Abstract
Objective
There is limited information regarding the benefits of Lenvatinib-transcatheter arterial chemoembolization (LEN-TACE) sequential therapy for unresectable hepatocellular carcinoma (u-HCC). We compared the efficacy and safety of LEN-TACE sequential therapy to LEN monotherapy and investigated the factors contributing to the LEN-TACE sequential therapy deep response.
Methods
We enrolled a multicenter cohort of 247 patients with u-HCC treated with LEN between 2018 and 2020. Propensity score matching identified 63 matching pairs of patients with well-balanced characteristics. We retrospectively compared the clinical outcomes, including overall survival (OS), progression-free survival (PFS), and incidence of adverse events (AEs), between the LEN-TACE and LEN monotherapy groups. Additionally, we evaluated the tumor response, change in albumin-bilirubin (ALBI) score, factors affecting PFS and OS, and independent predictors contributing to the LEN-TACE sequential therapy deep response. In this study, at eight weeks after resumption of LEN after initial TACE, “deep response” was defined as achieving complete response or partial response (PR) on modified Response Evaluation Criteria in Solid Tumors (mRECIST), and at least a 30% decrease in the sum of diameters of target lesions, taking the baseline sum diameters as the reference.
Results
The OS and PFS in the LEN-TACE group were significantly higher than those in the LEN monotherapy group (p = 0.002 and p = 0.037, respectively). The incidence of AEs related to LEN was not significantly different between the two groups. In LEN-TACE sequential therapy, the objective response rate was 61.9%, and the disease control rate was 74.6%, according to the mRECIST criteria. No significant change in the ALBI score was observed during sequential LEN-TACE therapy. Multivariable analyses revealed that deep response was independently associated with the outcome of the initial response to LEN by mRECIST: PR (odds ratio: 5.176, 95% confidence interval: 1.528–17.537, p < 0.001).
Conclusions
LEN-TACE sequential therapy may provide more clinical benefits than LEN monotherapy in u-HCC patients who responded to initial LEN treatment. Objective response according to mRECIST to initial LEN is an independent factor contributing to LEN-TACE sequential therapy deep response.
Keywords: Hepatocellular carcinoma, Objective response, Modified RECIST, Lenvatinib-transcatheter arterial chemoembolization sequential therapy
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and a leading cause of cancer-related deaths worldwide [1, 2]. Curative therapies, such as resection and ablation, can improve the prognosis of patients with early stage HCC [3, 4, 5]. In contrast, patients with intermediate- or advanced-stage unresectable HCC (u-HCC) are usually treated with transcatheter arterial chemoembolization (TACE), molecular-targeted agents (MTAs), or immunotherapy [6, 7, 8, 9]. Patients with intermediate-stage HCC represent a very heterogeneous population with respect to tumor burden and liver function status [10]. Although the prognosis for intermediate- or advanced-stage HCC has improved, it remains inadequate, and new treatment strategies are required.
Lenvatinib (Lenvima®; Eisai Co., Ltd., Tokyo, Japan) (LEN) is an approved MTA treatment for u-HCC patients [11]. LEN exerts antiangiogenic and direct antitumor effects by targeting multiple kinase receptors, including vascular endothelial growth factor (VEGF), fibroblast growth factor, and platelet-derived growth factor receptors [12]. Based on the REFLECT study results, the treatment options using LEN have been expanded to serve as a promising first-line therapy for patients with u-HCC, and this therapy was approved in Japan, the European Union, the United States of America, and China as a monotherapy for patients with u-HCC. The real-world efficacy of LEN has also been reported [13, 14, 15, 16]. However, an important drawback of LEN therapy is that some cases need drug interruptions or dose reductions at early phases of the treatment because of adverse events (AEs) [17, 18]. Moreover, it is not easy to achieve complete response (CR) with LEN monotherapy [11]. Even if a partial response (PR) can be achieved, tumor progression and new lesions may occur due to the presence of LEN-resistant issues. On the contrary, therapeutic strategies aimed at local control by adding selective locoregional therapy-on-demand to molecularly targeted drugs have been investigated.
Recently, LEN-TACE sequential therapy has been reported to improve the prognosis of u-HCC patients treated with LEN [19, 20, 21, 22, 23, 24, 25]. Kudo reported the concept and utility of the potential efficacy of upfront LEN with subsequent TACE for patients with intermediate-stage HCC beyond up-to-7 criteria [19]. Additionally, Kawamura et al. [20] reported the utility of LEN-TACE sequential therapy for prolonging survival during LEN treatment. Moreover, Ando et al. [21] showed that LEN-TACE sequential therapy resulted in better overall survival (OS) and progression-free survival (PFS) outcomes than LEN monotherapy. However, independent predictors contributing to the response to LEN-TACE sequential therapy are unclear. Consequently, LEN-TACE sequential therapy has not yet been established as a therapeutic strategy.
Therefore, in this multicenter study, we performed propensity score matching (PSM) to minimize potential confounding based on differences in the patients' characteristics and compared the safety, OS, and PFS of LEN-TACE to those of LEN monotherapy. Additionally, we attempted to identify the independent predictors that contribute to the deep response in LEN-TACE sequential therapy.
Materials and Methods
Patients
This retrospective study was conducted on a multicenter cohort in Japan. Patients were recruited from eight liver centers across Japan (Iwate Medical University Hospital, Hirosaki University Hospital, Aomori Prefectural Central Hospital, Akita University Hospital, Tohoku University Hospital, National Hospital Organization Sendai Medical Center, Yamagata University Hospital, and Fukushima Medical University Hospital). We enrolled 247 patients with intermediate- or advanced-stage u-HCC, who were treated with LEN between April 2018 and December 2020. The inclusion criteria for registration in the study were as follows: (i) the diagnosis of HCC was based on findings of tumor-targeted biopsy, ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI); (ii) measurable lesions on CT or MRI; (iii) liver function scored as Child-Pugh class A or B; and (iv) Eastern Cooperative Oncology Group performance status scores between 0 and 1 [26]. Patients were excluded if their initial response according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and/or modified RECIST (mRECIST) at 8 weeks after LEN administration was not evaluated. Patients with intolerance to LEN received second-line treatment with other MTAs or the best supportive care [27, 28]. The decision to perform LEN-TACE sequential therapy or LEN monotherapy was based on each facility's discretion. In the early stages of this study, we had more experience with LEN monotherapy than with LEN-TACE sequential therapy. Therefore, LEN-TACE sequential therapy was initially chosen carefully. We used PSM (1:1 ratio) to create two groups of matched patients who underwent LEN-TACE sequential therapy or LEN monotherapy. All protocols followed in this study were approved by the institutional review board of Iwate Medical University (approval number: MH2019-082). All the patients provided written informed consent before the study, and the study was conducted in accordance with the principles of the Declaration of Helsinki (revision of Fortaleza, 2013).
