are severely limited in the absence of any DMSO. This observed effect implies a synergistic mode of action between polyampholytes and permeating cryoprotectants like DMSO95,97. When combined with DMSO, a synthetically scalable polyampholyte (Fig. 4biv) enabled high post-thaw recovery of up to 90% for A549 monolayers98, as well as stem cells99 and red blood cells100. Polymers with a ‘PEG-like’ backbone and sulfoxide side chains (considering the electron distribution between the sulfur and oxygen atoms, these could be considered to have ‘mixed charges’) have also been evaluated. Under DMSO-free conditions, these polymers gave high immediate post-thaw viabilities, which were far lower than DMSO alone upon culture (60 h)101. The mode of action of these materials is not yet known and chemical probes are required to delineate this function.
As an alternative, trehalose side chain polymers have been evaluated for use in the stabilization of proteins (Fig. 4c) under heat and freeze-dry stress, but the utility of these for cells has not yet been reported102,103. Notably, polymers with trehalose in their main chain (by reaction with epichlorohydrin) do allow some recovery post-thaw, although the membrane integrity of cells stored in this manner was lower than DMSO alone104.
In addition to proteins, a polyxylomannan was reported to modulate ice growth without information regarding the structure–property relationships105. FucoPol, a fucose-rich anionic polysaccharide from Enterobacter A47 (Fig. 4d), has been found to have cryoprotective properties106. At 2.5 mg ml−1, FucoPol increased the post-thaw viabilities of DMSO-cryopreserved cells by 70% (ref.107). As with many emerging cryoprotectants, the exact mechanism is not yet clear and it has minimal effects on ice growth, but was suggested to reduce the extent of supercooling. Exopolysaccharides are frequently found in extremophilic microorganisms and, thus, are recognized as exciting platforms108, with the potential to be obtained in large scales using bioreactors, although their characterization remains challenging owing to their complex structure109.
Ice nucleators
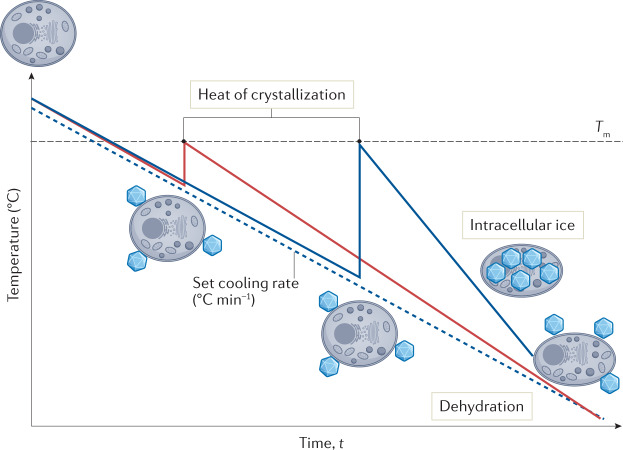
As discussed previously, supercooling, in which a solution will cool far below its equilibrium melting temperature prior to ice nucleation, is a common issue in cryopreservation (Fig. 5). This process is particularly true for small volumes, such as multiwell plates, cryovials and straws, where the probability of the solution containing a contaminant or site for heterogeneous nucleation is lower than for larger volumes110. The ability to control nucleation, which is a stochastic process, and induce nucleation at temperatures close to zero has been shown to increase both cell recovery and reproducibility20, by allowing cells sufficient time to dehydrate and consequently avoid IIF. There are two conceptual approaches to this: external induction (mechanical) and the use of ice-nucleating agents.
Fig. 5. Control of ice nucleation.
Cryopreservation samples generally supercool below the melting point of the solution before heterogeneous ice nucleation (ice formation catalysed by the presence of foreign particles) occurs. As an exothermic process, ice formation leads to heat release (heat of crystallization) and the supercooled solution warms up to close to the melting point. Thermodynamically stable extracellular ice drives dehydration of the cell, leading to a reduced chance of fatal intracellular ice formation. Nucleation at warmer sub-zero temperatures leads to a small heat of crystallization (red line) followed by a gradual return to the set cooling rate (dashed line) and more time for dehydration. This is generally achieved with the addition of ice nucleators. Nucleation at lower temperatures (more supercooling, blue line) leads to a large heat of crystallization, a higher subsequent cooling rate and less time for dehydration, resulting in intracellular ice formation.
Ice mist was used to induce nucleation during cryopreservation of adherent primary somatic cells in 96-well plates, increasing post-thaw viabilities from ~30% without ice mist to 60% (ref.111). Nucleation induced by liquid nitrogen vapour improved cryopreservation of alginate-encapsulated mesenchymal stem cells (MSCs)112 and enabled freezing of human fetal liver MSCs113. Liquid-nitrogen-cooled forceps114 and specific nucleation devices115 have also been used effectively to control nucleation. Despite the obvious benefits, controlled nucleation is often overlooked in cryopreservation protocols, potentially because procedures that include nucleation control can be cumbersome and non-trivial. For instance, physical methods such as ice seeding111,114, electrofreezing116 and shock cooling117 require direct intervention with the material, accurate timing for their use during the cooling profile and are also difficult to automate and scale up. Chemical nucleants, which could be added to the culture media, offer a promising alternative as they do not require specific timing of application, can be applied to automated workflows and would be compatible with a range of cryopreservation vessels, such as plates, vials and cell bags.
Crystalline cholesterol (2.8 mM), which has been shown to nucleate ice as warm as −4 °C (ref.118), was used to induce nucleation in encapsulated liver spheroids119. Cell viability and multiple functional outputs, such as albumin production, all increased when crystalline cholesterol was employed, an effect that was observed in extended culture — up to 72 h post-thaw. In a follow-up study using encapsulated liver spheroids, a strong correlation was identified between higher nucleation temperatures (less supercooling) and improved cell viabilities post-thaw120. Crystalline cholesterol has the benefit of being chemically well defined. Electron microscopy coupled with molecular simulations have suggested that the varied topography of cholesterol crystals may give rise to its broad temperature range (−4 to −20 °C) of ice nucleation activity118. Other steroids, including testosterone and androsterone, have also been shown to nucleate ice at relatively high temperatures (~−7 °C), although their use in cryopreservation applications has been limited121.
Several bacterial species are known to produce ice-nucleating proteins. One of the most studied bacterial protein extracts comes from Pseudomonas syringae, which is sold as the commercial product Snomax as an additive in the artificial production of snow. The cryopreservation outcomes of mouse embryos when frozen with Snomax as a nucleant were compared with using manual nucleation (via application of a liquid-nitrogen-cooled swab) and with no-seeding controls122. Embryos cryopreserved with Snomax in the medium had the same development success as manually seeded embryos (no embryos in the no-seeding group survived). However, there are questions surrounding the biocompatibility of Snomax, particularly in samples intended for clinical use. Attempts have been made to encapsulate Snomax in alginate beads to provide a barrier between the nucleant and the biologics to give a more effective means of removal123.
Feldspars (aluminium tectosilicates) can nucleate ice at temperatures close to zero and may contribute towards cloud formation in the atmosphere124,125. Different types of feldspar are found to have different ice nucleation activities, with K-feldspar (KAlSi3O8) reported to be the most active126, nucleating 50% of water droplets at −22.6 °C in a microlitre droplet assay124. Despite its promising nucleating activity, the low solubility of feldspar in aqueous solutions that means it is difficult to incorporate it into existing cryopreservation protocols, thereby restricting its practical use in cryopreservation.
Considering the potential impact of nucleants in cryopreservation, there is a real need and interest in discovering and developing new ice-nucleating agents. However, few tested compounds nucleate ice and the molecular descriptors for a good nucleator are largely unknown. This is, in part, because the timescale of ice nucleation (nanoseconds) makes it difficult to observe experimentally, and the variation between different known ice nucleators (small molecules versus ice-binding proteins) makes deciphering important characteristics challenging. A number of new materials have been assessed for nucleation control that have not been evaluated in cell cryopreservation protocols, including graphene-oxide-modified hydrophobic polymers127, diamond nanoparticles128 and oxidized carbon nanomaterials129.
As it stands, there is still an unmet need for soluble and sterilizable (can be filtered) chemically defined nucleants, and any material meeting these criteria could have wide-reaching benefits for the field of cryopreservation. As discussed earlier, inhibition of nucleation also has benefits for vitrification.
Methods that use chemical tools
The following sections describe strategies that use chemical tools to improve cryopreservation outcomes, specifically covering cell encapsulation, intracellular CPA delivery and the modulation of biochemical pathways.
Cell encapsulation
Cell encapsulation is now widely used to facilitate ready-to-use materials for tissue engineering applications130 or allow above-zero cell storage for short time periods (up to two weeks)131. In many cases, studies have shown superior outcomes in cryopreservation when cells are encapsulated, compared with non-encapsulated cells132–135.
Alginates are polysaccharides that can be crosslinked by divalent cations, such as calcium and barium, to form hydrogel networks. They are popular for cell encapsulation due to their cytocompatibility, mild crosslinking conditions and low cost. Primary rat neurospheres were cryopreserved in ultrahigh-viscosity calcium alginate microbeads and it was found that microencapsulation retained neurosphere shape, average diameter and membrane integrity, reduced cell disintegration and improved cell viabilities post-thaw136. The investigators attributed the success to reduced mechanical damage to neurospheres, such as loss of cell–cell contacts, during cryopreservation. Alginate–poly-L-lysine–alginate microbeads were generated to encapsulate PC12 cells and the latent heat released at different cooling rates was measured using DSC132. At lower cooling rates (0.5 °C min−1), latent heat release was lower and cell viabilities were higher for microencapsulated cells, compared with those in suspension, suggesting that the microcapsules reduced the degree of ice formation. Although alginate encapsulation is beneficial for the cryopreservation of some cell types, further research is required to elucidate direct structure–activity relationships and the mechanism of protection.
