Abstract
Background
Prone position is frequently used in patients with acute respiratory distress syndrome (ARDS), especially during the Coronavirus disease 2019 pandemic. Our study investigated the ability of pulse pressure variation (PPV) and its changes during a tidal volume challenge (TVC) to assess preload responsiveness in ARDS patients under prone position.
Methods
This was a prospective study conducted in a 25-bed intensive care unit at a university hospital. We included patients with ARDS under prone position, ventilated with 6 mL/kg tidal volume and monitored by a transpulmonary thermodilution device. We measured PPV and its changes during a TVC (ΔPPV TVC6–8) after increasing the tidal volume from 6 to 8 mL/kg for one minute. Changes in cardiac index (CI) during a Trendelenburg maneuver (ΔCITREND) and during end-expiratory occlusion (EEO) at 8 mL/kg tidal volume (ΔCI EEO8) were recorded. Preload responsiveness was defined by both ΔCITREND ≥ 8% and ΔCI EEO8 ≥ 5%. Preload unresponsiveness was defined by both ΔCITREND < 8% and ΔCI EEO8 < 5%.
Results
Eighty-four sets of measurements were analyzed in 58 patients. Before prone positioning, the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen was 104 ± 27 mmHg. At the inclusion time, patients were under prone position for 11 (2–14) hours. Norepinephrine was administered in 83% of cases with a dose of 0.25 (0.15–0.42) µg/kg/min. The positive end-expiratory pressure was 14 (11–16) cmH2O. The driving pressure was 12 (10–17) cmH2O, and the respiratory system compliance was 32 (22–40) mL/cmH2O. Preload responsiveness was detected in 42 cases. An absolute change in PPV ≥ 3.5% during a TVC assessed preload responsiveness with an area under the receiver operating characteristics (AUROC) curve of 0.94 ± 0.03 (sensitivity: 98%, specificity: 86%) better than that of baseline PPV (0.85 ± 0.05; p = 0.047). In the 56 cases where baseline PPV was inconclusive (≥ 4% and < 11%), ΔPPV TVC6–8 ≥ 3.5% still enabled to reliably assess preload responsiveness (AUROC: 0.91 ± 0.05, sensitivity: 97%, specificity: 81%; p < 0.01 vs. baseline PPV).
Conclusion
In patients with ARDS under low tidal volume ventilation during prone position, the changes in PPV during a TVC can reliably assess preload responsiveness without the need for cardiac output measurements.
Trial registration: ClinicalTrials.gov (NCT04457739). Registered 30 June 2020 —Retrospectively registered, https://clinicaltrials.gov/ct2/show/record/NCT04457739
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04087-w.
Keywords: Pulse pressure variation, Fluid responsiveness, ARDS, End-expiratory occlusion test
Background
Prone positioning is recommended in mechanically ventilated patients with acute respiratory distress syndrome (ARDS) and a ratio of partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) < 150 mmHg [1–3], for at least 16 h [1] and even more for some patients [4]. During the Coronavirus disease 2019 (COVID-19) pandemic, prone positioning has been widely applied in patients with ARDS [5–9]. Patients with ARDS, especially those with COVID-19, are characterized by lung edema mainly related to increased pulmonary endothelial permeability [10, 11]. Fluid management in such patients is challenging [12, 13]. On the one hand, ARDS patients could experience shock that might require fluid therapy and vasopressors [14, 15]. The objective of fluid therapy is to restore adequate organ perfusion in patients in the case of fluid responsiveness, a phenomenon that is generally present in 50% of patients [16]. On the other hand, fluid therapy may worsen lung edema due to the altered permeability of pulmonary microvessels [17]. Therefore, the prediction of fluid responsiveness is important to test in patients with ARDS to prevent fluid administration in those who are fluid unresponsive and for whom harmful consequences of fluid therapy would be maximal [18]. Pulse pressure variation (PPV), passive leg raising (PLR), and end-expiratory occlusion (EEO) are dynamic variables or tests that are commonly used to predict fluid responsiveness in mechanically ventilated patients [19, 20]. Nevertheless, PLR and EEO test require real-time cardiac output measurements, while PPV, which can be measured non-invasively [21, 22] or invasively by a simple arterial catheter, is less reliable in patients mechanically ventilated with a low tidal volume [23–25]. The tidal volume challenge (TVC) was suggested to compensate for the limitation of PPV during low tidal volume ventilation [26], though limited and controversial evidence exists in patients under prone position [27, 28]. The primary objective of our study was to investigate whether a one-minute TVC could assess preload responsiveness in patients with ARDS under prone position. The secondary objective was to investigate the predictive performance of EEO test at the tidal volume of 6 mL/kg predicted body weight (PBW) under prone position.
Methods
This is a prospective study conducted in an intensive care unit (ICU) of a tertiary hospital. Our study was approved by Comité de Protection des Personnes (2019-A00064-53) and was registered on ClinicalTrials (NCT04457739).
Patients
We included patients with ARDS according to the Berlin definition [29], under prone position and monitored by calibrated transpulmonary thermodilution device (PiCCO2, Getinge, Sweden). Patients for whom an assessment of preload responsiveness was required by the attending physician were included. Exclusion criteria were: age ≤ 18 years, pregnancy, presence of cardiac arrhythmia or venous compression stockings, presence of extracorporeal membrane oxygenation (ECMO) assistance at the time of inclusion, and contraindication to the Trendelenburg maneuver. Informed consent was obtained from the patient’s next of kin.
Transpulmonary thermodilution measurements
In all patients, a thermistor-tipped femoral artery catheter and a central venous catheter were already in place as part of the patient’s hemodynamic monitoring [30]. After calibrating the monitoring system by transpulmonary thermodilution (TPTD) [30], the continuous pulse contour analysis-derived cardiac index (CI) could be estimated [30]. The following TPTD variables were also collected at baseline: cardiac index, global end-diastolic volume indexed for body surface (GEDVI), extravascular lung water index for body weight (EVLWi), and pulmonary vascular permeability index (PVPI).
Ventilation settings
All the patients received protective ventilation with the settled tidal volume of 6 mL/kg PBW in the volume assist-controlled mode. The respiratory rate and the FiO2 were adjusted by the attending physician. Neuromuscular-blocking agents were used if required. Before prone positioning, we collected the blood lactate concentration and the arterial blood gases data, including PaO2/FiO2. The respiratory system compliance (Crs) was calculated under prone position by calculating the ratio of tidal volume over the difference between plateau pressure and total positive end-expiratory pressure (PEEP) at baseline.