LEN Therapy Protocol
The dose of LEN was set based on body weight and hepatic reserve and was administered at an initial dosage of 12 mg/day for those weighing over 60 kg and 8 mg/day for those weighing under 60 kg. According to the guidelines for administering LEN, the drug dose was reduced, or the treatment was interrupted in patients who developed grade ≥3 severe AEs or any unacceptable grade 2 drug-related AEs. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 [29]. The dose reduction of LEN was performed at the discretion of each facility. LEN was continued until disease progression, unacceptable toxicity, or the patient's withdrawal of consent. The relative dose intensity was calculated by dividing the actual dose by the ideal dose over the entire period of LEN. Treatment response to LEN was evaluated by dynamic CT or MRI, in accordance with the RECIST v1.1 and mRECIST criteria. Tumors were evaluated once within the first 8 weeks and then every 8 weeks thereafter. The objective response rate (ORR) was defined as CR plus PR. Disease control rate (DCR) was defined as the sum of CR, PR, and stable disease (SD).
LEN-TACE Sequential Therapy Protocol
TACE was performed at the facility's discretion after assessing the therapeutic effect 8 weeks after LEN administration. Digital subtraction angiography was performed to evaluate the feeding vessels of the target HCC. The tip of the catheter was selectively placed to nourish the segmental or subsegmental arteries using selective hepatic angiography and/or navigation imaging where possible. More than half of the LEN-TACE group underwent super-selective TACE in this study. Nonselective TACE was not performed because it may require a large amount of Lipiodol to achieve sufficient therapeutic effects, which could severely damage the liver. In conventional TACE (cTACE), an emulsion containing iodized oil (Lipiodol; Guerbet Japan) and 60–120 mg miriplatin (Miripla®; Sumitomo Dainippon Pharma, Osaka, Japan), 50–100 mg cisplatin (IAcall®; Nippon Kayaku, Tokyo, Japan), and 20–50 mg epirubicin (Epirubicin®; Nippon Kayaku, Tokyo, Japan) was injected super-selectively or segmentally until a high-grade stasis was reached, followed by embolization with absorbable gelatin sponge particles (Gelpart®; Nippon Kayaku or Gelfoam®; Upjohn, Kalamazoo, MI, USA). The study also included cases in which drug-eluting beaded TACE (DEB-TACE) was used instead of cTACE at the facility's discretion. DEB-TACE was performed super-selectively as a rule, but it was performed segmentally when not possible. The drug-eluting embolic agent in the DEB-TACE group was DC Beads® (Biocompatibles UK, Surrey, UK) or HepaSphereTM (Merit Medical, South Jordan, UT, USA).
In TACE for HCC with portal vein invasion, super-selective TACE catheterization was essential to reduce the total dose of Lipiodol and minimize liver toxicity associated with cTACE. Moreover, CT, cone-beam CT, or TACE guidance software were used for therapeutic support to identify the feeding vessels of the portal vein tumor thrombus. TACE was not performed in cases with severe hepatic arterioportal shunt, or super-selective catheterization was difficult. LEN was retained for at least 2 days before and after each TACE session and resumed at the same dose as before retention after confirming the patient's general condition and hepatic reserve. The therapeutic response of LEN-TACE sequential therapy was evaluated using dynamic CT or MRI at 8 weeks after restarting treatment with LEN according to mRECIST. LEN-TACE sequential therapy or LEN monotherapy was repeated when the tumor response was assessed as PR or SD. In this study, at eight weeks after resumption of LEN after initial TACE, “deep response” was defined as achieving CR or PR on mRECIST, and at least a 30% decrease in the sum of diameters of target lesions, taking the baseline sum diameters as the reference. We attempted to identify an independent factor contributing to the deep response to sequential LEN-TACE therapy.
PSM Analysis
We performed PSM to decrease the effects of selection bias on the survival analyses by creating matched groups of patients who had received LEN-TACE sequential therapy or LEN monotherapy. The propensity score model included age, sex, performance status score, etiology, naive or non-naive status, TACE refractoriness before the introduction of LEN, history of MTA treatment, modified albumin-bilirubin (ALBI) grade, serum α-fetoprotein concentration, largest tumor size, tumor number, macrovascular invasion, extrahepatic spread, initial dose of LEN, relative dose intensity, and treatment effect of LEN at eight weeks. Propensity scores were calculated by applying these variables to a logistic regression model, and C-statistics were calculated to evaluate the goodness of fit. One-to-one PSM was performed using a caliper width of <0.2 of the pooled standard deviation of the estimated propensity scores.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 23.0; IBM Corp., Armonk, NY, USA) or XLSTAT 2020 software (Microsoft Corp., Redmond, WA, USA). Based on previous reports, we calculated the ORR of LEN monotherapy as 40.6% and that of LEN-TACE sequential therapy as 68.3% in this study [11, 23]. We estimated the required sample size at a level of 5% and a power of 0.9 and, finally, selected a sample size of 102. Continuous variables were presented as the mean ± standard deviation or median (interquartile range) and were analyzed using Student's t test or Mann-Whitney U test. Categorical variables were presented as numbers (percentages) and analyzed using Pearson's χ2 test or Fisher's exact test. OS and PFS curves were created using the Kaplan-Meier method and compared using the log-rank test. Independent predictors contributing to deep response were evaluated using multivariable logistic regression analysis, which was adjusted for factors with a p value of <0.05 in the univariate analyses. Univariate and multivariate analyses based on the Cox regression model were performed to identify independent prognostic factors associated with OS or PFS. Differences were considered statistically significant at p < 0.05.
Results
Baseline Characteristics of the Patients
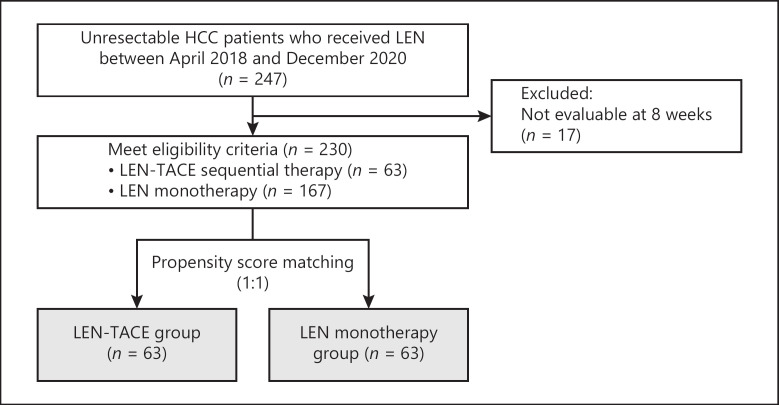
The retrospective study protocol is illustrated in Figure 1. During the study period, 247 u-HCC patients were treated with LEN. Seventeen patients were excluded because the initial response at eight weeks after LEN administration was not evaluable. The remaining 230 patients included in the study were divided into the LEN-TACE group (n = 63, 27.4%) and LEN monotherapy group (n = 167, 72.6%). The characteristics of the two groups are shown in Table 1, and we observed a few differences in the characteristics of the groups. Relative to the LEN monotherapy group, the LEN-TACE group had a significantly smaller proportion of patients with a history of MTA treatment (p = 0.002). Additionally, in the LEN-TACE group, the proportion of PR cases was high in both RECIST 1.1 and mRECIST in the tumor response at 8 weeks of precedence LEN (RECIST 1.1: p = 0.004, mRECIST: p = 0.008).
Fig. 1.
Flowchart of eligible patients with HCC. u-HCC, unresectable hepatocellular carcinoma; LEN, lenvatinib; TACE, transcatheter arterial chemoembolization.
Table 1.
