Abstract
Background
Thailand’s strong malaria elimination programme relies on effective implementation of its 1-3-7 surveillance strategy, which was endorsed and implemented nationwide in 2016. For each confirmed malaria patient, the Ministry of Public Health’s Division of Vector Borne Diseases (DVBD) ensures completion of case notification within 1 day, case investigation within 3 days, and foci investigation within 7 days. To date, there has not been a comprehensive assessment of the performance and achievements of the 1-3-7 surveillance strategy although such results could help Thailand’s future malaria elimination strategic planning.
Methods
This study examined adherence to the 1-3-7 protocols, tracked progress against set targets, and examined geographic variations in implementation of the 1-3-7 strategy in the programme’s initial 5 years. An auto-regressive integrated moving average (ARIMA) time series analysis with seasonal decomposition assessed the plausible implementation effect of the 1-3-7 strategy on malaria incidence in the programme’s initial 5 years. The quantitative analysis included all confirmed malaria cases from public health and non-governmental community facilities from October 2014 to September 2021 (fiscal year [FY] 2015 to FY 2021) (n = 77,405). The spatial analysis included active foci with known geocoordinates that reported more than five cases from FY 2018 to FY 2021.
Results
From FY 2017 to FY 2021, on-time case notification improved from 24.4% to 89.3%, case investigations from 58.0% to 96.5%, and foci investigations from 37.9% to 87.2%. Adherence to timeliness protocols did not show statistically significant variation by area risk classification. However, adherence to 1-3-7 protocols showed a marked spatial heterogeneity among active foci, and the ARIMA model showed a statistically significant acceleration in the reduction of malaria incidence. The 1-3-7 strategy national indicators and targets in Thailand have shown progressive success, and most targets were achieved for FY 2021.
Conclusion
The results of Thailand’s 1-3-7 surveillance strategy are associated with a decreased incidence in the period following the adoption of the strategy although there is notable geographic variation. The DVBD will continue to implement and adapt the 1-3-7 strategy to accelerate progress toward malaria elimination. This assessment may be useful for domestic strategic planning and to other countries considering more intensive case and foci investigation and response strategies.
Keywords: Elimination, Surveillance, 1-3-7 Strategy
Background
Thailand has a long history of conducting case investigations, case classifications, and focus investigation and response, as outlined in the Malaria Control Guidelines for Public Health Workers [1]. Between 2012 and 2015, the Division of Vector Borne Diseases (DVBD) in Thailand’s Ministry of Public Health (MOPH) reported significant reductions in the blood slide positivity rate to less than 5% among suspected cases with fever and in annual parasite incidence (API) to less than 1 per 1000 population (range 0.38–0.82) [2, 3]. Reaching these milestones allowed the DVBD to transition from a malaria control programme to a malaria elimination programme; details of this transition are presented in Lertpiriyasuwat et al. [4]. As part of the transition and based on results of a malaria programme review in 2015 [2, 3], the MOPH emphasized its commitment to surveillance as a core intervention by introducing the 1-3-7 malaria surveillance strategy in 2016.
The 1-3-7 malaria surveillance strategy is adapted from China, where it is considered a key factor in the country’s achievement of zero locally transmitted cases by 2017. The 1-3-7 strategy is operationalized with a simplified set of targets that outline responsibilities, actions, and the time frame for the crucial surveillance and response components of rapidly identifying infections and preventing them from spreading [2]. The time frame is delineated as follows: within 1 day of diagnosis (i.e., 24 h), local health staff are required to report the confirmed malaria case through the online Malaria Information System (MIS). Within 3 days, case investigation and classification must be completed to determine whether the case was locally acquired or imported. Finally, within 7 days, a focus investigation and tailored response based on the case investigation results and area stratification must be completed for each index case [5, 6]. Since the 1-3-7 strategy aims to reduce risk of onward transmission, response is similar for indigenous and imported cases except in areas without suitable vectors. Details on the implementation of the 1-3-7 strategy are documented in Lertpiriyasuwat et al. [4].
The 1-3-7 strategy builds on Thailand’s long-standing history of case notification, case classification, and focus investigation and response, with the addition of stricter time protocols to encourage adherence. Thailand’s National Malaria Elimination Strategy (NMES) 2017–2026 includes annual targets to support monitoring and evaluation of each component of the strategy. These targets were approved by the Cabinet of the Royal Thai Government in 2016 and aim to reach zero indigenous malaria cases by 2024 [2].
Since fiscal year (FY) 2015, the baseline year for the 1-3-7 strategy, malaria incidence in Thailand has declined from 0.37 per 1000 population to just 0.04 per 1000 population in FY 2021. The DVBD has also verified 42 of its 77 provinces as malaria-free, suggesting that the malaria elimination programme is progressing as intended. Remaining foci and high-transmission areas are concentrated in border provinces: Myanmar in the west, Cambodia in the east, and Malaysia in the South [7, 8]. The movement of people across international borders is a main challenge that continues to thwart malaria elimination in the Greater Mekong Subregion (GMS); districts bordering Thailand often have different strategies in place and, as seen in Fig. 1, may have higher malaria burden [9–12]. High population mobility and a large workforce of migrant workers make it difficult to track patients over time to ensure radical cure in this region of high Plasmodium vivax prevalence [6, 13–16]. Despite these challenges, 90.1% of Plasmodium vivax reportedly received radical cure and 81.5% of Plasmodium falciparum cases received single-dose primaquine in FY 2021, after steady improvement over time [17].
Fig. 1.
