Abstract
BACKGROUND
Wellens syndrome is an electrocardiogram (ECG) pattern seen in high-risk patients with unstable angina pectoris. It is characterized by inverted or biphasic T-waves that change into positive or pseudo-normalized waves at precordial leads when the patient experiences an angina attack; however, the mechanism for this condition remains unclear.
CASE SUMMARY
A 47-year-old male patient experienced repeated, unprovoked episodes of chest pain for > 20 d, with worsening during the previous day. On the day of admission, he experienced episodes of paroxysmal chest pain lasting more than 30 min, in addition to radiating pain to the left arm and exertional dyspnea. The patient presented to the emergency department with no chest pain or other discomfort at that time. ECG at presentation showed sinus tachycardia and T-wave changes, which were identified as Wellens syndrome when combined with previous ECG findings. ECGs and myocardial enzymology examinations were normal when angina was present, but the ECG showed inverted or biphasic T-waves when angina was absent. After percutaneous coronary intervention, the ECGs demonstrated inverted or biphasic T-waves in the anterior precordial leads on days 0, 1, and 2, but normal T-waves on day 3. The ECGs showed no subsequent ischemic ST-T-wave changes.
CONCLUSION
The Wellens syndrome pseudo-normalized T-waves likely reflect development of unstable angina pectoris into the hyperacute phase of ST-segment elevation myocardial infarction.
Keywords: Wellens syndrome, Pseudo-normalized T-waves, Unstable angina pectoris, Myocardial ischemia, Electrocardiogram, Case report
Core Tip: Wellens syndrome, an electrocardiogram (ECG) pattern seen in high-risk patients with unstable angina pectoris, is characterized by inverted or biphasic T-waves that change into positive or pseudo-normalized waves at precordial leads when the patient experiences an angina attack. We report a case of Wellens syndrome in which we recorded the entire process of ECG evolution. We found that pseudo-normalized T-waves reflected the hyperacute T-waves of ST-segment elevation myocardial infarction (STEMI), which are higher and more symmetrical than normal postoperative T-waves. These pseudo-normalized T-waves may be a manifestation of unstable angina pectoris developing into the hyperacute phase of STEMI.
INTRODUCTION
First described by de Zwaan et al[1] in 1982, Wellens syndrome is an important electrocardiogram (ECG) pattern, indicating severe stenosis in the left anterior descending (LAD) coronary artery. It is characterized by inverted or biphasic T-waves at precordial leads in angina-free periods, but shows positive or “normalized” T-waves during angina, which complicates the diagnosis. These positive or “normalized” T-waves are called pseudo-normalized T-waves[2] and might delay recognition of the emergency status of patients with chest pain. Here, we report a case of Wellens syndrome to deepen our understanding of these ECG signs.
CASE PRESENTATION
Chief complaints
Chest pain, dyspnea.
History of present illness
A 47-year-old male patient was admitted with the chief complaint of repeated unprovoked chest pain for more than 20 da, which was aggravated for 1 d. After getting up early in the morning on the day of admission, he experienced paroxysmal chest pain again, which lasted for more than 30 min, in addition to radiating pain to the left arm and exertional dyspnea. At 18:30, the patient presented to the emergency department but had no chest pain or other discomfort at that time.
History of past illness
The patient had a history of smoking > 20 cigarettes per day for over 20 years, type 2 diabetes for 5 years, and gout for 2 years.
Personal and family history
No remarkable personal and familial medical history was identified.
Physical examination
At admission, the patient’s temperature was 37.0 °C, pulse was 99 bpm, and blood pressure was 13.9/9.6 kPa. Cardiopulmonary and abdominal physical examinations showed no obvious abnormalities.
