Abstract
Introduction
Lenvatinib is the first-line treatment for advanced hepatocellular carcinoma (HCC). We aimed to compare the clinical outcomes of lenvatinib plus drug-eluting beads transarterial chemoembolization (DEB-TACE) versus lenvatinib alone in real-world practice.
Methods
This retrospective analysis included 142 consecutive patients who received lenvatinib plus DEB-TACE and 69 patients who received lenvatinib alone as first-line treatment from 15 Chinese academic centers from November 2018 to November 2019. Overall survival (OS), progression-free survival (PFS), objective response rate (ORR) were evaluated by modified Response Evaluation Criteria in Solid Tumors criteria, and safety profiles were compared between the two groups.
Results
The median OS and PFS were significantly longer in the combined therapy group than in the monotherapy group in whole cohort (median OS, 15.9 vs. 8.6 months, p = 0.0022; median PFS, 8.6 vs. 4.4 months, p < 0.001) and after propensity score matching analysis (median OS, 13.8 vs. 7.8 months, p = 0.03; median PFS, 7.8 vs. 4.5 months, p = 0.009). Moreover, the treatment option was an independent prognostic factor for OS and PFS with adjustment based upon baseline characteristics (adjusted hazard ratio [HR]: 0.53, 95% confidence interval [CI]: 0.36–0.78, p = 0.001, and adjusted HR: 0.42, 95% CI: 0.30–0.60, p < 0.001, respectively) and propensity score (adjusted HR: 0.52, 95% CI: 0.36–0.76, p = 0.001, and adjusted HR: 0.46, 95% CI: 0.33–0.64, p < 0.001, respectively). Moreover, a greater ORR was observed in the combined group (ORR: 46.48% vs. 13.05%, p < 0.001). Furthermore, the most common adverse events (AEs) were elevated aspartate aminotransferase (54.9%) and fatigue (46.4%) in the lenvatinib plus DEB-TACE group and lenvatinib group, respectively. Most AEs were mild-to-moderate and manageable.
Conclusions
With well-tolerated safety, lenvatinib plus DEB-TACE was more effective than lenvatinib monotherapy in improving OS, PFS, and ORR. Thus, it may be a promising treatment for advanced HCC. Future prospective studies confirming these findings are warranted.
Keywords: Lenvatinib, Drug-eluting beads transarterial chemoembolization, Hepatocellular carcinoma, Efficacy, Safety
Introduction
Hepatocellular carcinoma (HCC) remains a global health challenge, and its incidence is growing worldwide [1, 2]. The prognosis of HCC, especially for advanced cases, remains poor, due to underlying chronic liver disease, late diagnosis at advanced stages of disease, and frequent recurrence/progression after treatment [2]. Sorafenib is the first systemic therapy proven to prolong overall survival (OS) and has been recommended as first-line treatment, showing a median improvement in OS of approximately 3 months compared to placebo [3, 4, 5, 6]. Recently, based on the result of the practice-changing “REFLECT” trial in advanced HCC, lenvatinib was established as an alternative to sorafenib as first-line treatment, with a comparable median OS (13.6 vs. 12.3 months) [2, 6, 7]. Nevertheless, the efficacy of these drugs still remains suboptimal, and alternative strategies that can improve outcomes are still urgently needed.
In the past decade, we have seen the evolution of trials in which transarterial chemoembolization (TACE) combined with sorafenib [8, 9, 10, 11, 12], brivanib [13], and orantinib [14] have been pursued to improve outcomes of intermediate-to-advanced HCC. The hypothesis that antiangiogenics (including tyrosine kinase inhibitors [TKIs] or anti-vascular endothelial growth factor) might delay tumor revascularization and recurrence following TACE has been addressed in several randomized controlled trials [2]. However, all these trials have shown that adding a TKI to TACE is feasible and safe but not efficacious [15].
Compared with sorafenib, lenvatinib is a small molecular type V TKI, with more potent activity against vascular endothelial growth factor receptors and the fibroblast growth factor receptor family [16]. In the REFLECT trial, it was shown to significantly improve progression-free survival (PFS, 7.4 vs. 3.7 months; hazard ratio [HR]: 0.66, 95% confidence interval [CI]: 0.57–0.77; p < 0.001) and objective response rate (ORR) as assessed by the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria (24.1% vs. 9.2%; odds ratio: 3.13, 95% CI: 2.15–4.56; p < 0.0001) over sorafenib [7]. Notably, the study suggested lenvatinib may be more effective for patients infected with hepatic B virus (HBV) [7]. Given these possibilities, lenvatinib plus TACE may represent a promising combination therapy [17, 18, 19, 20]. In addition, the patients enrolled in the REFLECT trial were highly selected, as patients with a huge tumor burden, main portal vein invasion, or poor liver function were excluded [7]. However, many patients with these characteristics require systematic therapy and exhibit a large unmet need in daily clinical practice. Therefore, we conducted a real-world, multicenter study to compare the efficacy and safety of lenvatinib plus drug-eluting beads TACE (DEB-TACE) with those of lenvatinib alone as first-line treatment in Chinese patients with advanced HCC.
Materials and Methods
Study Design
A total of 383 consecutive patients with unresectable liver cancer who received lenvatinib plus DEB-TACE or lenvatinib alone between November 2018 and November 2019 at 15 academic Chinese centers were retrospectively screened. The inclusion criteria for the present study included: (1) diagnosis of HCC based on the European Association for the Study of the Liver or the American Association for the Study of Liver Diseases guidelines [5, 21], (2) treated with lenvatinib, (3) at least one measurable lesion ≥1 cm, (4) Child-Pugh grade A and B, and (5) Eastern Cooperative Oncology Group performance status score 0–1. The following exclusion criteria were applied: (1) metastatic malignancy from other organ, (2) unmeasurable lesions, (3) previously received any systemic therapy, (4) combined with other therapies, including radiofrequency ablation and immune checkpoint inhibitor, (5) absence of baseline radiological imaging, (6) concomitant with other malignancies, and (7) lenvatinib as second-line or third-line therapy. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institute's committee on human research of participating centers. Permission to use the corresponding data was obtained from all patients by written consent.
