Abstract
Portal vein tumor thrombus (PVTT) is very common and it plays a major role in the prognosis and clinical staging of hepatocellular carcinoma (HCC). We have published the first version of the guideline in 2016 and revised in 2018. Over the past several years, many new evidences for the treatment of PVTT become available, especially for the advent of new targeted drugs and immune checkpoint inhibitors which have further improved the prognosis of PVTT. So, the Chinese Association of Liver Cancer and Chinese Medical Doctor Association revised the 2018 version of the guideline to adapt to the development of PVTT treatment. Future treatment strategies for HCC with PVTT in China would depend on new evidences from more future clinical trials.
Keywords: Hepatocellular carcinoma, Portal vein tumor thrombus, Multidisciplinary therapy, Guideline
Introduction
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer worldwide, and China accounts for more than half of new cases and deaths related to HCC every year [1]. The latest data indicated that the morbidity and mortality rates of HCC ranked fourth and third, respectively, among all malignant tumors reported in China [2]. Given the advances in diagnosis and treatment strategies for different stages of HCC, the prognosis of HCC patients has improved. Unfortunately, 70%–80% of patients are still diagnosed at an advanced stage as there are no obvious clinical symptoms at early stages. At present, the overall prognosis of HCC is not satisfactory.
Owing to the biological characteristics of liver cancer and the anatomical characteristics of the liver, HCC is prone to invade intrahepatic vessels, especially the portal venous system. In China, the incidences of portal vein tumor thrombus (PVTT) have been reported to range from 44% to 62.2% [3]. Once developed, PVTT progresses rapidly to cause portal hypertension, hepatocellular jaundice, and intractable ascites. The median survival of HCC patients with main PVTT is 2.7 months [4]. PVTT plays a major role in the prognosis and clinical staging of HCC [5, 6].
There have been no worldwide consensuses or guidelines on the diagnosis and treatment of HCC with PVTT. Guidelines in Europe and America follow the Barcelona Clinic Liver Cancer Staging (BCLC) and regard HCC with PVTT to be at BCLC Stage C. The guidelines also recommend treating HCC patients with PVTT with systemic therapy [7]. On the contrary, experts from Southeast Asian countries, including China opine that multidisciplinary therapy, including surgery, transcatheter arterial chemoembolization (TACE), radiotherapy (RT), molecular-targeted drugs, and/or immune checkpoint inhibitor (ICIs) should be considered to achieve more satisfactory outcomes. But the difference is that Chinese doctors tend to use more curable treatments for the same subgroup of PVTT patients.
In May 2016, the Chinese National Research Cooperative Group for Diagnosis and Treatment of Hepatocellular Carcinoma with Tumor Thrombus launched The Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (version 2016) [8] based on the existing evidences published internationally and in China at that time. In 2018, we revised the 2016 version of the guideline to adapt to the development of PVTT treatment. This version (version 2018) [9] has been widely used and recognized clinically in China.
Over the past several years, many new evidences for the treatment of PVTT become available, especially for the advent of new targeted drugs and ICIs which have further improved the prognosis of PVTT. So, the Chinese Association of Liver Cancer revised the 2018 version of the guideline to adapt to the development of PVTT treatment.
Based on internationally accepted practice, the grades of evidence we use are presented in Table 1 [10]. We also adopted the United States Preventive Service Task Force recommendations to assign 5 alphabets (A, B, C, D, and I) to denote the strength of recommendation for clinical practice (Table 2) [11].
Table 1.
Grades of evidences
| Grades of evidences | Description |
|---|---|
| Ia | Evidences are originated from the meta-analysis results of various RCTs |
|
| |
| Ib | Evidences are originated from the results of at least one well-designed RCT |
|
| |
| IIa | Evidences are originated from the results of at least one well-designed perspective non-RCT |
|
| |
| IIb | Evidences are originated from the results of at least one well-designed interventional clinical research of other types |
|
| |
| III | Evidences are originated from the well-designed non-interventional clinical researches, such as descriptive researches and relevant researches |
|
| |
| IV | Evidences are originated from the reports made by the committee of experts or the clinical reports of authoritative experts |
RCT, randomized controlled trial.
Table 2.
Ranking of recommended opinion
| Grades of evidences | Description |
|---|---|
| A | Favorable scientific evidences indicate that the medical treatment can provide clear and definite benefits to the patients; physicians are strongly recommended to administer the medical treatment to eligible patients |
|
| |
| B | Existing evidences indicate that the medical treatment may provide moderate benefits that outweigh the potential risks; physicians may suggest or patients may carry out the said medical treatment |
|
| |
| C | Existing evidences indicate that the medical treatment may provide only little benefits, or the benefits do not outweigh the risks; physicians may suggest or administer the said medical treatment selectively based on the patient's condition |
|
| |
| D | Existing evidences indicate that the medical treatment would not benefit the patients, or the potential risks would outweigh the benefits; physicians are recommended not to administer the said medical treatment in patients |
|
| |
| I | There are not enough scientific evidences, or the existing evidences cannot be used, to evaluate the benefits and risks of the said medical treatment; physicians should help the patients understand well the uncertainty of this medical treatment |
Guideline Recommendations
Diagnosis and Classification of PVTT
PVTT is one of the most common complications of HCC. A diagnosis of HCC is a prerequisite to diagnose PVTT [12]. The imaging features of PVTT include solid lesions within the portal vein in all the phases of intravenous enhanced three-phase computed tomography, especially with an enhancement of contrast in the arterial phase and washout in the portal venous phase of the procedure [13, 14]. Clinically, PVTT should be distinguished from portal vein thrombosis (PVT), which occurs as a complication of cirrhosis or after splenectomy. PVT is not enhanced in the arterial phase. It occasionally disappears or improves after anticoagulant therapy [15].
The extent of PVTT is closely related to the prognosis of HCC. The HCC staging systems that are commonly used today are the TNM staging, BCLC staging, and staging of the liver cancer study group of Japan. All these staging systems accept the importance of PVTT. However, they do not further define the extent of PVTT. At present, there are two classifications for PVTT: the Japanese VP classification [16], and the Cheng's classification as suggested by Professor Cheng Shuqun of China [17, 18, 19].
The Cheng's classification comprises four levels based on the extent of tumor thrombus in the portal vein shown on medical imagings: type I, tumor thrombus involving segmental or sectoral branches of the portal vein or above; type II, tumor thrombus involving the right/left portal vein; type III, tumor thrombus involving the main portal vein; and type IV, tumor thrombus involving the superior mesenteric vein. Type I0, tumor thrombus found only under microscopy. Many studies have supported that the Cheng's classification to be more applicable than the VP classification for disease assessment, treatment selection, and prognostic judgment in patients with PVTT [18, 19, 20], and hence it is recommended to be used for classifying the extent of PVTT.