Comparison of the LEN-TACE and LEN monotherapy groups in the unmatched and propensity score-matched cohorts
| Characteristics | Unmatched cohort |
Propensity score-matched cohort |
||||
|---|---|---|---|---|---|---|
| LEN-TACE (n = 63) | LEN monotherapy (n = 167) | p value | LEN-TACE (n = 63) | LEN monotherapy (n = 63) | p value | |
| Gender, males/females | 51/12 | 139/28 | 0.684 | 51/12 | 53/10 | 0.639 |
| Age, years | 70.4±9.6 | 71.2±8.3 | 0.621 | 70.4±9.6 | 69.6±8.9 | 0.597 |
| BMI, kg/m2 | 23.7 (21.1–27.3) | 23.4 (21.2–25.6) | 0.887 | 23.7 (21.1–27.3) | 23.9 (22.2–27.4) | 0.973 |
| Etiology, HBV/HCV/nonviral | 11/19/33 | 40/47/80 | 0.571 | 11/19/33 | 15/17/31 | 0.673 |
| ECOG PS, 0/1 | 53/10 | 136/31 | 0.717 | 53/10 | 52/11 | 0.811 |
| Naive/non-naive | 16/47 | 30/137 | 0.209 | 16/47 | 15/48 | 0.836 |
| History of TKI treatment, n (%) | 2 (3.2) | 33 (19.6) | 0.002 | 2 (3.2) | 2 (3.2) | 1.000 |
| TACE refractoriness, n (%) | 22 (34.9) | 47 (27.9) | 0.317 | 22 (34.9) | 20 (31.7) | 0.705 |
| mALBI, 1/2a/2b | 24/14/25 | 65/48/68 | 0.897 | 24/14/25 | 21/10/21 | 0.910 |
| Child-Pugh score, 5/6/7 (points) | 36/20/7 | 101/50/16 | 0.812 | 36/20/7 | 39/18/6 | 0.860 |
| Tumor size, mm | 5.0 (3.5–7.6) | 4.8 (3.0–9.0) | 0.377 | 5.0 (3.5–7.6) | 4.9 (3.2–7.0) | 0.872 |
| Tumors, n single/multiple | 20/43 | 42/125 | 0.315 | 20/43 | 23/40 | 0.573 |
| MVI, Vp0/1/2/3/4 | 35/13/10/4/1 | 101/17/35/11/3 | 0.326 | 35/13/10/4/1 | 43/4/12/4/0 | 0.149 |
| EM, n (%) | 16 (25.3) | 51 (30.5) | 0.444 | 16 (25.3) | 17 (26.9) | 0.839 |
| BCLC stage, B/C | 27/36 | 66/101 | 0.649 | 27/36 | 32/31 | 0.372 |
| AFP,≥200/<200ng/mL | 21/42 | 60/107 | 0.713 | 21/42 | 23/40 | 0.709 |
| Initial dose of LEN, 8/12 mg | 37/26 | 111/56 | 0.275 | 37/26 | 34/29 | 0.590 |
| RDI,% | 59.4 (40.2–74.8) | 62.4 (37.2–89.8) | 0.829 | 59.4 (40.2–74.8) | 59.6 (35.5–88.1) | 0.881 |
| mRECIST (LEN 8 weeks), CR/PR/SD/PD | 0/35/17/11 | 1/53/57/56 | 0.008 | 0/35/17/11 | 0/35/16/12 | 0.964 |
| RECIST 1.1 (LEN 8 weeks), CR/PR/SD/PD | 0/22/30/11 | 0/28/84/55 | 0.004 | 0/22/30/11 | 0/21/30/12 | 0.967 |
LEN, lenvatinib; TACE, transarterial chemoembolization; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; Non-Viral, non-HBV and non-HCV; ECOG, Eastern Cooperative Oncology Group; PS, performance status; TKI, tyrosine kinase inhibitor; mALBI, modified albumin-bilirubin; MVI, microvascular invasion; Vp, portal vein invasion; EM, extrahepatic metastasis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; RDI, relative dose intensity; mRECIST, modified Response Evaluation Criteria in Solid Tumors; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease. The values represent the mean±standard deviation, median (25th-75th percentile), or patients, n.
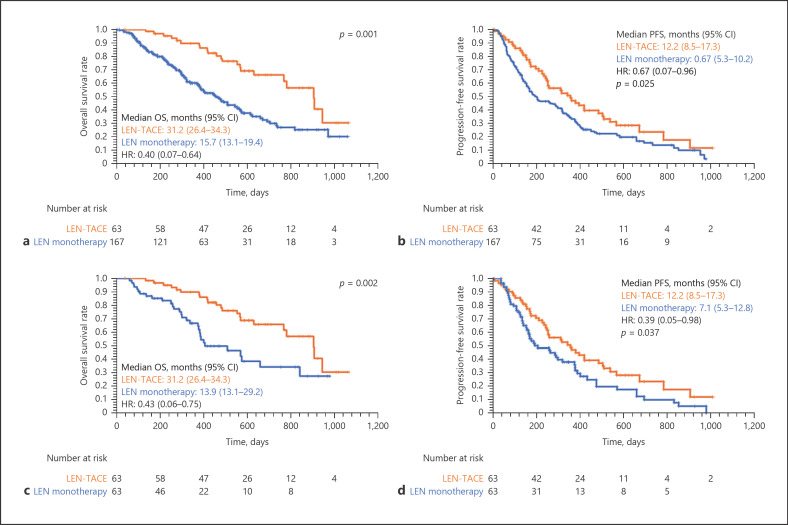
The median follow-up time for all patients was 15.7 months. The median OS for the LEN-TACE group was 31.2 (26.4–34.3) months, and that for the LEN monotherapy group was 15.7 (13.1–19.4) months (Fig. 2a). OS in the LEN-TACE group was significantly higher than in the LEN monotherapy group (p = 0.001). Furthermore, the median PFS for the LEN-TACE group was 12.2 (8.5–17.3) months, and that for the LEN monotherapy group was 6.7 (5.3–10.2) months (p = 0.025, Fig. 2b).
Fig. 2.
Kaplan-Meier curves for OS among all patients (a), PFS (b) among all patients, OS in the PSM cohort (c), and PFS in the PSM cohort (d). The median OS for the LEN-TACE group was 31.2 (26.4–34.3) months, and this for the LEN monotherapy group was 15.7 (13.1–19.4) months, respectively (p = 0.001, a). The median PFS for the LEN-TACE group was 12.2 (8.5–17.3) months, and this for the LEN monotherapy group was 6.7 (5.3–10.2) months, respectively (p = 0.025, b). The median OS for the LEN-TACE group was 31.2 (26.4–34.3) months, and that for the LEN monotherapy group was 13.9 (13.1–29.2) months, respectively (p = 0.002, c). The median PFS for the LEN-TACE group was 12.2 (8.5–17.3) months, and this for the LEN monotherapy group was 7.1 (5.3–12.8) months, respectively (p = 0.037, d). LEN, lenvatinib; TACE, transcatheter arterial chemoembolization; OS, overall survival; PFS, progression-free survival.