Medical Care
The generalized treatment protocol had been formulated by multidisciplinary teams. The patients who were concomitantly treated with DEB-TACE within 30 days before or after administration of lenvatinib were considered to have received combined therapy; otherwise, lenvatinib alone. All patients received lenvatinib, based on the hypothesis that TACE, inducing the upregulation of angiogenic factors by ischemic liver injury, plus lenvatinib may complementarily inhibit angiogenic factors and tumor growth, and an earlier disease control produced by TACE with reduction of variable tumor for those advanced patients with large tumor burden, TACE was conducted to control targeted liver nodule and regarded as the concomitant therapy recommended for lenvatinib, according to the condition of the tumor (especially for patients with large, hypervascularity tumor), liver function and performance status (physicians' judgment). The final treatment decision was consented by individual patients. For the patients with Child-Pugh class A, lenvatinib was initiated routinely at a dose of 8 mg/day for those with the weight of ≤60 kg, or 12 mg/day for the ones with >60 kg. While for limited Child-Pugh class B patients, the recommended 8 mg was given orally once per day based on the previous early-phase clinical trial [22, 23]. Since DEB-TACE might induce liver injury, we recommended lenvatinib was discontinued for 1–3 days before each TACE, and restored after TACE, if patients recovered from adverse events (AEs) caused by DEB-TACE (such as fever, vomit, nausea, etc.), and liver function/performance status permitted (median time: 8 days, range: 1–28 days). At the time of restarting lenvatinib, the dose was the same as that used before DEB-TACE. The dose of agents was to be reduced upon the development of a grade 3 or 4 severe AE, or the agent was discontinued when any unacceptable or serious AE occurred. AEs were graded using the Common Terminology Criteria for Adverse Events version 4.0. Typically, when clinical tumor progression was observed, the decision to continue or discontinue lenvatinib treatment was made at the discretion of the attending physicians, and due to lack of second-line treatment after lenvatinib, patients were encouraged to continue lenvatinib therapy unless an unmanageable or a life-threatening AE occurred.
For patients who received combined therapy, since without significant difference in prolonging OS and relatively better safety, compared to conventional TACE [24, 25, 26], DEB-TACE was performed by experienced investigators at each institution. Patients were treated either selectively or super-selectively with 100–300, 300–500 μm LC Beads (BTG, London, UK) loading epirubicin, doxorubicin, pirarubicin, with 15–37.5 mg/mL of beads. Up to 4 mL of DEBs with a total maximum dose of up to 120 mg agents were delivered to the targeted tissue to induce substantial arterial flow reduction. The diameter of DEBs, the type and the dose of chemo agent were selected according to the institutional practice, based on tumor burden, tumor vascularity, portal venous patency, liver function, etc. Indeed, there are no definitive re-TACE criteria in advanced HCC, subsequent DEB-TACE was usually performed if there was variable tumor (like new intrahepatic lesion [NIH], treated intrahepatic lesion) and liver function and performance status permitted. The retreat strategy was made by multidisciplinary teams, with the consent given by individuals.
Follow-Up and Outcome Assessments
OS was defined as the time from the date of first lenvatinib administration to the date of death from any cause or the date of last follow-up (December 1, 2020). PFS was defined as the time from the date of first lenvatinib administration to the date of confirming radiological progression according to the mRECIST criteria [27, 28] or the date of death. Specially, unlike the definition of progressive disease (PD) in the TACTICS trial [10], an NIH and 20% regrowth of treated tumor were regarded as PD in both groups in order to ensure comparability. Objective response assessment using multiphasic contrast-enhanced computed tomography or dynamic contrast-enhanced magnetic resonance imaging was recommended 4–8 weeks after treatment and every 2–3 months thereafter. We performed response assessment in the best response during the follow-up, complete response (CR), partial response (PR), stable disease (SD), and PD were defined by the mRECIST criteria and reviewed by two independent investigators (Bai W. and Wang E.). For analysis, the overall response, combined responses of target (a maximum of 2 target lesions per organ and 5 target lesions in total), and nontarget lesions was adopted in our study [27, 28]. The patterns of progression were defined as follows: ≥20% increase in tumor size against a known baseline lesion (intrahepatic growth or extrahepatic growth), NIH, or new extrahepatic lesion and/or vascular invasion [29]. Additionally, liver cirrhosis was defined by the previous liver biology after liver resection, clinical signs, laboratory, hemodynamic, and/or imaging tests. Child-Pugh classification [30] and albumin-bilirubin (ALBI) grade were used for the assessment of hepatic reserve function. All patients with ascites were judged from radiological imaging, with a small amount and no clinical signs existing. The ALBI score was calculated with serum albumin and total-bilirubin values using the following formula: ALBI score = log10 bilirubin (μmol/L) × 0.66 + albumin (g/L) × −0.085 (≤−2.60, ALBI grade 1; >−2.60 to ≤−1.39, grade 2; and >−1.39, grade 3) [31]. Since the upper limit values of alpha-fetoprotein (AFP) detection were different in participating centers, we regarded it as a categorical variable with cutoffs of 200 ng/mL [7, 32].
Statistical Analysis
Quantitative variables are presented as median with interquartile range and were compared by Student's t test or nonparametric Mann-Whitney U test, whereas categorical variables are presented as counts with percentages and were compared by χ2 test or Fisher's exact test. Survival curves were estimated using the Kaplan-Meier method and were compared by log-rank test. To identify prognostic factors for OS and PFS, we conducted two models with multivariate Cox regression. The initial multivariate models included the treatment group, the covariates deemed likely to influence the original treatment assignments (including tumor size [defined as the maximum diameter of the largest tumor], tumor number, liver function, and performance status), and other variables significantly associated with treatment outcomes according to univariate analyses at a significance level of 10%. The second multivariate model included the treatment group and a propensity score calculated from logistic regression using a set of covariates deemed likely to have affected the treatment decisions and all those significant prognostic factors at univariate analyses. Propensity score matching (PSM) was estimated using a logistic regression model fit with the following variables: gender, age, etiology, Eastern Cooperative Oncology Group score, tumor number and size, macrovascular invasion (MVI), extrahepatic spread, Child-Pugh class, blood urea nitrogen level, creatinine level, platelet count, international normalized ratio, and AFP level (≤200/>200 ng/mL). To create a propensity-matched cohort of patients treated with lenvatinib alone or combined with DEB-TACE (1:1 match), a nearest neighbor-matching algorithm with a greedy heuristic was used. Additionally, we tested the interaction between treatment and risk categories on multiplicative (HR) scales [33]. We imputed missing data for the covariates by using multiple imputations. All statistical analyses were performed using SPSS, version 23.0 software, and R v.4.0.5 with p < 0.05 defined as statistical significance.