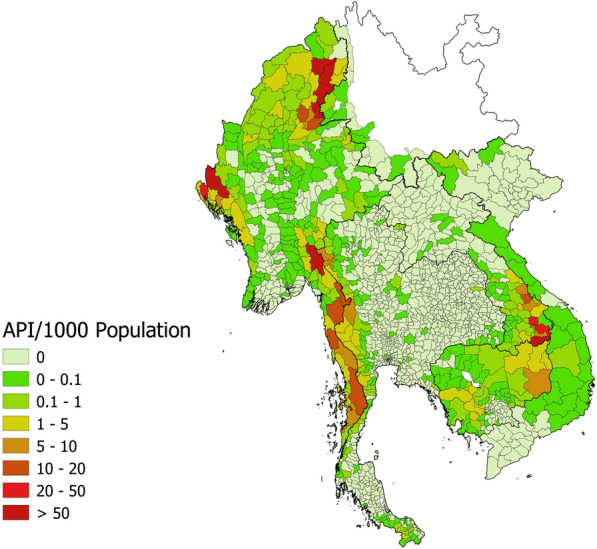
Malaria annual parasite incidence in GMS, FY 2021. Source: Malaria Elimination Database, Mekong Malaria Elimination Programme, 2021 (pers. commun.)
The DVBD has programmatically documented improvements in the timeliness of case reports and investigations [3, 4]. However, there has not been an in-depth examination of the performance and achievements of the 1-3-7 surveillance strategy in accelerating malaria elimination in Thailand. Adopting the 2024 elimination goal and related milestones without monitoring progress may lead to wasted resources and missed opportunities to enhance efficiency. This study examines adherence to the 1-3-7 protocols outlined in the Guidelines for Malaria Elimination for Public Health Workers, tracks progress against set targets in the NMES, and examines geographic variation in implementation and results [18]. This assessment of the strategy’s first 5 years of implementation will help Thailand’s future strategic planning and may be useful to other countries considering these more intensive case and foci investigation and response strategies.
Methods
The analysis included all parasitologically confirmed cases (by microscopy or rapid diagnostic test) from public health and non-governmental community facilities reported from October 2014 to September 2021, representing FY 2015 to FY 2021. The study utilized FYs because Thailand’s malaria programme and database are based on FY targets. Quantitative malaria data were extracted from the national routine malaria information system, reviewed, and analyzed using IBM SPSS Statistics version 22. Spatial analyses were performed using R [19], Quantum GIS [20], and GeoDa [21]. To ensure high quality data and meaningful results, the authors verified, cross-checked, and cleaned data; the final dataset is described in the Results section. The analysis was threefold: (1) examining adherence to 1-3-7 protocols, (2) determining achievement of results compared to nationally set targets, and (3) understanding geographic variations that could explain continued transmission patterns.
In line with the National Strategic Operational Plan 2017–2021, FY 2015 was treated as a baseline year, and FY 2017 was treated as the first year of full-coverage implementation, leaving FY 2016, as a “buffer year” in measurement analyses [22]. The exclusion of FY 2016 accounted for the extraordinary effort that was required to develop, pilot test, and roll out materials and interventions to subnational officers to launch the 1-3-7 strategy. The buffer year also accommodated a policy change of stricter reactive case detection (RACD) inclusion criteria. Whereas RACD was previously conducted for 100 to 150 people (approximately 20 to 30 households) or a radius 1 to 2 km, in FY 2017 a narrower screening of 50 people (approximately 10 households) within 1–2 km was adopted nationally based on reducing malaria burden [18, 23]. Spatial analyses were conducted for FY 2018–FY 2021 only, when 1-3-7 performance was more consistent.
In Thailand, cases classified as “indigenous” refer to patients who contracted malaria in the village where they lived during the infection period [18, 24]. New cases refer to patients with confirmed malaria for the first time or at least 90 days subsequent to a previous malaria infection [18]. Due to the operational difficulty in differentiating P. vivax relapses from reinfections, these cases are usually reported as recurrences and later analysed in detail [17]; recurrences were treated as new cases for the purpose of this study. Any focus that has recorded an indigenous case in the previous 3 years (based on an annual focus classification cycle) is considered to be an “active focus” [25]. It is worth noting that the number of active foci has decreased dramatically, from 2227 in FY 2013 to 700 in FY 2019, as reported in Sudathip et al. [25, 26] and has further dropped to 469 in FY 2021. Adherence to the 1-3-7 requirements was measured with descriptive statistics on malaria case notification, case investigation and classification, foci investigation and classification, and responses results, as outlined in the National Strategic Operational Plan (Table 1). Results were consolidated by month from FY 2017–FY 2021.
Table 1.
1-3-7 strategy’s indicators and outputs and NMES indicators
| 1-3-7 key and supplemental indicators | |
|---|---|
|
Day 1 |
Total malaria cases reported to the database |
| Number and percentage of malaria cases reported to the malaria database within 1 day | |
|
Day 3 |
Number and percentage of malaria cases reported to the malaria database that were investigated |
| Number and percentage of case investigations completed within 3 days | |
| Day 7 | Number of cases reported to the malaria database that require RACD screening |
| Number and percentage of RACD events conducted | |
| Number and percentage of RACD events conducted within 7 days | |
| Supplementary indicators without set targets | |
| Number of individuals targeted for RACD screening | |
| Number and percentage of individuals screened during RACD | |
| Number and percentage of positive cases newly detected during RACD |
| NMES outcome and impact indicators | |
|---|---|
| Percentage of districts with no indigenous malaria case transmission for at least 3 years | |
| Number of villages with indigenous malaria cases transmission |
To assess achievement, values for key indicators were compared with national targets from the NMES. Values for supplementary indicators on RACD, for which the NMES does not specify annual targets (Table 1), were also reported to show progress over time. Outcome and impact indicators in the NMES track the number of villages and districts free from reported indigenous cases (Table 1).
To assess the plausible effect of the 1-3-7 strategy’s implementation on malaria incidence toward the elimination target of 2024, an auto regressive integrated moving average (ARIMA) was used to fit the time series model with trend and random components for active foci. Other key prevention and elimination strategies stayed the same over the study period, making this an appropriate approach to assess plausible results of the new strategy [26].
Thailand’s 1-3-7 protocol is intended to be standardized across all subnational units. However, it is possible that the strategy’s components are operationalized differently or that provincial teams face unique challenges, which could influence the strategy’s effect [24]. Spatial heterogeneity of adherence to 1-3-7 protocols was investigated among all active foci with known geocoordinates that reported more than five cases from FY 2018 to FY 2021.
