Abstract
We report the case of a 70-year-old man coming to our attention for new onset refractory status epilepticus (NORSE) in a rapidly evolving CJD during SARS-CoV-2 co-infection. Our case report describes a fulminant CJD evolution associated with SARS-CoV-2 infection, which led to patient death after 15 days from admission. First EEG presented continuous diffuse spikes, sharp waves and sharp-and-slow wave complexes, pattern consistent with a non-convulsive status epilepticus (NORSE). Our case supports how CJD with SARS-CoV-2 co-infection could be characterized by an accelerated evolution, as already hypothesize for others microorganism infections, and how the diagnosis might be more challenging due to its uncommon presentations, such as NORSE.
Keywords: COVID-19, Creutzfeldt–Jakob, NORSE, Status epilepticus, Prion disease
Introduction
Creutzfeldt–Jakob Disease (CJD) may be challenging to diagnose, in particular in COVID-19 era [1]. Usually, the initial course of the disease is characterized by a subtle onset of psychiatric and neurological symptoms, with most of patients experiencing depression, anxiety, nervousness, autonomic disturbances, disruption of sleep–wakefulness rhythm, gait alterations, ataxia and myoclonus [2, 3]. Seizures are not a common manifestation in the first phase of the disease, and status epilepticus is rare, while still remaining possible [4]. Non-convulsive status epilepticus (NCSE) in patients with confusion may be difficult to differentiate from non-epileptic encephalopathies, potentially leading to a wrong diagnosis [5]. We report the case of a 70-year-old right-handed patient coming to our attention for new onset refractory status epilepticus (NORSE) in a rapidly evolving CJD during SARS-CoV-2 co-infection.
Case report
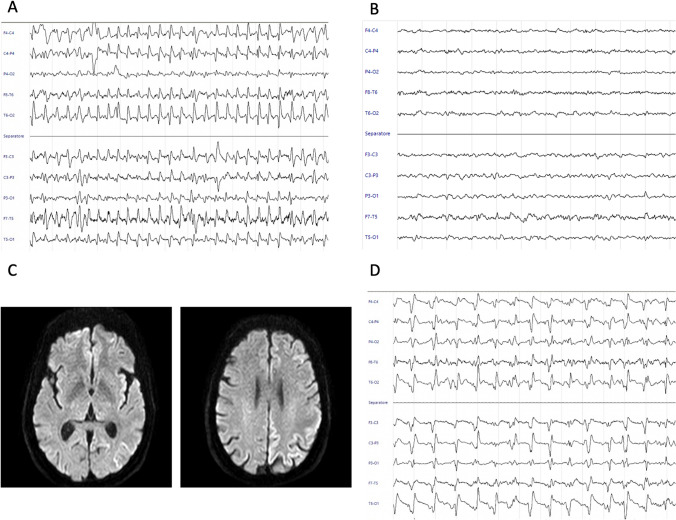
In the 40 days before the admission, the patient experienced non-specific ailment. The first complains were related to motor symptoms, including inability to sign, compromised coordination and paresis to the right leg. Owing to these initial symptoms, the patient was evaluated by a private practicing neurologist who prescribed a MRI scan, which reported no abnormalities. In the meantime, neurological symptoms rapidly worsened, including speech impairments, dystonia and myoclonus. Hence, the patient was admitted to the emergency department (ED). Neurological evaluation showed a mild right hemiparesis, non-fluent speech and bilateral myoclonus augmented by startle. A brain CT scan was performed, showing no acute alterations. Since the screening nasopharyngeal swab for SARS-CoV-2 previous to admission resulted positive, the patient was diverted to the COVID-19 ward. Comprehensive metabolic panel did not show abnormalities, while chest radiography showed mild bilateral interstitial pneumonia requiring low oxygen inspired fraction support. An EEG was performed, showing continuous diffuse spikes, sharp waves and sharp-and-slow wave complexes, pattern consistent with a non-convulsive status epilepticus (NORSE) (Fig. 1A). The patient was treated with 10 mg Diazepam e.v. with a complete resolution of the abnormal electric activity but without a clinical improvement (Fig. 1B). An antiepileptic therapy with Levetiracetam 3000 mg daily was started. The following day, EEG tracings showed the abnormal pattern previously recorded consistent with status epilepticus. Diagnosis of NORSE was made and an additional antiepileptic treatment with valproic acid 2400 mg daily was started, without clinical response. Based on the clinical history of a rapidly progressive dementia with epileptic seizures, a prion disorder or an autoimmune encephalitis were suspected and a lumbar puncture was performed. Standard CSF analysis showed only a mild glycorrhachia and further tested for autoimmune encephalitis, neurodegeneration markers and for RT-QuIC (Table 1). A trained neuroradiologist re-evaluated the first MRI scan and highlighted the presence of a diffuse cortical ribboning in DWI (Fig. 1C). An empirical therapy with high dose Methylprednisolone 1 g daily was started. In the following days, periodic triphasic complexes were observed at EEG consistent with the diagnosis of CJD (Fig. 1D). The clinical condition of the patient rapidly evolved into akinetic mutism and coma, leading to his death in two weeks. The RT-QuIC results confirmed the probable diagnosis of CJD.