Other variables
Demographic and other hemodynamic parameters, including heart rate, arterial blood pressure, and central venous pressure (CVP), were recorded. The intra-abdominal pressure (IAP) was recorded at each timepoint under prone position. The fluid balance during the last 24 h before prone positioning was collected. The use of norepinephrine and its dose were recorded.
Design of the study
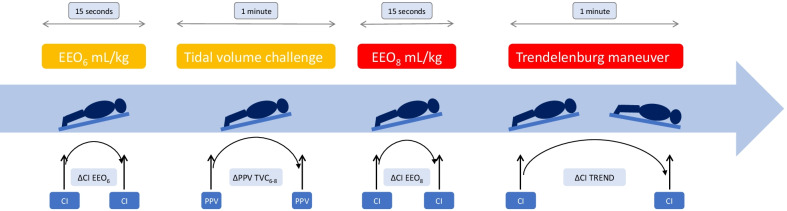
At the time of inclusion, patients were in the prone position with a 13° upward bed angulation [28] (Fig. 1). A set of TPTD measurements was performed, and related hemodynamic and respiratory variables were collected. Then, while tidal volume was at 6 mL/kg PBW, we performed an EEO test (EEO6) for 15 s as previously described [31] and the pulse contour analysis CI was recorded at the end of EEO6. The percent changes in CI during EEO (ΔCI EEO6) were then calculated. After the pulse contour analysis CI returned to the baseline value, we recorded the PPV value (PPVbase) before increasing the tidal volume from 6 to 8 mL/kg for one minute. We recorded the maximal value of PPV (PPVmax) during the maneuver and calculated the absolute changes in PPV (ΔPPV TVC6–8) during TVC (PPVmax–PPVbase). Then, we performed another EEO test for 15 s [16], while the tidal volume was kept at 8 mL/kg (EEO8). We calculated the percent changes in pulse contour analysis CI during the second EEO test (ΔCI EEO8), and then, we decreased the tidal volume to 6 mL/kg PBW. When the pulse contour analysis CI was stabilized again (CIbase), we performed a Trendelenburg maneuver by using the automatic moving function of the bed. This resulted in a patient’s position where the head and the trunk were lowered to a maximum of − 13° bed angulation. After one minute, the maximal value of pulse contour analysis CI (CImax) was recorded to calculate the percent changes in CI during Trendelenburg (ΔCITREND) = (CImax – CIbase)/CIbase. According to the decision of the attending physician, some patients received 500 mL of saline over 10 min. In this subgroup of patients, a new set of hemodynamic variables including TPTD measurements was obtained at the end of the saline infusion. Doses of norepinephrine and of sedative drugs, as well as respiratory rate and level of PEEP, were kept constant throughout the study period. Some patients were studied more than once. Nevertheless, in this case, the studies were never performed more than once during the same day or the same session of prone position.
Fig. 1.
Study protocol
Statistical analysis
The Trendelenburg test was defined as positive if ΔCITREND was ≥ 8% according to Yonis et al. [28]. The EEO test was defined as positive if ΔCI EEO8 ≥ 5% [32]. Since we did not assess the response to fluid administration in all patients, we defined preload responsiveness as when both ΔCITREND and ΔCI EEO8 were ≥ 8% and ≥ 5%, respectively. Preload unresponsiveness was defined as when both ΔCITREND and ΔCI EEO8 < 8% and < 5%, respectively. Results in terms of preload responsiveness and preload unresponsiveness were given according to cases and not to patients as some patients were studied more than once. Cases, where only ΔCITREND was ≥ 8% or only ΔCI EEO8 was ≥ 5%, were excluded from the final analysis. The main objective of this study was to investigate whether the changes in PPV (ΔPPV TVC6–8) during TVC could assess preload responsiveness under prone position with an area under the receiver operating characteristics (ROC) curve (AUROC) of at least 0.9. We considered that the null hypothesis was at 0.75 since an AUROC between 0.5 and 0.75 would have been too low in terms of sensitivity and specificity to draw a relevant conclusion. Considering an α risk at 5% and a β risk at 10%, and assuming that the prevalence of preload responsiveness is 50% [16]. We thus calculated that 84 cases were required by using the method of Hanley-McNeil [33]. We also tested the performance of PPVbase, of PPVmax, and of EEO6 to predict preload responsiveness.
Quantitative and categorical variables are presented as frequency (percentage) and mean ± SD or median (interquartile range) as appropriate. Histograms were used to verify the distribution of data. The ROC curve data are presented as the AUROC value ± standard error (with a 95% confidence interval), sensitivity (with a 95% confidence interval), and specificity (with a 95% confidence interval). The p values for the interaction effects among different timepoints, between baseline and maximal values, and between responsive cases and non-responsive cases, have been calculated by estimating a Generalized Linear Mixed Effect Model (GLMM). The model considers a random effect term on the repeated measurements accounting for the correlation between repeated measurements. The details of this model are presented in the additional file [34]. Moreover, the Benjamini and Hochberg corrections were performed for the multiple testing correction in Table 3 [35]. The p value adjustment method has been used instead of the false discovery rate definition [36].
Table 3.