Multidisciplinary Therapy Path for HCC with PVTT
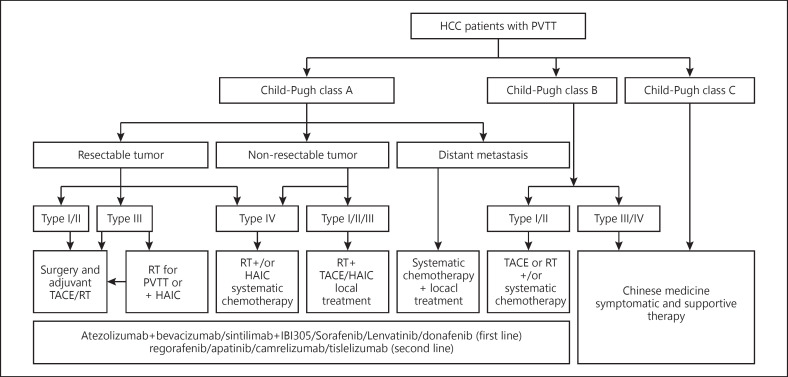
A multidisciplinary team to coordinate diagnosis and treatment of HCC patients with PVTT provides maximal benefits to patients. The therapeutic plan for the treatment of HCC with PVTT formulated by the National Research Cooperative Group for Diagnosis and Treatment of Hepatocellular Carcinoma with Tumor Thrombus is presented in Figure 1. Patients with Child-Pugh A liver function can undergo any treatment according to the PVTT type. When the lesion is resectable and when there is no extrahepatic metastasis, patients with type I/II PVTT should undergo surgical resection of the PVTT en bloc with the primary HCC. For patients with PVTT type III, the treatment choices include surgery, TACE, and/or RT depending on the patient's preference. For unresectable lesions, patients with type I/II/III PVTT should receive RT combined with TACE or (Hepatic Arterial Infusion Chemotherapy, HAIC), and patients with type IV PVTT should receive RT or systemic therapy. Patients with Child-Pugh B liver function should first receive antiviral treatment for HCC secondary to hepatitis B or C infections. If the liver function improves to Child-Pugh A, then these patient subgroups can be treated as mentioned above. Surgery and TACE are not recommended for Child-Pugh B patients. Child-Pugh C patients should only receive supportive care. Child-Pugh A patients who have extrahepatic metastases can receive systemic treatment and/or local treatment. Sorafenib, Lenvatinib, Donafenib, Atezolizumab plus Bevacizumab, and Sintilimab plus IBI305 can be used for patients with all extents of PVTT with Child-Pugh A liver function. Regorafenib, Apatinib, Camrelizumab, and Tislelizumab are the second-line treatments of PVTT patients.
Fig. 1.
Diagnosis and treatment of HCC with PVTT.
Recommended First-Line Treatment Options for PVTT
The treatment of HCC patients with PVTT is based on the patients' liver function, the stage of hepatic lesion, and the extent of PVTT. A strategy that can either eliminate or control HCC with PVTT using multimodality therapy can extend survival and improve quality of life of the patient.
Surgery
Recommendations
• Surgery is the preferred treatment in patients with Child-Pugh A, PVTT type I/II, and ECOG PS 0–1 (Evidence level IIb, Recommendation A); type III PVTT patients can undergo surgery directly (Evidence level IIb, Recommendation B) or after tumor down-staging using RT (Evidence level Ib, Recommendation A).
• Adjuvant TACE (Evidence level Ib, Recommendation A), RT (Evidence level Ib, Recommendation A), or molecular-targeted therapy (Evidence level IIb, Recommendation B) after surgery can be used to reduce recurrence.
Surgical treatment is considered to be potentially curative and is the preferred treatment option for HCC patients with type I/II PVTT. En bloc resection of the primary HCC and PVTT provides a potential for cure. Many studies reported that patients who had undergone surgery had a better prognosis than those treated with TACE [12, 21, 22] or TACE combined with RT [23].
Type I/II PVTT is more suitable for resection than type III/IV (Evidence level IIb) [18, 24, 25]. En-bloc resection can be performed in type I/II PVTT patients with partial hepatectomy or hemihepatectomy. For type III PVTT patients, as the PVTT has extended to the main portal vein, partial hepatectomy has to be combined with thrombectomy or main portal vein resection followed by reconstruction. At present, studies have revealed that there is no significant difference in prognosis among these surgical procedures (Evidence level IIb) [26]. Thrombectomy is by far the most commonly used surgical procedure.
The following are the recommendations for reducing recurrence rates and metastasis after surgery: (1) Preoperative small-dose RT has been reported to downstage some type III PVTT patients, reduce recurrence rate without increasing surgical risks, and reduce postoperative hepatic failure rates (Evidence level Ib) [27, 28]. (2) Adjuvant TACE after surgery has been reported to reduce recurrence rates and prolong survival of PVTT patients in a randomized controlled trial (RCT) (From January 1996 to December 2004, including 126 patients) (Evidence level Ib) [29]; but a recent meta-analysis revealed that adjuvant TACE can only increase the 1-year survival rate. (3) Adjuvant RT has been reported to reduce recurrence rates and prolong survival of PVTT patients [30] (Evidence level Ib).
Other treatment recommendations that are controversial include the following: postoperative portal vein chemotherapy [31] (Evidence level IIb), adjuvant HAIC [32] (Evidence level IIb), adjuvant molecular-targeted therapy [33] (Evidence level IIb), and postoperative intravenous chemotherapy [34] (Evidence level III).
Nonsurgical Therapies
Hepatic Artery Infusion Chemotherapy
Recommendations
• Patients with non-resectable primary tumor, type I/II/III/IV PVTT, and Child-Pugh A liver function may receive HAIC (Evidence level Ib, Recommendation B).
HAIC was developed to treat metastatic liver tumors and was known to be more effective than conventional systemic chemotherapy. Recently, HAIC was then applied to advanced HCC [35]. Commonly used chemotherapeutic agents for HAIC include platinum/oxaliplatin and 5-fluorouracil. Chemotherapeutic agents were administered every 2–4 weeks through the hepatic artery, and the patient's responses were usually evaluated every one or two cycles. A prospective randomized controlled study, including 58 HCC patients with PVTT in Korea revealed that the median OS of the HAIC group was 14.9 months, which was significantly higher than that of the Sorafenib group (7.2 months, p = 0.012) [36]. Lyu et al. [35] reported a single-center retrospective study demonstrating the higher objective remission rate (ORR) of HAIC compared with sorafenib (mRECIST, 47.8% vs. 9.1%, p < 0.01), and 26.1% of the patients in the HAIC group achieved remission to receive local treatment. HAIC may be more effective in combination with other treatments. Nagai et al. [37] studied the effect of HAIC combined with sorafenib on HCC with PVTT compared with Haic alone. The results showed that the OS of the combined treatment group was 4 months longer than that of HAIC alone (p < 0.05). Another study published by Onishi et al. [38] showed that the ORR in HAIC combined with RT for HCC with PVTT was significantly higher than HAIC alone (52% vs. 18%, p < 0.05).
