Abstract
The presence of isotopically light carbonates in association with fine-grained magnetite is considered to be primarily due to the reduction of Fe(III) by Fe(III)-reducing bacteria in the environment. Here, we report on magnetite formation by biooxidation of Fe(II) coupled to denitrification. This metabolism offers an alternative environmental source of biogenic magnetite.
The contribution of biogenic magnetite produced through reduction of Fe(III) by both dissimilatory Fe(III)-reducing bacteria (DIRB) and magnetotactic bacteria (MB) to natural remanent magnetization of deep-sea and other sediments is well recognized (4, 15, 25). Although the relative contribution of the two metabolic processes to natural remanent magnetization is controversial (4), the former process is thought to have resulted in the accumulation of large amounts of extracellular magnetite (Fe3O4) through the reduction of Fe(III) oxides as the terminal electron acceptor for organic matter oxidation (2). Magnetite is a mixed Fe(II)-Fe(III) mineral with magnetic properties. The discovery of biogenic magnetite at depths of ∼6.7 km in the subsurface (16) has been used as a marker of activity of DIRB in the deep subsurface. In addition, the presence of magnetite in association with isotopically light carbon in carbonate minerals in the Precambrian banded iron formations (BIFs), the world's oldest and largest iron deposits (26), suggests that these organisms played a role in the Precambrian biosphere (1). However, a satisfactory explanation for the oxidation of soluble Fe(II) to Fe(III) required for DIRB to produce magnetite in the anoxic Precambrian hydrosphere before the evolution of oxygenic photosynthesis is still not available (7, 8, 12, 22).
It is unanimously agreed that in Precambrian times, both the hydrosphere and the atmosphere were anoxic, life was restricted to prokaryotes, and deep ocean waters contained significant amounts of soluble Fe(II) that resulted from the chemical weathering of the continental crust and/or subseafloor hydrothermal convection processes (1, 7). According to the accepted reductive mechanisms of biogenic magnetite formation by both DIRB (15, 25, 31) and MB (4), iron would have to be present as Fe(III) or oxidized from existing Fe(II). The most widely accepted theory for the presence of Fe(III) is the interaction of Fe(II) with oxygen produced as a result of microbial oxygenic photosynthesis (9, 31). However Canfield (7) recently demonstrated that deep-ocean water did not become oxic until the Neoproterozoic era (1.0 to ∼0.54 Gyr.). In contrast, the isolation of Fe(II)-oxidizing anoxygenic phototrophs (14, 34) suggested that oxygen-independent biological oxidation of Fe(II) was possible before the evolution of oxygenic photosynthesis. Nonetheless, this type of microbial metabolism could be operative only in shallow seas that received unrestricted sunlight (2). Alternatively, the possibility of direct photooxidation of Fe(II) by UV radiation from sunlight near the surface of Precambrian oceans, prior to the formation of the ozone layer that today reduces UV radiation, has been suggested (8). However, the concentration of Fe(II) in ocean waters and its velocity of upwelling to the upper water column, where photooxidation occurred, are two factors that would have controlled the rate of photooxidation. The existence of upwelling velocities for transferring soluble Fe(II) to the upper oceans along specific basins for geologically extended periods is controversial (22). Moreover, the required high upwelling eddy velocities would hinder the formation of the finely laminated layered structure of BIFs.
The last massive deposition of BIFs around 1.8 Gyr (20) suggests that there would have been sufficient time for slow microbial oxidative precipitation of significant quantities of dissolved Fe(II) from ocean water. Moreover, it has been documented that in the primitive era before the development of oxygenic atmosphere, the net effect of lightning converted atmospheric N2 mainly to dissolved NO3, which remained in the ocean until the evolution of organisms capable of using it as a resource (28). If so, microorganisms that are capable of light-independent direct oxidation of soluble Fe(II) in the anoxic environs of the deep sea through nitrate reduction may offer an alternative explanation of Fe(III) formation (17). This microbial metabolism was only recently identified (5, 17, 32), but magnetite formation as a result of biooxidation of Fe(II) was never demonstrated.