The threshold of five cases was set arbitrarily to avoid inclusion of foci with very few investigated cases. Adherence was measured using the following four indicators: percentage of cases reported within 1 day, percentage of cases investigated within 3 days, percentage of cases for which RACD was completed within 7 days, and percentage of cases with the full 1-3-7 sequence performed without delays. A hot spot analysis based on the G* local spatial clustering test [27] was used to identify those areas with foci showing statistically significant lower adherence to the 1-3-7 schedules compared to all other foci. The significance of the results obtained by the G* local spatial test was computed by comparing observed values to a random case distribution (null hypothesis) by randomly re-assigning the values of the tested indicator across the foci. The statistical significance calculation was based on 10,000 Monte Carlo randomizations (p < 0.05, with Bonferroni correction).
Results
Data from 77,405 malaria cases reported to the Malaria Information System (MIS) were split into 24,332 (31.4%) cases for FY 2015; 17,578 (22.7%) cases for the FY 2016 transition period; and 35,495 (45.9%) cases for the FY 2017–FY 2021 post-intervention period. Adherence and achievement analyses included all cases with complete data on 1-3-7 indicators, which were available for 15,887 (90.4%) of FY 2016 cases and 32,957 (92.9%) of FY 2017–FY 2021 post-intervention cases; the remainder were not included for analysis due to missing or duplicate data.
Adherence to 1-3-7 protocols
Adherence to case notification within 1 day
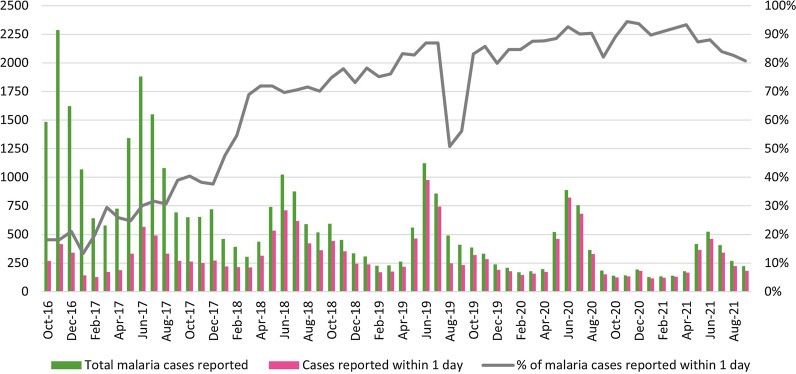
The monthly proportion of confirmed malaria cases reported within 24 h to the system increased over the study period, from 18.2% in October 2016 to 80.7% in September 2021 (Fig. 2). This increase was steady despite the fact that reporting rates vary based on malaria seasonality. During the peak transmission month of June, the proportion of timely notification increased steadily from 30.1% in FY 2017 (567/1881) to 92.6% in FY 2020 (823/889) and 88.0% in FY 2021 (462/525). Note that there was a temporary drop of the timely notification proportion, to 56.2% at the end of FY 2019, due to an MIS database security breach in August 2019 that temporarily disabled data entry; malaria officers inputted data retrospectively 2 months later [28].
Fig. 2.
Number and percentage of malaria cases reported within 1 day, by month, FY 2017–FY 2021
Adherence to case investigation within 3 days
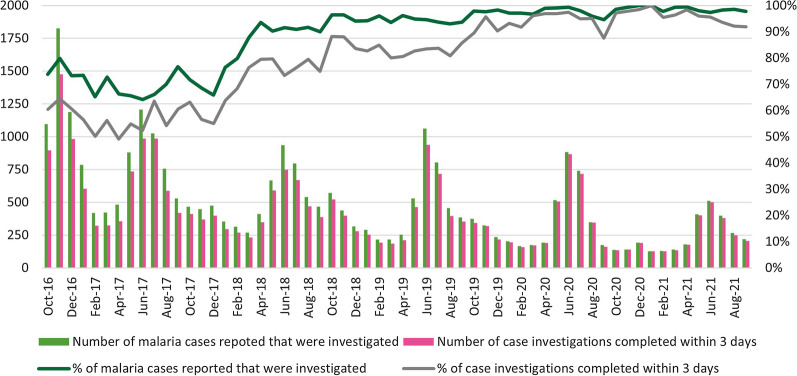
The monthly proportion of malaria cases investigated improved from 73.8% in October 2016 to 97.8% by September 2021, with substantial growth each year (Fig. 3). The proportion of case investigations within the 3-day requirement also improved over time, from 52.4% in June 2017 to 91.9% by September 2021, even after seasonality is considered. The proportion of malaria cases investigated during the peak season in June rose from 64.1% (1206/1881) in FY 2017 to 97.3% (511/525) in FY 2021. During the low transmission season, case investigation followed the same upward trend, rising from 72.7% (421/579) in FY 2017 to 99.3% (139/140) in FY 2021. Reporting timeliness also increased from 52.4% and 56.1% in the FY 2017 peak and low seasons, respectively, to 95.6% and 96.4% in the FY 2021 peak and low seasons, respectively.
Fig. 3.
Number and percentage of malaria cases investigated within 3 days, by month, FY 2017–FY 2021
Adherence to foci investigation and response within 7 days
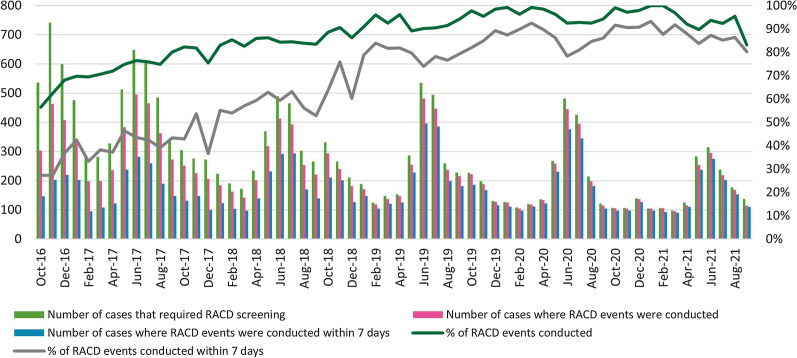
The monthly proportion of RACD conducted among the cases that required RACD improved substantially over the study period, from 56.5% in October 2016 to 83.2% by September 2021 (Fig. 4). The proportion of RACD events conducted on time (within 7 days of case notification) also made dramatic improvement over the study period, from 48.2% to 80.3%. These proportions did not vary much between high and low transmission seasons.