New materials are emerging to utilize encapsulation as a tool for cryopreservation. Alginate gels were modified with an arginine–glycine–aspartic acid–serine (RGDS) peptide, which is an essential recognition site for many adherent cells137. They hypothesized that inclusion of RGDS would mitigate apoptosis by increasing cell attachment and improve cellular responses to CPA loading, as RGDS plays a role in cell and tissue permeability. Mouse embryonic stem cell (mESC) survival, metabolic activity and stem cell markers were all increased post-thaw in RGDS-modified alginates compared with non-modified alginate or free-floating cells. Typical mESC characteristics such as cell spreading and actin remodelling were also observed with RGDS-alginate, but not with unmodified alginate. A thermoreversible supramolecular gel based on a Boc-O-dodecyl-L-tyrosine (BDT) gelator was prepared and self-assembled at 8.2 °C into a hydrogel network138. When used for the cryopreservation of two rat cell lines, PC12 and RSC96, post-thaw viabilities increased from 73% and 68% (without BDT gelator) to 81% and 76%, respectively. Calculations from DSC measurements indicated that the degree of water available for freezing was lower in the BDT gel than in the culture media, meaning that less ice was able to form. Analysis of gel swelling kinetics also revealed that the gel led to slower diffusion rates of DMSO, leading to decreased cell swelling and reduced osmotic shock. The thermoresponsive gel returned to a solution as the temperature was increased, providing a simple method for cell recovery. Dextran-based polyampholytes capable of forming hydrogel networks were made and evaluated for their ability to cryoprotect L929 cells89. Azido-dextran was combined with carboxylated poly-L-lysine to form dextran polyampholytes with azido functionality (azide-Dex-PA). Post-thaw cell viability was highest when 69% of the poly-L-lysine side chain was carboxylated. Increasing the number of amine groups on each dextran unit also led to increased cell viabilities. Similar to previous reports139, fluorescently tagged polyampholyte was found to adsorb onto the cell surface, potentially providing cryoprotection through membrane stabilization. Mixing azide-Dex-PA with dibenzocyclooctyne (DBCO)-modified dextran (DBCO-Dex) produced hydrogels via Cu-free strain-promoted azide–alkyne cycloaddition click chemistry. Further cryopreservation studies demonstrated that the Dex-PA hydrogel afforded cell viabilities of >90%. Cryopreservation tests without the polyampholyte motif produced 0% live cells post-thaw, highlighting that polyampholyte functionality is key to cryopreservation success.
Cryopreserving cells in an encapsulated format enables prefabricated, off-the-shelf materials to be manufactured and stored, such as stem-cell-laden alginate microgels used in the 3D bioprinting of living tissues140. Excitingly, encapsulation has shown success in clinical trials for diabetes treatment through transplantation of encapsulated β-islet cells to improve insulin production141.
Intracellular cryoprotectant delivery
The (non-reducing) disaccharide trehalose can protect mammalian cells during cryopreservation142,143 and is required to be present both extracellularly and intracellularly for optimum cryoprotection120. However, trehalose is largely impermeable to cell membranes and mammalian cells lack transporters to facilitate its intracellular delivery. Poly(L-lysine isophthalate) with pendant L-phenylalanine groups was synthesized to yield an amphipathic biopolymer (PP-50), intended to transiently permeabilize cell membranes to enable trehalose loading144. Application of PP-50 enabled an 85-fold increase in intracellular trehalose concentration of up to 120 mM in ovine red blood cells and improved cryosurvival by 20% compared with extracellular trehalose alone. The biopolymer’s hydrophobicity was key for efficient membrane permeabilization, with less hydrophobic amino acid side chains (leucine and valine) leading to lower trehalose loading. When applying PP-50 to human osteosarcoma cells for DMSO-free cryopreservation, 25 µg ml−1 was the optimum concentration of biopolymer for cryosurvival145. The results were comparable with those achieved with 10% DMSO and PP-50 led to less variation in cell-doubling time post-thaw.
Amphiphilic polymers have been used in applications where permeabilization or lysis of cell membranes is desirable (reviewed in detail by Marie et al.146) and cationic polymers are widely known to interact with cell membranes and lead to greater permeability147. Application of these polymers for intracellular cryoprotectant delivery could offer a useful general strategy; however, the specificity of this approach requires further development. Other emerging chemical methods for intracellular trehalose delivery include the use of nanoparticles148–150, application of metal-attenuated pore-forming proteins151 and engineering lipophilic trehalose152. A comprehensive review of intracellular trehalose delivery is provided by Stewart and He (ref.153). Although other sugars (and osmolytes) can also have a protective effect, the concept of delivery of a cryoprotectant, which would otherwise not function, is an interesting approach that synergizes with drug delivery strategies and the challenges of intracellular delivery of difficult cargoes154. The fate of trehalose after delivery inside of cells requires understanding to further develop this method.
Modulating biochemical pathways
Historically, cryopreservation of biologics has been viewed as a purely physical problem, with solutions focused on controlling and modulating ice growth/nucleation, addressing osmotic changes and cellular dehydration or altering freezing rates to provide superior outcomes. However, cryopreservation is increasingly being viewed from a biochemical perspective such that cold stress can be mitigated through modulation of biochemical pathways, in the same way that one would consider drug discovery for diseases. The following subsections describe compounds, both natural and synthetic, that have demonstrated an ability to modulate biochemical pathways, resulting in improved cryopreservation outcomes.
Osmolytes
Nature has developed biochemical tools for organisms to survive at sub-zero temperatures. Microorganisms are known to produce and accumulate small-molecule osmolytes, such as trehalose155 and proline156 (Fig. 6a) in response to cold temperature exposure. Osmolytes have a physical protective effect, but there is evidence that some of these also play a biochemical role.
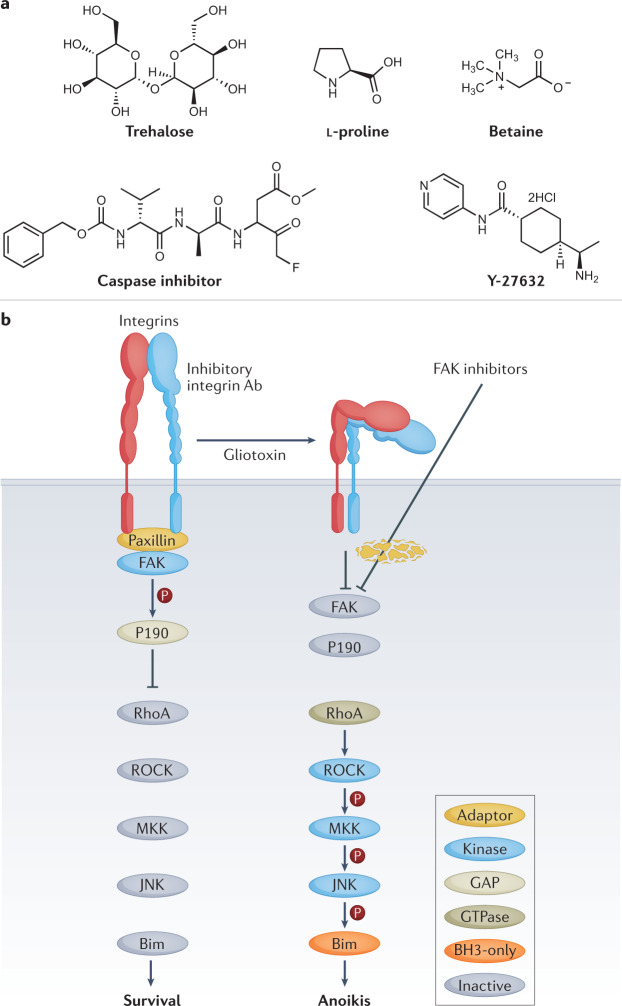
Fig. 6. Modulating biochemical pathways.
a | There is a growing understanding of how biochemical pathways can be modulated to improve cryopreservation outcomes. Naturally occurring osmolytes, such as trehalose, L-proline and betaine, have shown promise in protecting organisms and cells at low temperatures due to modulating biochemical pathways11,159. Improved cryopreservation outcomes have been achieved by applying specific inhibitors for biochemical pathways, such as caspase inhibitors (caspase-1 inhibitor V, also referred to as Z-VAD-FMK) for apoptosis166. b | Activity of Rho-associated coiled kinase (ROCK) has been linked to the biochemical pathway for anoikis174 (pathway diagram reproduced from ref.178) and inhibition of this pathway by ROCK inhibitor Y-27632 has shown benefits for embryonic stem cells after cryopreservation175. Panel b is reprinted from ref.178 under a Creative Commons licence CC BY 4.0.