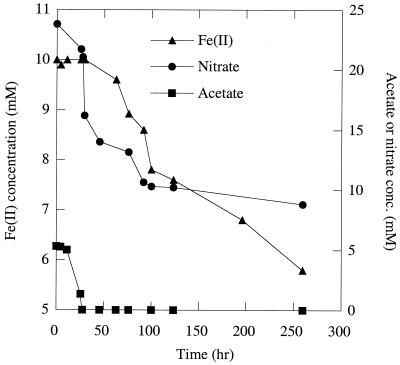
As part of a study of the metabolic diversity of organisms capable of growth by the anaerobic respiration of perchlorate, we isolated a novel organism, Dechlorosoma suillum strain PS, from a swine waste lagoon (10, 29). Physiological characterization revealed that D. suillum rapidly oxidized (10 mM) Fe(II) in the form of FeCl2 with nitrate as the electron acceptor under strict anaerobic conditions (21) (Fig. 1). With 10 mM acetate as a cosubstrate, more than 70% of the added iron was oxidized within 7 days. No Fe(II) was oxidized in the absence of cells or if the nitrate was omitted (data not shown). Fe(II) oxidation was initiated after complete mineralization of acetate to CO2, and growth was not associated with this metabolism (Fig. 1). Nitrate reduction was concomitant with Fe(II) oxidation throughout the incubation (Fig. 1), and the oxidation of 4.2 mM Fe(II) resulted in the reduction of 0.8 mM nitrate, which is 95% of the theoretical stoichiometry of nitrate reduction coupled to Fe(II) oxidation according to the equation
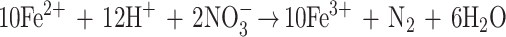 |
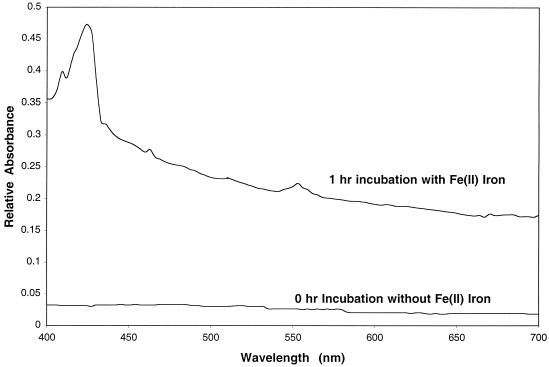
Difference spectrum studies performed as previously described (6, 10) on anaerobic washed whole cells in the presence of Fe(II) demonstrated that the oxidized c-type cytochrome content of D. suillum was reduced after 1 h of incubation in the presence of Fe(II) (Fig. 2), indicating that electrons from Fe(II) are transferred to the electron transport chain of D. suillum. In addition, the main product of nitrate reduction was N2. Chromatographic analysis (ion chromatography and gas chromatography [6, 10]) of headspace gases and culture broths throughout incubation revealed no detectable quantities of nitrite or N2O formed by D. suillum (29). These results suggest that Fe(II) oxidation by D. suillum is enzymatic and is not the result of an abiotic reaction with highly oxidized intermediates potentially formed transiently during the reductive metabolism of nitrate.
FIG. 1.
Oxidation of acetate and FeCl2 by D. suillum in bicarbonate-buffered anaerobic medium with 5 mM acetate as a cosubstrate and nitrate as the sole electron acceptor.
FIG. 2.
Difference spectra of the c-type cytrochrome content of an anoxic washed whole-cell suspension of D. suillum initially and after 1 h of incubation in the presence of Fe(II) at 30°C.
Immediately after the addition of Fe(II), in the form of FeCl2, to freshly prepared anoxic basal medium (6) inoculated with an active culture of D. suillum, Fe(II) ions reacted, probably with carbonate in the medium, to form a white fluffy precipitate similar to that observed in previous studies with anoxygenic phototrophs (34). The white precipitate was transformed into a greenish-gray substance within a week after the start of incubation. Previously reported enrichment and pure cultures of anaerobic Fe(II)-oxidizing organisms oxidized added Fe(II) directly to yellow-brown precipitates resembling amorphous Fe(III) oxides and hydroxides (5, 32). No greenish-gray mixed Fe(II)-Fe(III) hydroxides were formed in the course of those incubations (5, 32). In contrast, D. suillum formed greenish-gray mixed Fe(II)-Fe(III) hydroxides, known as carbonate-containing green rusts (13), as major metabolic products within a week after the start of incubation. Green rusts are generally unstable in the environment (11), and further slow oxidation can lead to the formation of magnetite (13, 30). On prolonged incubation (14 days), the green rusts gradually transformed into blackish brown-green. Previous studies have demonstrated that green rust will chemically react with nitrate to form magnetite and ammonia as the sole end products (18, 19). Since green rust is one of the precursors of magnetite formed by D. suillum with nitrate as the electron acceptor, it is possible that the biogenic green rusts are abiotically reacting with the remaining nitrate to form magnetite. In contrast, no transformation of the original white precipitate was observed in abiotic controls.