Transcatheter Arterial Chemoembolization
Recommendations
• Patients with non-resectable primary tumor, type I/II PVTT, and Child-Pugh A liver function may receive TACE (Evidence level IIb, Recommendation B) alone or in combination with RT (Evidence level Ib, Recommendation A) or molecular-targeted therapy (Evidence level IIb, Recommendation A).
• Patients with Child-Pugh B liver function or type III/IV PVTT are not recommended to receive TACE (Evidence level IIb, Recommendation C).
TACE is one of the most commonly used techniques to manage nonresectable HCC with PVTT [39]. Despite the possible benefit of TACE in prolonging overall survival (4–7 months) in patients with HCC and PVTT type III/IV, the use of TACE in patients is controversial due to the risk of liver infarction and hepatic failure [40]. At present, TACE is considered for PVTT patients with good liver function with adequate collateral circulation around the obstructed portal vein [41, 42]. The overall survival rate varies greatly among patients with PVTT after TACE. The patient survival rates decreased from 82% at 3 months to 71% at 6 months and 47% at 12 months, with a median survival of 10 months. Patients with Child-Pugh A liver function had better median survival when compared to patients with Child-Pugh B (15 vs. 13 months) [43], and the complete remission rate (CR), partial remission rate, and stable disease rate were reported to be 0, 19.5–26.3%, and 42.5–62.7%, respectively [44, 45, 46]. Lipiodol and gelatin sponge are common embolizing agents used in TACE [47]. Some reports have suggested that TACE, when combined with lipiodol, is more effective than TAI or conservative treatment [39, 48]. The effectiveness of the embolizing agents depends on their size. The smaller the diameter of an embolizing agent, the better is the effect on PVTT patients and the lower is its adverse side effects [49, 50]. The use of super-selective catheterization improves therapeutic effects and reduces damages to the normal liver when compared with conventional TACE. Recently, TACE with drug-eluting beads has been introduced into a clinical application; however, its effects on HCC patients with PVTT are controversial [51].
Radiotherapy
External Beam Radiation Therapy
Recommendations
• Patients with non-resectable HCC with all types of PVTT, with Child-Pugh A or B liver function, are recommended to receive RT with the target region containing both the primary tumors and PVTT − 3DCRT or intensify-modulated RT (IMRT) 95% PTV 40–60 Gy/2–3 Gy (Evidence level IIb, Recommendation B) or SBRT 36–40 Gy/5–6 Gy (Evidence level IIb, Recommendation A).
• Patients with Child-Pugh A liver function and types I, II, and III PVTT are recommended to receive combined RT and TACE (Evidence level Ib, Recommendation A) or HAIC (Evidence level IIb, Recommendation B). The RT target region includes the primary tumor and PVTT or only the PVTT.
With the development of newer technologies such as three-dimensional conformal RT, IMRT, and three-dimensional-oriented RT (SBRT), radiation dosage to the targeted regions can be increased while giving better protection to the adjacent healthy tissues [52, 53, 54]. This allows the maximum use of RT technologies and enables their use in HCC patients with all types of PVTT.
The use of RT alone or in combination with other treatments such as TACE improved survival and quality of life in HCC patients with PVTT. Yoon et al. [55] conducted a prospective randomized controlled study, including 90 HCC patients with PVTT and there were 45 cases in the TACE combined with RT group and 45 cases in the Sorafenib group. The results revealed that the median OS of TACE combined with RT group was 12.8 months, which was significantly higher than that of Sorafenib group (10.0 months, p = 0.04).
Target localization suggests the use of computed tomography and MRI image fusion technology based on the area of lipiodol deposition after TACE. The clinical target volume is 4 mm larger than the diameter of the tumor area [56]. The plan target volume should be determined on the basis of a moving target, set-up error, and random error. The designation of the irradiation area is still controversial, which should be determined individually. The hepatic lesion and PVTT should be irradiated simultaneously if the hepatic lesion is small and PVTT is nearby. If the volume of the primary tumor is large or PVTT is distant to the primary tumor, only the PVTT should receive irradiation [57].
There is not enough evidence to determine the best radiation and fraction doses. The existing evidence suggests a positive correlation between total radiation dose and tumor response [58]. However, multivariate analysis only showed response to RT to be associated with survival [58, 59]. Image-guided IMRT should be applied if available, which is better than conventional 3D-CRT [60].
Radiation-induced liver disease (RILD) or radiation hepatitis is a subacute form of liver injury, which occurs due to overexposure of the liver to radiation [53]. The key to prevent RILD is to keep the total dose within the tolerance range limit when designing the RT plan [61]. As most HCC patients in China have a cirrhotic background, the radiation tolerance dose of the liver in these patients is lower than that in patients from other countries. The liver tolerance dose (average dose of the liver) is 23 Gy for Child-Pugh A patients and only 6 Gy for Child-Pugh B patients [62]. The most common risk factors of RILD include pre-existing poor liver function, high irradiation volume, coexisting PVT, and acute liver toxicity due to other causes [61, 62]. It is reported that individualized adaptive RT based on a direct biomarker of liver function such as ICG 15 can be used to achieve both high rates of local control and a high degree of safety without sacrificing either (Evidence level IIa) [63].
Evidence from clinical studies has shown a combination of RT and TACE produces better clinical outcomes than TACE or RT alone. The time interval between TACE and RT should not exceed 1 month [64]. When TACE is combined with RT, the order of the treatments given should be decided clinically. As the effect on liver function is less in patients receiving RT first than those receiving TACE first, with similar treatment outcomes, RT should be given before TACE [65]. A combination of RT and HAIC might be more effective than HAIC alone [38], but it needs to be demonstrated by further RCTs.
Internal Radiation Therapy
Recommendations
• Patients with nonresectable primary tumors; types I, II, and III PVTT; and Child-Pugh A liver function could be treated with transarterial arterial radio-embolization (TARE) (Evidence level IIb, Recommendation C) or portal veins I125 seed implantation (Evidence level IIb, Recommendation B).
Patients treated with I125 particle seeds implanted in the portal vein and TACE have been reported to have better survival outcomes when compared to patients treated with TACE alone. This combination therapy also improved the reperfusion rate of portal vein significantly [66]. Another study showed I125 seeds followed by TACE significantly improved the median survival and progression-free survival rates when compared to I125 alone (p = 0.037 and 0.002, respectively) [67]. TARE with yttrium-90 (Y90) microspheres are considered to be a viable treatment option in HCC patients with PVTT. TARE has been shown to produce better long-term survival outcomes than TACE [68]. However, The SARAH trial revealed that the overall survival did not significantly differ between the Sorafenib group and TARE group for advanced HCC patients [69]. Furthermore, there is no uniform dosage standard at present for internal radiation therapy.
Systematic Therapy
Recommendations
• Nucleoside analogs are recommended in PVTT patients with HBsAg positive regardless of whether or not HBV DNA is positive (Evidence level Ia, Recommendation A).