Feeding drosophila fly larva (Chymomyza costata) with a proline-rich diet enabled their cryopreservation in liquid nitrogen, increasing adult-stage development of cryopreserved larva from 0 (larva fed a diet that is not proline-rich) to 36% (ref.157), with similar results obtained for previously chill-susceptible fruitfly larva (Drosophila melanogaster)158. Preincubation of mouse neuronal cell monolayers with either trehalose or L-proline 24 h prior to freezing led to a significant increase in cell recovery post-thaw, an effect that was improved further when the two osmolytes were combined11. Substituting either osmolyte for sucrose did not provide the same benefit, indicating that the mechanism of action was not simply due to an increase in total solute concentration, but more likely a biochemical one. This proposed mechanism is supported by recent work demonstrating that preincubation of A549 cell monolayers with 200 mM L-proline resulted in 50% cell recovery post-thaw, compared with just 25% in the absence of this L-proline pretreatment159. The proline pretreatment led to a significant decrease in cell metabolic activity before freezing, again suggesting that L-proline was exhibiting a biochemical effect. A similar benefit was observed when L-proline pretreatment was combined with polyproline as a cryoprotective agent56. Betaine (Fig. 6a) has been shown to improve cryopreservation outcomes160 and modulate cellular functions in response to stress, such as increasing proliferation rates of SV-3T3 cells in hyperosmotic environments161, although the exact mode of action is still unknown. Understanding the mechanism behind the biochemical effects of osmolytes will be key to future cryoprotectant development.
Caspase inhibitors
Apoptosis, the process of programmed cell death, has been widely reported as a consequence of cryopreservation, which results in delayed-onset cell death post-thaw10,97,162,163. Caspase proteins are known effectors of apoptosis, therefore the addition of a caspase inhibitor to cryopreservation media was hypothesized to enable improved outcomes164. Addition of 10 µM caspase-1 inhibitor V, also referred to as Z-VAD-FMK (Fig. 6a), to the cryopreservation media of Madin–Darby canine kidney (MDCK) cells led to a modest (10%) but significant increase in living cells post-thaw in optimized cryopreservation solutions165. Higher concentrations (>25 µM) of the inhibitor did not lead to further improvements, suggesting that additional pathways may also be involved. It has been found that caspase-3 is activated as a result of cryopreservation166 and that adding the Z-VAD-FMK to both freezing and thawing solutions of HeLa, Jurkat and 293 kidney carcinoma cells led to significantly enhanced survival. These findings have since been replicated in bovine embryos167, porcine hepatocytes168, primary human hepatocytes169 and ovarian tissue170. Other broad-spectrum caspase inhibitors, such as IDN-1965, have also shown efficacy in cryopreserved porcine hepatocytes171. The cryopreservation outcomes for human MSCs after treatment with Z-VAD-FMK compared with selective caspase inhibitors were investigated172. It appeared that both the intrinsic (mitochondrial) and the extrinsic (death receptor) pathways are activated in response to cryopreservation, as well as the calpain cascade, which likely explains why cell viability improved in the presence of the broad-spectrum inhibitor as opposed to specific caspase inhibitors.
These studies highlight the effectiveness of using a drug-discovery-type approach to cryopreservation. Although caspase inhibition with the broad-spectrum inhibitor Z-VAD-FMK appears to be the most viable approach in modulating apoptosis, care must be taken when considering future applications, particularly for cells destined for clinical use. Aberrant apoptosis inhibition is associated with malignancy in certain cancers173, among other diseases. Therefore, a greater understanding of the specific pathways involved in cryopreserved-induced apoptosis as well as the long-term effects of apoptosis inhibitors on cryopreserved cells would be incredibly useful.
ROCK inhibitors
The Rho-associated coiled kinase (ROCK) active site inhibitor Y-27632 (Fig. 6a) was able to decrease anoikis — dissociation-induced apoptosis — in hESC174. Given that adherent cell types cryopreserved in suspension need to reattach to a substrate post-thaw in order to survive, this finding offers an opportunity to target a ‘druggable’ pathway that could improve cell function post-thaw. When Y-27632 was supplemented into the freezing medium of hESCs, post-thaw cell recoveries increased by 50% compared with untreated controls175 and also led to increased cell adhesion. There was a marked increase in hESC colony formation when Y-27632 was added to the thawing media alone, suggesting that the main impact was not during the cryopreservation process but during thawing. These findings have been corroborated where Y-27632 increased post-thaw survival of hESCs in single suspension, even under challenging conditions such as serum-free and feeder-layer-free culture (feeder layers are typically used to support the growth of embryonic stem cells)176. Cells treated with Y-27632 required longer treatment with Accutase, an enzyme mixture required to remove cells from adherent culture, further suggesting that treatment with the ROCK inhibitor led to improved cell attachment and reduced anoikis. Y-27632 and another ROCK inhibitor, Fasudil, were both able to improve hESC growth post-thaw177. Recently, the fungal virulence factor gliotoxin was used to identify a specific signalling pathway for anoikis to better understand the role of ROCK in anoikis activation178. The investigators demonstrated that inhibition of focal adhesion kinase (FAK) led to activation of a kinase cascade involving the Rho-ROCK-MKK4/MKK7-JNK signalling pathway (Fig. 6b), resulting in anoikis.
Although ROCK inhibitors are not cryoprotectants in the traditional definition, they are a key example of how modulating a biochemical pathway, rather than the cryopreservation process itself, can lead to improved post-thaw outcomes of biological material. Similar concepts could be applied to modulate other pathways, such as oxidative damage through targeted application of antioxidants, as has been shown for spermatogonial stem cells179. It is worth noting that different cell types will have different biochemical pathways; therefore, biochemical approaches to cryopreservation will likely be cell-dependent and tissue-dependent, rather than a one-size-fits-all solution.
Emerging and future discovery methods
The approaches described in this Review outline some of the recent innovations in the pursuit of novel cryoprotectant strategies. However, more detailed structure–activity relationships are necessary in order to discover and develop new cryoprotectants. In addition, linking the mechanisms of protection (for example, ice growth inhibition or controlling nucleation) to the specific biological outcomes and mechanisms quantitatively would be of great value, but this is challenging owing to the complexity of the cryopreservation process.
The use of compound libraries for drug screening applications has revolutionized the process of drug discovery within the pharmaceutical industry180. The combination of intelligent library design, computer modelling, rapid screening assays and advances in genomics has led to a greater understanding of structure–function relationships, particularly when using phenotype or target-driven assays181. This combinatorial discovery approach, utilizing a liquid-handling system, was deployed to create a library of polyampholytes and screen them for cryoprotective activity using red blood cells94. Non-linear relationships between red blood cell recovery and the comonomer composition were identified that would have been missed in traditional targeted synthetic methods, which allowed selection of ‘hit’ polymers to be evaluated in nucleated cell cryopreservation. A matrix approach enabled by liquid-handling robotics was used to screen 54 combinations of DMSO and trehalose for red blood cell cryopreservation. This initial screen helped identify a cryoprotectant mixture at concentrations lower than either CPA is traditionally used, leading to synergistic cryoprotective benefit with minimal toxicity. Addition of a polyampholyte to the mixture mitigated damage further. Novel high-throughput discovery methods are also emerging; phage display coupled with an ice-affinity selection protocol was used to identify new ice-binding peptides182. A lead candidate consisting of a cyclic 14-amino-acid peptide was identified from a mixture of billions of potential peptides, but its use in cryopreservation was not determined. Specific amino acid residues for ice binding were successfully identified using this method.
Combining high-throughput testing with iterative computational algorithms has led to more intuitive cryopreservation screening. Differential evolution algorithms have been used to optimize the cooling rate and cryoprotectant composition for both Jurkat and MSCs183. Feeding experimental results into the algorithm for up to eight generations meant that a multiparametric space was screened efficiently using far fewer experiments than empirical methods. This approach has also been applied to determine optimum DMSO-free cryopreservation solutions for the cryopreservation of hiPSCs184. The total number of experiments was reduced from >1,000 to just eight using a differential evolution algorithm, which allowed for the exploration of a very large design space using minimal consumables and time.
Advances in microfluidics and lab-on-chip platforms have been applied to cryopreservation to investigate a broad spectrum of areas including cell membrane properties, CPA loading and removal, and effects of cooling and warming profiles (reviewed by Zhao and Fu)185. On-chip cell cryopreservation screening assays are developing, where different CPAs can be tested for toxicity and cryoprotection in low-consumable, computer-driven assays186. This technology could provide a method for rapid screening of numerous cryoprotectants to further inform structure–activity relationships.
Molecular modelling techniques offer an opportunity to probe processes that cannot be visualized experimentally due to limits in spatial and temporal resolution, such as capturing rates of water crystallization that lead to homogeneous nucleation187. Modelling also offers the chance to explore the structure–function relationships of cryoprotectants and their interactions with ice, such as probing the ice recrystallization inhibition mechanism of PVA and synthetic copolymers using molecular dynamic simulations188 and assessing the ice nucleation abilities of crystalline surfaces189. Properties of small-molecule cryoprotectants have also been investigated, including the interaction of DMSO with model lipid membranes190, as well as the hydrogen-bonding networks of water with glycerol191. Advances in computational complexity could drive innovation in this field and aid the design of new cryoprotectants.
For vitrification, a greater understanding of cryoprotectant toxicity is needed, beyond simply ‘more is worse’, which is not always the case. An innovative method for identifying genes involved in cryoprotectant toxicity resistance was developed by creating a library of mutant mESCs and exposing them to 9% M22 (a common vitrification solution)192. The investigators identified six mutants that were resistant to M22, with multiple possible pathways involved in cryoprotectant toxicity resistance. Although distinct mechanisms of action were not established, the identification of genes involved in cryoprotectant toxicity resistance provides an opportunity to utilize a pharmacological approach by using druggable targets.