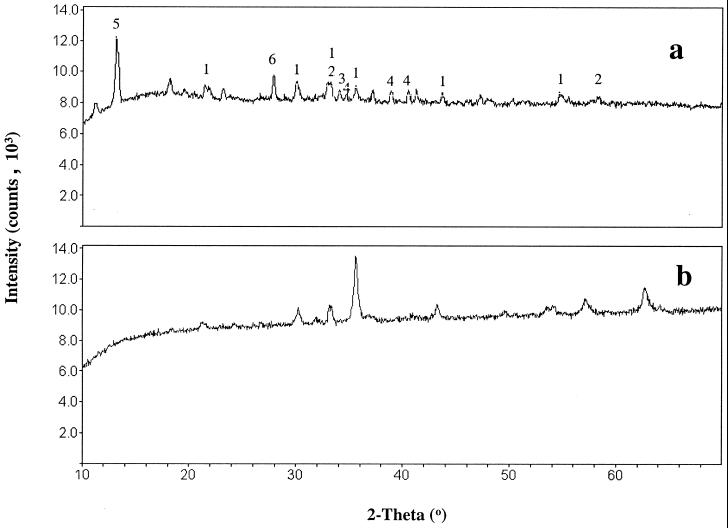
X-ray diffraction (XRD) analysis of the biologically produced Fe(III) oxides 1 week after precipitation showed initial development of various crystalline phases. Samples for XRD analysis were collected and centrifuged under an N2 gas phase and washed twice before being dried overnight in an anoxic glove bag containing a headspace of N2-H2 (95:5). In abiotic controls in which the FeCl2 was oxidized with air, crystalline phases did not develop for several weeks (data not shown). The peak intensities of the biogenic crystalline phases gradually increased with time as the precipitates aged. Figure 3a shows the XRD pattern of biogenic Fe(III) oxides that were 4 months old. The presence of peaks indicative of magnetite in Fig. 3a was confirmed by comparison with the XRD peaks of a known magnetite mineral (Fig. 3b). The dissolution of the precipitate using solutions of dithionite-citrate buffered with bicarbonate (DCB) resulted in a soft, insoluble, black residue. Aqueous solutions of DCB dissolve most common magnetic minerals except magnetite, including hematite, maghemite, goethite, and phyrrhotite (24). Assuming that the black residue was 100% magnetite, total iron analysis of the aged precipitate (4 months old) in DCB gave a magnetite yield of ∼215 to 255 g per kg of dry precipitate produced from biooxidation of soluble Fe(II).
FIG. 3.
X-ray diffractogram of biologically produced Fe(III) oxides (a) and magnetite mineral (b). 1, magnetite; 2, hematite; 3, iron hydrogen carbonate; 4, green rust; 5, vivianite; 6, maghemite.
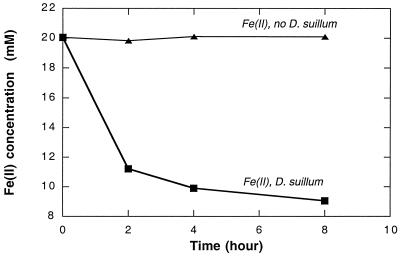
In most reduced environments, soluble Fe(II) represents only a small proportion of the total Fe(II) available (27). Most of the Fe(II) is present as insoluble carbonate or silicaceous mineral phases such as siderite (FeCO3), almandine [Fe3Al2(SiO4)3], or glauconite {[(Fe1.097Al0.849Mg0.442Ti0.003Mn0.001)(Si3.611Al0.389)O10(OH)2]K0.725Ca0.096}. If this iron is not bioavailable, abiotic oxidative reactions are more likely to have been the first step in the biogenic formation of magnetite, as previously suggested (9, 31). However, washed anaerobic whole-cell suspensions of D. suillum rapidly oxidized the Fe(II) content in various natural iron minerals including both siderite and almandine (Fig. 4; Table 1). Both the rate and extent of Fe(II) oxidation were different for the various minerals, probably due to differences in bioavailability of the Fe(II) in the mineral matrices. No oxidation of Fe(II) was observed in abiotic controls or in the absence of a suitable electron acceptor. These results demonstrate that Fe(II) oxidation is not limited to soluble Fe2+ ions and that direct oxidation of Fe(II) in insoluble minerals may also potentially result in the formation of magnetite.
FIG. 4.