Evolution of hemodynamic variables in preload responsive cases and non-responsive cases
| Variables | EEO6 | TVC | EEO8 | TREND | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | for PCCImax* | Baseline | for PPVmax† | Baseline | for PCCImax* | Baseline | PCCImax* | |
| Heart rate (beats/min) | ||||||||
| Responsive cases | 89 ± 20 | 90 ± 20 | 90 ± 20 | 90 ± 21 | 90 ± 21 | 91 ± 21 | 90 ± 21 | 90 ± 19 |
| Non-responsive cases | 81 ± 20 | 81 ± 20 | 81 ± 20 | 81 ± 20 | 81 ± 20 | 80 ± 19 | 82 ± 20 | 80 ± 20 |
| Systolic arterial pressure (mmHg) | ||||||||
| Responsive cases | 122 ± 17 | 126 ± 18 | 126 ± 19 | 122 ± 18 | 121 ± 17 | 125 ± 18 | 122 ± 19 | 144 ± 20abcd |
| Non-responsive cases | 125 ± 18 | 125 ± 18 | 124 ± 18 | 123 ± 17 | 124 ± 18 | 124 ± 19 | 124 ± 17 | 133 ± 18abcd |
| Diastolic arterial pressure (mmHg) | ||||||||
| Responsive cases | 58 ± 8 | 59 ± 9 | 60 ± 9 | 58 ± 9 | 58 ± 8 | 59 ± 9 | 58 ± 9 | 70 ± 1abcd |
| Non-responsive cases | 56 ± 9 | 56 ± 9 | 56 ± 9 | 55 ± 8 | 55 ± 9 | 55 ± 9 | 56 ± 9 | 62 ± 9abcd |
| Mean arterial pressure (mmHg) | ||||||||
| Responsive cases | 79 ± 11 | 82 ± 12 | 82 ± 13 | 79 ± 11 | 78 ± 11 | 81 ± 11 | 79 ± 12 | 96 ± 14abcd |
| Non-responsive cases | 80 ± 11 | 80 ± 11 | 79 ± 11 | 78 ± 11 | 79 ± 11 | 79 ± 11 | 79 ± 11 | 87 ± 12abcd |
| Pulse pressure (mmHg) | ||||||||
| Responsive cases | 64 ± 14 | 66 ± 15 | 67 ± 15 | 64 ± 14 | 63 ± 14 | 66 ± 15 | 64 ± 15 | 74 ± 16abcd |
| Non-responsive cases | 69 ± 16 | 70 ± 16 | 69 ± 16 | 68 ± 16 | 68 ± 16 | 69 ± 16 | 68 ± 15 | 71 ± 16abcd |
| Central venous pressure (mmHg) | ||||||||
| Responsive cases | 8 ± 4 | 7 ± 4 | 8 ± 4 | 8 ± 4 | 8 ± 4 | 7 ± 4 | 7 ± 4 | 13 ± 5abcd |
| Non-responsive cases | 11 ± 5 | 10 ± 5 | 11 ± 5 | 11 ± 5 | 11 ± 5 | 10 ± 5 | 10 ± 5 | 16 ± 8abcd |
| Pulse pressure variation (%) | ||||||||
| Responsive cases | 9 ± 5a | 8 ± 4a | 9 ± 4a | 13 ± 5ab | 12 ± 5ab | 11 ± 5ab | 9 ± 4ad | 9 ± 4acd |
| Non-responsive cases | 5 ± 2 | 4 ± 2 | 4 ± 2 | 6 ± 3b | 6 ± 3b | 5 ± 3b | 4 ± 2d | 5 ± 3 |
| PCCI (L/min/m2) | ||||||||
| Responsive cases | 2.66 ± 0.75a | 2.81 ± 0.78a | 2.70 ± 0.73a | 2.56 ± 0.66a | 2.53 ± 0.65a | 2.73 ± 0.69a | 2.59 ± 0.67a | 2.92 ± 0.74abcd |
| Non-responsive cases | 3.35 ± 0.99 | 3.39 ± 1.00 | 3.33 ± 0.95 | 3.25 ± 0.94 | 3.27 ± 0.97 | 3.32 ± 0.97 | 3.43 ± 1.13 | 3.31 ± 1.01 |
| Intra-abdominal pressure (mmHg)‡ | ||||||||
| Responsive cases | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 | 11 ± 4 |
| Non-responsive cases | 11 ± 4 | 11 ± 4 | 11 ± 4 | 12 ± 4 | 12 ± 4 | 11 ± 4c | 11 ± 4 | 10 ± 4 cd |
EEO6 end-expiratory occlusion performed at 6 mL/kg tidal volume, EEO8 end-expiratory occlusion performed at 8 mL/kg tidal volume, Max maximal value, PCCI pulse contour cardiac index, TREND Trendelenburg maneuver, TV tidal volume, TVC tidal volume challenge
*variables values collected for the maximal PCCI obtained during EEO6, EEO8 and TREND. †variables values collected for the maximal PPV obtained during TVC. ‡available in 78 cases (40 responsive vs. 38 non-responsive cases). ap < 0.05, responsive vs. non-responsive cases; bp < 0.05 compared with EEO6 at the same timepoint; cp < 0.05 compared with TVC at the same timepoint; dp < 0.05 compared with EEO8 at the same timepoint
The fivefold cross-validated AUROC estimates for a repeated measurements data set have been calculated by using the LeDell approach [37, 38]. A computationally efficient influence curve-based approach was used to obtain a variance estimate for cross-validated AUROC [37]. The stratified bootstrapped p value (10,000 runs) was calculated for the comparison with the gold standard of 0.75. Because of repeated measurements, 95% confidence intervals around the parameters were estimated using a 10,000 individual bootstrap by resampling subjects instead of measurements [39]. The same set bootstrapping of samples was used for comparison between AUROCs in each iteration, preserving the correlation between repeated measurements [40].
A p value < 0.05 was considered statistically significant. The gray zone analysis has been conducted as reported elsewhere [41, 42]. The Youden index (sensitivity + specificity − 1) was used to define the optimal cutoff values for each test and its calculation was conducted within 10,000 bootstraps resamples. By using a two-step procedure, the gray zone was then defined as the values presenting with either sensitivity less than 90% or specificity less than 90% [42].
Statistical analysis was performed using MedCalc 11.6.0 software (Mariakerke, Belgium), and cutpoint package in R software (version 3.4.1).
Results
Patient characteristics
Patients with ARDS ventilated with a 6 mL/kg tidal volume under prone position were prospectively included from January 2019 to May 2021. In total, we included 69 patients of whom 55 had COVID-19 (76%). The inclusions were conducted after prone positioning for 11 (2–14) hours. Data from eleven patients (13 cases) were excluded from the analysis since only one of the two preload responsiveness tests (Trendelenburg or EEO8) was positive (three cases with only ΔCITREND ≥ 8% and ten cases with only ΔCI EEO8 ≥ 5%). Therefore, data from 58 patients were included in the analysis (Table 1). Sixteen patients were studied more than once (maximal number: four times), and the delay between two inclusions was 5 (3–14) days. A total of 84 different cases were finally analyzed (Table 2). According to the increase in CI both during Trendelenburg and EEO8, preload responsiveness was found in 42 cases and preload unresponsiveness was found in 42 other cases.
Table 1.
General characteristics of patients with acute respiratory distress syndrome under prone position at the time of inclusion
| Variables | All patients (n = 58) |
|---|---|
| Age (years) | 65 ± 11 |
| Male (n, %) | 45 (76) |
| Body mass index (kg/m2) | 27.4 (24.5–30.7) |
| Hypertension (n, %) | 33 (57%) |
| COVID-19 (n, %) | 44 (76) |
| SAPS II at admission | 39 (32–52) |
| SOFA score at admission | 4 (4–6) |
| ICU length of stay (days) | 23 (12–35) |
| ICU mortality (n, %) | 32 (55) |
Values are expressed as mean ± SD or median (interquartile range) or number (percentage). COVID-19, Coronavirus disease 2019; ICU, intensive care unit; SAPS II, Simplified Acute Physiology Score II; and SOFA, Sequential Organ Failure Assessment
Table 2.