Considering the immense progress in drug discovery afforded using approaches across chemistry (synthesis), automation (robotics) and biochemistry (phage display/DNA-encoded libraries), there are clear opportunities to implement discovery programmes to identify advanced cryoprotectants able to target specific (or multiple) damage pathways. Further, the use of emerging machine learning/artificial intelligence tools will be essential to dissect the complex datasets and non-linear trends typically seen.
Conclusions and outlook
Cryopreservation is, and will remain, an essential tool for biomedical discovery and translational science. Building on the seminal contributions that identified (in particular) DMSO and glycerol, new and more advanced cell/tissue models and therapies will require equally advanced tools to: protect precious biological samples; maximize recovery/function; and ensure the cryopreservation methods align with the cold chain needs. The latter point has been specifically highlighted by the COVID-19 pandemic with the stringent storage requirements of mRNA vaccines.
In this Review, we have introduced how new chemical tools are being developed to address challenges in cryopreservation. We also describe the complex nature of this multivariate problem, in which multiple mechanisms of damage need to be addressed and subtle differences between cell types and freezing methods (slow versus fast) exist. There has been significant interest in learning from extremophiles and either applying their solutions (ice-binding proteins) or learning from these to develop molecules or polymers with advanced cryoprotective function. There is also the need for the analytical tools to enable dissection of function.
To address these challenges, a truly transdisciplinary approach is required, using structural and evolutionary biology, synthetic and computational chemistry, materials discovery and cell biology. There is also the need to find the right material for the problem of interest. For example, a new cryoprotectant that cannot be washed/removed before transfusion to a patient must meet strict regulatory and safety parameters. In contrast, a cryoprotectant used upstream, or in basic research, can be more readily applied and used. In this evolved field, it is clear that chance discoveries alone cannot be relied upon. Therefore, a rational discovery science approach where specific mechanisms (both biochemical and biophysical) are addressed is imperative.
Acknowledgements
K.A.M. thanks the Wellcome Trust and the University of Warwick for supporting a Translational Partnership Fellowship (WT-219429/Z/19/Z). This project (M.I.G.) has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 866056) and the Royal Society for an Industry Fellowship (M.I.G., 191037) with Cytiva.
Glossary
- Phenotypic drift
A gradual change in the observable traits of a cell due to prolonged time in culture.
- Cell therapies
Using a patient’s own cells, or donated cells, to elicit a medicinal response to disease.
- Immunotherapies
Treatments that use a person’s immune system to fight diseases, such as cancer.
- Cold chain
A low-temperature supply chain.
- Cryoprotective agents
(CPAs). Compounds added to freezing solutions to provide a protective effect to the material being frozen.
- Ice recrystallization
An increase in ice crystal size over time in an already frozen material.
- Glass transition temperature
The temperature at which materials transition from rigid to flexible.
- Super-flash freezing
Cooling at an incredibly high cooling rate, so the liquid freezes almost instantly.
- Differential scanning calorimetry
(DSC). A thermal analysis technique used to study the heat flow of a sample.
- Ice-binding proteins
Molecules found in a diverse range of organisms that bind to the interface between ice and water.
- Thermal hysteresis
A difference in the melting and freezing temperatures.
- Exopolysaccharides
Macromolecules secreted from microorganisms.
- Extremophilic
A propensity for extreme environments, such as organisms that survive at very high or very low temperatures.
- Heterogeneous ice nucleation
Ice crystallization induced by the presence of foreign particles.
- Ice seeding
Introducing microscopic ice crystals to induce nucleation in a supercooled liquid.
- Electrofreezing
Applying an electric current to induce ice nucleation in a supercooled liquid.
- Shock cooling
Applying external force or rapid temperature changes to induce nucleation in a supercooled liquid.
- Cryosurvival
The ability to survive exposure to cold temperatures, often referring to temperatures far below 0 °C.
- Caspase
Protease enzyme involved in the activation and execution of apoptosis.
- Calpain cascade
A signalling cascade driven by calcium-dependent cysteine proteases, known as calpains.
- Lab-on-chip
A miniature microchip device used to integrate several processes that are typically performed in a laboratory, such as chemical or biological analyses.
Peer review
Peer review information
Nature Reviews Chemistry thanks J. Elliott, A. Higgins and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
M.I.G. is co-founder and shareholder of Cryologyx Ltd, which is developing cryopreservation technology.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giwa S, et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 2017;35:530–542. doi: 10.1038/nbt.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler C. The Svalbard seed vault and crop security. Bioscience. 2008;58:190–191. doi: 10.1641/B580302. [DOI] [Google Scholar]
- 3.Geraghty RJ, et al. Guidelines for the use of cell lines in biomedical research. Br. J. Cancer. 2014;111:1021–1046. doi: 10.1038/bjc.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swioklo S, Constantinescu A, Connon C. Alginate-encapsulation for the improved hypothermic preservation of human adipose-derived stem cells. Stem Cell Transl. Med. 2016;5:339–349. doi: 10.5966/sctm.2015-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polge C, Smith AU, Parkes A. Revival of spermatozoa after vitrification and dehydration. Nature. 1949;164:666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 6.Lovelock JE, Bishop MW. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183:1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- 7.Scott KL, Lecak J, Acker JP. Biopreservation of red blood cells: past, present, and future. Transfus. Med. Rev. 2005;19:127–142. doi: 10.1016/j.tmrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Lysak D, et al. Long-term cryopreservation does not affect quality of peripheral blood stem cell grafts: a comparative study of native, short-term and long-term cryopreserved haematopoietic stem cells. Cell Transpl. 2021;30:09636897211036004. doi: 10.1177/09636897211036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panch SR, et al. Effect of cryopreservation on autologous chimeric antigen receptor T cell characteristics. Mol. Ther. 2019;27:1275–1285. doi: 10.1016/j.ymthe.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, et al. The roles of apoptotic pathways in the low recovery rate after cryopreservation of dissociated human embryonic stem cells. Biotechnol. Prog. 2009;26:827–837. doi: 10.1002/btpr.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey TL, et al. Protective effects of osmolytes in cryopreserving adherent neuroblastoma (Neuro-2a) cells. Cryobiology. 2015;71:472–480. doi: 10.1016/j.cryobiol.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Eskandari N, Marquez-Curtis LA, McGann LE, Elliott JAW. Cryopreservation of human umbilical vein and porcine corneal endothelial cell monolayers. Cryobiology. 2018;85:63–72. doi: 10.1016/j.cryobiol.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Marquez-Curtis LA, Bokenfohr R, McGann LE, Elliott JAW. Cryopreservation of human cerebral microvascular endothelial cells and astrocytes in suspension and monolayers. PLoS One. 2021;16:e0249814. doi: 10.1371/journal.pone.0249814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagerberg, J. W. in Cryopreservation and Freeze-Drying Protocols (eds Wolkers, W. F. & Oldenhof, H.) 353–367 (Springer, 2015).