Anaerobic oxidation of Fe(II) in almandine, an insoluble crystalline Fe(II) mineral, by anoxic washed whole-cell suspensions of D. suillum strain PS in the presence of nitrate as the electron acceptor.
TABLE 1.
Microbial oxidation of Fe(II) present in different natural iron minerals by anoxic washed whole-cell suspensions of D. suillum coupled to the reduction of nitrate
| Mineral | Chemical formula | Amt of Fe(II) oxidizeda (mmol kg−1) | % of total Fe(II) in starting mineral that was oxidized |
|---|---|---|---|
| Almandine | Fe3Al2(SiO4)3 | 10.32 | 52.00 |
| Arsenopyrite | FeAsS | 18.27 | 31.00 |
| Chromite | FeCr2O4 | 9.42 | 95.00 |
| Siderite | FeCO3 | 288.91 | 30.42 |
| Staurolite | (Fe,Mg,Zn)2Al9(Si,Al)4O22(OH)2 | 0.96 | 16.67 |
The Fe(II) content was determined by a ferrozine assay after dissolution of the insoluble mineral in 6 N HCl over 24 h.
The results presented here demonstrate that the presence of biogenic magnetite is not necessarily indicative of the activity of DIRB or other similar reductive metabolisms. Here we show that anaerobic microbial oxidation may also account for the geological evidence. The present model of magnetite formation by nitrate-reducing bacteria through biooxidation of Fe(II) can also account for the presence of isotopically light carbon observed in carbonate minerals (3) as well as in BIFs (33), since these organisms can cometabolize and completely oxidize organic substrates such as acetate. Such heterotrophic metabolisms would result in the isotopic fractionation of carbon, leading to the formation of CO2 and ultimately carbonates with a lighter isotope signature. Although the evolutionary timescale of microbial nitrate reduction is unknown, it is hypothesized that microbial nitrate reduction arose prior to the end of the Precambrian era. The presence of nitrate and Fe(II) in Precambrian ocean waters could have driven organisms capable of Fe(II) oxidation to form magnetite in anoxic sediments. The confinement of the process of bacterial magnetite formation to a zone between the levels of nitrate reduction and Fe(III) reduction in neoteric aquatic sediments (23) supports the above mechanism.
Acknowledgments
We thank L. A. Achenbach for critical review of the manuscript.
Support for this research was provided by grant DE-FG02-98ER62689 from the Department of Energy.
REFERENCES
- 1.Ahn J H, Buseck P R. Hematite Nanospheres of possible colloidal origin from a Precambrian banded iron formation. Science. 1990;250:111–113. doi: 10.1126/science.250.4977.111. [DOI] [PubMed] [Google Scholar]
- 2.Ann Brown D, Gross G A, Sawicki J A. A review of the microbial geochemistry of banded iron-formation. Can Mineral. 1995;33:1321–1333. [Google Scholar]
- 3.Baur M, Hayes J, Studley S, Walter M. Millimeter-scale variations of stable isotope abundances in carbonates from banded iron-formations in the Hamersley Group of Wastern Australia. Econ Geol. 1985;80:270. doi: 10.2113/gsecongeo.80.2.270. [DOI] [PubMed] [Google Scholar]
- 4.Bazylinski D A, Frankel R B, Jannasch H W. Anarobic magnetite production by a marine, magnetotactic bacterium. Nature. 1988;334:518–519. [Google Scholar]
- 5.Benz M, Brune A, Schink B. Anaerobic and aerobic oxidation of ferrous iron at neutral pH by chemoheterotrophic nitrate-reducing bacteria. Arch Microbiol. 1998;169:159–165. doi: 10.1007/s002030050555. [DOI] [PubMed] [Google Scholar]
- 6.Bruce R A, Achenbach L A, Coates J D. Reduction of (per)chlorate by a novel organism isolated from a paper mill waste. Environ Microbiol. 1999;1:319–331. doi: 10.1046/j.1462-2920.1999.00042.x. [DOI] [PubMed] [Google Scholar]
- 7.Canfield D E. A new model for Proterozoic ocean chemistry. Nature. 1998;396:450–452. [Google Scholar]
- 8.Castro L O. Genesis of banded iron-formation. Econ Geol. 1994;89:1384–1397. [Google Scholar]
- 9.Cloud P. Paleoecological significance of the banded iron-formation. Econ Geol. 1973;68:1135–1145. [Google Scholar]