Baseline cardiovascular and respiratory parameters of all the cases at the time of inclusion
| Studied variables | All cases (n = 84) |
Non-responsive cases (n = 42) |
Responsive cases (n = 42) |
p value |
|---|---|---|---|---|
| Inclusion time after admission (days) | 7 (4–11) | 8 (5–15) | 6 (3–10) | 0.145 |
| Inclusion time after prone positioning (hours) | 11 (2–14) | 12 (5–15) | 8 (1–12) | 0.033 |
| Hemodynamic variables | ||||
| Number of patients with norepinephrine (n, %) | 70 (83) | 35 (83) | 35 (83) | 0.770 |
| Dose of norepinephrine (µg/kg/min) | 0.25 (0.15–0.42) | 0.23 (0.14–0.35) | 0.32 (0.17–0.56) | 0.382 |
| Heart rate (beats/min) | 83 (69–100) | 82 (67–94) | 94 (74–107) | 0.014 |
| Systolic arterial pressure (mmHg) | 127 ± 18 | 127 ± 18 | 127 ± 18 | 1.000 |
| Diastolic arterial pressure (mmHg) | 59 ± 10 | 57 ± 8 | 60 ± 11 | 0.131 |
| Mean arterial pressure (mmHg) | 82 ± 12 | 82 ± 11 | 83 ± 13 | 0.594 |
| Central venous pressure (mmHg) | 10 ± 4 | 11 ± 5 | 8 ± 4 | 0.012 |
| Intra-abdominal pressure (mmHg) | 11 ± 4 | 11 ± 4 | 11 ± 4 | 0.924 |
| CITPTD (L/min/m2) | 3.06 ± 0.90 | 3.38 ± 0.95 | 2.73 ± 0.74 | 0.001 |
| GEDVI (mL/m2) | 744 ± 173 | 780 ± 202 | 708 ± 130 | 0.055 |
| EVLW (mL/kg) | 17 (15–20) | 17 (15–20) | 17 (14–21) | 0.936 |
| PVPI | 3.4 (2.9–4.1) | 3.2 (2.6–4.1) | 3.5 (2.9–4.1) | 0.293 |
| Fluid balance of previous 24 h (mL) | 562 (-224–1660) | 500 (-183–1720) | 693 (-280–1717) | 0.831 |
| Lactate (mmol/L) | 1.6 (1.3–2.3) | 1.5 (1.1–1.8) | 2.1 (1.3–2.8) | 0.005 |
| Respiratory variables | ||||
| Respiratory rate (breaths/min) | 30 (26–33) | 30 (28–35) | 28 (25–30) | 0.035 |
| PaO2/FiO2 before prone positioning (mmHg) | 104 ± 27 | 105 ± 24 | 102 ± 29 | 0.684 |
| Tidal volume before TVC (mL/kg PBW) | 6.0 (6.0–6.0) | 6.0 (6.0–6.0) | 6.0 (6.0–6.0) | 0.267 |
| Tidal volume during TVC (mL/kg PBW) | 8.0 (8.0–8.0) | 8.0 (8.0–8.0) | 8.0 (8.0–8.0) | 0.321 |
| Total PEEP (cmH2O) | 14 (11–16) | 14 (12–16) | 14 (10–16) | 0.946 |
| Driving pressure before TVC (cmH2O) | 12 (10–17) | 13 (10–18) | 12 (10–17) | 0.723 |
| Plateau pressure before TVC (cmH2O) | 27 (25–31) | 27 (25–30) | 27 (25–31) | 0.882 |
| Crs before TVC (mL/cmH2O) | 32 (22–41) | 31 (21–39) | 34 (22–41) | 0.591 |
| Driving pressure at the end of TVC (cmH2O)* | 19 (15–22) | 19 (15–21) | 20 (15–24) | 0.473 |
| Plateau pressure at the end of TVC (cmH2O)* | 32 (29–37) | 33 (30–38) | 32 (28–35) | 0.229 |
| Crs at the end of TVC (mL/cmH2O)* | 28 (22–39) | 28 (24–39) | 27 (21–39) | 0.715 |
CI cardiac index, Crs compliance of the respiratory system, EVLW extravascular lung water, GEDVI global end-diastolic volume index, PVPI pulmonary vascular permeability index, PaO2/FiO2 the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen, PBW predicted body weight, PEEP positive end-expiratory pressure, TPTD transpulmonary thermodilution, TVC tidal volume challenge; Bold font indicates statistical significance
*Available in 78 cases
The demographic characteristics of all the 58 analyzed patients are detailed in Table 1. Table 2 shows the detailed general characteristics of all the 84 analyzed cases. The mean arterial pressure was 82 (75–90) mmHg [under norepinephrine in 70 (83%) cases at a dose of 0.25 (0.15–0.42) µg/kg/min] at the time of inclusion. The PEEP was 14 (11–16) cmH2O. The driving pressure was 12 (10–17) cmH2O before the TVC versus 19 (15–22) cmH2O at the end of the TVC (p < 0.001), the plateau pressure was 27 (25–31) cmH2O before the TVC versus 32 (29–37) cmH2O at the end of the TVC (p < 0.001), and the Crs was 32 (21–41) mL/cmH2O before the TVC versus 28 (22–39) mL/cmH2O at the end of the TVC (p = 0.366). The evolution of hemodynamic variables in both preload responsive cases and preload unresponsive cases is shown in Table 3. The PPVbase was significantly increased in cases of preload responsiveness than in cases of preload unresponsiveness. In twelve cases, the patients (n = 11) received fluids after performing all the tests, and in all of them, CI increased by more than 15%.