Results
Patient Characteristics
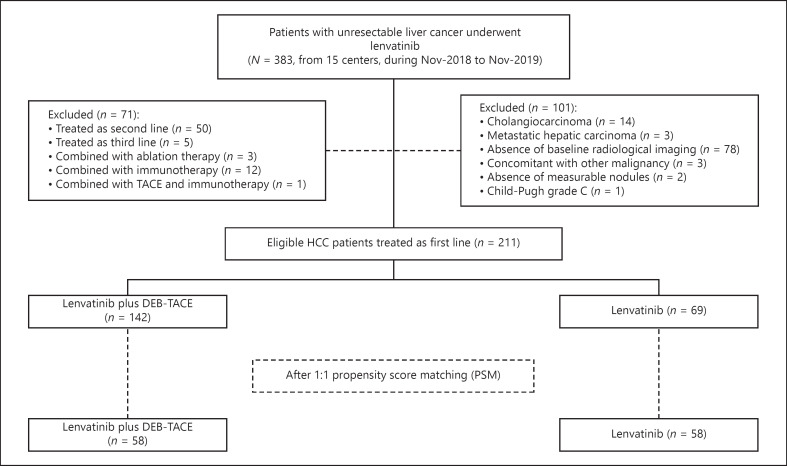
A total of 211 patients were finally included in this study, consisting of 142 patients who received lenvatinib plus DEB-TACE (LEN + DEB-TACE group) and 69 patients who received lenvatinib alone (LEN group) (Fig. 1). The baseline characteristics were well-balanced between the two groups, with the exceptions of blood urea nitrogen level and white blood cell count (Table 1). Additionally, the duration of lenvatinib administration was significantly longer in the LEN + DEB-TACE group than in the LEN group (mean 7.6 months vs. mean 5.8 months, p = 0.047). Patterns of missing values were shown in online supplementary Figure 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000523849). There were 13 patients and 26 patients of Barcelona Clinic Liver Cancer (BCLC) stages A and B, respectively, enrolled for the reasons summarized in online supplementary Table 1. Notably, there were 17 patients who previously received TACE in the LEN group, and among them, 15 patients received TACE as initial treatment at an early-to-intermediate stage and then progressed to the advanced stage. Although 2 patients received lenvatinib alone at BCLC stage B, they had previously undergone multiple sessions of TACE and were regarded as likely to have limited benefit from further TACE. As shown in online supplementary Table 2, there were 49 (34.5%) patients and 18 (26%) patients who received subsequent treatment in the LEN and DEB-TACE groups. In LEN + DEB-TACE group, DEB-TACE was conducted before the date of lenvatinib initiation (103, 72.5%), and after the date of lenvatinib initiation (39, 27.5%). In total, 133 (93.7%) patients received super-selective procedures, and 24 patients received subsequent DEB-TACE (19 for one session, and 5 for two sessions until the date of the last follow-up, online suppl. Table 2). The median (range) relative dose intensity of lenvatinib was 95.5% (80%–150%) at 4 weeks, 85.5% (50%–150%) at 8 weeks, and 76.6% (30%–150%) at 12 weeks in DEB-TACE-LEN group, and 92% (73%–150%) at 4 weeks, 83.3% (53.3%–150%) at 8 weeks, and 73.3% (33.3%–150%) at 12 weeks in LEN group.
Fig. 1.
Flow chart of the current study.
Table 1.
Baseline demographics and clinical characteristics in 211 patients
| Characteristics | Total (N = 211) | LEN + TACE (N1 = 142) | LEN (N2 = 69) | p value |
|---|---|---|---|---|
| Age, years, median (IQR) | 53 (45–61) | 52.5 (43–61.3) | 53 (46–62) | 0.734 |
| Gender, n (%) | ||||
| Male | 184 (87.2) | 127 (89.4) | 57 (82.6) | 0.164 |
| Female | 27 (12.8) | 15 (10.6) | 12 (17.4) | |
| Etiology, n (%) | ||||
| HBV | 195 (92.4) | 131 (92.3) | 64 (92.8) | 0.898 |
| Others | 16 (7.6) | 11 (7.7) | 5 (7.8) | |
| BCLC stage, n (%) | ||||
| A | 13 (6.2) | 11 (7.7) | 2 (2.9) | 0.281 |
| B | 26 (12.3) | 19 (13.4) | 7 (10.1) | |
| C | 172 (81.5) | 112 (78.9) | 60 (87) | |
| ECOG PS score, n (%) | ||||
| 0 | 116 (55) | 80 (56.3) | 36 (52.8) | 0.568 |
| 1 | 95 (45) | 62 (43.7) | 33 (47.2) | |
| Tumor number, n (%) | ||||
| 1 | 77 (36.5) | 53 (37.3) | 24 (34.8) | 0.932 |
| 2 | 35 (16.6) | 23 (16.2) | 12 (17.4) | |
| ≥3 | 99 (46.9) | 66 (46.5) | 33 (47.8) | |
| Tumor size, cm, median (IQR) | 8.4 (4.7–12.3) | 9 (5.1–12.4) | 7.3 (3.6–11.9) | 0.211 |
| MVI, n (%) | ||||
| Absence | 96 (45.5) | 62 (43.7) | 34 (49.7) | 0.442 |
| Presence | 115 (55.5) | 80 (56.3) | 35 (50.3) | |
| Vp2 | 49 (42.6) | 37 (46.3) | 12 (34.3) | 0.486 |
| Vp3 | 54 (46.9) | 35 (47.7) | 19 (54.3) | |
| Vp4 | 12 (10.5) | 8 (10) | 4 (11.4) | |
| EHS, n (%) | ||||
| Absence | 128 (60.7) | 92 (64.8) | 36 (52.2) | 0.078 |
| Presence | 83 (39.3) | 50 (35.2) | 33 (47.8) | |
| Cirrhosis, n (%) | ||||
| Absence | 39 (18.5) | 28 (19.7) | 11 (15.9) | 0.507 |
| Presence | 172 (81.5) | 114 (80.3) | 58 (84.1) | |
| Ascites, n (%) | ||||
| Absence | 159 (75.4) | 111 (78.2) | 48 (69.6) | 0.174 |
| Presence | 52 (24.6) | 31 (21.8) | 21 (30.4) | |
| Child-Pugh score, n (%) | ||||