Laboratory examinations
Upon admission, the patient’s serum high-sensitivity cardiac troponin I concentration was 0.317 ng/mL (normal range: 0-0.034 ng/mL). A serum lipid panel showed that the total cholesterol concentration was 5.44 mmol/L↑ (normal range: 2.6-5.2 mmol/L), low-density lipoprotein was 3.78 mmol/L↑ (normal range: ≤ 3.37 mmol/L), triglycerides were 1.65 mmol/L (normal range: 0.34-1.70 mmol/L), and high-density lipoprotein was 1.10 mmol/L (normal range: > 1.04 mmol/L).
Imaging examinations
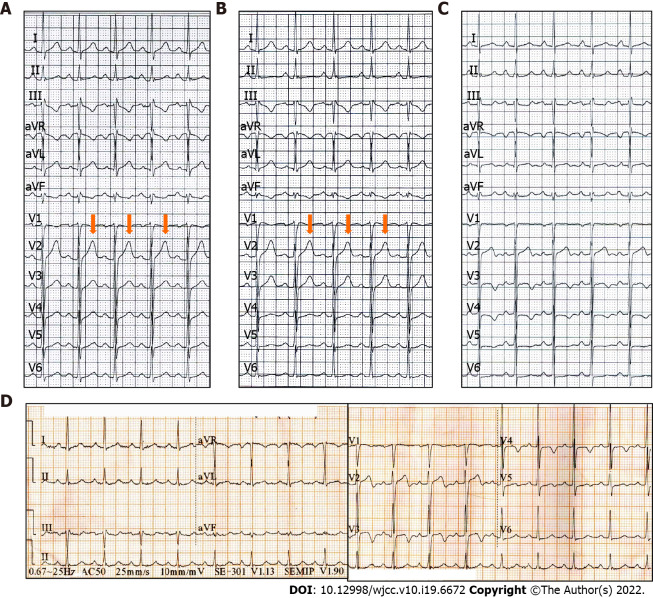
The ECG (Figure 1A and B) and myocardial enzymology examinations were normal when angina was present, while the ECG showed inverted or biphasic T-waves when angina was absent (Figure 1C). The ECG at presentation (Figure 1D) showed sinus tachycardia and T-wave changes, which were identified as Wellens syndrome when combined with previous ECG findings. Coronary angiography (CAG) showed localized stenosis of the proximal LAD (90%-95%; Figure 2), the D1 ostium (60%-70%), and the middle portion of the left circumflex artery.
Figure 1.
Preoperative electrocardiogram findings in a patient presenting with chest pain and exertional dyspnea. A and B: The electrocardiograms (ECGs) are normal in the presence of angina; C: The ECG shows inverted or biphasic T-waves in the absence of angina; D: The ECG at admission in the absence of angina showing sinus tachycardia and T-wave changes.
Figure 2.
Coronary angiography findings in a patient presenting with chest pain and exertional dyspnea. A and B: Coronary angiography (CAG) showing 90%-95% localized stenosis of the proximal left anterior descending (LAD) artery; C: CAG showing recovery of LAD flow after the percutaneous coronary intervention.
FINAL DIAGNOSIS
Wellens syndrome.
TREATMENT
Owing to frequent angina pectoris and the ECG pattern of Wellens syndrome, the patient was administered aspirin (300 mg), clopidogrel (300 mg), and atorvastatin (40 mg) for dual-loading antiplatelet and statin therapy, followed by percutaneous coronary intervention (PCI). Subsequently, a 3.0 mm × 23 mm drug-eluting stent was successfully implanted into the proximal LAD.
OUTCOME AND FOLLOW-UP
The patient’s chest pain fully resolved after the PCI. Postoperative ECGs demonstrated inverted or biphasic T-waves at the extensive anterior precordial leads on the day of PCI (Figure 3A), and 1 and 2 d after PCI (Figure 3B and C, respectively), but normal T-waves 3 d after PCI (Figure 3D). His ECGs recorded no subsequent ischemic ST-T-wave changes. Postoperative transthoracic echocardiography indicated a left ventricular ejection fraction of 69%. The patient’s ECG records at 1 (Figure 3E), 3, and 6 mo after hospital discharge were all normal, and he experienced no further chest pains during follow-up.