• Atezolizumab plus Bevacizumab, Sorafenib, Lenvatinib, Donafenib, and Sintilimab plus IBI305 are recommended as the basic drug for PVTT patients with Child-Pugh A liver function (Evidence level Ib, Recommendation A). Regorafenib, Apatinib, Camrelizumab, and Tislelizumab are recommended as the second-line treatment for PVTT patients with Child-Pugh A liver function (Evidence level Ib, Recommendation A).
• Chemotherapy is recommended in PVTT patients (Evidence level IIb, Recommendation B) with extrahepatic metastasis and Child-Pugh A liver function.
Persistent HBV infection is an important poor risk factor for occurrence, progression, recurrence, and death in patients with HCC secondary to HBV infection. Antiviral therapy reduces postoperative recurrence and improves survival of HCC patients [70]. Antiviral therapy should also be given to PVTT patients [71, 72].
Sorafenib, Lenvatinib, and Donafenib are universally accepted therapy that effectively prolong survival in patients with advanced HCC (Evidence level Ib) [73, 74, 75]. All have been listed by the National Medical Products Administration (NMPA) as the first-line treatment option in patients with advanced HCC. The STORM was a phase 3, double-blind, randomized, placebo-controlled study, which evaluated the effectiveness of sorafenib as adjuvant therapy to surgery. When compared to placebo, sorafenib did not show any significant improvement in the median recurrence-free survival (33.3 vs. 33.7 months, p = 0.26), suggesting that adjuvant sorafenib to be ineffective [76]. The effectiveness of Sorafenib and TACE combination has also been controversial [77, 78, 79, 80]. Regorafenib, Apatinib is recommended as the second-line treatment for PVTT patients (Evidence level Ib) [81, 82]. Cabozantinib [83] and Ramucirumab (AFP >400 μg/L) [84] were only approved by FDA as second-line targeted drugs.
The application of ICIs has created a new era in the systematic treatment of advanced HCC, especially in combination with TKI. In the global multicenter phase III clinical trial (IMBrave 150) study [85], the ORR of Atezolizumab plus Bevacizumab (T+A) was 30%, which was significantly higher than that of Sorafenib group, while the risk of death and disease progression decreased by 35% and 34%, respectively. In another multicenter phase III clinical trial (ORIENT-032) [86, 87], the ORR of Sintilimab plus IBI305 was 21%, and the risk of death and disease progression were reduced by 43.1% and 43.5% respectively when compared with Sorafenib group. T+A (evidence level Ib) and Sintilimab plus IBI305 (evidence level Ib) have been approved by NMPA as the first-line treatment of advanced HCC. Camrelizumab [88] and Tislelizumab [89] are recommended as the second-line treatment for PVTT patients (Evidence level Ib). Pembrolizumab [90, 91] and Nivolumab Plus Ipilimumab [92] were only approved by FDA as second-line targeted drugs. At present, the clinical research on immunotherapy has made rapid progress [93, 94, 95, 96, 97, 98], including Camrelizumab plus Apatinib (RESCUE), Lenvatinib plus Pembrolizumab, Tremelimumab (T) plus Durvalumab (D), Regorafenib plus Pembrolizumab, Regorafenib plus Pembrolizumab, Penpulimab with Anlotinib. Based on current evidences, Atezolizumab plus Bevacizumab should be a priority and positioned as 1st-line therapy among all systemic drugs.
Before the treatment of ICIs, the medical history taking, physical examination, laboratory, and imaging examination must be done to evaluate the tumor burden and organ function (heart, lung, liver, kidney, endocrine system, and so on) of the patients comprehensively. Immune-related adverse events should be monitored during ICIs treatment, including delayed toxicity after treatment. In case of immune-related adverse events, please refer to the NCCN Guidelines for Management of Immunotherapy-Related Toxicities [99]. The research on ICIs is very active in the field of HCC. The future version of this guideline will also be modified according to the corresponding research results.
The EACH study demonstrated that FOLFOX 4 (an oxaliplatin-containing chemotherapy) provided partial cure in patients with advanced HCC (including PVTT patients). FOLFOX 4 might be administered in patients with good liver function and tolerance (Evidence level Ib) [100]. A phased II prospective study revealed that mFOLFOX4 combined with Sorafenib would be more effective, but the results need further validation [101].
Local Treatment
Recommendations
• Local ablation therapies should be recommended in PVTT patients with caution; further studies are warranted (Evidence level III, Recommendation C). Local ablation therapies may be combined with TACE and molecular-targeted therapy (Evidence level IIb, Recommendation B).
The local ablation therapies include percutaneous ethanol injection, radiofrequency ablation, and laser ablation. These therapies may be adopted to reduce tumor load and recanalization of portal vein. However, local therapies must be used cautiously as there is a risk of damaging the portal vein wall and bile duct. In addition, a high recurrence rate of PVTT has been reported within a short period of time (Level III evidence) [102, 103]. Therefore, it is suggested to combine local ablation therapies with other treatments such as TACE and molecular-targeted therapy to improve the curative effect (Level II evidence) [104, 105, 106].
Symptomatic and Supportive Treatment
Recommendations
• Symptomatic and supportive treatment is recommended in patients with Child-Pugh C liver function, with massive ascites or gastrointestinal bleeding due to esophageal varices and hepatic encephalopathy (Evidence level Ia, Recommendation A).
Portal vein stenting may be adopted to recanalize blood flow in the portal veins of PVTT patients, with resultant increase in blood flow to the liver, but without reducing the tumor load. In patients with PVTT, portal vein stenting can result in improved liver functions, reduced portal vein pressure, and at the same time, win time for other therapies such as RT and TACE to act (Evidence level III) [107, 108].
Most complications of PVTT result from portal hypertension. The common complications include upper gastrointestinal hemorrhage, ascites, hypersplenism, hepatorenal syndrome, and hepatic failure. For therapeutic methods, please refer to the article on treatment of portal hypertension [109]. In addition, Chinese medicine [110, 111] such as Huaier granule and Cidan could also be used for PVTT patients with nonresectable primary tumors.
Tumor Down-Staging of HCC with PVTT
Recommendations
• Tumor down-staging is suggested for non-resectable HCC patients with all types of PVTT, with Child-Pugh A liver function and more clinical trials need to be carried out (Evidence level IIb, Recommendation A).
Tumor down-staging of HCC with PVTT is an important way to improve the survival of PVTT patients. Especially in recent years, with the significant progress of various non-surgical treatment methods, such as immunotherapy, molecular-targeted therapy, RT, and HAIC, the down-staging success rate of PVTT patients has been significantly improved, which has greatly prolonged the survival time of certain PVTT patients. It is one of the main directions of PVTT clinical research. At present, the most reported down-staging therapy is based on RT, ICIs, and molecular-targeted therapy.