| 5 | 112 (53.1) | 79 (55.6) | 33 (47.8) | 0.385 |
| 6 | 54 (25.6) | 36 (25.4) | 18 (26.1) | |
| 7 | 29 (13.7) | 18 (12.7) | 11 (15.9) | |
| 8 | 12 (5.7) | 8 (5.6) | 4 (5.8) | |
| 9 | 4 (1.9) | 1 (0.7) | 3 (4.3) | |
| AFP, n (%) | ||||
| ≤200 ng/mL | 94 (45.5) | 67 (47.2) | 27 39.1) | 0.27 |
| >200 ng/mL | 117 (55.5) | 75 (52.8) | 42 (60.1) | |
| ALB, g/L, median (IQR) | 38.2 (34.2–42.4) | 38.4 (33.9–42.1) | 37.5 (34.3–43.7) | 0.418 |
| TBIL, µmol/mL, median (IQR) | 19.0 (13.4–27) | 19.1 (13.5–26.1) | 17.9 (13.1–28.5) | 0.394 |
| ALBI grade, n (%) | ||||
| 1 | 79 (39.4) | 52 (36.6) | 27 (39.1) | 0.797 |
| 2 | 127 (60.2) | 86 (60.6) | 47 (59.4) | |
| 3 | 5 (2.4) | 4 (2.8) | 1 (1.4) | |
| AST, U/L, median (IQR) | 52 (34–80) | 50.5 (33.8–80.3) | 58 (34–87) | 0.61 |
| ALT, U/L, median (IQR) | 38.1 (23–61) | 38 (24–60.3) | 40 (19–68.5) | 0.719 |
| BUN, mmol/L, median (IQR) | 4.53 (3.6–5.6) | 4.4 (3.6–5.2) | 5.1 (3.75–5.95) | 0.006 |
| Cr, µmol/L, median (IQR) | 66 (56–76) | 67 (57–76.3) | 62 (53–76.5) | 0.777 |
| WBC, ×109/L, median (IQR) | 5.7 (4.16–7.1) | 6.0 (4.2–7.8) | 5.1 (3.8–6.4) | 0.01 |
| PLT, ×109/L, median (IQR) | 145 (95–222) | 156.5 (96.8–225) | 129 (90–220) | 0.551 |
| INR, median (IQR) | 1.07 (1.00–1.13) | 1.06 (1.00–1.12) | 1.08 (1.02–1.15) | 0.084 |
| Previous treatment, n (%) | ||||
| No | 106 (50.2) | 77 (54.2) | 29 (42) | 0.096 |
| Yes | ||||
| Liver resection | 40 (19) | 24 (16.9) | 16 (23.2) | |
| TACE | 51 (24.2) | 34 (23.9) | 17 (24.6) | |
| LT | 5 (2.4) | 0 | 5 (7.2) | |
| Ablation | 8 (3.8) | 6 (4.2) | 2 (2.9) | |
| Ablation + TACE | 1 (0.5) | 1 (0.7) | 0 | |
| Duration of lenvatinib, months, mean ± SE | 7±5.1 | 7.6±5.4 | 5.8±4.3 | 0.047 |
AFP, alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; BUN, blood urea nitrogen; Cr, creatinine; ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; HBV, hepatic B virus; INR, international normalized ratio; IQR, interquartile range; LEN, lenvatinib; LT, liver transplantation; MVI, macrovascular invasion; PLT, platelet; PS, performance status; SE, standard error; TACE, transarterial chemoembolization; TBIL, total bilirubin; WBC, white blood cell.
Efficacy
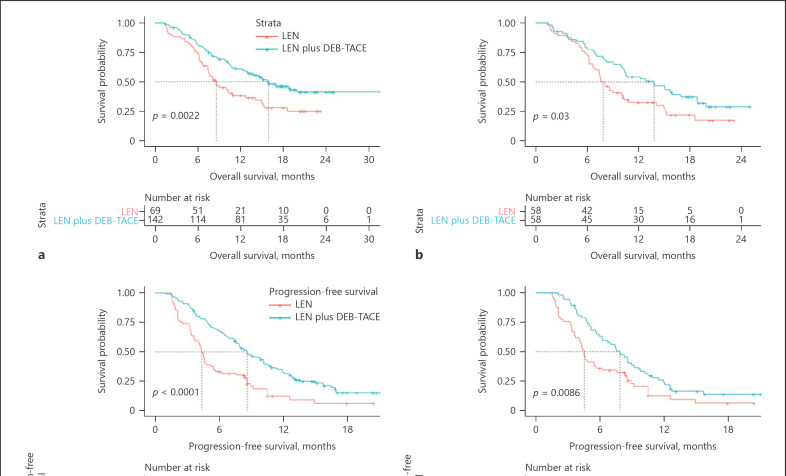
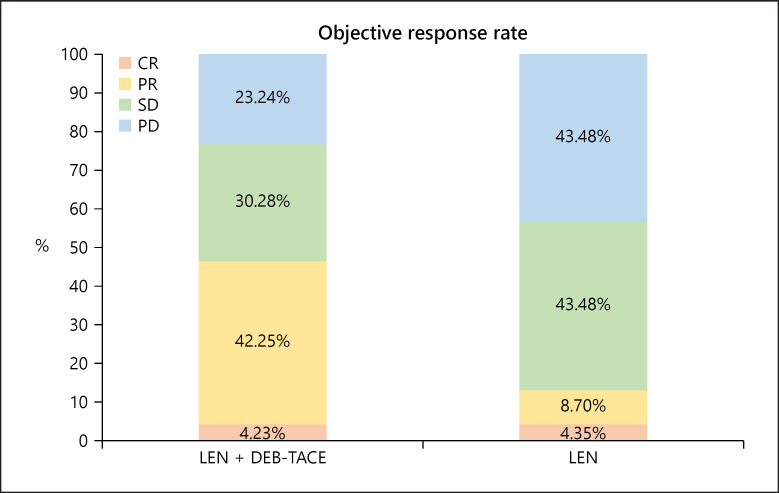
Median follow-up time was 13.5 (7.4–18) months for the LEN + DEB-TACE group and 8.2 (5.8–13.9) months for the LEN group. 127 patients presented radiological progression (81 for LEN + DEB-TACE group, 46 for LEN group, online suppl. Fig. 2). The main pattern of radiological progression between the combined and monotherapy groups was intrahepatic growth (29.6% and 19.6%, respectively, online suppl. Table 3). Median OS was 15.9 (95% CI, 12.3–19.5) months in the LEN + DEB-TACE group, which was significantly longer than that in the LEN group (8.6 [95% CI, 6.3–10.9] months, p = 0.002; Fig 2a). A significantly prolonged median PFS was also observed in patients treated with combined therapy compared with monotherapy (8.6 [95% CI, 7.2–10.0] vs. 4.4 [4.0–4.7] months, p < 0.001; Fig 2c). After PSM, a total of 116 patients (58 patients in each group) were analyzed (Fig. 1), and the features were well-balanced between the two groups (online suppl. Table 4). Median OS in the combination therapy group was still greater than that in the monotherapy group (13.8 [95% CI: 9.2–18.4] vs. 7.8 [95% CI: 6.5–8.8] months, p = 0.03; Fig 2b), and a similar result was revealed in PFS analysis (7.8 [95% CI: 6.2–9.52] months vs. 4.5 [95% CI: 4.1–4.9] months, p = 0.009; Fig 2d). Additionally, based on the mRECIST criteria, CR, PR, SD, and PD were observed in 4.23%, 42.25%, 30.28%, and 23.24% of cases in the LEN + DEB-TACE group, respectively, whereas these percentages were 4.35%, 8.7%, 43.48%, and 43.48% in the LEN group, respectively. Moreover, the ORR and disease control rates in the LEN + TACE group were 46.48% and 76.76%, respectively, and these rates were significantly higher than those of 13.05% and 56.52% in the monotherapy group (both p < 0.001; Fig. 3).