- 15.Verheijen M, et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019;9:4641–4653. doi: 10.1038/s41598-019-40660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler S, Pellizzer C, Paparella M, Hartung T, Bremer S. The effects of solvents on embryonic stem cell differentiation. Toxicol. In Vitro. 2006;20:265–271. doi: 10.1016/j.tiv.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi R, et al. Plasticizer concentration in cord blood cryopreserved with DMSO. Bone Marrow Transpl. 2014;49:157–158. doi: 10.1038/bmt.2013.135. [DOI] [PubMed] [Google Scholar]
- 18.Morris C, et al. Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. Transfusion. 2014;54:2514–2522. doi: 10.1111/trf.12759. [DOI] [PubMed] [Google Scholar]
- 19.Maral S, et al. Dimethyl sulfoxide-induced tonic-clonic seizure and cardiac arrest during infusion of autologous peripheral blood stem cells. Cell Tissue Bank. 2018;19:831–832. doi: 10.1007/s10561-018-9718-x. [DOI] [PubMed] [Google Scholar]
- 20.John Morris G, Acton E. Controlled ice nucleation in cryopreservation–a review. Cryobiology. 2013;66:85–92. doi: 10.1016/j.cryobiol.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 22.Deller RC, Vatish M, Mitchell DA, Gibson MI. Synthetic polymers enable non-vitreous cellular cryopreservation by reducing ice crystal growth during thawing. Nat. Commun. 2014;5:3244. doi: 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- 23.Cao E, Chen Y, Cui Z, Foster PR. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng. 2003;82:684–690. doi: 10.1002/bit.10612. [DOI] [PubMed] [Google Scholar]
- 24.Rienzi L, et al. Polscope analysis of meiotic spindle changes in living metaphase II human oocytes during the freezing and thawing procedures. Hum. Reprod. 2004;19:655–659. doi: 10.1093/humrep/deh101. [DOI] [PubMed] [Google Scholar]
- 25.Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo-Lett. 2004;25:375–388. [PubMed] [Google Scholar]
- 26.Elliott GD, Wang S, Fuller BJ. Cryoprotectants: a review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74–91. doi: 10.1016/j.cryobiol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil. Steril. 2020;113:241–247. doi: 10.1016/j.fertnstert.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Rienzi L, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahy GM, et al. Physical and biological aspects of renal vitrification. Organogenesis. 2009;5:167–175. doi: 10.4161/org.5.3.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuleshova LL, Gouk SS, Hutmacher DW. Vitrification as a prospect for cryopreservation of tissue-engineered constructs. Biomaterials. 2007;28:1585–1596. doi: 10.1016/j.biomaterials.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 31.Jomha NM, et al. Vitrification of intact human articular cartilage. Biomaterials. 2012;33:6061–6068. doi: 10.1016/j.biomaterials.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Yong KW, Laouar L, Elliott JAW, Jomha NM. Review of non-permeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology. 2020;96:1–11. doi: 10.1016/j.cryobiol.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Fahy GM, Wowk B, Wu J, Paynter S. Improved vitrification solutions based on the predictability of vitrification solution toxicity. Cryobiology. 2004;48:22–35. doi: 10.1016/j.cryobiol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Fahy GM. Cryoprotectant toxicity neutralization. Cryobiology. 2010;60:S45–S53. doi: 10.1016/j.cryobiol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Warner RM, et al. Rapid quantification of multi-cryoprotectant toxicity using an automated liquid handling method. Cryobiology. 2021;98:219–232. doi: 10.1016/j.cryobiol.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demirci U, Montesano G. Cell encapsulating droplet vitrification. Lab Chip. 2007;7:1428–1433. doi: 10.1039/b705809h. [DOI] [PubMed] [Google Scholar]
- 37.Dou R, Saunders RE, Mohamet L, Ward CM, Derby B. High throughput cryopreservation of cells by rapid freezing of sub-Μl drops using inkjet printing-cryoprinting. Lab Chip. 2015;15:3503–3513. doi: 10.1039/C5LC00674K. [DOI] [PubMed] [Google Scholar]
- 38.Akiyama Y, Shinose M, Watanabe H, Yamada S, Kanda Y. Cryoprotectant-free cryopreservation of mammalian cells by superflash freezing. Proc. Natl Acad. Sci. USA. 2019;116:7738–7743. doi: 10.1073/pnas.1808645116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manuchehrabadi N, et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017;9:eaah4586. doi: 10.1126/scitranslmed.aah4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito A, et al. Magnetic heating of nanoparticles as a scalable cryopreservation technology for human induced pluripotent stem cells. Sci. Rep. 2020;10:13605. doi: 10.1038/s41598-020-70707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Z, et al. Preparation of scalable silica-coated iron oxide nanoparticles for nanowarming. Adv. Sci. 2020;7:1901624. doi: 10.1002/advs.201901624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Zhao G, Chen Z, Panhwar F, He X. Dual suppression effect of magnetic induction heating and microencapsulation on ice crystallization enables low-cryoprotectant vitrification of stem cell–alginate hydrogel constructs. ACS Appl. Mater. Interfaces. 2018;10:16822–16835. doi: 10.1021/acsami.8b04496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wowk B, et al. Vitrification enhancement by synthetic ice blocking agents. Cryobiology. 2000;40:228–236. doi: 10.1006/cryo.2000.2243. [DOI] [PubMed] [Google Scholar]
- 44.Biggs CI, et al. Polymer mimics of biomacromolecular antifreezes. Nat. Commun. 2017;8:1546. doi: 10.1038/s41467-017-01421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang HY, Inada T, Funakoshi K, Lu SS. Inhibition of nucleation and growth of ice by poly(vinyl alcohol) in vitrification solution. Cryobiology. 2009;59:83–89. doi: 10.1016/j.cryobiol.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Matsumura K, Bae JY, Kim HH, Hyon SH. Effective vitrification of human induced pluripotent stem cells using carboxylated ε-poly-l-lysine. Cryobiology. 2011;63:76–83. doi: 10.1016/j.cryobiol.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Matsumura K, Hayashi F, Nagashima T, Rajan R, Hyon S-H. Molecular mechanisms of cell cryopreservation with polyampholytes studied by solid-state NMR. Commun. Mater. 2021;2:15. doi: 10.1038/s43246-021-00118-1. [DOI] [Google Scholar]
- 48.Ota A, Matsumura K, Lee JJ, Sumi S, Hyon SH. StemCell Keep™ is effective for cryopreservation of human embryonic stem cells by vitrification. Cell Transpl. 2017;26:773–787. doi: 10.3727/096368916X692654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamoshita M, et al. Successful vitrification of pronuclear-stage pig embryos with a novel cryoprotective agent, carboxylated ε-poly-l-lysine. PLoS One. 2017;12:e0176711. doi: 10.1371/journal.pone.0176711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumura K, et al. Molecular design of polyampholytes for vitrification-induced preservation of three-dimensional cell constructs without using liquid nitrogen. Biomacromolecules. 2020;21:3017–3025. doi: 10.1021/acs.biomac.0c00293. [DOI] [PubMed] [Google Scholar]
- 51.Bar Dolev M, Braslavsky I, Davies PL. Ice-binding proteins and their function. Annu. Rev. Biochem. 2016;85:515–542. doi: 10.1146/annurev-biochem-060815-014546. [DOI] [PubMed] [Google Scholar]
- 52.Budke C, et al. Quantitative efficacy classification of ice recrystallization inhibition agents. Cryst. Growth Des. 2014;14:4285–4294. doi: 10.1021/cg5003308. [DOI] [Google Scholar]
- 53.Biggs CI, et al. Mimicking the ice recrystallization activity of biological antifreezes. When is a new polymer “active”? Macromol. Biosci. 2019;19:1900082. doi: 10.1002/mabi.201900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carpenter JF, Hansen TN. Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proc. Natl Acad. Sci. USA. 1992;89:8953–8957. doi: 10.1073/pnas.89.19.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Y, Liu J, Li Z, Wang J, Huang Y. Nonionic and water-soluble poly(d/l-serine) as a promising biomedical polymer for cryopreservation. ACS Appl. Mater. Interfaces. 2021;13:18454–18461. doi: 10.1021/acsami.0c22308. [DOI] [PubMed] [Google Scholar]
- 56.Graham B, et al. Polyproline as a minimal antifreeze protein mimic that enhances the cryopreservation of cell monolayers. Angew. Chem. Int. Ed. 2017;56:15941–15944. doi: 10.1002/anie.201706703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin Q, et al. Bioinspired l-proline oligomers for the cryopreservation of oocytes via controlling ice growth. ACS Appl. Mater. Interfaces. 2020;12:18352–18362. doi: 10.1021/acsami.0c02719. [DOI] [PubMed] [Google Scholar]
- 58.Mochizuki K, Molinero V. Antifreeze glycoproteins bind reversibly to ice via hydrophobic groups. J. Am. Chem. Soc. 2018;140:4803–4811. doi: 10.1021/jacs.7b13630. [DOI] [PubMed] [Google Scholar]
- 59.Graham B, Fayter AER, Houston JE, Evans RC, Gibson MI. Facially amphipathic glycopolymers inhibit ice recrystallization. J. Am. Chem. Soc. 2018;140:5682–5685. doi: 10.1021/jacs.8b02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Georgiou PG, et al. Polymer self-assembly induced enhancement of ice recrystallization inhibition. J. Am. Chem. Soc. 2021;143:7449–7461. doi: 10.1021/jacs.1c01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balcerzak AK, Febbraro M, Ben RN. The importance of hydrophobic moieties in ice recrystallization inhibitors. RSC Adv. 2013;9:3232–3236. doi: 10.1039/c3ra23220d. [DOI] [Google Scholar]