Figure 3.
Electrocardiogram findings after percutaneous coronary intervention in a patient presenting with chest pain and exertional dyspnea. A: Postoperative electrocardiograms (ECGs) showing inverted or biphasic T-waves at the extensive anterior precordial leads on the day of percutaneous coronary intervention (PCI); B: 1 d after PCI; C: 2 d after PCI; D: Postoperative ECGs showing normal T-waves on 3 d after PCI; E: 30 d after PCI.
DISCUSSION
An increasing number of special ECG patterns are found to correlate highly with acute myocardial infarction (AMI), and these ECG patterns are even capable of predicting the exact site of the culprit lesions. For example, ST-segment elevation in lead aVR indicates an acute left main coronary artery occlusion[3] and leads V2 and aVL ST elevations indicate occlusion of the first diagonal artery[4]. Besides, de Winter pattern[5], Wellens syndrome, and Aslanger syndrome[6] among other ECG patterns, also draw the attention of clinic practitioners. However, due to the absence of classic ECG manifestations of AMI, identification of high-risk patients with these special ECG patterns is often delayed. In this paper, we report a case of Wellens syndrome to emphasize its value in the early identification of AMI.
Wellens syndrome, a specific ECG manifestation in high-risk patients with unstable angina pectoris, has an incidence of 0.1%[7] and a prevalence of 14%-18%[8] in patients with unstable angina pectoris. A history of angina, inverted or biphasic T-waves in the precordial ECG leads during the pain-free period, and little or no abnormality in cardiac biomarkers are key diagnostic indicators[9]. Another feature of Wellens syndrome is that in the presence of angina, the inverted or biphasic T-waves develop into positive T-waves[10], called pseudo-normalized T-waves[2] with an unknown mechanism. Additionally, Wellens syndrome is categorized into type A (biphasic) and type B (inverted) T-waves, which account for approximately 75% and 25% of the cases, respectively.
Previous studies have documented common etiologies of Wellens syndrome, including myocardial bridge[11], Tako-Tsubo cardiomyopathy[12], pulmonary embolus[13], and coronary spasm caused by drug abuse[14]. The pathophysiological mechanism underlying the characteristic T-wave changes in Wellens syndrome is debatable. Some studies have suggested that dynamic T-wave changes are caused by myocardial stunning or ventricular systolic dysfunction[15,16], whereas others have suggested that the pathophysiology is myocardial edema rather than myocardial dysfunction[17]. However, these studies have not clarified the mechanisms underlying the inverted or biphasic T-waves during the pain-free period, or the positive T-waves in the presence of angina.
Wellens syndrome plays an important role in the early recognition of the pre-infarction state and severe stenosis of the LAD. A study conducted by Haines et al[18] indicated that the Wellens syndrome ECG pattern has a sensitivity of 69%, specificity of 89%, and positive predictive value of 86% for significant LAD stenosis. Among patients with Wellens syndrome that accepted medicinal treatment alone, 75% reportedly developed myocardial infarction within 8.5 d[1]. Once Wellens syndrome is diagnosed, patients should undergo primary PCI, instead of exercise stress testing. In patients with Wellens syndrome, exercise stress testing induces AMI, malignant arrhythmias, or even sudden death[19].
The ECGs in our patient showed positive T-waves during angina, but inverted or biphasic T-waves when angina was relieved, thereby meeting the diagnostic criteria of Wellens syndrome. CAG showed a 90%-95% localized stenosis of the proximal LAD, which indicates that critical myocardial ischemia was present in this patient before he underwent PCI. Additionally, the patient’s angina resolved fully after PCI, and the T-waves normalized. These findings imply that the abnormal T-waves associated with Wellens syndrome resulted from severe fixed coronary stenosis, which could be repaired by PCI.