Patient Characteristics in the PSM Cohort
PSM analysis identified 63 matched pairs of patients. The matched groups of patients had similar baseline characteristics (Table 1) (all p > 0.05). The median follow-up time for the PSM cohort was 15.6 months. The median follow-up time for all patients was 14.7 months. The median OS for the LEN-TACE group was 31.2 (26.4–34.3) months and that for the LEN monotherapy group was 13.9 (13.1–29.2) months (Fig. 2c). OS in the LEN-TACE group was significantly higher than in the LEN monotherapy group (p = 0.002). Furthermore, the median PFS for the LEN-TACE group was 12.2 (8.5–17.3) months, and that for the LEN monotherapy group was 7.1 (5.3–12.8) months (p = 0.037, Fig. 2d).
Treatment Efficacy of the LEN-TACE Sequential Therapy
The treatment parameters for LEN-TACE sequential therapy are presented in Table 2. The reasons for additional TACE during LEN treatment were divided into two major groups: local curative treatment and disease control. Achieving CR (n = 33, 52.4%) or tumor downstaging (n = 14, 22.2%) with local curative treatment by TACE were the treatment goals. On the other hand, in cases of progressive disease (PD) with LEN treatment (n = 11, 17.5%) and temporary discontinuation of LEN due to AE (n = 5, 7.9%), the primary therapeutic goal was disease control. The median time from the introduction of LEN to initial TACE was 120.5 days. The response of LEN by mRECIST when TACE was added was PR (n = 33), SD (n = 19), PD (n = 11). TACE was performed using cTACE (n = 55) and DEB-TACE (n = 8). The median resting time of LEN before TACE was 2 days. The median time to readminister LEN after TACE was 8 days. The total number of TACE procedures was 1 (n = 34), 2 (n = 20), 3 (n = 7), and 4 (n = 2). The median interval between TACE procedures was 110 days. In this study, 93.7% (59/63) cases resumed LEN after TACE. Tolerability of LEN was the main reason LEN could not be restarted after TACE. These cases received repeated TACE (n = 2), second-line treatment with other tyrosine kinase inhibitors (n = 1), or the best supportive care (n = 1).
Table 2.
Treatment parameters of LEN-TACE sequential therapy
| Variables | |
|---|---|
| Reasons for additional TACE, achievement of CR/downstage/discontinuation of LEN due to AEs/PD with LEN treatment | 33/14/5/11 |
| Median time from the introduction of LEN to initial TACE, days | 120.5 (82.8–251.3) |
| TACE technique, cTACE/DEB-TACE | 55/8 |
| TACE chemo agent, MPT/CDDP/EPI | 35/18/10 |
| Median rest time of LEN before TACE, days | 2.0 (2.0–5.0) |
| Median time to readminister LEN after TACE, days | 8.0 (4.0–13.3) |
| Number of TACE procedures, 1/2/3/4 times | 34/20/7/2 |
| Median interval between TACE, days | 111.0 (101.0–131.8) |
| Major complications of TACE, ascites/liver abscess | 2/1 |
| Minor complications of TACE, post-embolization syndrome/liver function decline/fever/abdominal pain | 10/9/9/6 |
LEN, lenvatinib; TACE, transarterial chemoembolization; AEs, adverse events; PD, progressive disease; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beaded transarterial chemoembolization; MPT, miriplatin; CDDP, cisplatin; EPI, epirubicin. The values represent the median (25th-75th percentile) or patients, n.
TACE-derived major complications had an incidence of 4.7% (3/63) and included ascites (n = 1) and liver abscesses (n = 1). In addition, minor complications, including post-embolization syndrome (n = 10), liver function decline (n = 9), fever (n = 9), and abdominal pain (n = 6) were observed. However, when we analyzed the adverse effects, most patients were asymptomatic or had mild symptoms.
Based on the mRECIST criteria, the proportion of all patients with CR, PR, SD, and PD after 8 weeks of TACE in the LEN-TACE group was 20.6%, 41.3%, 12.7%, and 25.4%, respectively. The ORR and DCR were 61.9% and 74.6%, respectively. Subgroup analysis for both Barcelona Clinic Liver Cancer (BCLC) stage B (n = 22), the proportions of patients with CR, PR, SD, and PD were 14.8%, 59.3%, 14.8%, and 11.1%, respectively. The ORR and DCR were 74.1% and 88.9%, respectively (Table 3). Subgroup analysis for BCLC B within up-to-7 criteria (n = 11), BCLC B beyond up-to-7 criteria (n = 16), and BCLC C (n = 36) indicated that the benefit trend was generally consistent with the total population (online suppl. Table 1; see www.karger.com/doi/10.1159/000522424 for all online suppl. material).
Table 3.
The tumor response after 8 weeks of initial TACE in LEN-TACE sequential therapy according to mRECIST
| Total (n = 63) | BCLC B (n = 27) | |
|---|---|---|
| CR | 13 (20.6) | 4 (14.8) |
| PR | 26 (41.3) | 16 (59.3) |
| SD | 8 (12.7) | 4 (14.8) |
| PD | 16 (25.4) | 3 (11.1) |
| ORR | 61.9 | 74.1 |
| DCR | 74.6 | 88.9 |
Data are presented as n (%). LEN, lenvatinib; TACE, transarterial chemoembolization; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate; BCLC, Barcelona Clinic Liver Cancer; mRECIST, modified Response Evaluation Criteria in Solid Tumors.
Safety Outcomes
AEs that occurred more frequently in the LEN-TACE group than in the LEN monotherapy group included fatigue, hypertension, reduced appetite, hypothyroidism, liver dysfunction, diarrhea, hoarseness, decreased weight, and pyrexia. Only liver dysfunction was significantly higher in the LEN-TACE group than in the LEN monotherapy group (p = 0.04), but it soon resolved for most patients. As shown in Table 4, the most common AEs of any grade in the LEN-TACE group were fatigue (47.6%), hypertension (46.0%), and decreased appetite (42.9%). In addition, the most common grade ≥3 AE was hypertension (11.1%). Thus, the LEN-TACE group had an acceptable safety profile without unexpected safety signals. The most common AEs in the LEN monotherapy group were proteinuria (42.9%), hand-foot skin reaction (34.9%), fatigue (38.1%), and hypertension (38.1%). The most common grade ≥3 AE was hypertension (7.9%).
Table 4.
AEs of LEN-TACE sequential therapy and LEN monotherapy
| AEs | LEN-TACE (n = 63) |
LEN monotherapy (n = 63) |
|||
|---|---|---|---|---|---|
| any grade, n (%) | grade ≥3, n (%) | any grade, n (%) | grade ≥3, n (%) | ||
| Fatigue | 30 (47.6) | 1 (1.6) | 24 (38.1) | 4 (6.3) | |
| Hypertension | 29 (46.0) | 7 (11.1) | 24 (38.1) | 5 (7.9) | |
| Decreased appetite | 27 (42.9) | 1 (1.6) | 21 (33.3) | 4 (6.3) | |
| Proteinuria | 25 (39.7) | 1 (1.6) | 27 (42.9) | 3 (4.8) | |
| Hypothyroidism | 22 (34.9) | 0 (0.0) | 23 (36.5) | 1 (1.6) | |
| Hand-foot skin reaction | 22 (34.9) | 4 (6.3) | 22 (34.9) | 2 (3.2) | |
| Liver dysfunction | 22 (34.9)* | 5 (7.9) | 12 (19.0) | 2 (3.2) | |
| Diarrhea | 13 (20.6) | 1 (1.6) | 7 (11.1) | 1 (1.6) | |
| Hoarseness | 10 (15.9) | 0 (0.0) | 10 (15.9) | 0 (0.0) | |
| Decreased weight | 10 (15.9) | 0 (0.0) | 7 (11.1) | 0 (0.0) | |
| Pyrexia | 7 (11.1) | 4 (6.3) | 4 (6.3) | 1 (1.6) | |
| Thrombocytopenia | 1 (1.6) | 1 (1.6) | 1 (1.6) | 1 (1.6) | |
| Encephalopathy | 1 (1.6) | 1 (1.6) | 4 (6.3) | 0 (0.0) | |
| Pneumonia | 0 (0.0) | 0 (0.0) | 1 (1.6) | 1 (1.6) | |
Data are presented as n (%). LEN, lenvatinib; TACE, transarterial chemoembolization.
p < 0.05, compared with LEN alone.