Performance of PPVbase, ΔPPV TVC6–8, and ΔCI EEO6 to assess preload responsiveness
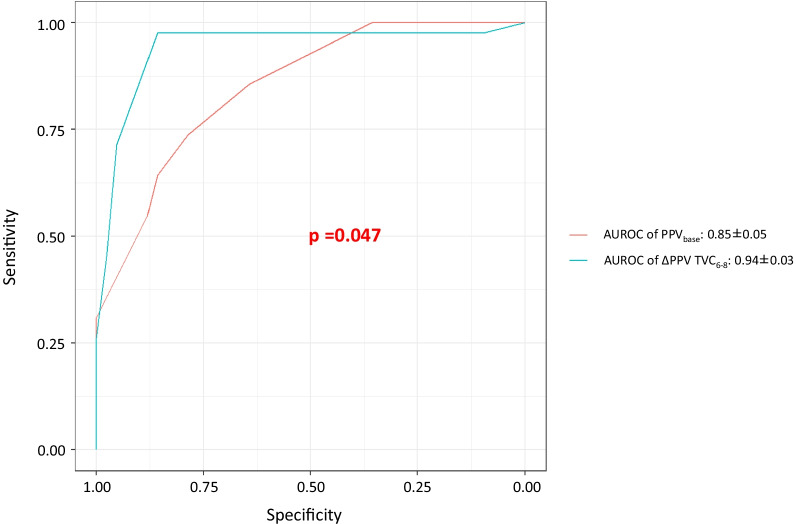
A PPVbase ≥ 6.5% enabled to assess preload responsiveness with an AUROC of 0.85 ± 0.05 (0.77–0.92), sensitivity: 74% (57–95%), specificity: 79% (56–96%), p < 0.01 versus 0.75; gray zone: 5–8% (32/84 cases) (Fig. 2, Additional file 1: Fig. S1). A detailed analysis of the 84 cases shows that all cases (n = 15) with PPVbase < 4% were non-responsive cases and that all cases (n = 13) with PPVbase ≥ 11% were responsive cases. In 56 cases, PPVbase was between ≥ 4 and < 11%.
Fig. 2.
The comparison of the receiver operating characteristics (ROC) curves of baseline pulse pressure variation (PPVbase) at a tidal volume of 6 mL/kg predicted body weight versus changes in pulse pressure variation during a tidal volume challenge (ΔPPV TVC6–8)
A ΔPPV TVC6–8 ≥ 3.5% enabled to assess preload responsiveness with an AUROC of 0.94 ± 0.03 (0.88–0.99), sensitivity: 98% (89–99%), specificity: 86% (75–97%); p < 0.01 versus 0.75; p = 0.047 versus AUROC for PPVbase; gray zone: 3.0–4.5% (28/84 cases) (Fig. 2, Additional file 1: Fig. S2). In the 56 cases where PPVbase was between ≥ 4 and < 11%, a ΔPPV TVC6–8 ≥ 3.5% enabled to assess preload responsiveness with an AUROC of 0.91 ± 0.05 (0.80–1.00), sensitivity: 97% (87–99%), specificity: 81% (65–96%), p < 0.01 versus. AUROC for PPVbase: 0.66 ± 0.09 (0.49–0.83) (Additional file 1: Fig. S3).
An increase in CI ≥ 3.2% during EEO6 assessed preload responsiveness with an AUROC of 0.93 ± 0.06 (0.87–0.98), sensitivity: 88% (73–93%), specificity: 93% (90–99%); p = 0.911 versus AUROC for ΔPPV TVC6–8; gray zone: 2.2–4.6% (20/82 cases) (Additional file 1: Figs. S4 and S5).
The effect of intra-abdominal pressure
In 34 cases, including 16 cases with preload responsiveness, IAP was ≥ 12 mmHg [43]. In these cases, PPVbase assessed preload responsiveness with an AUROC of 0.76 ± 0.09 (0.58–0.90); p = 0.90 versus 0.75 (Additional file 1: Fig. S6). A ΔPPV TVC6–8 ≥ 3% assessed preload responsiveness with an AUROC of 0.93 ± 0.05 (0.87–0.98), sensitivity: 97% (90–99%), specificity: 84% (63–99%); p < 0.01 versus 0.75; p = 0.06 versus AUROC for PPVbase (Additional file 1: Fig. S6). An increase in CI ≥ 3.2% during EEO6 assessed preload responsiveness with an AUROC of 0.93 ± 0.05 (0.83–0.99), sensitivity: 94% (68–99%), specificity: 87% (80–99%); p < 0.01 versus 0.75, p = 0.06 versus AUROC for PPVbase; p = 0.90 versus AUROC for ΔPPV TVC6–8 (Additional file 1: Fig. S6).
Discussion
Our study showed that in patients with ARDS under prone position, an increase in PPV ≥ 3.5% during one-minute TVC could reliably assess preload responsiveness and its predictive value was better than that of PPV alone.
The PPV is one of the most utilized dynamic indices to predict fluid responsiveness [44]. One of the reasons is that it can be easily obtained [21, 22]. However, interpretation of PPV is limited in many circumstances such as low tidal volume ventilation [25], arrhythmia, low respiratory compliance, and spontaneous breathing activity [24]. In patients who receive low tidal volume ventilation, respiratory changes in intrathoracic pressure might be insufficient to produce significant changes in preload and therefore PPV may lack sensitivity to predict fluid responsiveness [45]. This was illustrated by studies performed in patients with or without ARDS and who were mechanically ventilated with a tidal volume of ≤ 8 mL/kg [25, 46]. To compensate for the limitations of PPV at low tidal volume, it has been suggested to evaluate the response of PPV to a TVC [26]. An increase in PPV > 3.5% during a TVC was shown to predict fluid responsiveness reliably in patients who were ventilated with 6 mL/kg tidal volume in supine position (AUROC curve of 0.99) [26]. Another study [47] and a recent meta-analysis [20] have confirmed such excellent results in supine patients ventilated with low tidal volume. The findings of our present study confirmed that the TVC was still valid in patients with ARDS who underwent prone position with a threshold value (3.5%) which is the same as that previously reported [26]. The least significant change of PPV according to De Courson et al. was 8.9% [48]. Thus, for a given value of PPV, for example, 7 (which is the mean baseline value of PPV in our study), the smallest change in absolute value that can be trusted as a real PPV change would be small (less than 1 in absolute value). In our study, the cutoff value of absolute PPV change for TVC (+ 3.5) is thus higher than the least significant change value found in the previous literature [48].
Our study also confirmed that ΔPPV TVC6–8 could perform better than PPVbase to assess preload responsiveness. Importantly, there were many cases (56/84 cases) where PPV was inconclusive (between 4 and 11%) and where the ΔPPV TVC6–8 still reliably assessed preload responsiveness. The plateau pressure and hence the driving pressure increased significantly during the one-minute TVC, although there was no difference regarding respiratory compliance. Since the TVC is quite short, the effects on the driving pressure are expected to be transient and reversible. Nevertheless, caution should be taken in using this test in patients with markedly increased driving pressure.