Regarding the evolution of the inverted or biphasic T-waves to positive T-waves during angina, we believe this was caused by the aggravation of myocardial ischemia. It is well known that flat, biphasic, or inverted T-waves are ECG manifestations of endocardial ischemia, whereas tall and symmetrical positive T-waves are features of transmural injury in the hyperacute phase of ST-segment elevation myocardial infarction (STEMI). For patients with Wellens syndrome, coronary stenosis, coronary spasm, unstable plaque rupture, or myocardial oxygen supply-demand imbalance, would aggravate myocardial ischemia and even cause acute epicardial injury, resulting in angina attacks and abnormal T-waves becoming positive or pseudo-normalized. We found that the T-waves on this patient’s ECGs during angina attacks, which were previously considered pseudo-normalized, were higher and more symmetrical than the normal T-waves on ECGs at 3 and 30 days after PCI. Since Wellens syndrome has the potential to develop into AMI, we propose that the pseudo-normalized T-waves of Wellens syndrome in the presence of angina probably reflect its deterioration into the hyperacute phase of STEMI.
One limitation of this study is that we did not obtain enough cases to quantify and compare the symmetry of the T-waves in patients with Wellens syndrome before and after PCI.
CONCLUSION
Our report demonstrates the ECG manifestations of Wellens syndrome before and after PCI, and during long-term postoperative follow-up, which were not considered in previous studies. The follow-up ECGs indicate that PCI can resolve the abnormal T-waves of Wellens syndrome. The abnormal T-waves during the pain-free period of Wellens syndrome likely result from endocardial ischemia based on significant coronary stenosis, while the pseudo-normalized T-waves reflect epicardial ischemia caused by stenosis aggravation. Without timely and effective treatment, pseudo-normalized T-waves are likely to develop into ST-segment elevation. Further studies are required to quantify and compare the symmetry of the T-waves in a larger number of patients with Wellens syndrome before and after PCI.
ACKNOWLEDGEMENTS
The author would like to express their gratitude to Dr. Yang LL and Dr. Yu X for their help on the revise of this paper.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 4, 2022
First decision: February 8, 2022
Article in press: May 13, 2022
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fazilat-Panah D, Iran; Gulel O, Turkey; He J, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
Contributor Information
Na Tang, The First Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China.
Yi-Hua Li, The First Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China.
Liang Kang, The First Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China.
Rong Li, Department of Cardiovascular Disease, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China.
Qing-Min Chu, Department of Cardiovascular Disease, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou 510000, Guangdong Province, China. 13929504676@163.com.
References
- 1.de Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982;103:730–736. doi: 10.1016/0002-8703(82)90480-x. [DOI] [PubMed] [Google Scholar]
- 2.Nastasi M. Intermittent Typical Angina: Remember Wellens' Syndrome. Adv J Emerg Med. 2019;3:e30. doi: 10.22114/ajem.v0i0.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikus KC, Eskola MJ. Electrocardiogram patterns in acute left main coronary artery occlusion. J Electrocardiol. 2008;41:626–629. doi: 10.1016/j.jelectrocard.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Gülel O, Ciçekçioğlu H, Tekin M, Aydoğdu S, Diker E. Specific electrocardiographic findings due to occlusion of the first diagonal artery. Anadolu Kardiyol Derg. 2006;6:79–80. [PubMed] [Google Scholar]