A retrospective single-arm study conducted by Serenari et al. [112] showed that up to 29.4% of PVTT patients treated with TARE had achieved down-staging and had the opportunity to receive liver transplantation. Another retrospective study [113] on PVTT patients compared the efficacy of TARE with Sorafenib. The results showed that the down-staging rate of TARE was 24.4%, which was significantly higher than that of Sorafenib. The efficacy of HAIC on PVTT has been mentioned above, and it may obtain a higher down-staging rate when combined with RT. The retrospective study of Hamaoka et al. [114] showed that the down-staging rate of RT combined with HAIC was 14%, and the survival time of patients undergoing surgery was significantly longer than that of non-surgical patients. Another retrospective study [115] showed that the down-staging rate of PVTT by RT combined with HAIC was 26.5%, and the pathology of PVTT of all patients undergoing surgery showed complete necrosis.
Tumor down-staging based on ICIS and targeted drugs is an important research direction to improve the down-staging rate of PVTT. At present, more clinical trials need to be carried out using various new schemes. A retrospective single-arm study conducted by Huang et al. [116] showed that the ORR of Lenvatinib combined with PD-1 was 54.5% for PVTT and 32.8% for hepatic tumors. Of the 17 PVTT patients who achieved ORR, 6 (18.1%) underwent surgery. Postoperative pathology showed that 66.7% of PVTT achieved pathological complete necrosis. A real-world study by Tsai et al. [117] included 28 patients with PVTT. The ORR of PD-1 combined with TKI was 50%, including 2 cases of CR and 1 case underwent surgery. He et al. [118] reported an RCT study that compared the efficacy of HAIC combined with Sorafenib and Sorafenib monotherapy in the treatment of PVTT. The results showed that the effective rate of the combined treatment group was significantly better than that of Sorafenib monotherapy, and 12.8% of the patients in the combined treatment group were successfully downstaged.
For patients who were successfully down-staged, it is suggested that targeted drugs should be stopped for more than 1–2 weeks, ICIs should be stopped for more than 2–4 weeks, and Bevacizumab should be stopped for more than 6 weeks before surgery. If TACE is performed, the operation should be performed 4 weeks after the last treatment and if low-dose RT is performed, surgery should be performed 3 weeks after the last RT.
Future Outlook
It is necessary to develop a treatment guideline in China as HCC patients with PVTT in China are different from those in Europe and America in terms of etiology and biological behavior. Although treatment of HCC patients with PVTT is still controversial, new evidences are being gathered. Similar to the multidisciplinary approach of HCC treatment in the United States (the American Association for the Study of Liver Diseases practice guidelines) and Europe (the European Association for the Study of the Liver − European Organization for Research and Treatment of Cancer) for HCC management, we have adopted a multidisciplinary approach for HCC with PVTT. This treatment approach when combined with early diagnosis, will enable a larger number of patients to receive an appropriate treatment based on the stage of the disease.
In our Guide meetings, the following principles in clinical practice are emphasized: (1) Multidisciplinary treatment should be used in HCC patients with PVTT to achieve better results. (2) Prolongation of overall survival is the most important target and the chance of cure is low. Emphasis should also be given to the quality of life of these patients. The treatment complication rate should be kept at a minimum. (3) The targeted and immunotherapy of advanced HCC has made rapid progress, which needs to be extended to the clinical application of PVTT for the first time, and carry out relevant clinical trials. (4) Tumor downstaging can greatly prolong the survival time of PVTT patients. It is also one of the research hotspots of HCC at present. More clinical trials need to be carried out by using new technologies and medicines.
There are a huge number of PVTT patients in China, and the evidence-based level of the existing guideline recommendations is still low. Therefore, in the future, we should make full use of China's case resources, update the new stage of PVTT (such as Liu-Cheng's PVTT stage system [119]) in combination with the latest treatment progress, such as targeted and immune therapy, and carry out more randomized controlled studies to verify more effective diagnosis and treatment methods of PVTT. The molecular mechanisms underlying the genesis and development of PVTT also need to be studied to lay the foundation for more future effective treatment. The role of Chinese traditional medicine in the treatment of PVTT as an adjuvant to other therapeutic options such as surgical treatment, TACE, or RT should be evaluated.
Statement of Ethics
Our manuscript complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. In the manuscript, all authors state that subjects have given their written informed consent and that the study protocol was approved by the institute's committee on human research. The paper is approved by the Chinese Association of Liver Cancer (20210928).
Conflict of Interest Statement
The authors have no conflicts of interest to declare. Pro. Jia Fan is an Associate Editor of Liver Cancer.
Funding Sources
This research received no external funding.
Author Contributions
All the authors planned the study and contributed to the interpretation of the data, revisions, and gave input at all stages of the study. All the authors have approved the final version of the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Prof. Mengchao Wu, Prof. Zhaoyou Tang, Prof. Wanyee Lau, Prof. Xiaoping Chen, Prof. Xuehao Wang, Prof. Jinming Yu, Prof. Shusen Zheng, Prof. Jiahong Dong, and Prof. Jia Fan for their contribution to the guideline.
Sun Juxian, Guo Rongping, and Bi Xinyu contributed equally to this study.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65((2)):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66((2)):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z, Wan BJ, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumorthrombus. Int J Surg. 2015;20:8–16. doi: 10.1016/j.ijsu.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21((4)):386–91. doi: 10.1097/00000421-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Li SH, Wei W, Guo RP, Shi M, Guo ZX, Chen ZY, et al. Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med Oncol. 2013;30((4)):696. doi: 10.1007/s12032-013-0696-3. [DOI] [PubMed] [Google Scholar]
- 6.Li SH, Guo ZX, Xiao CZ, Wei W, Shi M, Chen ZY, et al. Risk factors for early and late intrahepatic recurrence in patients with single hepatocellular carcinoma without macrovascular invasion after curative resection. Asian Pac J Cancer Prev. 2013;14((8)):4759–63. doi: 10.7314/apjcp.2013.14.8.4759. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Cheng S, Chen M, Cai J, National Research Cooperative Group for Diagnosis and Treatment of Hepatocellular Carcinoma with Tumor Thrombus Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus: 2016 edition. Oncotarget. 2017;8((5)):8867–76. doi: 10.18632/oncotarget.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 Edition) Liver Cancer. 2020;9((1)):28–40. doi: 10.1159/000503685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryder SD, British Society of Gastroenterology Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52((Suppl 3)):iii1–8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Preventive Services Task Force Grade definitions and suggestions for practice. 2012. Available from: http://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions.