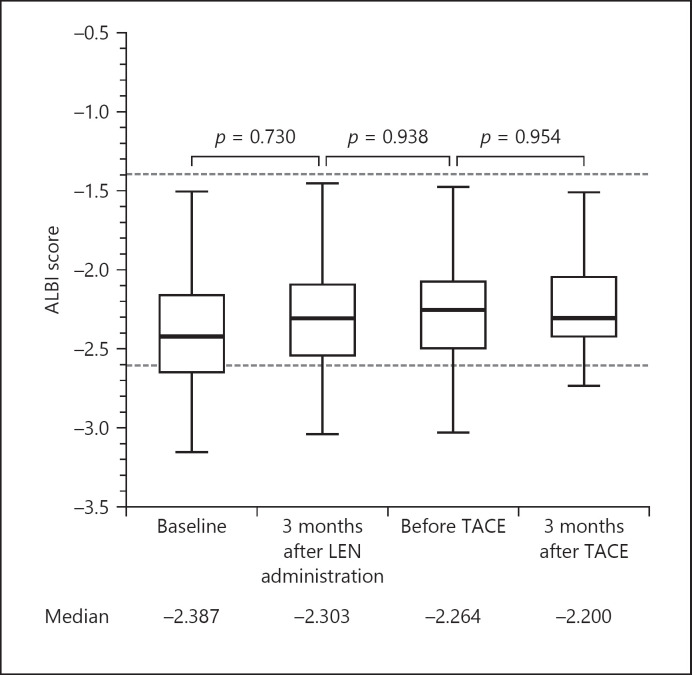
The median ALBI scores of patients in the LEN-TACE group before LEN administration, 3 months after LEN administration, before TACE, and two months after TACE were −2.38, −2.30, −2.26, and −2.20, respectively, with no statistically significant difference between any of these time points (Fig. 3).
Fig. 3.
Changes in ALBI score before and after LEN-TACE sequential therapy. The median ALBI scores of patients in the LEN-TACE group at baseline, 3 months after LEN administration, before TACE, and three months after TACE were −2.387, −2.303, −2.264, and −2.200, respectively. No statistically significant difference was observed between any of the time points. ALBI, albumin-bilirubin; LEN, lenvatinib; TACE, transcatheter arterial chemoembolization.
Univariable and Multivariable Analysis of Factors Associated with OS and PFS
The univariate analyses revealed that OS was significantly associated with mALBI grade 1+2a (p = 0.004), Child-Pugh score 5 points (p = 0.007), MVI-Vp0 (p = 0.002), AFP <200 ng/mL (p = 0.029), and the addition of TACE (p = 0.003). The multivariable analyses revealed that OS was independently associated with mALBI grade 1+2a (p = 0.006), MVI-Vp0 (p < 0.001), and the addition of TACE (p < 0.001) (Table 5). The univariate analyses revealed that PFS was significantly associated with mALBI grade 1+2a (p = 0.013), Child-Pugh score 5 points (p = 0.018), and addition of TACE (p = 0.039). The multivariable analyses revealed that RFS was independently predicted by mALBI grade 1+2a (p = 0.007) and the addition of TACE (p = 0.023). The OS and PFS outcomes were independently associated with the addition of TACE.
Table 5.
Univaliable and multivaliable analysis of factors associated with OS and PFS
| Parameter | OS |
PFS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| univariate analysis |
multivariate analysis |
univariate analysis |
multivariate analysis |
|||||||||
| HR | (95% CI) | p value | HR | (95% CI) | p value | HR | (95% CI) | p value | HR | (95% CI) | p value | |
| Gender, male versus female | 0.890 | (0.554–1.430) | 0.631 | 0.779 | (0.553–1.198) | 0.236 | ||||||
| Age, <70 versus ≥70 years | 0.84 | (0.487–1.448) | 0.531 | 0.767 | (0.512–1.149) | 0.199 | ||||||
| Etiology, viral versus nonviral | 0.850 | (0.588–1.226) | 0.384 | 1.025 | (0.686–1.532) | 0.903 | ||||||
| ECOG PS, 0 versus 1 | 0.697 | (0.245–1.095) | 0.082 | 0.721 | (0.439–1.183) | 0.196 | ||||||
| Naive, yes versus no | 1.358 | (0.850–2.168) | 0.199 | 1.134 | (0.704–1.824) | 0.605 | ||||||
| TACE refractoriness, yes versus no | 0.743 | (0.395–1.395) | 0.356 | 1.203 | (0.779–1.857) | 0.403 | ||||||
| RDI, ≥60 versus <60% | 1.250 | (0.724–2.155) | 0.423 | 1.060 | (0.702–1.600) | 0.780 | ||||||
| mALBI grade, 1 + 2a versus 2b | 0.447 | (0.236–0.775) | 0.004 | 0.460 | (0.225–0.808) | 0.006 | 0.595 | (0.334–0.896) | 0.013 | 0.570 | (0.331–0.861) | 0.007 |
| Child-Pugh score, 5 versus 6 + 7 | 0.473 | (0.255–0.815) | 0.007 | 0.611 | (0.311–0.918) | 0.018 | ||||||
| BCLC, B versus C | 0.612 | (0.347–1.077) | 0.089 | 0.917 | (0.610–1.376) | 0.675 | ||||||
| Tumor size, <51 versus ≥51 cm | 0.582 | (0.327–1.035) | 0.066 | 0.867 | (0.569–1.321) | 0.508 | ||||||
| Number of tumors, single versus multiple | 0.875 | (0.489–1.566) | 0.654 | 0.875 | (0.489–1.566) | 0.654 | ||||||
| MVI, Vp0 versus Vp1–4 | 0.507 | (0.314–0.716) | 0.002 | 0.318 | (0.207–0.586) | <0.001 | 0.806 | (0.534–1.214) | 0.302 | |||
| EM, yes versus no | 1.147 | (0.627–2.099) | 0.656 | 1.171 | (0.747–1.832) | 0.490 | ||||||
| AFP, <200 versus ≥200 ng/mL | 0.528 | (0.345–0.935) | 0.029 | 0.822 | (0.458–1.474) | 0.511 | 0.841 | (0.549–1.287) | 0.426 | |||
| Addition of TACE, with versus without | 0.430 | (0.212–0.750) | 0.003 | 0.287 | (0.158–0.530) | <0.001 | 0.652 | (0.369–0.977) | 0.039 | 0.622 | (0.354–0.936) | 0.023 |
OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; TACE, transarterial chemoembolization; RDI, relative dose intensity; mALBI, modified albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; MVI, microvascular invasion; EM, extrahepatic metastasis; AFP, alpha-fetoprotein.