Fig. 4.
Number and percentage of foci investigated and responded to within 7 days, FY 2017–FY 2021
Comparison by area classification
Comparative t-tests showed that adherence to the DVBD’s timeliness protocols did not show statistically significant variation by area classification. Before and during the buffer year of 1-3-7 implementation, timely case reporting was higher among active foci than among cleared foci with an index case (p < 0.05). Although active foci continued to show more timely surveillance and response than cleared foci, in the post-intervention period, these differences were not statistically significant.
Achievements of the 1-3-7 surveillance strategy compared to national targets
The second part of this analysis compared actual results to targets laid out by FY, divided into output results and outcome/impact results (see Table 2 for summarized results). The NMES uses FY 2015 as a baseline year.
Table 2.
Achievement of 1-3-7 strategy, national indicators, and targets for FY 2017–FY 2020
| Indicators | FY 2015 | FY 2017 | FY 2018 | FY 2019 | FY 2020 | FY 2021 | |
|---|---|---|---|---|---|---|---|
| Output indicators | |||||||
| Percentage of malaria cases reported in the MIS within 24 h | Target | 50% | 60% | 70% | 80% | 90% | |
| Achievement | 18.0% | 24.4% | 59.6% | 77.1% | 87.8% | 89.3% | |
| Total number of malaria cases reported to the database | 24,332 | 14,954 | 7368 | 5845 | 4421 | 2835 | |
| Number of malaria cases reported to the malaria database within 1 day | 4383 | 3643 | 4388 | 4508 | 3881 | 2531 | |
| Percentage of malaria cases investigated within 3 days | Target | 85% | 90% | 95% | 95% | 95% | |
| Achievement | 44.1% | 58.0% | 70.4% | 84.0% | 94.7% | 96.5% | |
| Number of malaria cases reported to the malaria database | 24,332 | 14,954 | 7368 | 5845 | 4421 | 2,835 | |
| Number of malaria cases reported to the malaria database that were investigated | 15,992 | 10,614 | 6140 | 5532 | 4331 | 2785 | |
| Number of case investigations completed within 3 days | 10,742 | 8674 | 5185 | 4907 | 4185 | 2735 | |
| Percentage of malaria cases investigated with foci response conducted within 7 days | Target | 30% | 50% | 60% | 70% | 80% | |
| Achievement | 35.7% | 37.9% | 55.2% | 75.3% | 84.1% | 87.2% | |
| Number of cases reported to the malaria database that require RACD screening | 7704 | 5848 | 3566 | 3225 | 2557 | 1936 | |
| Number of RACD events conducted | 5410 | 4119 | 2973 | 2928 | 2433 | 1824 | |
| Number of RACD events conducted within 7 days | 2750 | 2214 | 1969 | 2428 | 2150 | 1689 | |
| Outcome and impact indicators | |||||||
| Number and percentage of districts with no indigenous malaria case transmission for at least 3 years (N = 928) | Target | 696 (75%) | 743 (80%) | 789 (85%) | 836 (90%) | 882 (95%) | |
| Achievement | 632 | 748 (80%) | 767 (83%) | 788 (85%) | 798 (86%) | 813 (88%) | |
| Number of villages with indigenous malaria case transmission | Target | 3610 | 2707 | 1895 | 1232 | 739 | |
| Achievement | 5552 | 2310 | 1676 | 1382 | 1187 | 469 | |
Output indicators
Overall, results on output indicators were strong. First, malaria cases reported in the MIS within the 1-day (or 24-h) period showed consistent improvement over time, with rates reaching targets in FY 2019, FY 2020, and FY 2021 (77.1%, 87.8%, and 89.3%, respectively). Second, the proportion of cases investigated within 3 days dramatically increased from baseline to 96.5%, exceeding the FY 2021 target (95%). Lastly, for foci investigation and response conducted within 7 days, the outputs met the targets in every FY, with the FY 2020 and FY 2021 proportions of 84.1% and 87.2% both surpassing the FY 2021 target.
The authors also examined the supplemental output indicators for which targets were not defined in the NMES, with results summarized in Table 3. During FY 2017–FY 2021, 14,277 index cases triggered RACD (83.3% of total cases eligible for RACD), leading to 866,920 people being screened: 1513 (0.17%) were positive for malaria infection. Each year, the number of individuals screened during RACD events dropped, with the proportional yield of positive cases also decreasing from 0.23% (507 cases) in FY 2017 to 0.11% (133 cases) in FY 2021. RACD methods produced nearly equal yields for both active foci and cleared foci with a confirmed index case (0.19% versus 0.16%).
Table 3.
Performance of RACD events conducted among foci investigations for FY 2017–FY 2021
| Indicator | Achievement | ||||
|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | |
| Number of individuals screened during RACD | 222,991 | 182,275 | 176,162 | 159,892 | 125,600 |
| Number of positive cases newly identified using RACD | 507 | 311 | 314 | 248 | 133 |
| Percent found positive from RACD events | 0.23% | 0.17% | 0.18% | 0.16% | 0.11% |
Outcome/impact indicators
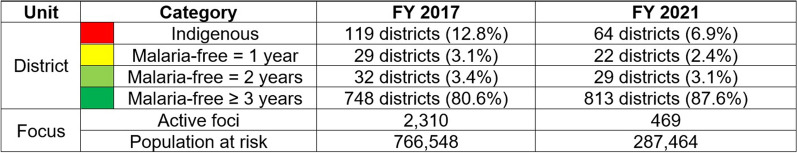
For the outcome indicator of percentage of districts without local transmission for at least 3 years (among a total of 928 districts in Thailand), targets were reached every FY between 2017 and 2019 (80%, 83%, and 85% respectively). Progress plateaued in FY 2020 and FY 2021, whereby district achievement reached 86% and 85%, missing the 90% and 95% targets, respectively. The second outcome indicator is the number of villages with malaria transmission, which dropped from 2310 in FY 2017 to 469 in FY 2021 (Table 2). This indicator has shown better-than-expected results, with substantial reductions beyond the set target each year, showing strides in interrupting community transmission.