- 12.Wang K, Guo WX, Chen MS, Mao YL, Sun BC, Shi J, et al. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus: a large-scale, multicenter, propensity mathching score analysis. Medicine. 2016;95((11)):e3015. doi: 10.1097/MD.0000000000003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin S, Primary Liver Cancer Diagnosis and Treatment Expert Panel of the Chinese Ministry of Health Guidelines on the diagnosis and treatment of primary liver cancer (2011 edition) Chin Clin Oncol. 2012;1((1)):10. doi: 10.3978/j.issn.2304-3865.2012.07.01. [DOI] [PubMed] [Google Scholar]
- 14.Hennedige T, Venkatesh SK. Advances in computed tomography and magnetic resonance imaging of hepatocellular carcinoma. World J Gastroenterol. 2016;22((1)):205–20. doi: 10.3748/wjg.v22.i1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponziani FR, Zocco MA, Campanale C, Rinninella E, Tortora A, Di Maurizio L, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol. 2010;16((2)):143–55. doi: 10.3748/wjg.v16.i2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikai I, Yamamoto Y, Yamamoto N, Terajima H, Hatano E, Shimahara Y, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12((1)):65–75. doi: 10.1016/s1055-3207(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 17.Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54((74)):499–502. [PubMed] [Google Scholar]
- 18.Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17((8)):2073–80. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18((1)):74–80. doi: 10.1007/s00534-010-0314-0. [DOI] [PubMed] [Google Scholar]
- 20.Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li ZK, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellularcarcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29((4)):2992–7. doi: 10.1007/s12032-011-0145-0. [DOI] [PubMed] [Google Scholar]
- 21.Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118((19)):4725–36. doi: 10.1002/cncr.26561. [DOI] [PubMed] [Google Scholar]
- 22.Liang L, Chen TH, Li C, Xing H, Han J, Wang MD, et al. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB. 2018;20((12)):1119–29. doi: 10.1016/j.hpb.2018.06.1804. [DOI] [PubMed] [Google Scholar]
- 23.Yu JI, Choi GS, Lim DH, Lee E, Joh JW, Kwon CHD, et al. Treatment of Naïve HCC combined with segmental or subsegmental portal vein tumor thrombosis: liver resection versus TACE followed by radiotherapy. Anticancer Res. 2018;38((8)):4919–25. doi: 10.21873/anticanres.12808. [DOI] [PubMed] [Google Scholar]
- 24.Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13((7)):940–6. doi: 10.1245/ASO.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Wang Z, Wu S, Yu Y, Zhu C, Wu L. Analysis of surgical treatment and prognostic factors for hepatocellular carcinoma with portal vein tumor thrombus. Transl Cancer Res. 2017;6((1)):247–53. [Google Scholar]
- 26.Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg. 2014;38((2)):490–6. doi: 10.1007/s00268-013-2290-4. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Feng S, Xue J, Wei XB, Shi J, Guo WX, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB. 2016;18((6)):549–56. doi: 10.1016/j.hpb.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectablehepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37((24)):2141–51. doi: 10.1200/JCO.18.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198((3)):313–8. doi: 10.1016/j.amjsurg.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Yang L, Shi J, Liu C, Zhang X, Chai Z, et al. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: an open-label randomized controlled trial. Radiother Oncol. 2019;140:20–5. doi: 10.1016/j.radonc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Wang PX, Cheng JW, Sun YF, Hu B, Guo W, et al. Chemotherapeutic perfusion of portal vein after tumor thrombectomy and hepatectomy benefits patients with advancedhepatocellular carcinoma: a propensity score-matched survival analysis. Cancer Med. 2019;8((16)):6933–44. doi: 10.1002/cam4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatano E, Uemoto S, Yamaue H, Yamamoto M, Japanese Society of Hepato-Biliary-Pancreatic Surgery Significance of hepatic resection and adjuvant hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombus in the first branch of portal vein and the main portal trunk: a project study for hepatic surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2018;25((9)):395–402. doi: 10.1002/jhbp.574. [DOI] [PubMed] [Google Scholar]
- 33.Sun HC, Zhu XD, Zhou J, Gao Q, Shi YH, Ding ZB, et al. Adjuvant apatinib treatment after resection of hepatocellular carcinoma with portal vein tumor thrombosis: a phase II trial. Ann Transl Med. 2020;8((20)):1301. doi: 10.21037/atm-20-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai T, Chen J, Xie ZB, Wu FX, Wang SD, Liu JJ, et al. The efficacy and safety of postoperative adjuvant transarterial embolization and radiotherapy in hepatocellular carcinoma patients with portal vein tumor thrombus. Onco Targets Ther. 2016;9:3841–8. doi: 10.2147/OTT.S104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyu N, Kong Y, Mu L, Lin Y, Li J, Liu Y, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin versus sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69((1)):60–9. doi: 10.1016/j.jhep.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Choi JH, Chung WJ, Bae SH, Song DS, Song MJ, Kim YS, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib versus hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82((3)):469–78. doi: 10.1007/s00280-018-3638-0. [DOI] [PubMed] [Google Scholar]
- 37.Nagai H, Mukozu T, Ogino YU, Matsui D, Matsui T, Wakui N, et al. Sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombus. Anticancer Res. 2015;35((4)):2269–77. [PubMed] [Google Scholar]
- 38.Onishi H, Nouso K, Nakamura S, Katsui K, Wada N, Morimoto Y, et al. Efficacy of hepatic arterial infusion chemotherapy in combination with irradiation for advanced hepatocellular carcinoma with portal vein invasion. Hepatol Int. 2015;9((1)):105–12. doi: 10.1007/s12072-014-9592-y. [DOI] [PubMed] [Google Scholar]
- 39.Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. doi: 10.1186/1471-230X-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol. 2016;22((32)):7289–300. doi: 10.3748/wjg.v22.i32.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258((2)):627–34. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 42.Kim HC, Chung JW, Lee W, Jae HJ, Park JH. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005;25((Suppl 1)):S25–39. doi: 10.1148/rg.25si055508. [DOI] [PubMed] [Google Scholar]
- 43.Ajit Y, Sudarsan H, Saumya G, Abhishek A, Navneet R, Piyush R, et al. Transarterial chemoembolization in unresectable hepatocellular carcinoma with portal vein thrombosis: a perspective on survival. Oman Med J. 2014;29((6)):430–6. doi: 10.5001/omj.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L, Zhang C, Zhao Y, Qi X, Chen H, Bai W, et al. Transarterial chemoembolization for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis: prognostic factors in a single-center study of 188 patients. Biomed Res Int. 2014;2014:194278. doi: 10.1155/2014/194278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jang JW, Bae SH, Choi JY, Oh HJ, Kim MS, Lee SY, et al. A combination therapy with transarterial chemo-lipiodolization and systemic chemo-infusion for large extensive hepatocellular carcinoma invading portal vein in comparison with conservative management. Cancer Chemother Pharmacol. 2007;59((1)):9–15. doi: 10.1007/s00280-006-0239-0. [DOI] [PubMed] [Google Scholar]
- 46.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18((2)):413–20. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 47.Liu YS, Ou MC, Tsai YS, Lin XZ, Wang CK, Tsai HM, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015;16((1)):125–32. doi: 10.3348/kjr.2015.16.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu YM, Qin H, Wang CB, Fang XH, Ma QY. Comparision of different interventional therapies for primary liver cancer. Zhonghua Zhong Liu Za Zhi. 2007;29((3)):232–5. [PubMed] [Google Scholar]