Univariate and Multivariable Analyses of Deep Response in the LEN-TACE Sequential Therapy
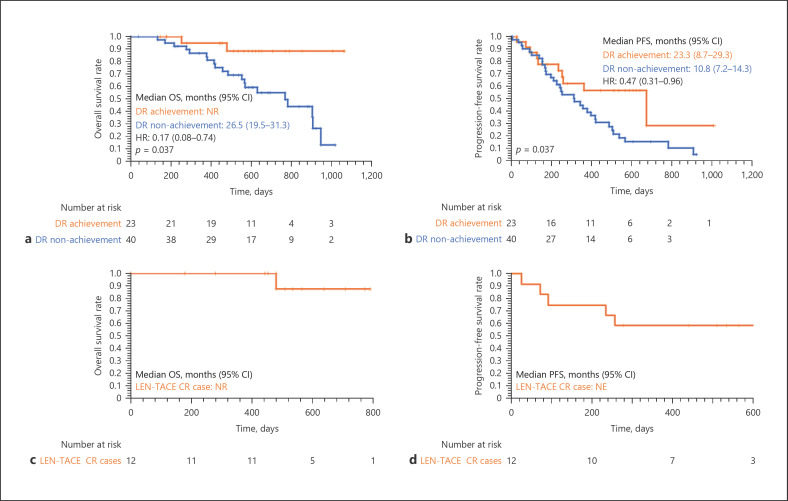
We attempted to identify an independent factor contributing to the deep response to sequential LEN-TACE therapy. Twenty-three patients (36.5%) achieved a deep response in the LEN-TACE group. The median OS for the deep response achievement group was not reached, and for the deep response nonachievement group, it was 26.5 (19.5–31.3) months (Fig. 4a). OS in the deep response achievement group was significantly higher than that in the deep response nonachievement group (p = 0.037). Furthermore, the median PFS for the deep response achievement group was 23.3 (8.7–29.3) months, and for the deep response nonachievement group, it was 10.8 (7.2–14.3) months (p = 0.037, Fig. 4b).
Fig. 4.
Kaplan-Meier curves for OS (a) and PFS (b) by the achievement of deep response in the LEN-TACE group. Kaplan-Meier curves for OS (c) and PFS (d) in the LEN-TACE CR case. The median OS for the deep response achievement group was not reached, and this for the deep response nonachievement group was 26.5 (19.5–31.3) months, respectively (p = 0.037, a). The median PFS for the deep response achievement group was 23.3 (8.7–29.3) months, and this for the deep response nonachievement group was 10.8 (7.2–14.3) months, respectively (p = 0.037, b). 1-year OSR of 100% and a 2-year OSR of 87.5% in the LEN-TACE CR case (c). The PFSR was 83.3% at 3 months, 75.0% at 6 months, and 58.3% at 12 months in the LEN-TACE CR case (d). DR, deep response; NR, not reached; OS, overall survival; PFS, progression-free survival; OSR, overall survival rate; PFSR, progression-free survival rate.
Characteristics of patients with or without deep response in the LEN-TACE group are shown in Table 6, and we observed a few differences in the characteristics of the groups. In the deep response achievement group, the proportion of PR cases by mRECIST was high in the tumor response at eight weeks of precedence LEN (p < 0.001). Moreover, relative to the deep response nonachievement group, the deep response achievement group had a more significant proportion of naive patients (p = 0.012). In the deep response achievement group, significantly more cases had TACE timing shorter than 120 days after LEN introduction than the timing of TACE after introducing LEN (p = 0.021). Multivariable analyses revealed that deep response was independently associated with the outcome of the initial tumor response to LEN by mRECIST: PR (odds ratio: 5.176, 95% confidence interval: 1.528–17.537, p < 0.001) (Table 7).
Table 6.
Characteristics of patients with or without deep response in the LEN-TACE group
| Parameter | DR achievement group | DR nonachievement group | p value |
|---|---|---|---|
| Gender, males/females | 20/3 | 31/9 | 0.357 |
| Age, <70/≥70 years | 7/16 | 18/22 | 0.255 |
| Etiology, HBV/HCV/nonviral | 3/7/13 | 8/12/20 | 0.789 |
| ECOG PS, 0/1 | 20/3 | 53/7 | 0.641 |
| Naive/nonnaive | 10/13 | 6/34 | 0.012 |
| History of TKI treatment, yes/no | 1/22 | 1/39 | 0.687 |
| TACE refractoriness, yes/no | 7/16 | 15/25 | 0.571 |
| mALBI grade, 1/2a/2b | 7/5/11 | 17/9/14 | 0.558 |
| Child-Pugh score, 5/6/7 points | 12/6/5 | 24/14/2 | 0.122 |
| Tumor size, <51/≥51 cm | 12/11 | 28/12 | 0.157 |
| Number of tumors, single/multiple | 9/14 | 11/29 | 0.341 |
| MVI, Vp0/1/2/3/4 | 12/9/1/1 | 23/14/3/0 | 0.794 |
| EM, yes/no | 4/19 | 12/28 | 0.268 |
| BCLC stage, B/C | 10/13 | 17/23 | 0.941 |
| Up-to-7 criteria, within/others | 3/20 | 8/32 | 0.484 |
| AFP, ≥200/<200 ng/mL | 8/15 | 13/27 | 0.713 |
| Initial dose of LEN, 8/12 mg | 15/8 | 22/18 | 0.428 |
| RDI, ≥60/<60% | 12/11 | 17/23 | 0.458 |
| mRECIST (LEN 8 week), CR/PR/SD/PD | 0/21/2/0 | 0/14/15/11 | <0.001 |
| RECIST 1.1 (LEN 8 week), CR/PR/SD/PD | 0/11/11/1 | 0/11/19/10 | 0.071 |
| Timing of TACE after introducing LEN, <120/≥120 days | 15/8 | 14/26 | 0.021 |
| TACE technique, cTACE/DEB-TACE | 4/19 | 4/36 | 0.396 |
| TACE chemo agent, MPT/CDDP/EPI | 12/7/4 | 23/11/6 | 0.918 |
| Number of TACE, 1/2/3/4 times | 11/11/1/0 | 23/9/6/2 | 0.119 |
| Rest period of LEN, <10/≥10 days | 14/9 | 26/14 | 0.743 |
The values represent the patients, n. LEN, lenvatinib; TACE, transarterial chemoembolization; DR, deep response; HBV, hepatitis B virus; HCV, hepatitis C virus; ECOG, Eastern Cooperative Oncology Group; PS, performance status; TKI, Tyrosine Kinase Inhibitor; mALBI, modified albumin-bilirubin; MVI, microvascular invasion; EM, extrahepatic metastasis; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; RDI, relative dose intensity; mRECIST, modified Response Evaluation Criteria in Solid Tumors; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; cTACE, conventional transarterial chemoembolization; DEB-TACE, drug-eluting beaded transarterial chemoembolization; MPT, miriplatin; CDDP, cisplatin; EPI, epirubicin.