- 10.Coates J D, Michaelidou U, Bruce R A, O'Connor S M, Crespi J N, Achenbach L A. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl Environ Microbiol. 1999;65:5234–5241. doi: 10.1128/aem.65.12.5234-5241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domingo C, Rodriguez-Clemente R, Blesa M. Morphological properties of alpha-FeOOH and gamma-FeOOH obtained by oxidation of aqueous Fe(II) solutions. J Collord Interface Sci. 1994;165:244–252. [Google Scholar]
- 12.Drever J I. Geochemical model for the origin of Precambrian banded iron formations. Geol Soc Am Bull. 1974;85:1099–1106. [Google Scholar]
- 13.Drissi S H, Refait P, Abdelmoula M, Genin J M R. The preparation and thermodynamic properties of Fe(II) Fe(III) hydroxide-carbonate (green rust): Pourbaix diagram of iron in carbonate-containing aqueous media. Corrosion Sci. 1995;37:2025–2041. [Google Scholar]
- 14.Ehrenreich E, Widdel F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl Environ Microbiol. 1994;60:4517–4526. doi: 10.1128/aem.60.12.4517-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs-Eggar Z, Jude B, Dominik J, Loizeau J L, Oldfield F. Possible evedience for dissimilatory bacterial magnetite dominating the magnetite properties of recent lake sediments. Earth Planet Sci Lett. 1999;168:1–6. [Google Scholar]
- 16.Gold T. The deep, hot biosphere. Proc Natl Acad Sci USA. 1992;89:6045–6049. doi: 10.1073/pnas.89.13.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hafenbradl D, Keller M, Dirmeier R, Rachel R, RoBnagel P, Burggraf S, Huber H, Stetter K O. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe+2at neutral pH under anoxic conditions. Arch Microbiol. 1996;166:308–314. doi: 10.1007/s002030050388. [DOI] [PubMed] [Google Scholar]
- 18.Hansen H C B, Koch C B. Reduction of nitrate to ammonium by sulfate green rust—activation energy and reaction mechanism. Clay Miner. 1998;33:87–101. [Google Scholar]
- 19.Hansen H C B, Koch C B, Nancke-Krogh H, Borggaard O K, Sorensen J. Abiotic nitrate reduction to ammonium: key role of green rust. Environ Sci Technol. 1996;30:2053–2056. [Google Scholar]
- 20.Holland H D. The Chemical Evolution of the Atmosphere and Oceans. Princeton, N.J: Princeton University Press; 1984. [Google Scholar]
- 21.Hungate R E. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 1969;3B:117–132. [Google Scholar]
- 22.Isley A E. Hydrothermal plumes and the delivery of iron to banded iron formation. J Geol. 1995;103:169–185. [Google Scholar]
- 23.Karlin R, Lyle M, Heath G R. Authigenic magnetite formation in suboxic marine sediments. Nature. 1987;326:490–493. [Google Scholar]
- 24.Krischvink J L, Chang S B R. Ultrafine-grained magnetite in deep-sea sediments: possible bacterial magnetofossils. Geology. 1984;12:559–562. [Google Scholar]
- 25.Lovely D R, Stolz J F, Nord G L, Jr, Phillips E J P. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature. 1987;330:252–254. [Google Scholar]
- 26.Lovely D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 27.Lovely D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancinelli R L, McKay C P. The evolution of nitogen cycle. Origins Life Evol Biosci. 1988;18:311–325. doi: 10.1007/BF01808213. [DOI] [PubMed] [Google Scholar]
- 29.Michaelidou U, Achenbach L A, Coates J D. Isolation and characterization of two novel (per)chlorate-reducing bacteria from swine waste lagoons. In: Urbansky E D, editor. Perchlorate in the environment. New York, N.Y: Kluwer Academic/Plenum; 2000. pp. 271–283. [Google Scholar]
- 30.Molinier M, Price D J, Wood P T, Powell A K. Biomimetic control of iron oxide and hydroxide phases in the iron oxalate system. J Chem Soc Dalton Trans. 1997;1997:4061–4068. [Google Scholar]
- 31.Nealson K H, Myers C R. Iron reduction by bacteria: a potential role in the genesis of banded iron formations. Am J Sci. 1990;290-A:35–45. [Google Scholar]
- 32.Straub K L, Benz M, Schink B, Widdel F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol. 1996;62:1458–1460. doi: 10.1128/aem.62.4.1458-1460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker J C G. Suboxic diagenesis in banded iron formations. Nature. 1984;309:340–342. doi: 10.1038/309340a0. [DOI] [PubMed] [Google Scholar]
- 34.Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 1993;362:834–836. [Google Scholar]