- 62.Voets IK. From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter. 2017;13:4808–4823. doi: 10.1039/C6SM02867E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruneberg AK, et al. Ice recrystallization inhibition activity varies with ice-binding protein type and does not correlate with thermal hysteresis. Cryobiology. 2021;99:28–39. doi: 10.1016/j.cryobiol.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Olijve LLC, et al. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl Acad. Sci. 2016;113:3740–3745. doi: 10.1073/pnas.1524109113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eniade A, Purushotham M, Ben RN, Wang JB, Horwath K. A serendipitous discovery of antifreeze protein-specific activity in C-linked antifreeze glycoprotein analogs. Cell Biochem. Biophys. 2003;38:115–124. doi: 10.1385/CBB:38:2:115. [DOI] [PubMed] [Google Scholar]
- 66.Tachibana Y, et al. Antifreeze glycoproteins: elucidation of the structural motifs that are essential for antifreeze activity. Angew. Chem. Int. Ed. 2004;43:856–862. doi: 10.1002/anie.200353110. [DOI] [PubMed] [Google Scholar]
- 67.Briard JG, et al. Small molecule ice recrystallization inhibitors mitigate red blood cell lysis during freezing, transient warming and thawing. Sci. Rep. 2016;6:23619. doi: 10.1038/srep23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capicciotti CJ, et al. Potent inhibition of ice recrystallization by low molecular weight carbohydrate-based surfactants and hydrogelators. Chem. Sci. 2012;3:1408–1416. doi: 10.1039/c2sc00885h. [DOI] [Google Scholar]
- 69.Congdon T, Notman R, Gibson MI. Antifreeze (glyco)protein mimetic behavior of poly(vinyl alcohol): detailed structure ice recrystallization inhibition activity study. Biomacromolecules. 2013;14:1578–1586. doi: 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- 70.Chaytor JL, Ben RN. Assessing the ability of a short fluorinated antifreeze glycopeptide and a fluorinated carbohydrate derivative to inhibit ice recrystallization. Bioorg. Med. Chem. Lett. 2010;20:5251–5254. doi: 10.1016/j.bmcl.2010.06.148. [DOI] [PubMed] [Google Scholar]
- 71.Poisson JS, Acker JP, Briard JG, Meyer JE, Ben RN. Modulating intracellular ice growth with cell-permeating small-molecule ice recrystallization inhibitors. Langmuir. 2019;35:7452–7458. doi: 10.1021/acs.langmuir.8b02126. [DOI] [PubMed] [Google Scholar]
- 72.Tomas RMF, Bailey TL, Hasan M, Gibson MI. Extracellular antifreeze protein significantly enhance the cryopreservation of cell monolayers. Biomacromolecules. 2019;20:3864–3872. doi: 10.1021/acs.biomac.9b00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acker JP, Larese A, Yang H, Petrenko A, McGann LE. Intracellular ice formation is affected by cell interactions. Cryobiology. 1999;38:363–371. doi: 10.1006/cryo.1999.2179. [DOI] [PubMed] [Google Scholar]
- 74.Geng H, et al. Graphene oxide restricts growth and recrystallization of ice crystals. Angew. Chem. Int. Ed. 2017;56:997–1001. doi: 10.1002/anie.201609230. [DOI] [PubMed] [Google Scholar]
- 75.Chaytor JL, et al. Inhibiting ice recrystallization and optimization of cell viability after cryopreservation. Glycobiology. 2012;22:123–133. doi: 10.1093/glycob/cwr115. [DOI] [PubMed] [Google Scholar]
- 76.Lautner L, Himmat S, Acker JP, Nagendran J. The efficacy of ice recrystallization inhibitors in rat lung cryopreservation using a low cost technique for ex vivo subnormothermic lung perfusion. Cryobiology. 2020;97:93–100. doi: 10.1016/j.cryobiol.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Li T, Zhao Y, Zhong Q, Wu T. Inhibiting ice recrystallization by nanocelluloses. Biomacromolecules. 2019;20:1667–1674. doi: 10.1021/acs.biomac.9b00027. [DOI] [PubMed] [Google Scholar]
- 78.Mitchell DE, et al. Antifreeze protein mimetic metallohelices with potent ice recrystallization inhibition activity. J. Am. Chem. Soc. 2017;139:9835–9838. doi: 10.1021/jacs.7b05822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drori R, et al. A supramolecular ice growth inhibitor. J. Am. Chem. Soc. 2016;138:13396–13401. doi: 10.1021/jacs.6b08267. [DOI] [PubMed] [Google Scholar]
- 80.Xue B, et al. Bioinspired ice growth inhibitors based on self-assembling peptides. ACS Macro Lett. 2019;8:1383–1390. doi: 10.1021/acsmacrolett.9b00610. [DOI] [PubMed] [Google Scholar]
- 81.MacDonald MJ, Cornejo NR, Gellman SH. Inhibition of ice recrystallization by nylon-3 polymers. ACS Macro Lett. 2017;6:695–699. doi: 10.1021/acsmacrolett.7b00396. [DOI] [PubMed] [Google Scholar]
- 82.He Z, Liu K, Wang J. Bioinspired materials for controlling ice nucleation, growth, and recrystallization. Acc. Chem. Res. 2018;51:1082–1091. doi: 10.1021/acs.accounts.7b00528. [DOI] [PubMed] [Google Scholar]
- 83.Fayter AER, Hasan M, Congdon TRTR, Kontopoulou I, Gibson MIMI. Ice recrystallisation inhibiting polymers prevent irreversible protein aggregation during solvent-free cryopreservation as additives and as covalent polymer-protein conjugates. Eur. Polym. J. 2020;140:110036. doi: 10.1016/j.eurpolymj.2020.110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas MJG, Parry ES, Nash SG, Bell SH. A method for the cryopreservation of red blood cells using hydroxyethyl starch as a cryoprotectant. Transfus. Sci. 1996;17:385–396. doi: 10.1016/0955-3886(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 85.Park S, Lee DR, Nam JS, Ahn CW, Kim H. Fetal bovine serum-free cryopreservation methods for clinical banking of human adipose-derived stem cells. Cryobiology. 2018;81:65–73. doi: 10.1016/j.cryobiol.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 86.Halberstadt M, Athmann S, Hagenah M. Corneal cryopreservation with dextran. Cryobiology. 2001;43:71–80. doi: 10.1006/cryo.2001.2342. [DOI] [PubMed] [Google Scholar]
- 87.O’Neil L, Paynter SJ, Fuller BJ, Shaw RW. Vitrification of mature mouse oocytes in dimethylsulphoxide: improved results following the addition of polyethylene glycol but not dextran. Cryobiology. 1997;34:295–301. doi: 10.1006/cryo.1997.2007. [DOI] [PubMed] [Google Scholar]
- 88.Matsumura K, Hyon SH. Polyampholytes as low toxic efficient cryoprotective agents with antifreeze protein properties. Biomaterials. 2009;30:4842–4849. doi: 10.1016/j.biomaterials.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 89.Jain M, Rajan R, Hyon SH, Matsumura K. Hydrogelation of dextran-based polyampholytes with cryoprotective properties via click chemistry. Biomater. Sci. 2014;2:308–317. doi: 10.1039/C3BM60261C. [DOI] [PubMed] [Google Scholar]
- 90.Mitchell DE, Cameron NR, Gibson MI. Rational, yet simple, design and synthesis of an antifreeze-protein inspired polymer for cellular cryopreservation. Chem. Commun. 2015;51:12977–12980. doi: 10.1039/C5CC04647E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stubbs C, Lipecki J, Gibson MI. Regioregular alternating polyampholytes have enhanced biomimetic ice recrystallization activity compared to random copolymers and the role of side chain versus main chain hydrophobicity. Biomacromolecules. 2017;18:295–302. doi: 10.1021/acs.biomac.6b01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maehara M, et al. Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnol. 2013;13:58. doi: 10.1186/1472-6750-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajan R, Hayashi F, Nagashima T, Matsumura K. Toward a molecular understanding of the mechanism of cryopreservation by polyampholytes: cell membrane interactions and hydrophobicity. Biomacromolecules. 2016;17:1882–1893. doi: 10.1021/acs.biomac.6b00343. [DOI] [PubMed] [Google Scholar]
- 94.Stubbs C, Murray KA, Ishibe T, Mathers RT, Gibson MI. Combinatorial biomaterials discovery strategy to identify new macromolecular cryoprotectants. ACS Macro Lett. 2020;9:290–294. doi: 10.1021/acsmacrolett.0c00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao J, Johnson MA, Fisher R, Burke NAD, Stöver HDH. Synthetic polyampholytes as macromolecular cryoprotective agents. Langmuir. 2019;35:1807–1817. doi: 10.1021/acs.langmuir.8b01602. [DOI] [PubMed] [Google Scholar]
- 96.Stubbs C, Bailey TL, Murray K, Gibson MI. Polyampholytes as emerging macromolecular cryoprotectants. Biomacromolecules. 2020;21:7–17. doi: 10.1021/acs.biomac.9b01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murray KA, Gibson MI. Post-thaw culture and measurement of total cell recovery is crucial in the evaluation of new macromolecular cryoprotectants. Biomacromolecules. 2020;21:2864–2873. doi: 10.1021/acs.biomac.0c00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bailey TL, et al. A synthetically scalable poly(ampholyte) which dramatically enhances cellular cryopreservation. Biomacromolecules. 2019;20:3104–3114. doi: 10.1021/acs.biomac.9b00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murray KA, Tomás RMF, Gibson MI. Low DMSO cryopreservation of stem cells enabled by macromolecular cryoprotectants. ACS Appl. Bio Mater. 2020;3:5627–5632. doi: 10.1021/acsabm.0c00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murray A, Congdon TR, Tomás RMF, Kilbride P, Gibson MI. Red blood cell cryopreservation with minimal post-thaw lysis enabled by a synergistic combination of a cryoprotecting polyampholyte with DMSO/trehalose. Biomacromolecules. 2022;23:467–477. doi: 10.1021/acs.biomac.1c00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burkey AA, et al. Mechanism of polymer-mediated cryopreservation using poly(methyl glycidyl sulfoxide) Biomacromolecules. 2020;21:3047–3055. doi: 10.1021/acs.biomac.0c00392. [DOI] [PubMed] [Google Scholar]
- 102.Mancini RJ, Lee J, Maynard HD. Trehalose glycopolymers for stabilization of protein conjugates to environmental stressors. J. Am. Chem. Soc. 2012;134:8474–8479. doi: 10.1021/ja2120234. [DOI] [PubMed] [Google Scholar]
- 103.Lee J, et al. Trehalose glycopolymers as excipients for protein stabilization. Biomacromolecules. 2013;14:2561–2569. doi: 10.1021/bm4003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diaz-Dussan D, et al. Trehalose-based polyethers for cryopreservation and three-dimensional cell scaffolds. Biomacromolecules. 2020;21:1264–1273. doi: 10.1021/acs.biomac.0c00018. [DOI] [PubMed] [Google Scholar]