These results were supplemented by other data collected by the DVBD on the population at risk in active foci, which from FY 2017 to FY 2021 dropped from 766,548 to 287,464 (Fig. 5), and a decrease in the number of active foci from 2310 to 469 for the same period. Because the DVBD’s unit of analysis for daily assessments of routine data was the focus level, the programme’s MIS collated more granular geotemporal data on malaria-free area classification than what was required for NMES reporting.
Fig. 5.
District-level improvements in malaria free status from FY 2017 and FY 2021. Source: DVBD, MOPH, 2021 (pers. commun.)
Observed and predicted trend in incidence
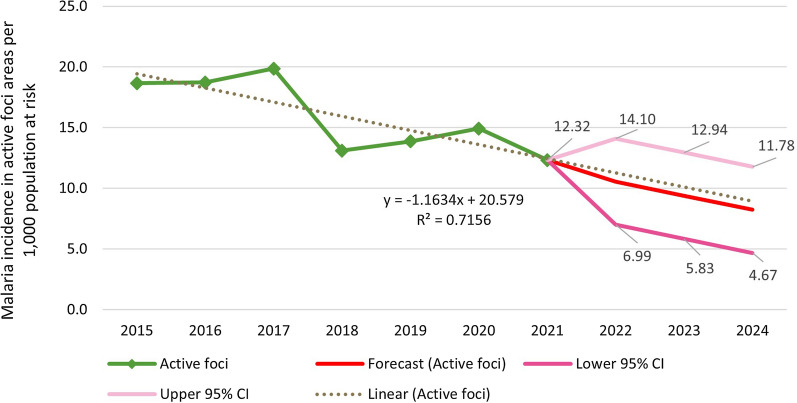
The annual positive rates of all malaria species were used to establish an ARIMA model in active foci areas (R2 = 0.72) (Fig. 6). Prior to the 1-3-7 strategy’s launch, malaria incidence among active foci was decreasing annually by 1.02 per 1000 population at risk. After the launch in FY 2016, the ARIMA model showed an additional reduction to 1.31 cases per 1000 population at risk per year. Each subsequent year has seen further reductions, averaging 1.36 (p = 0.62) annually, indicating an acceleration in the reduction of malaria incidence during the implementation period. The trend is forecasted to continue through FY 2024.
Fig. 6.
Observed and forecast malaria incidence per 1000 population at risk in active foci areas FY 2015–FY 2024
Spatial analyses among active foci
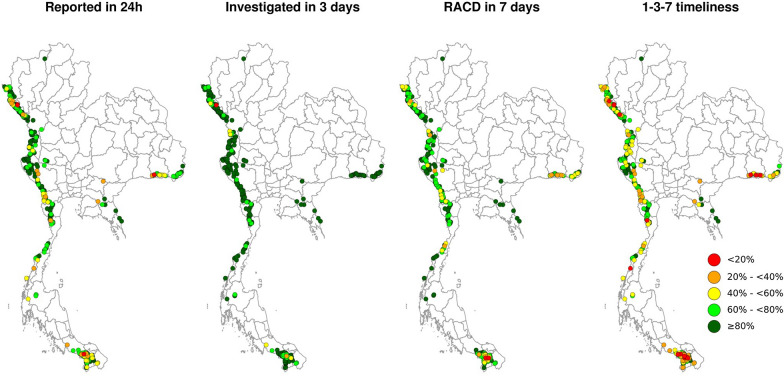
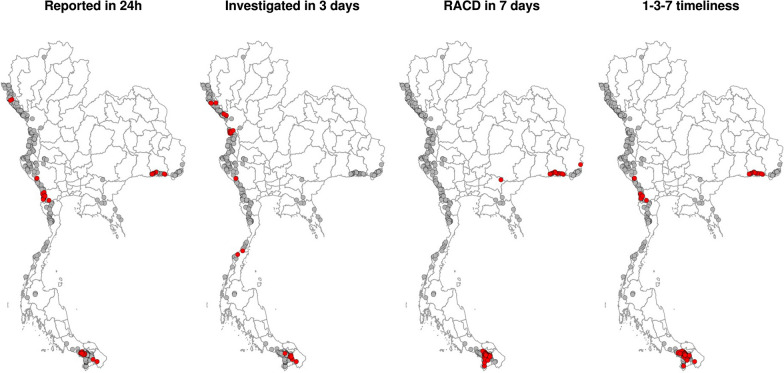
During the study period, active foci were clustered at Thailand’s international borders. Adherence to 1–3-7 protocols showed a marked spatial heterogeneity among active foci (Fig. 7), with southern and eastern foci reporting lowest adherence. Among the indicators examined, timely case investigation showed the highest results and lowest spatial heterogeneity (Fig. 7); however, some foci in high-burden areas in the northwest showed poor adherence (p < 0.05). The G* test identified foci with significantly (p < 0.05) lower adherence to overall 1-3-7 timeliness, mostly in Sisaket province in the east, Kanchanaburi and Ratchaburi provinces in the west, and Yala province in the south (Fig. 8).
Fig. 7.
Spatial pattern of adherence to 1-3-7 protocols among active foci in Thailand, FY 2018–FY 2021
Fig. 8.
Active foci with significantly lower adherence to 1-3-7 protocols, FY 2018–FY 2021 The red dots indicate significant hot spots (p < 0.05) of low adherence to 1-3-7 timing identified by the G* local spatial test
Discussion
The 1-3-7 strategy is an innovative surveillance and response intervention for accelerating progress toward Thailand’s malaria elimination goal by 2024. Even if only one malaria case is detected, local health personnel must respond immediately per standard operating procedures, making the strategy quite sensitive to epidemiological changes [3, 4]. Adherence rates show that the protocols are being successfully implemented, with case notifications within 1 day increasing from 24.4% to 89.3%, case investigations within 3 days increasing from 58.0% to 96.5%, and foci investigations and response within 7 days increasing from 37.9% to 87.2% during the first 5 years of implementation.