Fig. 1.
EEG raw data and MRI assessment. (A) EEG at admission which showed continuous diffuse spikes, sharp waves and sharp-and-slow wave complexes, pattern consistent with a NCSE. (B) EEG after 10 mg Diazepam ev infusion with resolution of epileptic activity. (C) DWI MRI assessment in ganglionic and supraganglionic plane that highlighted the presence of cortical ribboning. (D) EEG after 12 days from admission showed the presence of periodic triphasic sharp-wave complexes consistent with the diagnosis of CJD
Table 1.
Cerebrospinal fluid analysis and degeneration markers
| Laboratory results | Reference range | |
|---|---|---|
| Aspect | Clear | – |
| Color | Colorless | – |
| Glucose | 112.0 mg/dL | 40.0–70.0 |
| Proteins | 42.3 mg/dL | 15.0–45.0 |
| CSF/blood Glucose | 0.5 | 0.5–0.6 |
| White blood cells | 0 WBC/µL | < 5 |
| Autoimmune encephalitis antibodies research | Negative | – |
| Tau-T | > 2000 pg/mL | 146–410 |
| Tau-P | 59.9 pg/mL | 21.5–59.0 |
| Amyloid-β 1–42 | 513 pg/mL | 725–1777 |
| Amyloid-β 1–40 | 9667 pg/mL | 7755–16715 |
| AB42/AB40 | 0.053 | 0.068–0.115 |
| SARS-CoV-2 | Negative | – |
Discussion
CJD is a lethal condition with a survival expectancy of about 1 year from symptoms onset and 4 months post diagnosis [3]. Our case report describes a fulminant CJD associated with SARS-CoV-2 infection, which led to patient death after 15 days from the ED admission. To the best of our knowledge, only one study has previously reported a patient with rapidly progressive CJD with COVID-19 concomitant infection, suggesting a role of SARS-CoV-2 in spurring the progression of prion spreading [6]. This case report is in line with previous observations supporting the implication of SARS-CoV-2 as a co-factor in accelerating the progression of CJD, as already hypothesized also for other microorganisms [8]. However, the demonstration of cause-effect correlation cannot be proved by case reports. In addition, in our case NORSE presented as an atypical manifestation of early CJD. We hypothesized that this atypical presentation could be associated to systemic inflammatory changes, potentially leading to blood–brain damage, altered cerebral electric homeostasis and abnormal neurotransmission lowering the epileptic threshold [9]. Recent studies showed the role of SARS-CoV-2 in favoring the initiation of protein aggregation (including Aβ, α-synuclein, tau, prion, and TDP-43 RRM) leading to neurodegeneration [7] and a link between neurodegeneration and cerebral inflammation. Indeed, high levels of pro-inflammatory cytokines are linked to a faster progression for a wide range of neurodegenerative disorders, such as Parkinson’s disease, Alzheimer Disease, and fronto-temporal dementia [10–12]. Increased levels of IL-13 and TNF-α have been found to be associated to Alzheimer’s Disease, with the highest levels in rapidly progressive forms (rpAD) [13]. Similarly to other viral infections like Dengue fever or Ebola [14, 15], COVID-19 is characterized by a cytokine storm with increased levels of IL-1β, IL-6, IL-8, IL-10, TNF-α [16] and we can speculate that an increased levels cytokines may contribute to the rapid progression. Unfortunately, in our patient, we didn’t perform a cytokines panel which could have supported our hypothesis of neuroinflammation leading to accelerated neurodegeneration.