- 105.Walters KR, et al. A nonprotein thermal hysteresis-producing xylomannan antifreeze in the freeze-tolerant Alaskan beetle Upis ceramboides. Proc. Natl Acad. Sci. USA. 2009;106:20210–20215. doi: 10.1073/pnas.0909872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guerreiro BM, et al. Demonstration of the cryoprotective properties of the fucose-containing polysaccharide FucoPol. Carbohydr. Polym. 2020;245:116500. doi: 10.1016/j.carbpol.2020.116500. [DOI] [PubMed] [Google Scholar]
- 107.Guerreiro BM, et al. Development of a cryoprotective formula based on the fucose-containing polysaccharide FucoPol. ACS Appl. Bio Mater. 2021;4:4800–4808. doi: 10.1021/acsabm.1c00007. [DOI] [PubMed] [Google Scholar]
- 108.Wang J, Salem DR, Sani RK. Extremophilic exopolysaccharides: a review and new perspectives on engineering strategies and applications. Carbohydr. Polym. 2019;205:8–26. doi: 10.1016/j.carbpol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 109.Casillo A, et al. Structure-activity relationship of the exopolysaccharide from a psychrophilic bacterium: a strategy for cryoprotection. Carbohydr. Polym. 2017;156:364–371. doi: 10.1016/j.carbpol.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bigg EK. The supercooling of water. Proc. Phys. Soc. B. 1953;66:688–694. doi: 10.1088/0370-1301/66/8/309. [DOI] [Google Scholar]
- 111.Daily MI, et al. Cryopreservation of primary cultures of mammalian somatic cells in 96-well plates benefits from control of ice nucleation. Cryobiology. 2020;93:62–69. doi: 10.1016/j.cryobiol.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pravdyuk AI, Petrenko YA, Fuller BJ, Petrenko AY. Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology. 2013;66:215–222. doi: 10.1016/j.cryobiol.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 113.Skorobogatova NG, Novikov AN, Fuller BJ, Petrenko AY. Importance of a three-stage cooling regime and induced ice nucleation during cryopreservation on colony-forming potential and differentiation in mesenchymal stem/progenitor cells from human fetal liver. Cryo Lett. 2010;31:371–379. [PubMed] [Google Scholar]
- 114.Ware CB, Nelson AM, Blau CA. Controlled-rate freezing of human ES cells. Biotechniques. 2005;38:879–883. doi: 10.2144/05386ST01. [DOI] [PubMed] [Google Scholar]
- 115.Lauterboeck L, Hofmann N, Mueller T, Glasmacher B. Active control of the nucleation temperature enhances freezing survival of multipotent mesenchymal stromal cells. Cryobiology. 2015;71:384–390. doi: 10.1016/j.cryobiol.2015.10.145. [DOI] [PubMed] [Google Scholar]
- 116.Petersen A, Schneider H, Rau G, Glasmacher B. A new approach for freezing of aqueous solutions under active control of the nucleation temperature. Cryobiology. 2006;53:248–257. doi: 10.1016/j.cryobiol.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 117.Kilbride P, et al. Recovery and post-thaw assessment of human umbilical cord blood cryopreserved as quality control segments and bulk samples. Biol. Blood Marrow Transpl. 2019;25:2447–2453. doi: 10.1016/j.bbmt.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Sosso GC, et al. Unravelling the origins of ice nucleation on organic crystals. Chem. Sci. 2018;9:8077–8088. doi: 10.1039/C8SC02753F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Massie I, Selden C, Hodgson H, Fuller B. Cryopreservation of encapsulated liver spheroids for a bioartificial liver: reducing latent cryoinjury using an ice nucleating agent. Tissue Eng. Part C Methods. 2011;17:765–774. doi: 10.1089/ten.tec.2010.0394. [DOI] [PubMed] [Google Scholar]
- 120.Massie I, et al. GMP cryopreservation of large volumes of cells for regenerative medicine: active control of the freezing process. Tissue Eng. Part C Methods. 2014;20:693–702. doi: 10.1089/ten.tec.2013.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Head RB. Steroids as ice nucleators. Nature. 1961;191:1058–1059. doi: 10.1038/1911058a0. [DOI] [PubMed] [Google Scholar]
- 122.Teixeira M, et al. Ice nucleating agents allow embryo freezing without manual seeding. Theriogenology. 2017;104:173–178. doi: 10.1016/j.theriogenology.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 123.Weng L, Tessier SN, Swei A, Stott SL, Toner M. Controlled ice nucleation using freeze-dried Pseudomonas syringae encapsulated in alginate beads. Cryobiology. 2017;75:1–6. doi: 10.1016/j.cryobiol.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Atkinson JD, et al. The importance of feldspar for ice nucleation by mineral dust in mixed-phase clouds. Nature. 2013;498:355–358. doi: 10.1038/nature12278. [DOI] [PubMed] [Google Scholar]
- 125.Yakobi-Hancock JD, Ladino LA, Abbatt JPD. Feldspar minerals as efficient deposition ice nuclei. Atmos. Chem. Phys. 2013;13:11175–11185. doi: 10.5194/acp-13-11175-2013. [DOI] [Google Scholar]
- 126.Zolles T, et al. Identification of ice nucleation active sites on feldspar dust particles. J. Phys. Chem. A. 2015;119:2692–2700. doi: 10.1021/jp509839x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Biggs CI, et al. Impact of sequential surface-modification of graphene oxide on ice nucleation. Phys. Chem. Chem. Phys. 2017;19:21929–21932. doi: 10.1039/C7CP03219F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han X, Ma HB, Wilson C, Critser JK. Effects of nanoparticles on the nucleation and devitrification temperatures of polyol cryoprotectant solutions. Microfluid. Nanofluidics. 2008;4:357–361. doi: 10.1007/s10404-007-0186-z. [DOI] [Google Scholar]
- 129.Whale TF, Rosillo-Lopez M, Murray BJ, Salzmann CG. Ice nucleation properties of oxidized carbon nanomaterials. J. Phys. Chem. Lett. 2015;6:3012–3016. doi: 10.1021/acs.jpclett.5b01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cheng Y, Yu Y, Zhang Y, Zhao G, Zhao Y. Cold-responsive nanocapsules enable the sole-cryoprotectant-trehalose cryopreservation of β cell–laden hydrogels for diabetes treatment. Small. 2019;15:1904290. doi: 10.1002/smll.201904290. [DOI] [PubMed] [Google Scholar]
- 131.Canton I, et al. Mucin-inspired thermoresponsive synthetic hydrogels induce stasis in human pluripotent stem cells and human embryos. ACS Cent. Sci. 2016;2:65–74. doi: 10.1021/acscentsci.5b00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsumoto Y, Morinaga Y, Ujihira M, Oka K, Tanishita K. Improvement in the viability of cryopreserved cells by microencapsulation. JSME Int. J. Ser. C. 2001;44:580–587. doi: 10.1299/jsmec.44.937. [DOI] [Google Scholar]
- 133.Hardikar AA, Risbud MV, Bhonde RR. Improved post-cryopreservation recovery following encapsulation of islets in chitosan-alginate microcapsules. Transplant. Proc. 2000;32:824–825. doi: 10.1016/S0041-1345(00)00995-7. [DOI] [PubMed] [Google Scholar]
- 134.Cao K, et al. Hydrogel microfiber encapsulation enhances cryopreservation of human red blood cells with low concentrations of glycerol. Biopreserv. Biobank. 2020;18:228–234. doi: 10.1089/bio.2020.0003. [DOI] [PubMed] [Google Scholar]
- 135.Cui ZK, et al. Characteristics of neural growth and cryopreservation of the dorsal root ganglion using three-dimensional collagen hydrogel culture versus conventional culture. Neural Regen. Res. 2021;16:1856–1864. doi: 10.4103/1673-5374.306097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Malpique R, et al. Alginate encapsulation as a novel strategy for the cryopreservation of neurospheres. Tissue Eng. Part C Methods. 2010;16:965–977. doi: 10.1089/ten.tec.2009.0660. [DOI] [PubMed] [Google Scholar]
- 137.Sambu S, Xu X, Schiffter HA, Cui ZF, Ye H. RGDS-fuctionalized alginates improve the survival rate of encapsulated embryonic stem cells during cryopreservation. Cryo Lett. 2011;32:389–401. [PubMed] [Google Scholar]
- 138.Zeng J, et al. A supramolecular gel approach to minimize the neural cell damage during cryopreservation process. Macromol. Biosci. 2016;16:363–370. doi: 10.1002/mabi.201500277. [DOI] [PubMed] [Google Scholar]
- 139.Matsumura K, Hayashi F, Nagashima T, Hyon SH. Long-term cryopreservation of human mesenchymal stem cells using carboxylated poly-l-lysine without the addition of proteins or dimethyl sulfoxide. J. Biomater. Sci. Polym. Ed. 2013;24:1484–1497. doi: 10.1080/09205063.2013.771318. [DOI] [PubMed] [Google Scholar]
- 140.Jeon O, Lee YB, Hinton TJ, Feinberg AW, Alsberg E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019;12:61–70. doi: 10.1016/j.mtchem.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jacobs-Tulleneers-Thevissen D, et al. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56:1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- 142.Eroglu A, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat. Biotechnol. 1999;18:163–167. doi: 10.1038/72608. [DOI] [PubMed] [Google Scholar]
- 143.Eroglu A, Toner M, Toth TL. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil. Steril. 2002;77:152–158. doi: 10.1016/S0015-0282(01)02959-4. [DOI] [PubMed] [Google Scholar]
- 144.Lynch AL, et al. Biopolymer mediated trehalose uptake for enhanced erythrocyte cryosurvival. Biomaterials. 2010;31:6096–6103. doi: 10.1016/j.biomaterials.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 145.Sharp DMC, et al. Amphipathic polymer-mediated uptake of trehalose for dimethyl sulfoxide-free human cell cryopreservation. Cryobiology. 2013;67:305–311. doi: 10.1016/j.cryobiol.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marie E, Sagan S, Cribier S, Tribet C. Amphiphilic macromolecules on cell membranes: from protective layers to controlled permeabilization. J. Membr. Biol. 2014;247:861–881. doi: 10.1007/s00232-014-9679-3. [DOI] [PubMed] [Google Scholar]