Fig. 2.
Comparison of OS and PFS between LEN plus DEB-TACE and LEN by Kaplan-Meier method in patients before and after PSM (a, c refer to OS and PFS analysis before PSM, respectively; b, d refer to OS and PFS analysis after PSM, respectively). DEB-TACE, drug-eluting bead transarterial chemoembolization; LEN, lenvatinib; PSM, propensity score matching; OS, overall survival; PFS, progression-free survival.
Fig. 3.
ORR according to modified response evaluation criteria in solid tumors criteria between combined therapy and monotherapy.
Prognostic Factors for OS and PFS
From the univariate and multivariate Cox analyses for OS, significant predictors among the included patients included etiology (HR = 0.26 [95% CI, 0.10–0.71], p = 0.009), number of tumors (HR = 1.30 [95% CI, 1.06–1.59], p = 0.012), ascites (HR = 1.70 [95% CI, 1.14–2.55], p = 0.01), AFP level (HR = 1.93 [95% CI, 1.31–2.85], p = 0.001), total-bilirubin level (HR = 1.014 [95% CI, 1.001–1.03], p = 0.032), and treatment modality (HR = 0.53 [95% CI, 0.36–0.78], p = 0.001; Table 2, model 1). From the second model, treatment modality was still a significant predictor of OS (HR = 0.52 [95% CI, 0.36–0.76], p = 0.001; Table 2, model 2). In addition, number of tumors (HR = 1.23 [95% CI, 1.03–1.46], p = 0.024), MVI (HR, 1.56 [95% CI, 1.10–2.21], p = 0.012, AFP level (HR = 1.56 [95% CI, 1.13–2.15], p = 0.007), and treatment modality (HR = 0.42 [95% CI, 0.30–0.60], p < 0.001) were independent predictors of PFS by multivariable Cox regression analysis (online suppl. Table 5, model 1). With adjustment for propensity score, treatment option (HR = 0.46 [95% CI, 0.33–0.64], p < 0.001) was a significant predictor of PFS (online suppl. Table 5, model 2). Moreover, as expected, the multivariate analysis identified treatment modality as a robust predictor of OS (HR = 0.47 [95% CI, 0.30–0.76], p = 0.002; online suppl. Table 6) and PFS (HR = 0.48 [95% CI, 0.31–0.75], p = 0.001; online suppl. Table 7) after PSM. These findings were confirmed by the analyses of the treatment effect in most subgroups of patients according to the risk category of different clinical settings (online suppl. Tables 8, 9).
Table 2.
Univariate and multivariate Cox regression analyses for OS in the whole cohort
| Variables | Univariate analyses |
Multivariate analyses (model 1) |
Multivariate analyses (model 2) |
|||
|---|---|---|---|---|---|---|
| HR (95%CI) | p value | HR (95% CI) | p value | HR (95% CI) | adjusted p value | |
| Age, per 1 year increase | 0.99 (0.98–1.01) | 0.196 | ||||
| Gender (refer to male) | 0.71 (0.40–1.26) | 0.241 | ||||
| Etiology (refer to HBV) | 0.32 (0.12–0.87) | 0.026 | 0.26 (0.10–0.71) | 0.009 | ||
| ECOG (refer to score of 0) | 1.20 (0.84–1.72) | 0.312 | ||||
| Tumor number (refer to single) | 1.25 (1.02–1.52) | 0.033 | 1.30 (1.06–1.59) | 0.012 | ||
| The largest tumor diameter, per 1 cm increase | 1.03 (0.99–1.07) | 0.111 | ||||
| MVI (refer to absent) | 1.43 (0.99–2.05) | 0.054 | 1.42 (0.97–2.10) | 0.075 | ||
| EHS (refer to absent) | 1.38 (0.97–1.99) | 0.078 | 1.41 (0.97–2.05) | 0.071 | ||
| Ascites (refer to absent) | 1.88 (1.27–2.77) | 0.002 | 1.70 (1.14–2.55) | 0.01 | ||
| AFP (refer to ≤200 ng/mL) | 2.04 (1.40–3.00) | <0.001 | 1.93 (1.31–2.85) | 0.001 | ||
| ALB, per 1 g/L increase | 0.98 (0.95–1.02) | 0.232 | ||||
| TBIL, per 1 µmol/L increase | 1.01 (1.00–1.02) | 0.029 | 1.014 (1.001–1.03) | 0.032 | ||
| AST, per 1 U/L increase | 1.00 (0.999–1.001) | 0.851 | ||||
| ALT, per 1 U/L increase | 1.00 (0.997–1.003) | 0.849 | ||||
| BUN, perl mmol/L increase | 1.00 (0.914–1.098) | 0.968 | ||||
| Cr, per 1 µmol/L increase | 1.00 (0.99–1.01) | 0.966 | ||||
| WBC, per 1.0 × 109/L increase | 0.98 (0.91–1.06) | 0.614 | ||||
| INR, per 1% increase | 2.09 (0.61–7.14) | 0.240 | ||||
| LEN + DEB-TACE (refer to LEN) | 0.57 (0.39–0.82) | 0.003 | 0.53 (0.36–0.78) | 0.001 | 0.52 (0.36–0.76) | 0.001 |
AFP.alpha-fetoprotein; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; BUN, blood urea nitrogen; Cr, creatinine; DEB-TACE, drug-eluting bead transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; HBV, hepatic B virus; HR, hazard ratio; INR, international normalized ratio; IQR, interquartile range; LEN, lenvatinib; LT, liver transplantation; MVI, macrovascular invasion; PLT, platelet; PS, performance status; TBIL, total bilirubin; WBC, white blood cell.
Factors Affecting Treatment Decisions for Improving OS
To further analyze covariates that may have affected the treatment decisions, we tested the interaction between treatment and risk categories. The results showed that MVI (interaction p = 0.019) and ascites (interaction p = 0.045) were associated with treatment decisions (online suppl. Tables 8). From the Kaplan-Meier survival analysis, median OS was not significantly different between the two treatment groups in patients absent MVI (16.9 months, 95% CI, 13.4–20.4 months for the combined therapy group and 15.1 months, 95% CI 10.2–20.0 months for the monotherapy group, p = 0.72) (online suppl. Fig. 3a). However, for the patients who presented with MVI, median OS was significantly better in the combination group (15 months, 95% CI, 9.8–20.3 months) than in the monotherapy group (7.4 months, 95% CI, 6.2–8.6 months, p < 0.001; online suppl. Fig. 3b). Similarly, in patients absent ascites, no significant difference in median OS was observed between groups (15.9 months, 95% CI, 12.1–19.7 months and 14.1 months, 95% CI, 8.4–19.7% months) for the combined therapy and monotherapy groups, respectively (p = 0.232; online suppl. Fig. 3c). However, patients who presented with ascites had a significantly prolonged OS after undertaking the combination treatment (10.5 months, 95% CI 5.5–15.5 months and 6.4 months, 95% CI 4.4–8.5 months) for combined therapy and monotherapy groups, respectively (p = 0.001; online suppl. Fig. 3d).