Until now, only one study addressed the issue of assessment of preload responsiveness in patients with ARDS under prone position. Yonis et al. found that an increase in cardiac output greater than 8% during a Trendelenburg maneuver well assessed fluid responsiveness in a series of 33 patients under prone position and ventilated with 6 mL/kg tidal volume [28]. They also found that the changes in PPV during TVC did not well predict fluid responsiveness, which is in disagreement with our present results. It has to be noted that in this study [28], PPV and its changes in response to TVC were assessed in only 19/33 patients since 14 patients with cardiac arrhythmia were excluded from the analysis. There is no clear reason to substantiate the argument that heart–lung interactions cannot apply to patients in prone position as they apply to patients in the supine position. Therefore, there is no clear argument to support the fact that the hemodynamic effects of TVC are undermined during prone position. In this regard, in patients under prone position for neurosurgery and who received 6 mL/kg tidal volume, the response of PPV to a TVC was shown to predict fluid responsiveness with excellent accuracy (AUROC of 0.96; sensitivity: 95%; specificity: 95%) [27]. It has to be noted that the Crs was normal (around 65 mL/cmH2O on average) in the latter study, whereas it was low in the Yonis et al. study (around 30 mL/cmH2O on average), and this might account for the discrepancies between the findings of these two studies. Nevertheless, in the study by Myatra et al. [26], where the TVC performed very well to predict fluid responsiveness, the mean Crs was low (28 mL/cmH2O on average), and thus comparable with the Crs values reported by Yonis et al. [28]. In our present study, the Crs values were also low (around 30 mL/cmH2O on average) as we included patients with severe ARDS. An important difference between our study and that by Yonis et al. is the definition of preload responsiveness. As we did not administer fluids to all our patients with ARDS as they did, we defined preload responsiveness by the positivity of two preload responsiveness tests (Trendelenburg maneuver and EEO8). To minimize risks of uncertain interpretation, we excluded cases where one of the two tests was positive and the other one negative, a situation that occurred in 15% of cases. Therefore, in our study, we considered the presence of preload responsiveness when both ΔCITREND and ΔCI EEO8 were ≥ 8% and ≥ 5%, respectively, and the presence of preload unresponsiveness when both ΔCITREND and ΔCI EEO8 were < 8% and < 5%, respectively. It is noteworthy that our definition could identify the same proportion of preload responsive cases (50%) vs. preload unresponsive cases (50%) as reported in previous studies [16] including those where low tidal volume ventilation was used [20]. However, this does not totally exclude that both tests could be positive—according to our definition—while the patient would be fluid unresponsive and vice versa. Nevertheless, in all the cases with preload responsiveness—according to our definition—where fluid was administered, CI increased by ≥ 15% in response to fluid infusion, suggesting that our definition was appropriate at least in terms of specificity.
The predictive performance of PPVbase in our present study was better than that reported in some previous studies performed in patients receiving low tidal volume ventilation in supine position [25, 46]. Nevertheless, a recent meta-analysis that investigated the performance of PPV in patients under mechanical ventilation with tidal volume ≤ 8 mL/kg without arrhythmia and respiratory effort (22 studies) showed an AUROC of 0.82 (sensitivity 74% and specificity 77%) [20]. It is noteworthy that in patients with ARDS and ventilated with 6 mL/kg, Freitas et al. showed that PPV could predict fluid responsiveness with an AUROC of 0.91 (0.82–1.0), the sensitivity of 89%, and specificity of 90% [49]. Nevertheless, in our study, in two-thirds of cases, the PPVbase fell in a range of uncertainty (between 4 and 11%). Interestingly, the TVC might be helpful in the subgroup of cases where the intra-abdominal pressure was > 12 mmHg (n = 34) and where PPVbase failed to predict the preload responsiveness, although further studies are warranted to confirm this finding. All the above findings limit a broad application of PPV during prone position under low tidal volume ventilation and justify performing another test such as the TVC.
Since varied results were reported regarding the predictive performance of the EEO6 [26, 28, 50–52], we chose the EEO8 as one of the tests to definite the preload responsiveness in our current study in order to minimize uncertainty. Our results showed that the EEO test performed at 6 mL/kg tidal volume reliably assessed preload responsiveness in patients under prone position, which is consistent with our previous studies in the supine position [32, 51, 52] but in disagreement with some other studies showing a less reliable predictive performance of the EEO test at 6 mL/kg tidal volume in supine [19, 43] or in prone position [20, 21]. Nevertheless, although EEO6 seems to be reliable in prone position in our population, the cutoff value of CI change defining the preload responders was quite low (3.2%). Whereas the TVC only requires an arterial catheter to track the changes in PPV, the EEO6 has thus the disadvantage to require a real-time cardiac output monitor with a very high precision [31], a condition that is uncommon in resource-limited settings [53].
Our study has some limitations. Firstly, not all our patients received the standard fluid challenge, since administering fluid is not routinely performed by attending clinicians in patients with ARDS, even when preload responsiveness is present. Nevertheless, a postural maneuver (i.e., PLR) had been previously used to replace fluid administration in order to evaluate the validity of preload responsiveness tests [54–56]. Secondly, to interpret the analysis more straightforwardly, we did not include the cases where only one of the two reference tests of preload responsiveness was positive (i.e., either ΔCITREND ≥ 8% or ΔCI EEO8 ≥ 5%). This occurred, nevertheless in only 15% of cases. Thirdly, 16 patients were included more than once. It is noteworthy that these patients were never included twice during the same day or during the same prone position session. For these 16 patients, the delay between two inclusions was five days, which could be considered long enough for patients to present different hemodynamic profiles. Nevertheless, our statistical analysis took into account the effects of the repeated measurements on the same subject.
Conclusion
In conclusion, the changes in PPV during a TVC can reliably assess preload responsiveness in patients with ARDS under prone position and low tidal volume ventilation. The advantage of this test, which was demonstrated to be superior to PPV, is that it does not require any cardiac output monitor to assess its effects.