Table 7.
Multivariable analysis of factors contributing to deep response for the LEN-TACE sequential therapy
| Parameter | Multivariate analysis |
||
|---|---|---|---|
| OR | (95% CI) | p value | |
| Naive, yes versus no | 2.311 | (0.598–11.705) | 0.128 |
| mRECIST (LEN 8 weeks), PR versus SD + PD | 5.176 | (1.528–17.537) | <0.001 |
| Timing of TACE after introducing LEN, <120 versus ≥120 days | 2.132 | (0.799–10.758) | 0.144 |
LEN, lenvatinib; TACE, transarterial chemoembolization; OR, odds ratio; CI, confidence interval; mRECIST, modified Response Evaluation Criteria in Solid Tumors; PR, partial response; SD, stable disease; PD, progressive disease.
CR Achievement and Maintenance Rate in the LEN-TACE Sequential Therapy
In this study, a total of 12 patients (19.0%) achieved CR with the LEN-TACE sequential therapy, including 2 patients who underwent conversion surgery. The median follow-up period for the CR cases was 17.4 months. The prognosis of CR patients was excellent, with a 1-year OS rate (OSR) of 100% and a 2-year OSR of 87.5% (Fig. 4c). New lesions were identified in 5 cases; the PFS rate was 83.3% at three months, 75.0% at 6 months, and 58.3% at 12 months (Fig. 4d).
Discussion
In this multicenter retrospective study, we examined the efficacy and safety of sequential LEN-TACE therapy. Our results showed that LEN-TACE sequential therapy was superior to LEN monotherapy. The incidence of grade ≥3 severe AEs did not differ significantly between LEN-TACE sequential therapy and LEN monotherapy. The prognosis of patients who achieved a deep response or CR to LEN-TACE sequential therapy was excellent. This study showed that the objective response by mRECIST of initial LEN is an independent prognostic factor for the deep response in HCC patients treated with LEN-TACE sequential therapy. To our knowledge, the present study is the first to clarify the independent prognostic factors for LEN-TACE sequential therapy.
LEN was identified through exploratory research of agents with various tyrosine kinase inhibitory activities related to angiogenesis [12, 30, 31, 32]. In a recent phase III trial for patients with previously untreated u-HCC (REFLECT study), the ORR of LEN was 40.6% [11]. Furthermore, 61.3% of the Japanese population with intermediate-stage HCC [33], and an extremely high response rate of 73.3% were observed in the proof-of-concept study [19]. LEN improved the prognosis of u-HCC compared with sorafenib and was approved as the first-line treatment for u-HCC in March 2018 in Japan. However, it is not easy to achieve CR with LEN monotherapy, and even if PR is achieved, tumor progression and new lesions may eventually occur due to resistance issues [34]. For such cases, the local control of intrahepatic lesions by the addition of TACE followed by LEN may lead to a long-term continuation of LEN. Moreover, the addition of TACE may provide transient and complete control of HCC. Endo et al. [22] reported 2 cases in which u-HCC was locally controlled by LEN-TACE sequential therapy, and conversion surgery was achieved.
Therapeutic strategies aimed at local control by adding selective TACE to molecular-targeted drugs have been studied. The TACTICS trial showed the benefit of sorafenib followed by TACE as a treatment option to improve the clinical outcome of patients with intermediate-stage HCC [35]. Kudo [36] proposed that prior administration of LEN was better for prolonging prognosis than TACE for patients with HCC unsuitable for TACE, such as multiple liver lesions. Pre-administration of LEN normalizes tumor vessels, lowers vascular permeability and intertumoral interstitial pressure, and improves drug delivery, resulting in radical, highly selective TACE [37, 38]. In addition, selective TACE also contributes to the preservation of liver function. TACE induces ischemic conditions in tumor tissue, upregulates the expression of hypoxia-inducing factor 1, and increases the production of VEGF, fibroblast growth factor, and other angiogenic factors in tumor tissue [39, 40, 41]. Pre- and post-administration of LEN may suppress recurrence and metastasis by suppressing the hypoxia-induced release of VEGF [42].
Shimose et al. [24] reported the efficacy of alternating LEN and transarterial therapy in patients with intermediate-stage HCC. Kawamura et al. [20] reported the utility of LEN-TACE sequential therapy for prolonging post-progression survival during LEN treatment. Ando et al. [21] reported that the ORR of patients in the LEN-TACE group and LEN-alone group were equivalent, but patients in the LEN-TACE group had better PFS and OS than those in the LEN-alone group in a retrospective cohort study. Fu et al. [23] reported that combination treatment with LEN and TACE significantly prolonged OS and PFS compared with TACE monotherapy. Consistent with these previous reports, our results showed that ORR and DCR in LEN-TACE sequential therapy yielded promising results. Among them, the tumor response in BCLC stage B had a significant impact on performance. Moreover, our results showed that both OS and PFS of patients in the LEN-TACE group were significantly extended.
A recent study reported that patients with advanced HCC treated with LEN maintained or improved their liver function reserve at 4 and 12 weeks [43]. In combination with TACE, LEN may reduce the need for repeat TACE and maintain liver function, which may contribute to better clinical outcomes. Our results showed that liver function was well maintained during LEN-TACE sequential therapy, and this result supports previous findings. Together, these results indicated that LEN-TACE sequential therapy provided more clinical benefits than LEN monotherapy in u-HCC patients with a manageable safety profile.
We showed that the objective response by mRECIST of initial LEN is an independent factor contributing to the response in LEN-TACE sequential therapy. In addition, the objective response to mRECIST was a prognostic factor for LEN-TACE sequential therapy. In other words, it is inferred that there is a complementary relationship between LEN and TACE rather than a competitive relationship. If systemic chemotherapy with LEN results in a 30% or greater reduction in viable (enhancement in the arterial phase) target lesions, we recommend additional selective TACE. These therapeutic strategies that aim for local control with the addition of selective TACE may receive more attention in the future.
On the other hand, in recent years, atezolizumab and bevacizumab treatment is expected to be the first-line treatment for patients with HCC. This treatment offers a long-term prognosis while maintaining the quality of life. However, the IMbrave 150 trial reported a post-progression survival of 6.8 months [9]. It is often difficult to predict the onset of immune-related AEs. The fact that changes in tumor blood flow are observed early in the treatment is considered an advantage of LEN treatment. Kuzuya et al. [44] reported that LEN indicates a therapeutic response on CT 2 weeks after administration. Moreover, Kuorda et al. [45] reported that contrast-enhanced ultrasound tested seven days after starting LEN administration might serve as a valuable indicator of therapeutic outcomes for u-HCC. In this study, univariate analyses revealed that deep response was significantly associated with the timing of TACE after the introduction of LEN, with the optimal time being <120 days. In LEN-TACE sequential therapy, it is necessary to determine the therapeutic effect of LEN at an early stage and plan the timing of selective TACE. A detailed analysis of the timing of TACE is essential for future studies.
This study has several limitations. First, the study design was retrospective, and the sample size was small. Large-scale randomized controlled trials are needed to confirm these findings. Second, the influence of selection bias cannot be ruled out. Finally, a more evident conclusion requires a more extended observation period. Nonetheless, our data suggest that LEN-TACE sequential therapy may improve the prognosis of patients with u-HCC.