We also tested the outcomes in patients who met the enrollment criteria of the REFLECT trial or exceeded them [7]. In 69 patients within the REFLECT criteria, median OS in the combined group was not reached at the date of the last follow-up but was significantly longer than that in the monotherapy group (15.0 months, 95% CI 4.7–25.3 months, p = 0.05, online suppl. Fig. 4a). As shown in online supplementary Fig 4c, median PFS was significantly longer in the LEN + DEB-TACE group than in the LEN group (11.9 months, 95% CI 9.7–14.2 months vs. 7.6 months, 95% CI 1.2–14 months). For the 142 patients who did not meet the REFLECT criteria, consistent results were observed, with a median OS of 13.8 months (95% CI 8.6–19.1 months) and 8.0 months (95% CI 6.9–9.2 months, p = 0.034; online suppl. Fig. 4b) and median PFS of 7.5 months (95% CI 6.2–8.7 months) and 4.3 months (95% CI, 3.4–5.2 months) for patients in the combined therapy and monotherapy groups, respectively (p < 0.001; online suppl. Fig. 4d). To eliminate the bias produced by previous treatments, especially TACE, we analyzed the treatment-naïve patients. Online supplementary Tables 11 and 12 showed the baseline characteristic of these treatment-naïve patients before and after PSM. As shown in online supplementary Figure 5, compared to LEN group, better outcomes in LEN + DEB-TACE group were revealed consistently before and after PSM (29 matches reached). More importantly, treatment modality was significantly prognostic factor for OS and PFS in multivariable analyses before and after PSM (online suppl. Tables 13, 14).
In BCLC-B patients, no significant differences were observed regarding OS and PFS between LEN + DEB-TACE and LEN group (median OS: not reached vs. 15 months, p = 0.63; median PFS: 12.7 vs. 7.6 months, p = 0.096, respectively), since limited populations and inadequate follow-up time, we still found a trend of relative advantages of the combination in prolonging OS and PFS (online suppl. Fig. 6).
Safety Profiles
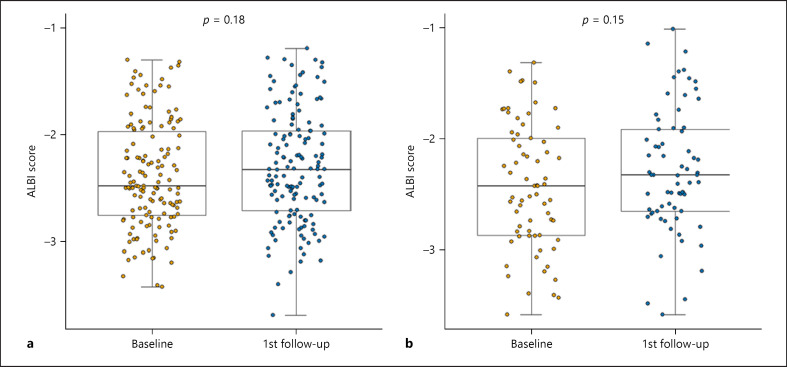
The common AEs that had been reported at the time of analysis in both groups are summarized in Table 3. The predominant AEs were aspartate aminotransferase elevation (54.9%) and fatigue (46.4%) in these two cohorts. The most common grade 3/4 AEs were proteinuria (10.6%) and hypertension (8.5%) in the LEN + DEB-TACE group. Comparably, proteinuria and hypertension occurred in 8.6% and 10.1% of patients in the lenvatinib monotherapy group. Moreover, drug interruption or dose reduction occurred in 35 patients (24.6%) in the LEN + TACE group due to an AE, which was comparable with the percentage of patients in the monotherapy group (23.1%). Additionally, no treatment-related deaths occurred in either group. The ALBI scores [31] prior to treatment and after the first follow-up period did not show a difference in either group (p = 0.18 and 0.15 for combined therapy and monotherapy, respectively; Fig. 4). Besides, we also documented the TACE-specific AEs (online suppl. Table 15).
Table 3.
Safety profiles and treatment discontinuations
| AE | Total (211) |
Child-Pugh grade A (166) |
Child-Pugh grade B (45) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LEN + DEB-TACE (142) |
LEN (69) |
LEN + DEB-TACE (115) |
LEN (51) |
LEN + DEB-TACE (27) |
LEN (18) |
|||||||
| any grade | grade ≥3 | any grade | grade ≥3 | any grade | grade ≥3 | any grade | grade ≥3 | any grade | grade ≥3 | any grade | grade ≥3 | |
| Hypertension, n (%) | 48 (33.8) | 12 (8.5) | 21 (30.4) | 7 (10.1) | 35 (30.4) | 9 (7.83) | 15 (29.4) | 5 (9.8) | 13 (48.1) | 3 (11.1) | 6 (33.3) | 2 (11.1) |
| Proteinuria, n (%) | 44 (30.9) | 15 (10.6) | 16 (23.2) | 6 (8.6) | 33 (28.7) | 11 (9.6) | 13 (25.5) | 5 (9.80) | 11 (40.7) | 4 (14.8) | 3 (16.7) | 1 (5.60) |
| Hand-foot syndrome, n (%) | 12 (8.40) | 0 | 6 (8.70) | 1 (1.4) | 10 (8.7) | 0 | 4 (7.8) | 1 (1.90) | 2 (7.4) | 0 | 2 (11.1) | 0 |
| Diarrhea, n (%) | 52 (36.6) | 12 (8.4) | 21 (30.4) | 8 (11.6) | 41 (35.7) | 10 (8.7) | 15 (29.4) | 6 (11.7) | 11 (40.7) | 2 (7.40) | 6 (33.3) | 2 (11.1) |
| Anorexia, n (%) | 68 (47.8) | 12 (8.5) | 31 (44.9) | 5 (7.2) | 54 (47.0) | 8 (7.0) | 21 (41.2) | 2 (3.90) | 14 (51.9) | 4 (14.8) | 10 (55.6) | 3 (16.7) |
| Nausea, n (%) | 54 (38.0) | 10 (7.0) | 28 (40.7) | 9 (13.4) | 42 (36.5) | 7 (6.1) | 23 (45.1) | 6 (11.7) | 12 (44.4) | 3 (11.1) | 5 (27.8) | 3 (16.7) |
| Fatigue, n (%) | 57 (40.1) | 1 (0.70) | 32 (46.4) | 2 (2.90) | 38 (33.0) | 0 | 23 (45.1) | 0 | 19 (70) | 1 (3.70) | 9 (50) | 2 (11.1) |
| AST elevation, n (%) | 78 (54.9) | 8 (5.60) | 17 (24.6) | 1 (1.4) | 66 (57.4) | 2 (1.7) | 14 (27.5) | 0 | 12 (44.4) | 6 (22.2) | 3 (16.7) | 1 (5.60) |
| ALT elevation, n (%) | 48 (33.8) | 3 (2.10) | 13 (18.8) | 2 (2.90) | 32 (27.8) | 2 (1.7) | 10 (19.6) | 0 | 16 (59.3) | 1 (3.70) | 3 (16.7) | 1 (5.60) |