Supplementary Information
Additional file 1: Additional file on further results.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- AUROC
Area under the receiver operating characteristic
- CI
Cardiac index
- COVID-19
Coronavirus disease 2019
- Crs
Compliance
- CVP
Central venous pressure
- EEO
End expiratory occlusion
- FiO2
Fraction of inspired oxygen
- IAP
Intra-abdominal pressure
- ICU
Intensive care unit
- PaO2
Arterial partial pressure of oxygen
- PBW
Predicted body weight
- PEEP
Positive end-expiratory pressure
- PLR
Passive leg raising
- PPV
Pulse pressure variation
- ROC
Receiver operating characteristic
- TPTD
Transpulmonary thermodilution
- TVC
Tidal volume challenge
Author contributions
RS and JLT conceived the idea. RS and JLT designed the study. JLT supervised the study. RS, SA, FM, NDV, FG, SC, AP, and CL collected data. RS and DA analyzed data. RS and JLT prepared the first draft of the manuscript. DA, FM, NDV, FG, SC, AP, CL, and XM helped in the interpretation of data, drafting of the manuscript, and revising the manuscript. All authors reviewed and revised drafts of the manuscript and approved the final version. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of supporting data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by Comité de Protection des Personnes (2019-A00064-53) and was registered on ClinicalTrials.gov (NCT04457739). Informed consent was obtained from the patient’s next of kin.
Consent for publication
Not applicable.
Competing interests
Drs. Jean-Louis Teboul and Xavier Monnet are members of the Medical Advisory Board of Getinge, Sweden. The other authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensiv Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 3.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 4.Jochmans S, Mazerand S, Chelly J, Pourcine F, Sy O, Thieulot-Rolin N, et al. Duration of prone position sessions: a prospective cohort study. Ann Intensiv Care. 2020;10:66. doi: 10.1186/s13613-020-00683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer T, Brioni M, Guzzardella A, Carlesso E, Cabrini L, Castelli G, et al. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. 2021;25:128. doi: 10.1186/s13054-021-03552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaramuzzo G, Gamberini L, Tonetti T, Zani G, Ottaviani I, Mazzoli CA, et al. Sustained oxygenation improvement after first prone positioning is associated with liberation from mechanical ventilation and mortality in critically ill COVID-19 patients: a cohort study. Ann Intensiv Care. 2021;11:63. doi: 10.1186/s13613-021-00853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greco M, De Corte T, Ercole A, Antonelli M, Azoulay E, Citerio G, et al. Clinical and organizational factors associated with mortality during the peak of first COVID-19 wave: the global UNITE-COVID study. Intensive Care Med. 2022;48:690–705. doi: 10.1007/s00134-022-06705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira JC, Ho YL, Besen B, Malbouisson LMS, Taniguchi LU, Mendes PV, et al. Protective ventilation and outcomes of critically ill patients with COVID-19: a cohort study. Ann Intensiv Care. 2021;11:92. doi: 10.1186/s13613-021-00882-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaldi D, Aissaoui N, Blonz G, Carbutti G, Courcelle R, Gaudry S, et al. Characteristics and outcomes of acute respiratory distress syndrome related to COVID-19 in Belgian and French intensive care units according to antiviral strategies: the COVADIS multicentre observational study. Ann Intensiv Care. 2020;10:131. doi: 10.1186/s13613-020-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 11.Shi R, Lai C, Teboul JL, Dres M, Moretto F, De Vita N, et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: the PiCCOVID study. Crit Care. 2021;25:186. doi: 10.1186/s13054-021-03594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S, et al. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensiv Care Med. 2016;42:739–749. doi: 10.1007/s00134-016-4326-3. [DOI] [PubMed] [Google Scholar]
- 13.Hajjar LA, Costa I, Rizk SI, Biselli B, Gomes BR, Bittar CS, et al. Intensive care management of patients with COVID-19: a practical approach. Ann Intensiv Care. 2021;11:36. doi: 10.1186/s13613-021-00820-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 15.Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensiv Care Med. 2016;42:862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 16.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 17.Clark WR, Jr, Nieman GF, Goyette D, Gryzboski D. Effects of crystalloid on lung fluid balance after smoke inhalation. Ann Surg. 1988;208:56–64. doi: 10.1097/00000658-198807000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendes RS, Pelosi P, Schultz MJ, Rocco PRM, Silva PL. Fluids in ARDS: more pros than cons. Intensiv Care Med Exp. 2020;8:32. doi: 10.1186/s40635-020-00319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi R, Monnet X, Teboul JL. Parameters of fluid responsiveness. Curr Opin Crit Care. 2020;26:319–326. doi: 10.1097/MCC.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 20.Alvarado Sánchez JI, Caicedo Ruiz JD, Diaztagle Fernández JJ, Amaya Zuñiga WF, Ospina-Tascón GA, Cruz Martínez LE. Predictors of fluid responsiveness in critically ill patients mechanically ventilated at low tidal volumes: systematic review and meta-analysis. Ann Intensiv Care. 2021;11:28. doi: 10.1186/s13613-021-00817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biais M, Stecken L, Ottolenghi L, Roullet S, Quinart A, Masson F, et al. The ability of pulse pressure variations obtained with CNAP™ device to predict fluid responsiveness in the operating room. Anesth Analg. 2011;113:523–528. doi: 10.1213/ANE.0b013e3182240054. [DOI] [PubMed] [Google Scholar]
- 22.Monnet X, Dres M, Ferré A, Le Teuff G, Jozwiak M, Bleibtreu A, et al. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth. 2012;109:330–338. doi: 10.1093/bja/aes182. [DOI] [PubMed] [Google Scholar]
- 23.Jozwiak M, Monnet X, Teboul JL. Prediction of fluid responsiveness in ventilated patients. Ann Transl Med. 2018;6:352. doi: 10.21037/atm.2018.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michard F, Chemla D, Teboul JL. Applicability of pulse pressure variation: How many shades of grey? Crit Care. 2015;19:144. doi: 10.1186/s13054-015-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensiv Care Med. 2005;31:517–523. doi: 10.1007/s00134-005-2586-4. [DOI] [PubMed] [Google Scholar]
- 26.Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul JL. The Changes in pulse pressure variation or stroke volume variation after a "tidal volume challenge" reliably predict fluid responsiveness during low tidal volume ventilation. Crit Care Med. 2017;45:415–421. doi: 10.1097/CCM.0000000000002183. [DOI] [PubMed] [Google Scholar]