- 49.Chern MC, Chuang VP, Liang CT, Lin ZH, Kuo TM. Transcatheter arterial chemoembolization for advanced hepatocellular carcinoma with portal vein invasion: safety, efficacy, and prognostic factors. J Vasc Interv Radiol. 2014;25((1)):32–40. doi: 10.1016/j.jvir.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Tsochatzis EA, Fatourou E, O'Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20((12)):3069–77. doi: 10.3748/wjg.v20.i12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34((17)):2046–53. doi: 10.1200/JCO.2015.64.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh CH, Liu CY, Shueng PW, Chong NS, Chen CJ, Chen MJ, et al. Comparison of coplanar and noncoplanar intensity-modulated radiation therapy and helical tomotherapy for hepatocellular carcinoma. Radiat Oncol. 2010;5:40. doi: 10.1186/1748-717X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang QH, Li AJ, Yang GM, Lai EC, Zhou WP, Jiang ZH, et al. Surgical resection versus conformal radiotherapy combined with TACE for resectable hepatocellular carcinoma with portal vein tumor thrombus: a comparative study. World J Surg. 2013;37((6)):1362–70. doi: 10.1007/s00268-013-1969-x. [DOI] [PubMed] [Google Scholar]
- 54.Kang J, Nie Q, DU R, Zhang L, Zhang J, Li Q, et al. Stereotactic body radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Mol Clin Oncol. 2014;2((1)):43–50. doi: 10.3892/mco.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy versus sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4((5)):661–9. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang MH, Ji Y, Zeng ZC, Tang ZY, Fan J, Zhou J, et al. Impact factors for microinvasion in patients with hepatocellular carcinoma: possible application to the definition of clinical tumor volume. Int J Radiation Oncol Biol Phys. 2010;76((2)):467–76. doi: 10.1016/j.ijrobp.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 57.Yu JI, Park HC. Radiotherapy as valid modality for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2016;22((30)):6851–63. doi: 10.3748/wjg.v22.i30.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang BS, Tsang NM, Lin SM, Lin DY, Lien JM, Lin CC, et al. High-dose hypofractionated X-ray radiotherapy for hepatocellular carcinoma: tumor responses and toxicities. Oncol Lett. 2013;6((5)):1514–20. doi: 10.3892/ol.2013.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, et al. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8((5)):e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou JZ, Zeng ZC, Wang BL, Yang P, Zhang JY, Mo HF. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT. Jpn J Clin Oncol. 2016;46((4)):357–62. doi: 10.1093/jjco/hyv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benson R, Madan R, Kilambi R, Chander S. Radiation induced liver disease: a clinical update. J Egypt Natl Canc Inst. 2016;28((1)):7–11. doi: 10.1016/j.jnci.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD, Lu HJ, et al. Radiation-induced liver disease in three dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65((2)):426–34. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 63.Feng M, Suresh K, Schipper MJ, Bazzi L, Ben-Josef E, Matuszak MM, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. 2018;4((1)):40–7. doi: 10.1001/jamaoncol.2017.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li XL, Guo WX, Hong XD, Yang L, Wang K, Shi J, et al. Efficacy of the treatment of transarterial chemoembolization combined with radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score analysis. Hepatol Res. 2016;46((11)):1088–98. doi: 10.1111/hepr.12657. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Guo W, Guo L, Lau WY, Ge N, Wang K, et al. Should transarterial chemoembolization be given before or after intensity-modulated radiotherapy to treat patients with hepatocellular carcinoma with portal vein tumor thrombus? a propensity score matching study. Oncotarget. 2018;9((36)):24537–47. doi: 10.18632/oncotarget.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang M, Fang Z, Yan Z, Luo J, Liu L, Zhang W, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seedstrand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: a two-arm, randomised clinical trial. J Cancer Res Clin Oncol. 2014;140((2)):211–9. doi: 10.1007/s00432-013-1568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li WW, Dai ZY, Wan HG, Yao LZ, Zhu J, Li CL, et al. Endovascular implantation of iodine-125 seeds strand and portal vein stenting followed by transcatheter arterial chemoembolization combined therapy with sorafenib for hepatocellular carcinoma with main portal vein tumor thrombus. Zhonghua Yi Xue Za Zhi. 2016;96((23)):1838–42. doi: 10.3760/cma.j.issn.0376-2491.2016.23.011. [DOI] [PubMed] [Google Scholar]
- 68.Lau WY, Sangro B, Chen PJ, Cheng SQ, Chow P, Lee RC, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013;84((5)):311–8. doi: 10.1159/000348325. [DOI] [PubMed] [Google Scholar]
- 69.Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18((12)):1624–36. doi: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 70.Yin J, Li N, Han Y, Xue J, Deng Y, Shi J, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31((29)):3647–55. doi: 10.1200/JCO.2012.48.5896. [DOI] [PubMed] [Google Scholar]
- 71.Tsuda Y, Kobayashi S, Tomimaru Y, Akita H, Hama N, Wada H, et al. Long-term survival of a patient with hepatocellular carcinoma with portal vein tumor thrombus treated with interferon-α and 5-fluorouracil combination therapy. Gan To Kagaku Ryoho. 2013;40((12)):1804–6. [PubMed] [Google Scholar]
- 72.Huang G, Lai EC, Lau WY, Zhou WP, Shen F, Pan ZY, et al. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257((3)):490–505. doi: 10.1097/SLA.0b013e318262b218. [DOI] [PubMed] [Google Scholar]
- 73.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57((4)):821–9. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 75.Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II–III trial. J Clin Oncol. 2021;39((27)):3002–11. doi: 10.1200/JCO.21.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16((13)):1344–54. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 77.Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib a retrospective controlled study. Radiology. 2014;272((1)):284–93. doi: 10.1148/radiol.14131946. [DOI] [PubMed] [Google Scholar]
- 78.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64((5)):1090–8. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 79.Fan W, Yuan G, Fan H, Li F, Wu Y, Zhao Y, et al. Apatinib combined with transarterial chemoembolization in patients with hepatocellular carcinoma and portal vein tumor thrombus: a multicenter retrospective study. Clin Ther. 2019;41((8)):1463–76. doi: 10.1016/j.clinthera.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 80.Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127((20)):3782–93. doi: 10.1002/cncr.33677. [DOI] [PubMed] [Google Scholar]
- 81.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389((10064)):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 82.Qin S, Li Q, Gu S, Chen X, Lin L, Wang Z, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. 2021;6((7)):559–68. doi: 10.1016/S2468-1253(21)00109-6. [DOI] [PubMed] [Google Scholar]
- 83.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379((1)):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20((2)):282–96. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 85.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W, Bi X, Sun Y, Yu Y, Zhou A. Preliminary results of sintilimab plus different dose of IBI305 (anti-VEGF monoclonal antibody) in patients with advanced hepatocellular carcinoma: a phase Ib study. J Clin Oncol. 2020;38((Suppl 15)):3079. [Google Scholar]
- 87.Ren ZG, Xu JM, Bai YX, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22((7)):977–90. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 88.Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21((4)):571–80. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 89.Ducreux M, Abou-Alfa G, Ren Z, Edeline J, Cheng A. Results from a global phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with previously treated advanced hepatocellular carcinoma. 2021 ESMO WCGIC Oral-1. [Google Scholar]
- 90.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19((7)):940–52. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 91.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 92.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6((11)):e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma(RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27((4)):1003–11. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 94.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38((26)):2960–70. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelley RK, Sangro B, Harris WP, Ikeda M, K Abou-Alfa G. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC) J Clin Oncol. 2020;38((15 Suppl l)):4508. [Google Scholar]
- 96.Galle PR, Kim RD, Sung MW, Harris WP, El-Khoueiry AB. Updated results of a phase 1b study of regorafenib plus pembrolizumab for first-line treatment of advanced hepatocellular carcinoma [R/QL] ESMO. 2020. E-Poster 990P.