| Bilirubin elevation, n (%) | 27 (19.0) | 3 (2.10) | 10 (14.5) | 1 (1.4) | 20 (17.4) | 2 (1.7) | 7 (13.7) | 0 | 7 (25.9) | 1 (3.70) | 3 (16.7) | 1 (5.60) |
| Thrombocytopenia, n (%) | 33 (23.2) | 12 (8.5) | 8 (11.6) | 2 (2.9) | 28 (24.3) | 9 (7.80) | 6 (11.8) | 2 (3.90) | 5 (18.5) | 3 (11.1) | 1 (5.60) | 0 |
| Hepatic encephalopathy, n (%) | 2 (1.40) | 1 (0.7) | 1 (1.40) | 1 (1.40) | 1 (0.90) | 0 | 0 | 0 | 1 (3.70) | 1 (3.70) | 1 (5.60) | 1 (5.60) |
| Interruption and/or dose reduction, n (%) | 35 (24.6) | 16 (23.1) | 27 (23.4) | 10 (19.6) | 8 (29.6) | 6 (33.3) | ||||||
AST, aspartate aminotransferase; ALT, alanine aminotransferase; DEB-TACE, drug-eluting bead transarterial chemoembolization; LEN, lenvatinib.
Fig. 4.
Liver function change according to ALBI score in patients receiving LEN plus DEB-TACE (a) and LEN alone (b). DEB-TACE, drug-eluting bead transarterial chemoembolization; LEN, lenvatinib.
Discussion
Unlike previous studies comparing TACE plus sorafenib or sorafenib alone in patients with unresectable HCC [8, 9, 10, 11, 12, 13, 14], this multicenter, nationwide, real-world study demonstrated, for the first time, that compared to lenvatinib alone, lenvatinib plus DEB-TACE achieved the expected endpoints of significantly improved OS, PFS, and ORR according to the mRECIST criteria in Chinese patients with advanced HCC, with an acceptable and well-tolerated safety profile. The strengths of the current study lie in: (1) its multicenter consecutive dataset, (2) the real-world and expanded study population meeting the inclusion criteria for the REFLECT trial or not, (3) the extended observation period and comprehensive outcome analyses, and (4) comprehensive reporting of the short- and long-term outcomes of patients with advanced HCC treated with lenvatinib plus DEB-TACE.
In the present study, patients treated with lenvatinib alone showed a median OS of 8.6 months, a median PFS of 4.4 months, and an ORR of 13.05% according to mRECIST criteria, which were similar to the values observed in previous real-world studies with a median OS of 8.7–13.9 months, a median PFS of 4.6–6.9 months, and an ORR of 14.1%–18.9% [34, 35, 36, 37]. However, these appear to be less than median OS of 13.6 months, median PFS of 7.4 months, and ORR of 40% by the masked independent imaging review according to the mRECIST criteria in phase III REFLECT trial [7]. It is noteworthy that 67.3% (142/211) of the population in our study were out of the REFLECT criteria, with extensive tumor burden and poor liver function and performance status. Actually, median OS and PFS of patients who fulfilled the REFLECT trial criteria (15 and 7.6 months) were comparable to the results of the REFLECT trial (13.6 and 7.4 months) [7]. More importantly, compared to monotherapy, the addition of DEB-TACE to lenvatinib reduced the risk of death by 48% (HR = 0.52, 95% CI 0.36–0.76) and the risk of progression by 58% (HR = 0.42, 95% CI 0.30–0.60). Such differences in OS and PFS might be related to the achievement of an improved treatment response (46.48% vs. 13.05%, p < 0.001). As reported in recent RCTs [12, 38] (online suppl. Table 16), DEB-TACE could provide an earlier disease control, but not local cure, than lenvatinib with reduction of variable tumor, which may induce a higher antitumor effect of lenvatinib under the released tumor burden and have contributed to the survival benefit by delaying the deleterious effect of tumor progression. Furthermore, similar results were obtained after adjusting for propensity score and with PSM, indicating that combined therapy offers a promising efficacy for advanced HCC.
Generally, clinical trials set strict enrollment criteria, and those patients might not be representative of the populations who receive the agents in real-world practice. Indeed, as illustrated by our study and other real-world studies [34, 35, 36, 37], heterogeneity exists among patients treated with lenvatinib at different centers. Only one-third of patients (69/211) in our study fulfilled the REFLECT trial criteria, but the benefit of combined treatment was consistently observed in patients who met the REFLECT trial criteria and in those who did not (online suppl. Fig. 4). Therefore, lenvatinib plus DEB-TACE should not be withheld in patients out of pre-specified criteria of the REFLECT trial. It is likely that this intervention may not benefit a subset of patients, such as patients with the absence of MVI or ascites, as well as BCLC-B stage patients (online suppl. Table 8; online suppl. Fig. 3A–D, 6). The population we analyzed was a heterogeneous class with a limited sample size and relatively short follow-up time, and the characteristics of these subgroups may not be well-balanced. Therefore, the potential use of combination therapy should be approached cautiously in those patients, and it is still necessary to validate its benefit in different settings to elucidate which patients can benefit from this combination treatment.