Data quality is high, particularly in terms of completeness and timeliness; in fact, the DVBD’s coordination and communication across subnational units has been cited as a success factor for the 1-3-7 strategy [3, 4]. The national team provides close operational support to subnational teams on data quality and compliance with 1-3-7 protocols. The DVBD regularly monitors for unusual data, such as an increase in cases compared to historic data or a case identified in a province already verified as malaria free. The programme immediately and directly communicates queries to specific subnational officers via its malaria group mobile chat application with more than 400 malaria members from across the country from every level of the health system. Subnational officers respond publicly, describing investigation efforts, providing contextual information and photographs, and following up to share subsequent results or requesting further guidance. The DVBD also distributes a monthly report collating unusual events and data trends to all provincial offices, in addition to a monthly report tracking progress on national indicators to leadership in the Department of Disease Control.
To ensure continued progress toward the ultimate goal of zero malaria, the DVBD is conducting further analyses on each component of the 1-3-7 strategy to better understand reasons behind the documented geographic variation. Thailand’s routine surveillance data show that high-incidence provinces have many more active foci per province and more cases per focus. However, the provinces with low adherence identified by the spatial analysis have unique challenges related to drug efficacy (Sisaket in the east), migration patterns (Kanchanaburi and Ratchaburi in the west), and civic unrest (Yala in the south). Assessing areas of persistent transmission can help target resources to accelerate elimination, so the DVBD is currently developing a comprehensive statistical model that includes 1-3-7 implementation, environmental factors, and social and behavioral characteristics.
The 1-3-7 strategy national indicators and targets in Thailand have shown progressive success—more than 80% achievement in every component. Most targets were achieved for FY 2021, suggesting that the DVBD set out ambitious but realistic goals in the NMES. Because few other changes were implemented in the malaria control programme at the time of the 1-3-7 adoption, the results of this study suggest it is plausible that the strategy is associated with the documented 80% decline in malaria burden from FY 2017 to FY 2021 and the notable increase in malaria-free foci and districts.
Of course, the context also evolves, particularly as the protracted COVID-19 pandemic poses a serious additional challenge to global progress toward malaria elimination [29]. In January 2020, Thailand became the second country in the world to confirm a COVID-19 case. Several protective movement restrictions were implemented in the subsequent months, resulting in just 3553 cases of COVID-19 recorded in FY 2020 [30]. These cases were mostly limited to urban areas, contrary to malaria cases that are concentrated in forested border areas, so there was limited overlap in epidemiology. However, in FY 2021, over 1.6 million cases were reported as the epidemiology widened to affect most areas of the country [30]. COVID-19 cases peaked during the high malaria transmission period from July to September 2021 and may have affected malaria interventions by overwhelming health systems, altering population movement and health seeking behaviors, and diverging resources and frontline workers. As seen in this study’s results, 1-3-7 adherence rates were higher in FY 2020 than FY 2021, particularly for focus investigation and response, thereby meriting further analyses. Overall, Thailand has maintained its results for 1-3-7 indicators despite COVID-19, likely due to high-level support and the presence of a strong vertical programme complemented by a wide community health worker network.
Thailand’s declining malaria burden supports its collaborative regional goals and programmes. Since 2000, GMS countries have successfully reduced the reported number of all malaria species cases by 90% and P. falciparum cases by 97% [29]. This is coupled with a decline in malaria deaths from 6000 in 2000 to 10 in 2020 [31] in the GMS. Altogether, this is remarkable progress toward the region’s collective goal of malaria elimination by 2030. Following China and Thailand, Myanmar, Cambodia, and Lao PDR began piloting the 1-3-7 strategy [32, 33]. Continuation of the close partnership among GMS countries will help support regional goals and address some of the most challenging border hot spots identified by the spatial analysis. There is also historical success of cross-border and civilian-military cooperation in Sisaket province [34]. A starting point for expanded programming could be cross-border notification and foci response, utilizing the networks of community health workers that are acutely familiar with local populations, and providing additional training to boost 1-3-7 adherence in those areas.
Limitations
This assessment is not a true impact evaluation of the malaria elimination programme or the 1-3-7 surveillance intervention of Thailand. There is substantial guidance on conducting robust impact evaluations for full-scale malaria interventions; however, these models have been designed for contexts with substantial malaria burden [33, 35, 36]. Even the guidance issued for lower transmission settings is designed for API that is higher than those in Thailand [37]. The ARIMA model is based on case burden and is a useful method to assess the 1-3-7 strategy’s plausible effect on incidence. However, the model does not account for important programmatic and contextual factors.
This study used robust routine data from Thailand’s national MIS [36]. Data completeness, as reported in the results section, is less than 100%, which is normal for routine data but requires appropriate interpretation. The cases not captured in the MIS are likely to be patients that have a deliberate reason to avoid the surveillance system or the formal public health system, and it is difficult to assess how many cases may be missed. Furthermore, response data are particularly challenging to interpret. RACD results are reported in aggregate at the foci level so coverage up to the required 50 individuals, 10 households, or 2 km is difficult to ascertain, as are case-level geolocation or demographics. The DVBD and its partners are working to ensure that the MIS is a complete and comprehensive source of malaria data before the 2024 elimination target.
Next steps and road to elimination
Although the 1-3-7 strategy has been successfully implemented and results have shown steady improvements, efforts must be sustained to address emerging challenges due to COVID-19, migration patterns, or sociopolitical dynamics in the GMS [7, 16, 38]. Preventing resurgence and reintroduction of malaria are also growing priorities for the DVBD, based on lessons learned elsewhere [39, 40]. These investments in malaria surveillance can enhance Thailand’s health security and preparedness in crucial areas such as rapid detection, alert, and response, thereby building resilience against future infectious disease threats.
The DVBD has been intensifying its strategies to ensure appropriate surveillance for very low-transmission settings [41, 42]. Such strategies include enhancements to foci response and active surveillance protocols, advocacy for policy legislation, resources for additional staff trainings on accurate case management, support for improved data recording and reporting, and involvement of the private sector and military to ensure that the surveillance system is capturing all cases [34, 43]. Effectively reaching marginalized populations may require innovative solutions, particularly as malaria burden and risk perception falls [5, 44].