- 5.de Winter RJ, Verouden NJ, Wellens HJ, Wilde AA Interventional Cardiology Group of the Academic Medical Center. A new ECG sign of proximal LAD occlusion. N Engl J Med. 2008;359:2071–2073. doi: 10.1056/NEJMc0804737. [DOI] [PubMed] [Google Scholar]
- 6.Aslanger E, Yıldırımtürk Ö, Şimşek B, Sungur A, Türer Cabbar A, Bozbeyoğlu E, Karabay CY, Smith SW, Değertekin M. A new electrocardiographic pattern indicating inferior myocardial infarction. J Electrocardiol. 2020;61:41–46. doi: 10.1016/j.jelectrocard.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Arshad S, Ferrick NJ, Monrad ES, Fisher JD, Krumerman A, Ferrick KJ. Prevalence and association of the Wellens' sign with coronary artery disease in an ethnically diverse urban population. J Electrocardiol. 2020;62:211–215. doi: 10.1016/j.jelectrocard.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Mao L, Jian C, Wei W, Tianmin L, Changzhi L, Dan H. For physicians: never forget the specific ECG T-wave changes of Wellens' syndrome. Int J Cardiol. 2013;167:e20–e21. doi: 10.1016/j.ijcard.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 9.Donahue B, Chan SB, Bhandarkar S. Rapid progression of Wellens syndrome in the emergency department. J Emerg Med. 2012;43:667–670. doi: 10.1016/j.jemermed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 10.de Zwaan C, Bär FW, Janssen JH, Cheriex EC, Dassen WR, Brugada P, Penn OC, Wellens HJ. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J. 1989;117:657–665. doi: 10.1016/0002-8703(89)90742-4. [DOI] [PubMed] [Google Scholar]
- 11.Avram A, Chioncel V, Guberna S, Cuciureanu I, Brezeanu RC, Andrei CL, Sinescu C. Myocardial bridging-an unusual cause of Wellens syndrome: A case report. Medicine (Baltimore) 2020;99:e22491. doi: 10.1097/MD.0000000000022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor RS, Skjerli L, Ashurst J. Takotsubo Cardiomyopathy Presenting as Wellens' Syndrome. Clin Pract Cases Emerg Med. 2017;1:175–178. doi: 10.5811/cpcem.2017.1.32297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedhai YR, Basnyat S, Bhattacharya PT. Pseudo-Wellens' syndrome in pulmonary embolism. BMJ Case Rep. 2018;11 doi: 10.1136/bcr-2018-227464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandah F, Mikulic S, Patel P, Dhruva P. Because I Got High: Marijuana Induced Pseudo-Wellen's Syndrome. Cureus. 2020;12:e10390. doi: 10.7759/cureus.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chioncel V, Avram A, Sinescu C. A particular case of Wellens' Syndrome. Med Hypotheses. 2020;144:110013. doi: 10.1016/j.mehy.2020.110013. [DOI] [PubMed] [Google Scholar]
- 16.Stankovic I, Kafedzic S, Janicijevic A, Cvjetan R, Vulovic T, Jankovic M, Ilic I, Putnikovic B, Neskovic AN. T-wave changes in patients with Wellens syndrome are associated with increased myocardial mechanical and electrical dispersion. Int J Cardiovasc Imaging. 2017;33:1541–1549. doi: 10.1007/s10554-017-1181-4. [DOI] [PubMed] [Google Scholar]
- 17.Migliore F, Zorzi A, Marra MP, Basso C, Corbetti F, De Lazzari M, Tarantini G, Buja P, Lacognata C, Thiene G, Corrado D, Iliceto S. Myocardial edema underlies dynamic T-wave inversion (Wellens' ECG pattern) in patients with reversible left ventricular dysfunction. Heart Rhythm. 2011;8:1629–1634. doi: 10.1016/j.hrthm.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Haines DE, Raabe DS, Gundel WD, Wackers FJ. Anatomic and prognostic significance of new T-wave inversion in unstable angina. Am J Cardiol. 1983;52:14–18. doi: 10.1016/0002-9149(83)90061-9. [DOI] [PubMed] [Google Scholar]
- 19.Tandy TK, Bottomy DP, Lewis JG. Wellens' syndrome. Ann Emerg Med. 1999;33:347–351. doi: 10.1016/s0196-0644(99)70373-2. [DOI] [PubMed] [Google Scholar]