Conclusions
Taken together, these observations lead to two major concerns. First, CJD with SARS-CoV-2 co-infection could be characterized by an accelerated evolution. Second, in this type of patients, CJD diagnosis might be more challenging due to its uncommon presentations, such as NORSE.
Acknowledgements
The authors thank Matteo di Franza for editorial assistance.
Funding
This study did not receive any funding.
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mcmurran CE, Chaggar GH, Ugoya SO. Case report a patient with sporadic Creutzfeldt–Jakob disease : challenges of rare diseases in the COVID-19 era. Published online. 2020;2020:443–446. doi: 10.1093/omcr/omaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray K. Creutzfeldt-Jakob disease mimics, or how to sort out the subacute encephalopathy patient. Postgrad Med J. 2011;87(1027):369–378. doi: 10.1136/pgmj.2010.235721rep. [DOI] [PubMed] [Google Scholar]
- 3.Uttley L, Carroll C, Wong R, Hilton DA, Stevenson M. Creutzfeldt-Jakob disease: a systematic review of global incidence, prevalence, infectivity, and incubation. Lancet Infect Dis. 2020;20(1):e2–e10. doi: 10.1016/S1473-3099(19)30615-2. [DOI] [PubMed] [Google Scholar]
- 4.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus – Report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Torre JL. Sporadic Creutzfeldt-Jakob disease mimicking nonconvulsive status epilepticus. Neurology. 2011;76(12):1111–1112. doi: 10.1212/WNL.0b013e31820a9535. [DOI] [PubMed] [Google Scholar]
- 6.Young MJ, O’Hare M, Matiello M, Schmahmann JD. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav Immun. 2020;89(June):601–603. doi: 10.1016/j.bbi.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idrees D, Kumar V. SARS-CoV-2 spike protein interactions with amyloidogenic proteins: potential clues to neurodegeneration. Biochem Biophys Res Commun. 2020;554(January):94–98. doi: 10.1016/j.bbrc.2021.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-Sanmiguel J, Schuh CMAP, Muñoz-Montesino C, Contreras-Kallens P, Aguayo LG, Aguayo S. Complex interaction between resident microbiota and misfolded proteins: role in neuroinflammation and neurodegeneration. Cells. 2020;9(11):1–28. doi: 10.3390/cells9112476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Chen Y. Inflammation : a network in the pathogenesis of status. Epilepticus. 2018;11(October):1–5. doi: 10.3389/fnmol.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bright F, Werry EL, Dobson-Stone C, et al. Neuroinflammation in frontotemporal dementia. Nat Rev Neurol. 2019;15(9):540–555. doi: 10.1038/s41582-019-0231-z. [DOI] [PubMed] [Google Scholar]
- 11.Tan EK, Chao YX, West A, Chan LL, Poewe W, Jankovic J. Parkinson disease and the immune system — associations, mechanisms and therapeutics. Nat Rev Neurol. 2020;16(6):303–318. doi: 10.1038/s41582-020-0344-4. [DOI] [PubMed] [Google Scholar]
- 12.Holmes C, Cunningham C, Perry VH. Systemic Inflammation and disease progression in Alzheimer Disease. Neurology. 2010;74(14):1157–1158. doi: 10.1016/S0028-3878(10)60344-5. [DOI] [PubMed] [Google Scholar]
- 13.Stoeck K, Schmitz M, Ebert E, Schmidt C, Zerr I. Immune responses in rapidly progressive dementia: a comparative study of neuroinflammatory markers in Creutzfeldt-Jakob disease, Alzheimer’s disease and multiple sclerosis. J Neuroinflammation. 2014;11(1):1–8. doi: 10.1186/s12974-014-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikiatkhachorn A, Mathew A, Rothman AL. Immune-mediated cytokine storm and its role in severe dengue. Published online. 2017 doi: 10.1007/s00281-017-0625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younan P, Iampietro M, Nishida A. Crossm ebola virus binding to tim-1 on t lymphocytes induces a cytokine storm. MBio. 2017 doi: 10.1128/mBio.00845-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]