Conclusions
The encouraging results of Thailand’s 1-3-7 surveillance strategy are in line with the dramatically decreased incidence seen in the time period following the strategy’s adoption. The malaria programme will continue its emphasis on implementing and applying Thailand’s 1-3-7 strategy, perhaps with additionally tailored activities for varying zones of transmission or that capitalize on community health networks and cross-border collaboration. The 1-3-7 strategy supports the DVBD’s goals to achieve and sustain malaria elimination and could be a useful example for other countries in the GMS aiming to eliminate malaria.
Acknowledgements
The authors recognize all local public health teams and partners who have contributed to planning and implementing the 1-3-7 strategy at the national and field levels under the leadership of Thailand’s Division of Vector Borne Diseases, Department of Disease Control, Ministry of Public Health.
Abbreviations
- ARIMA
Auto-regressive integrated moving average
- COVID-19
Coronavirus disease 2019
- DVBD
Division of Vector Borne Diseases
- FY
Fiscal year
- GMS
Greater Mekong Subregion
- MIS
Malaria Information System
- MOPH
Ministry of Public Health
- NMES
National Malaria Elimination Strategy
- PMI
President’s Malaria Initiative
- RACD
Reactive case detection
- USAID
United States Agency for International Development
Author contributions
PS, DG, DS, and JAS conceptualized the manuscript. SN analyzed the quantitative results and DB analyzed the spatial results. SN and JAS interpreted the results and led the writing of the manuscript. All authors, including SK and NP, provided input and review. All authors read and approved the final manuscript.
Disclaimer
The contents of this article are the responsibility of the authors and do not necessarily reflect the views of USAID, PMI, or the U.S. Government. DG is a staff member of the World Health Organization (WHO) and is responsible for the views expressed in this publication, which do not necessarily reflect the decisions or policies of the WHO.
Funding
This study was made possible by the generous support of the American people through the U.S. President’s Malaria Initiative (PMI) and United States Agency for International Development (USAID), under the terms of Cooperative Agreement AID-486-LA-15-00002 for Inform Asia: USAID’s Health Research Program.
Availability of data and materials
The visualizations supporting the conclusions of this article are available in the Malaria Online repository, http://malaria.ddc.moph.go.th/.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prayuth Sudathip and Sathapana Naowarat contributed equally to this work
References
- 1.Bureau of Vector Borne Disease . Guidelines on malaria control for public health personnel. Nonthaburi: Bureau of Vector Borne Disease; 2009. [Google Scholar]
- 2.Bureau of Vector Borne Disease . National Malaria Elimination Strategy, Thailand 2017–2026. Nonthaburi: Bureau of Vector Borne Disease; 2016. [Google Scholar]
- 3.WHO. Programmatic Review of the National Malaria Programme in Thailand: 31st August – 11th September 2015 . Summary Report. Geneva: World Health Organization; 2016. [Google Scholar]
- 4.Lertpiriyasuwat C, Sudathip P, Kitchakarn S, Areechokchai D, Naowarat S, Shah JA, et al. Implementation and success factors from Thailand’s 1-3-7 surveillance strategy for malaria elimination. Malar J. 2021;20:201. doi: 10.1186/s12936-021-03740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu G, Liu Y, Beiersmann C, Feng Y, Cao J, Müller O. Challenges in and lessons learned during the implementation of the 1-3-7 malaria surveillance and response strategy in China: a qualitative study. Infect Dis Poverty. 2016;5:94. doi: 10.1186/s40249-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng J, Liu J, Feng X, Zhang L, Xiao H, Xia Z. Towards malaria elimination: monitoring and evaluation of the “1-3-7” approach at the China-Myanmar border. Am J Trop Med Hyg. 2016;95:806–810. doi: 10.4269/ajtmh.15-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudathip P, Kongkasuriyachai D, Stelmach R, Bisanzio D, Sine J, Sawang S, et al. The investment case for malaria elimination in Thailand: a cost-benefit analysis. Am J Trop Med Hyg. 2019;100:1445–1453. doi: 10.4269/ajtmh.18-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tipmontree R, Fungladda W, Kaewkungwal J, Tempongko S, Schelp F. Migrants and malaria risk factors: a study of the Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 2009;40:1148–1157. [PubMed] [Google Scholar]
- 9.Wangdi K, Gatton ML, Kelly GC, Clements AC. Cross-border malaria: a major obstacle for malaria elimination. Adv Parasitol. 2015;89:79–107. doi: 10.1016/bs.apar.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Wangdi K, Clements CAA. Ending malaria transmission in the Asia Pacific Malaria Elimination Network (APMEN) countries challenges and the way forward. In: Manguin S, Dev V, editors. Towards malaria elimination - A leap forward. London: InTech; 2018. [Google Scholar]
- 11.Hlongwana K, Tsoka-Gwegweni J. Towards the implementation of malaria elimination policy in South Africa: the stakeholders' perspectives. Glob Health Action. 2017;10:1288954. doi: 10.1080/16549716.2017.1288954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Zahrani H, Omar IA, Abdoon MOA, Ibrahim AA, Alhogail A, Elmubarak M, et al. Cross-border movement, economic development and malaria elimination in the Kingdom of Saudi Arabia. BMC Med. 2018;16:98. doi: 10.1186/s12916-018-1081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wangdi K, Banwell C, Gatton ML, Kelly GC, Namgay R, Clements AC. Development and evaluation of a spatial decision support system for malaria elimination in Bhutan. Malar J. 2016;15:180. doi: 10.1186/s12936-016-1235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang F, Zhang L, Xue J, Zhou N, Thi A, Zhang J, et al. From control to elimination: a spatial-temporal analysis of malaria along the China-Myanmar border. Infect Dis Poverty. 2020;9:158. doi: 10.1186/s40249-020-00777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delacollette C, D’Souza C, Christophel E, Thimasarn K, Abdur R, Bell D, et al. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health. 2009;40:674–691. [PubMed] [Google Scholar]
- 16.Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. 2012;121:227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudathip P, Saejeng A, Khantikul N, Thongrad T, Kitchakarn S, Sugaram R, et al. Progress and challenges of integrated drug efficacy surveillance for uncomplicated malaria in Thailand. Malar J. 2021;20:261. doi: 10.1186/s12936-021-03791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bureau of Vector Borne Disease . Guidelines for malaria elimination operation for medical and health personnel in Thailand. Nonthaburi: Division of Vector Borne Diseases; 2019. [Google Scholar]
- 19.R Core Team. R. 2021.A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing.