- 147.Hong S, et al. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug. Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 148.Rao W, et al. Nanoparticle-mediated intracellular delivery enables cryopreservation of human adipose-derived stem cells using trehalose as the sole cryoprotectant. ACS Appl. Mater. Interfaces. 2015;7:5017–5028. doi: 10.1021/acsami.5b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, et al. Cold-responsive nanoparticle enables intracellular delivery and rapid release of trehalose for organic-solvent-free cryopreservation. Nano Lett. 2019;19:9051–9061. doi: 10.1021/acs.nanolett.9b04109. [DOI] [PubMed] [Google Scholar]
- 150.Stefanic M, et al. Apatite nanoparticles strongly improve red blood cell cryopreservation by mediating trehalose delivery via enhanced membrane permeation. Biomaterials. 2017;140:138–149. doi: 10.1016/j.biomaterials.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 151.Acker JP, et al. Measurement of trehalose loading of mammalian cells porated with a metal-actuated switchable pore. Biotechnol. Bioeng. 2003;82:525–532. doi: 10.1002/bit.10599. [DOI] [PubMed] [Google Scholar]
- 152.Abazari A, et al. Engineered trehalose permeable to mammalian cells. PLoS One. 2015;10:e0130323. doi: 10.1371/journal.pone.0130323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stewart S, He X. Intracellular delivery of trehalose for cell banking. Langmuir. 2019;35:7414–7422. doi: 10.1021/acs.langmuir.8b02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fu A, Tang R, Hardie J, Farkas ME, Rotello VM. Promises and pitfalls of intracellular delivery of proteins. Bioconjug. Chem. 2014;25:1602–1608. doi: 10.1021/bc500320j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.D’Amore T, Crumplen R, Stewart GG. The involvement of trehalose in yeast stress tolerance. J. Ind. Microbiol. 1991;7:191–195. doi: 10.1007/BF01575882. [DOI] [Google Scholar]
- 156.Morita Y, Nakamori S, Takagi H. l-proline accumulation and freeze tolerance in Saccharomyces cerevisiae are caused by a mutation in the PRO1 gene encoding γ-glutamyl kinase. Appl. Environ. Microbiol. 2003;69:212–219. doi: 10.1128/AEM.69.1.212-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koštál V, Zahradníèková H, Šimek P. Hyperprolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc. Natl Acad. Sci. USA. 2011;108:13041–13046. doi: 10.1073/pnas.1107060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Koštál V, Šimek P, Zahradníčková H, Cimlová J, Štětina T. Conversion of the chill susceptible fruit fly larva (Drosophila melanogaster) to a freeze tolerant organism. Proc. Natl Acad. Sci. USA. 2012;109:3270–3274. doi: 10.1073/pnas.1119986109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bailey TL, Hernandez-Fernaud JR, Gibson MI. Proline pre-conditioning of cell monolayers increases post-thaw recovery and viability by distinct mechanisms to other osmolytes. RSC Med. Chem. 2021;12:982–993. doi: 10.1039/D1MD00078K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang J, et al. Natural zwitterionic betaine enables cells to survive ultrarapid cryopreservation. Sci. Rep. 2016;6:37458. doi: 10.1038/srep37458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petronini PG, De Angelis EM, Borghetti P, Borghetti AF, Wheeler KP. Modulation by betaine of cellular responses to osmotic stress. Biochem. J. 1992;282:69–73. doi: 10.1042/bj2820069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boon CH, et al. Loss of viability during freeze–thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. J. Biomed. Sci. 2006;13:433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- 163.Paasch U, et al. Cryopreservation and thawing is associated with varying extent of activation of apoptotic machinery in subsets of ejaculated human spermatozoa. Biol. Reprod. 2004;71:1828–1837. doi: 10.1095/biolreprod.103.025627. [DOI] [PubMed] [Google Scholar]
- 164.Baust JM, Van Buskirk R, Baust JG. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev. Biol. - Anim. 2000;36:262–270. doi: 10.1290/1071-2690(2000)036<0262:CVIFIO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 165.Baust JM, Vogel MJ, Van Buskirk R, Baust JG. A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transpl. 2001;10:561–571. doi: 10.3727/000000001783986413. [DOI] [PubMed] [Google Scholar]
- 166.Stroh C, et al. The role of caspases in cryoinjury: caspase inhibition strongly improves the recovery of cryopreserved hematopoietic and other cells. FASEB J. 2002;16:1651–1653. doi: 10.1096/fj.02-0034fje. [DOI] [PubMed] [Google Scholar]
- 167.Pero ME, et al. Inhibition of apoptosis by caspase inhibitor Z-VAD-FMK improves cryotolerance of in vitro derived bovine embryos. Theriogenology. 2018;108:127–135. doi: 10.1016/j.theriogenology.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 168.Yagi T, et al. Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology. 2001;33:1432–1440. doi: 10.1053/jhep.2001.24560. [DOI] [PubMed] [Google Scholar]
- 169.Ölander M, et al. A simple approach for restoration of differentiation and function in cryopreserved human hepatocytes. Arch. Toxicol. 2019;93:819–829. doi: 10.1007/s00204-018-2375-9. [DOI] [PubMed] [Google Scholar]
- 170.Lee S, Cho HW, Kim B, Lee JK, Kim T. The effectiveness of anti-apoptotic agents to preserve primordial follicles and prevent tissue damage during ovarian tissue cryopreservation and xenotransplantation. Int. J. Mol. Sci. 2021;22:2534. doi: 10.3390/ijms22052534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Matsushita T, et al. Apoptotic cell death and function of cryopreserved porcine hepatocytes in a bioartificial liver. Cell Transpl. 2003;12:109–121. doi: 10.3727/000000003108746696. [DOI] [PubMed] [Google Scholar]
- 172.Bissoyi A, Pramanik K. Role of the apoptosis pathway in cryopreservation-induced cell death in mesenchymal stem cells derived from umbilical cord blood. Biopreserv. Biobank. 2014;12:246–254. doi: 10.1089/bio.2014.0005. [DOI] [PubMed] [Google Scholar]
- 173.Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23:620–633. doi: 10.1016/j.tcb.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Watanabe K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 175.Martin-Ibañez R, et al. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum. Reprod. 2008;23:2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- 176.Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum. Reprod. 2009;24:580–589. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- 177.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol. Reprod. Dev. 2009;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haun F, et al. Identification of a novel anoikis signalling pathway using the fungal virulence factor gliotoxin. Nat. Commun. 2018;9:3524. doi: 10.1038/s41467-018-05850-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ha SJ, et al. Effect of antioxidants and apoptosis inhibitors on cryopreservation of murine germ cells enriched for spermatogonial stem cells. PLoS One. 2016;11:e0161372. doi: 10.1371/journal.pone.0161372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 181.Mayer TU, et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 182.Stevens CA, et al. A minimalistic cyclic ice-binding peptide from phage display. Nat. Commun. 2021;12:2675. doi: 10.1038/s41467-021-22883-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pollock K, Budenske J, McKenna D, Dosa P, Hubel A. Algorithm-driven optimization of cryopreservation protocols for transfusion model cell types including Jurkat cells and mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017;11:2806–2815. doi: 10.1002/term.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li R, Hornberger K, Dutton JR, Hubel A. Cryopreservation of human IPS cell aggregates in a DMSO-free solution — an optimization and comparative study. Front. Bioeng. Biotechnol. 2020;8:1. doi: 10.3389/fbioe.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhao G, Fu J. Microfluidics for cryopreservation. Biotechnol. Adv. 2017;35:323–336. doi: 10.1016/j.biotechadv.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park S, Wijethunga PAL, Moon H, Han B. On-chip characterization of cryoprotective agent mixtures using an EWOD-based digital microfluidic device. Lab Chip. 2011;11:2212–2221. doi: 10.1039/c1lc20111e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moore EB, Molinero V. Structural transformation in supercooled water controls the crystallization rate of ice. Nature. 2011;479:506–508. doi: 10.1038/nature10586. [DOI] [PubMed] [Google Scholar]
- 188.Bachtiger F, Congdon TR, Stubbs C, Gibson MI, Sosso GC. The atomistic details of the ice recrystallisation inhibition activity of PVA. Nat. Commun. 2021;12:1323. doi: 10.1038/s41467-021-21717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pedevilla P, Fitzner M, Sosso GC, Michaelides A. Heterogeneous seeded molecular dynamics as a tool to probe the ice nucleating ability of crystalline surfaces. J. Chem. Phys. 2018;149:072327. doi: 10.1063/1.5029336. [DOI] [PubMed] [Google Scholar]
- 190.Notman R, Noro M, O’Malley B, Anwar J. Molecular basis for dimethylsulfoxide (DMSO) action on lipid membranes. J. Am. Chem. Soc. 2006;128:13982–13983. doi: 10.1021/ja063363t. [DOI] [PubMed] [Google Scholar]
- 191.Towey JJ, Soper AK, Dougan L. Molecular insight into the hydrogen bonding and micro-segregation of a cryoprotectant molecule. J. Phys. Chem. B. 2012;116:13898–13904. doi: 10.1021/jp3093034. [DOI] [PubMed] [Google Scholar]
- 192.Cypser JR, Chick WS, Fahy GM, Schumacher GJ, Johnson TE. Genetic suppression of cryoprotectant toxicity. Cryobiology. 2019;86:95–102. doi: 10.1016/j.cryobiol.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 194.Capicciotti CJ, et al. O-aryl-glycoside ice recrystallization inhibitors as novel cryoprotectants: a structure–function study. ACS Omega. 2016;1:656–662. doi: 10.1021/acsomega.6b00163. [DOI] [PMC free article] [PubMed] [Google Scholar]