- 97.Kudo M, Ikeda M, Motomura K, Okusaka T, Kobayashi M. A phase 1b study of lenvatinib (LEN) plus nivolumab (NIV) in patients with unresectable hepatocellular carcinoma (uHCC): study 117. J Clin Oncol. 2020;38((Suppl 4)):513. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiao S, Bai L, Dong J, Shen L, Qin Q, Bai Y, et al. Clinical activity and safety of penpulimab (Anti-PD-1) with anlotinib as first-line therapy for advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2020;38((Suppl 15)):4952. doi: 10.3389/fonc.2021.684867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. 2020;18((3)):230–41. doi: 10.6004/jnccn.2020.0012. [DOI] [PubMed] [Google Scholar]
- 100.Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31((28)):3501–8. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 101.Goyal L, Zheng H, Abrams TA, Miksad R, Bullock AJ, Allen JN, et al. A phase II and biomarker study of sorafenib combined with FOLFOX in patients with advanced hepatocellular carcinoma (HCC) Clin Cancer Res. 2019;25((1)):80–9. doi: 10.1158/1078-0432.CCR-18-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng JS, Long J, Sun B, Lu NN, Fang D, Zhao LY, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation can improve survival ofpatients with hepatocellular carcinoma with portal vein tumour thrombosis: extending the indication forablation? Clin Radiol. 2014;69((6)):e253–263. doi: 10.1016/j.crad.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 103.Lu ZH, Shen F, Yan ZL, Li J, Yang JH, Zong M, et al. Treatment of portal vein tumor thrombus of hepatocellular carcinoma with percutaneous laser ablation. J Cancer Res Clin Oncol. 2009;135((6)):783–9. doi: 10.1007/s00432-008-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, Li Y, Gao F, Zhang Q, Yang X, Zhu B, et al. Comparison of transcatheter arterial chemoembolization-radiofrequency ablation and transcatheter arterial chemoembolization alone for advanced hepatocellular carcinoma with macrovascular invasion using propensity score analysis: a retrospective cohort study. J Oncol. 2020;2020:1341863. doi: 10.1155/2020/1341863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ding X, Sun W, Chen J, Li W, Shen Y, Guo X, et al. Percutaneous radiofrequency ablation combined with transarterial chemoembolization plus sorafenib for large hepatocellular carcinoma invading the portal venous system: a prospective randomized study. Front Oncol. 2020;10:578633. doi: 10.3389/fonc.2020.578633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao WP, Li H, Guo J, Cai L, Duan Y, Hou X, et al. Hepatocellular carcinoma with type II–III portal vein tumour thrombosis: treatment using transarterial chemoembolisation and microwave ablation. Br J Radiol. 2021;94((1117)):20200415. doi: 10.1259/bjr.20200415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vibert E, Azoulay D, Cunha AS, Adam R, Samuel D, Castaing D, et al. Portal stenting for hepatocellular carcinoma extending into the portal vein in cirrhotic patients. J Surg Oncol. 2013;107((7)):696–701. doi: 10.1002/jso.23306. [DOI] [PubMed] [Google Scholar]
- 108.Lu J, Guo JH, Zhu HD, Zhu GY, Chen L, Teng GJ. Safety and efficacy of irradiation stent placement for malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: a single-center experience. J Vasc Interv Radiol. 2017;28((6)):786–e3. doi: 10.1016/j.jvir.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 109.de Franchis R, Baveno VI Faculty Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63((3)):743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 110.Chen Q, Shu C, Laurence AD, Chen Y, Peng BG, Zhen ZJ, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomized clinical trial. Gut. 2018;67((11)):2006–16. doi: 10.1136/gutjnl-2018-315983. [DOI] [PubMed] [Google Scholar]
- 111.Li N, Zheng D, Xue J, Guo W, Shi J, Sun J, et al. Cidan inhibits liver cancer cell growth by reducing COX-2 and VEGF expression and cell cycle arrest. Exp Ther Med. 2015;9((5)):1709–18. doi: 10.3892/etm.2015.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serenari M, Cappelli A, Cucchetti A, Mosconi C, Strigari L, Monari F, et al. Deceased donor liver transplantation after radioembolization for hepatocellular carcinoma and portal vein tumoral thrombosis: a pilot study. Liver Transpl. 2021 Dec;27((12)):1758–66. doi: 10.1002/lt.26257. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martelletti C, Ricotti A, Gesualdo M, Carucci P, Gaia S, Rolle E, et al. Radioembolization vs sorafenib in locally advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score and Bayesian analysis. J Dig Dis. 2021;22((8)):496–502. doi: 10.1111/1751-2980.13030. [DOI] [PubMed] [Google Scholar]
- 114.Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, et al. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg. 2017;44:223–8. doi: 10.1016/j.ijsu.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 115.Chong JU, Choi GH, Han DH, Kim KS, Seong J, Han KH, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2018;25((11)):3308–15. doi: 10.1245/s10434-018-6653-9. [DOI] [PubMed] [Google Scholar]
- 116.Huang C, Zhu XD, Shen YH, Wu D, Ji Y, Ge NL, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res. 2021;9((1)):19. doi: 10.1186/s40364-021-00274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsai HM, Han MZ, Lin YJ, Chang TT, Chen CY, Cheng PN, et al. Real-world outcome of immune checkpoint inhibitors for advanced hepatocellular carcinoma with macrovascular tumor thrombosis. Cancer Immunol Immunother. 2021;70((7)):1929–37. doi: 10.1007/s00262-020-02845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5((7)):953–60. doi: 10.1001/jamaoncol.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lau WY, Wang K, Zhang XP, Li LQ, Wen TF, Chen MS, et al. A new staging system for hepatocellular carcinoma associated with portal vein tumor thrombus. Hepatobiliary Surg Nutr. 2021;10((6)):782–95. doi: 10.21037/hbsn-19-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.