- 27.Messina A, Montagnini C, Cammarota G, Giuliani F, Muratore L, Baggiani M, et al. Assessment of fluid responsiveness in prone neurosurgical patients undergoing protective ventilation: role of dynamic indices, tidal volume challenge, and end-expiratory occlusion test. Anesth Analg. 2020;130:752–761. doi: 10.1213/ANE.0000000000004494. [DOI] [PubMed] [Google Scholar]
- 28.Yonis H, Bitker L, Aublanc M, Perinel Ragey S, Riad Z, Lissonde F, et al. Change in cardiac output during Trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit Care. 2017;21:295. doi: 10.1186/s13054-017-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 30.Monnet X, Teboul JL. Transpulmonary thermodilution: advantages and limits. Crit Care. 2017;21:147. doi: 10.1186/s13054-017-1739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavelli F, Teboul JL, Monnet X. The end-expiratory occlusion test: please, let me hold your breath! Crit Care. 2019;23:274. doi: 10.1186/s13054-019-2554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavelli F, Shi R, Teboul JL, Azzolina D, Monnet X. The end-expiratory occlusion test for detecting preload responsiveness: a systematic review and meta-analysis. Ann Intensiv Care. 2020;10:65. doi: 10.1186/s13613-020-00682-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 34.Dean CB, Nielsen JD. Generalized linear mixed models: a review and some extensions. Lifetime Data Anal. 2007;13:497–512. doi: 10.1007/s10985-007-9065-x. [DOI] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B. 1995;57:289–300. [Google Scholar]
- 36.Benjamini Y, Heller R, Yekutieli D. Selective inference in complex research. Philos Trans R Soc A Math Phys Eng Sci. 2009;367:4255–4271. doi: 10.1098/rsta.2009.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeDell E, Petersen M, van der Laan M. Computationally efficient confidence intervals for cross-validated area under the ROC curve estimates. Electron J Stat. 2015;9(1583–607):25. doi: 10.1214/15-EJS1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geisser S. The predictive sample reuse method with applications. J Am Stat Assoc. 1975;70:320–328. doi: 10.1080/01621459.1975.10479865. [DOI] [Google Scholar]
- 39.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–1164. doi: 10.1002/(SICI)1097-0258(20000515)19:9<1141::AID-SIM479>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray P, Le Manach Y, Riou B, Houle TT. Statistical evaluation of a biomarker. Anesthesiology. 2010;112:1023–1040. doi: 10.1097/ALN.0b013e3181d47604. [DOI] [PubMed] [Google Scholar]
- 42.Le Manach Y, Hofer CK, Lehot JJ, Vallet B, Goarin JP, Tavernier B, et al. Can changes in arterial pressure be used to detect changes in cardiac output during volume expansion in the perioperative period? Anesthesiology. 2012;117:1165–1174. doi: 10.1097/ALN.0b013e318275561d. [DOI] [PubMed] [Google Scholar]
- 43.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the world society of the abdominal compartment syndrome. Intensiv Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monnet X, Teboul JL. Assessment of fluid responsiveness: recent advances. Curr Opin Crit Care. 2018;24:190–195. doi: 10.1097/MCC.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 45.Teboul JL, Monnet X, Chemla D, Michard F. Arterial pulse pressure variation with mechanical ventilation. Am J Respir Crit Care Med. 2019;199:22–31. doi: 10.1164/rccm.201801-0088CI. [DOI] [PubMed] [Google Scholar]
- 46.Muller L, Louart G, Bousquet PJ, Candela D, Zoric L, de La Coussaye JE, et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensiv Care Med. 2010;36:496–503. doi: 10.1007/s00134-009-1686-y. [DOI] [PubMed] [Google Scholar]
- 47.Taccheri T, Gavelli F, Teboul JL, Shi R, Monnet X. Do changes in pulse pressure variation and inferior vena cava distensibility during passive leg raising and tidal volume challenge detect preload responsiveness in case of low tidal volume ventilation? Crit Care. 2021;25:110. doi: 10.1186/s13054-021-03515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Courson H, Ferrer L, Cane G, Verchère E, Sesay M, Nouette-Gaulain K, et al. Evaluation of least significant changes of pulse contour analysis-derived parameters. Ann Intensiv Care. 2019;9:116. doi: 10.1186/s13613-019-0590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freitas FG, Bafi AT, Nascente AP, Assunção M, Mazza B, Azevedo LC, et al. Predictive value of pulse pressure variation for fluid responsiveness in septic patients using lung-protective ventilation strategies. Br J Anaesth. 2013;110:402–408. doi: 10.1093/bja/aes398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messina A, Montagnini C, Cammarota G, De Rosa S, Giuliani F, Muratore L, et al. Tidal volume challenge to predict fluid responsiveness in the operating room: an observational study. Eur J Anaesthesiol. 2019;36:583–591. doi: 10.1097/EJA.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 51.Dépret F, Jozwiak M, Teboul JL, Alphonsine JE, Richard C, Monnet X. Esophageal doppler can predict fluid responsiveness through end-expiratory and end-inspiratory occlusion tests. Crit Care Med. 2019;47:e96–e102. doi: 10.1097/CCM.0000000000003522. [DOI] [PubMed] [Google Scholar]
- 52.Jozwiak M, Depret F, Teboul JL, Alphonsine JE, Lai C, Richard C, et al. Predicting fluid responsiveness in critically Ill patients by using combined end-Expiratory and end-inspiratory occlusions with echocardiography. Crit Care Med. 2017;45:e1131–e1138. doi: 10.1097/CCM.0000000000002704. [DOI] [PubMed] [Google Scholar]
- 53.Cecconi M, Hernandez G, Dunser M, Antonelli M, Baker T, Bakker J, et al. Fluid administration for acute circulatory dysfunction using basic monitoring: narrative review and expert panel recommendations from an ESICM task force. Intensiv Care Med. 2019;45:21–32. doi: 10.1007/s00134-018-5415-2. [DOI] [PubMed] [Google Scholar]
- 54.Vignon P, Repessé X, Bégot E, Léger J, Jacob C, Bouferrache K, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 2017;195:1022–1032. doi: 10.1164/rccm.201604-0844OC. [DOI] [PubMed] [Google Scholar]
- 55.Adda I, Lai C, Teboul JL, Guerin L, Gavelli F, Monnet X. Norepinephrine potentiates the efficacy of volume expansion on mean systemic pressure in septic shock. Crit Care. 2021;25:302. doi: 10.1186/s13054-021-03711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamzaoui O, Shi R, Carelli S, Sztrymf B, Prat D, Jacobs F, et al. Changes in pulse pressure variation to assess preload responsiveness in mechanically ventilated patients with spontaneous breathing activity: an observational study. Br J Anaesth. 2021;127:532–538. doi: 10.1016/j.bja.2021.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Additional file on further results.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.