- 20.QGIS Development Team. 2021. QGIS Geographic Information System. Open source geospatial foundation project.
- 21.Anselin L, Syabri I, Kho Y. GeoDa: an introduction to spatial data analysis. Geogr Anal. 2006;38:5–22. doi: 10.1111/j.0016-7363.2005.00671.x. [DOI] [Google Scholar]
- 22.Bureau of Vector Borne Disease . National Strategic Operation Plan, Thailand 2017–2021. Nonthaburi: Bureau of Vector Borne Disease; 2016. [Google Scholar]
- 23.WHO . Disease surveillance for malaria elimination: an operational manual. Geneva: World Health Organization; 2012. [Google Scholar]
- 24.WHO . A framework for malaria elimination. Geneva: World Health Organization; 2017. [Google Scholar]
- 25.Sudathip P, Kitchakarn S, Shah JA, Bisanzio D, Young F, Gopinath D, et al. A foci cohort analysis to monitor successful and persistent foci under Thailand's Malaria elimination strategy. Malar J. 2021;20:118. doi: 10.1186/s12936-021-03648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fretheim A, Zhang F, Ross-Degnan D, Oxman AD, Cheyne H, Foy R, et al. A reanalysis of cluster randomized trials showed interrupted time-series studies were valuable in health system evaluation. J Clin Epidemiol. 2015;68:324–333. doi: 10.1016/j.jclinepi.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Ord J, Getis A. Local spatial autocorrelation statistics. Distributional issues and an application. Geogr Anal. 1995;27:286–306. doi: 10.1111/j.1538-4632.1995.tb00912.x. [DOI] [Google Scholar]
- 28.Division of Vector Borne Disease . Malaria Online: the digital surveillance system for Thailand Malaria elimination. Nonthaburi: Division of Vector Borne Disease; 2019. [Google Scholar]
- 29.WHO . World malaria report 20 years of global progress and challenges. Geneva: World Health Organization; 2020. p. 2020. [Google Scholar]
- 30.Digital Government Development Agency (DGA). Daily report for COVID-19, Thailand dataset, 2021 https://data.go.th/dataset/covid-19-daily. Accessed 21 June 2021.
- 31.Mekong Malaria Elimination Programme. Countries of the Greater Mekong ready for the “last mile” of malaria elimination. 2020. https://www.who.int/publications-detail-redirect/WHO-UCN-GMP-MME-2020.05. Accessed 21 Dec 2021.
- 32.Aung P, Thein WZ, Hein NMZ, Aung TK, Mon ON, Linn YYN, et al. Challenges in early phase of implementing the 1-3-7 surveillance and response approach in malaria elimination setting: a field study from Myanmar. Infect Dis Poverty. 2020;9:18. doi: 10.1186/s40249-020-0632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kheang S, Sovannaroth S, Barat LM, Dysoley L, Kapella BK, Po L, et al. Malaria elimination using the 1-3-7 approach: lessons from Sampov Loun. Cambodia BMC Public Health. 2020;20:544. doi: 10.1186/s12889-020-08634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roh ME, Lausatianragit K, Chaitaveep N, Jongsakul K, Sudathip P, Raseebut C, et al. Civilian-military malaria outbreak response in Thailand: an example of multi-stakeholder engagement for malaria elimination. Malar J. 2021;20:458. doi: 10.1186/s12936-021-03995-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hershey C, Bhattarai A, Florey LS, McElroy PD, Nielsen CF, Ye Y, et al. Implementing impact evaluations of malaria control interventions: process, lessons learned, and recommendations. Am J Trop Med Hyg. 2017;97(3):20–31. doi: 10.4269/ajtmh.17-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe A, Steketee RW, Arnold F, Wardlaw T, Basu S, Bakyaita N, et al. Viewpoint: evaluating the impact of malaria control efforts on mortality in sub-Saharan Africa. Trop Med Int Health. 2007;12:1524–39. doi: 10.1111/j.1365-3156.2007.01961.x. [DOI] [PubMed] [Google Scholar]
- 37.Ashton A, Prosnitz D, Andrada A, Herrera S, Ye Y. Evaluating malaria programmes in moderate- and low-transmission settings: practical ways to generate robust evidence. Malar J. 2020;19:75. doi: 10.1186/s12936-020-03158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng J, Malla P, Zhang J, Xu S, Li C, Zhao Y, et al. Increasing trends of malaria in a border area of the Greater Mekong Subregion. Malar J. 2019;18:309. doi: 10.1186/s12936-019-2924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Premaratne R, Wickremasinghe R, Ranaweera D, Gunasekera WKA, Hevawitharana M, Pieris L, Fernando D, Mendis K. Technical and operational underpinnings of malaria elimination from Sri Lanka. Malar J. 2019;18:256. doi: 10.1186/s12936-019-2886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nkumama N, O’Meara PW, Osier HAF. Changes in malaria epidemiology in Africa and new challenges for elimination. Trends Parasitol. 2017;33:128–140. doi: 10.1016/j.pt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO . An operation manual: disease surveillance for malaria control. Geneva: World Health Organization; 2012. [Google Scholar]
- 42.Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;2008(8):369–78. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO . Malaria surveillance, monitoring and evaluation: a reference manual. Geneva: World Health Organization; 2018. [Google Scholar]
- 44.Bureau of Vector Borne Disease . Guide to malaria elimination for Thailand's local administrative organizations and the health network. Nonthaburi: Division of Vector Borne Diseases; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The visualizations supporting the conclusions of this article are available in the Malaria Online repository, http://malaria.ddc.moph.go.th/.