In conclusion, LEN-TACE sequential therapy may provide more clinical benefits than LEN monotherapy in u-HCC patients who responded to initial LEN treatment. The objective response by initial LEN assessed by mRECIST is an independent factor contributing to the response to LEN-TACE sequential therapy.
Statement of Ethics
This study followed the principles of the Declaration of Helsinki (revision of Fortaleza, 2013). This study was reviewed and approved by the institutional review board of Iwate Medical University (approval number: MH2019-082). All patients provided written informed consent before the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
Study concept and design: H.K. Acquisition of data: all authors. Analysis and interpretation of the data: H.K. and T.O. Drafting of the manuscript: H.K. Revision of the manuscript for intellectual content: all authors. Statistical analysis: H.K. and T.O. Study supervision: Y.U. and Y.T. All authors contributed substantially to critically reviewing or revising the manuscript for important intellectual content, provided approval of the final version of the manuscript to be submitted and published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Acknowledgment
The authors would like to thank Ms. Koko Motodate for providing excellent technical assistance.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019 Oct;16((10)):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M. Surveillance, diagnosis, and treatment outcomes of hepatocellular carcinoma in Japan: 2021 update. Liver Cancer. 2021 Jun;10((3)):167–80. doi: 10.1159/000516491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021 Jun;10((3)):181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakeji Y, Takahashi A, Udagawa H, Unno M, Endo I, Kunisaki C, et al. Surgical outcomes in gastroenterological surgery in Japan: report of national clinical database 2011–2016. Ann Gastroenterol Surg. 2018 Jan;2((1)):37–54. doi: 10.1002/ags3.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimose S, Kawaguchi T, Iwamoto H, Niizeki T, Shirono T, Tanaka M, et al. Indication of suitable transarterial chemoembolization and multikinase inhibitors for intermediate stage hepatocellular carcinoma. Oncol Lett. 2020 Apr;19((4)):2667–76. doi: 10.3892/ol.2020.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8,510 patients. Gastroenterology. 2006 Aug;131((2)):461–9. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019 Jul 1;5((7)):953–60. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 10.Brar G, Kesselman A, Malhotra A, Shah MA. Redefining intermediate-stage HCC treatment in the era of immune therapies. JCO Oncol Pract. 2021 Jul 13;:Op2100227. doi: 10.1200/OP.21.00227. [DOI] [PubMed] [Google Scholar]
- 11.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013 Oct 28;340((1)):97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multi-center analysis. Hepatol Res. 2019 Jan;49((1)):111–7. doi: 10.1111/hepr.13243. [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, et al. Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: multi-center analysis. Cancer Med. 2019 Jan;8((1)):137–46. doi: 10.1002/cam4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka A, Kumada T, Tada T, Tani J, Kariyama K, Fukunishi S, et al. Efficacy of lenvatinib for unresectable hepatocellular carcinoma based on background liver disease etiology: multi-center retrospective study. Sci Rep. 2021 Aug 17;11((1)):16663. doi: 10.1038/s41598-021-96089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodama K, Kawaoka T, Namba M, Uchikawa S, Ohya K, Morio K, et al. Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology. 2019;97((2)):75–81. doi: 10.1159/000499715. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016 Mar 15;22((6)):1385–94. doi: 10.1158/1078-0432.CCR-15-1354. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017 Apr;52((4)):512–9. doi: 10.1007/s00535-016-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child-Pugh a liver function: a proof-of-concept study. Cancers. 2019 Jul 31;11((8)):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Tominaga L, et al. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer. 2020 Dec;9((6)):756–70. doi: 10.1159/000510299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, et al. Efficacy and safety of lenvatinib-transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;99((8)):507–17. doi: 10.1159/000515865. [DOI] [PubMed] [Google Scholar]
- 22.Endo K, Kuroda H, Abe T, Sato H, Kooka Y, Oikawa T, et al. Two hepatectomy cases for initially unresectable hepatocellular carcinoma after achieving a radiological complete response to sequential therapy with lenvatinib and transcatheter arterial chemoembolization. Hepatol Res. 2021 May 13; doi: 10.1111/hepr.13665. [DOI] [PubMed] [Google Scholar]
- 23.Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021 Jun;15((3)):663–75. doi: 10.1007/s12072-021-10184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimose S, Iwamoto H, Tanaka M, Niizeki T, Shirono T, Noda Y, et al. Alternating lenvatinib and trans-arterial therapy prolongs overall survival in patients with inter-mediate stage hepatocellular carcinoma: a propensity score matching study. Cancers. 2021 Jan 5;13((1)):160. doi: 10.3390/cancers13010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A Changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-pacific primary liver cancer expert consensus statements. Liver Cancer. 2020 Jun;9((3)):245–60. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982 Dec;5((6)):649–55. [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45((2)):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010 Feb;30((1)):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 29.United States Department Of Health And Human Services, National Institutes of Health and National Cancer Institute Common terminology criteria for adverse events (CTCAE) Version 5.0. 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf.
- 30.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008 Sep 1;14((17)):5459–65. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 31.Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008 Feb 1;122((3)):664–71. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020 Jan;55((1)):113–22. doi: 10.1007/s00535-019-01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatanaka T, Naganuma A, Kakizaki S. Lenvatinib for hepatocellular carcinoma: a literature review. Pharmaceuticals. 2021 Jan 6;14((1)):36. doi: 10.3390/ph14010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020 Aug;69((8)):1492–501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudo M. Extremely high objective response rate of lenvatinib: its clinical relevance and changing the treatment paradigm in hepatocellular carcinoma. Liver Cancer. 2018 Sep;7((3)):215–24. doi: 10.1159/000492533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005 Jan 7;307((5706)):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 38.Kano MR, Komuta Y, Iwata C, Oka M, Shirai YT, Morishita Y, et al. Comparison of the effects of the kinase inhibitors imatinib, sorafenib, and transforming growth factor-beta receptor inhibitor on extravasation of nanoparticles from neovasculature. Cancer Sci. 2009 Jan;100((1)):173–80. doi: 10.1111/j.1349-7006.2008.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008 Jun;49((5)):523–9. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004 Oct 1;10((19)):2878–82. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008 Apr;103((4)):914–21. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Zhang Y, Iwamoto H, Hosaka K, Seki T, Andersson P, et al. Discontinuation of anti-VEGF cancer therapy promotes metastasis through a liver revascularization mechanism. Nat Commun. 2016 Sep 1;7:12680. doi: 10.1038/ncomms12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terashima T, Yamashita T, Takata N, Toyama T, Shimakami T, Takatori H, et al. Comparative analysis of liver functional reserve during lenvatinib and sorafenib for advanced hepatocellular carcinoma. Hepatol Res. 2020 Jul;50((7)):871–84. doi: 10.1111/hepr.13505. [DOI] [PubMed] [Google Scholar]
- 44.Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, et al. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol Res. 2020 Mar;50((3)):374–81. doi: 10.1111/hepr.13452. [DOI] [PubMed] [Google Scholar]
- 45.Kuorda H, Abe T, Fujiwara Y, Okamoto T, Yonezawa M, Sato H, et al. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2019 May 21;25((19)):2365–7. doi: 10.3748/wjg.v25.i19.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.