Indeed, the emergence of immune checkpoint inhibitors is revolutionizing the treatment of HCC. With a mature survival benefit in the breakthrough IMbrave 150 trial [39], atezolizumab-bevacizumab has been adopted as first-line therapy [2, 40]. However, it was not reimbursed by the national health insurance in China, which placed a greater financial burden on patients compared to sorafenib or lenvatinib. In this regard, lenvatinib still remains a preferred choice in clinical practice, and our study provides novel insight into the potential benefit of systematic therapy combined with TACE. Additionally, although several agents have been approved as second-line treatments [41, 42, 43, 44, 45], it should be noted that all of them were tested in patients who progressed and were tolerant to sorafenib. Thus, the relative benefit of these drugs in patients previously treated with lenvatinib remains uncertain [46]. This is the main reason why we encourage lenvatinib administration as long as no serious AEs occurred. A recent study demonstrated that the prognosis of unresectable HCC patients for whom lenvatinib treatment was continued after PD was significantly preferable to the prognosis of those who discontinued lenvatinib beyond PD [46]. Future studies are needed to determine the benefit of second-line treatments in patients previously treated with lenvatinib.
The present study supports the safety of lenvatinib plus DEB-TACE and lenvatinib alone, as no serious complications happened during the treatment period and the incidences of AEs were comparable in the two groups. Regarding TACE-related issues, temporary increases in alanine aminotransferase and aspartate aminotransferase were most common in the combination group, but most cases were graded as 1 or 2 and could be managed. The most frequent AEs were fatigue (46.4%), anorexia (44.9%), and nausea (40.7%), in monotherapy group, which were comparable to those observed in other real-life studies (online suppl. Table 10). In Child-Pugh B patients, the incidence of AEs was relatively higher than that among Child-Pugh A patients, and a potential underestimation should not be ignored considering the limited sample size with a lower starting dose in those patients and the nature of our retrospective study design. TACE might induce a deteriorated liver function; however, the ALBI score was not significantly altered at the first follow-up in LEN + DEB-TACE group (Fig. 4), the main reasons might be as follows: First, 90% of patients who present MVI were Vp2 and Vp3. As for MVI, the NCCN and BCLC guidelines recommend against TACE due to concerns that arterial embolization will result in severe ischemia and compromise remaining liver function, in the setting of preexisting occlusion of the liver's primary blood supply. In fact, the likelihood of this depends on multiple factors, including the degree of cavernous transformation, the location of portal vein thrombus, and the location of the tumor. Multiple retrospective studies have demonstrated the safety of TACE in the presence of both segmental and main portal vein tumor thrombosis [47, 48, 49, 50], and a recent RCT by Ding et al. [38] confirmed the safety of lenvatinib plus TACE for the patient with MVI. Second, 93.7% patients received super-selective procedure in combined group. In patients with MVI, a higher percentage of patients (95%) underwent this fine procedure. Advantages of super-selective embolization over nonselective embolization are less damage to the liver parenchyma and a stronger anticancer effect than those of nonselective embolization. Third, another factor was DEB-TACE we performed, since it's lower rate of AEs, comparing with conventional TACE [47]. The dose of agent per milliliter of beads in patients with Vp3 and Vp4 was significantly lower than that in patients with no MVI and Vp2 (Mann-Whitney U test, p < 0.001, online suppl. Fig. 7). These relatively lower doses of chemotherapy agents might be possible to reduce liver damage. Finally, antiviral therapy was encouraged to reduce the liver damage caused by HBV/HCV infections, which might be contributing to protecting liver function. Indeed, a maintained liver function could increase the treatment duration of lenvatinib along with DEB-TACE (mean 7.6 ± 5.4 months vs. mean 5.8 ± 4.3 months, p = 0.047) and a high relative dose intensity of agent. These factors might contribute to favorable outcomes.
Recently, Kudo et al. [18, 20] proposed the concept and utility of LEN-TACE sequential therapy for patients with intermediate-stage HCC beyond up-to-7 criteria. Ando et al. [51] demonstrated LEN-TACE sequential therapy may provide a deep response and favorable prognosis for intermediate-stage HCC. Kawamura et al. [52] reported the utility of LEN-TACE sequential therapy for prolonging post-progression survival during LEN treatment. In real-life practice, the optimal treatment strategy for intermediate and advanced patients still remains an unmet need, since its heterogeneous characteristics. Therefore, along with the previous studies, the current study sought to explore an effective and safe method to solve these needs as possible. Unlike LEN-TACE sequential therapy, the present study demonstrated lenvatinib combined DEB-TACE provided a novel approach in improving the prognosis of advanced HCC, and its strengths lied in: on the one hand, DEB-TACE provided a deeper and earlier disease control, which might provide a higher antitumor effect of lenvatinib under the released tumor burden. On the other hand, super-selectively DEB-TACE did not increase the risk of liver function damage, which could maintain a high relative dose intensity and a long treatment duration of the agent, which suggests a favorable prognosis [52, 53].
We acknowledge some limitations of our study. Firstly, selection and indication bias are inherent in retrospective observational studies, and although we performed a multivariate regression and PSM to adjust for potential confounders, unidentified biases may have acted significantly. Second, although the obvious advantage of lenvatinib plus DEB-TACE can be clearly observed in most clinical settings, the multiple subgroup analyses might have decreased the sample size, and therefore, the conclusion should be interpreted with caution. Third, other endpoints are becoming essential, such as duration of response/CR, etc. However, these endpoints need an extremely regular and rigorous follow-up protocol to capture survival benefits. As a matter of fact, the majority of patients might not follow it completely in real-world practice, and much radiologic information after treatment was hard to obtain because of the retrospective nature, and it is advisable to use it to capture benefit in high quality, prospective studies. Finally, since most study patients had HBV-related etiology and still the sample size was limited, additional prospective studies are highly warranted to generalize and extrapolate our findings.
In conclusion, lenvatinib plus DEB-TACE is expected to be promising and beneficial for patients with advanced HCC in the real-world setting, offering significant improvements in OS, PFS and ORR compared to lenvatinib alone, with acceptable adverse effects. Future prospective studies are required to confirm the efficacy and safety of the combination therapy.
Statement of Ethics
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Xi'an Internatioanl Medical Center Hospotal, No. 2021029. Patients' informed consent was waived or not required, as this is a retrospective study, and the analysis used anonymous clinical data that were obtained after the patient or their guardians had agreed to treatment with written consent.
Conflict of Interest Statement
All authors have no conflicts of interest related to this article.
Funding Sources
This study was supported by the National Natural Science Foundation of China (81420108020).
Author Contributions
Study concept and design: Dongdong Xia, Enxin Wang, Wei Bai, and Guohong Han. Data collection and follow-up: Dongdong Xia, Wei Bai, Enxin Wang, Zhexuan Wang, Qiaoyi Yang, Jiaping Li, Xiaoming Chen, Mingsheng Huang, Ming Huang, Junhui Sun, Weizhu Yang, Zhengyu Lin, Jianbing Wu, Zixiang Li, Shufa Yang, Xu Zhu, Zaizhong Chen, Yanfang Zhang, Wenzhe Fan, Qicong Mai, Rong Ding, Chunhui Nie, Long Feng, Xueda Li, Wukui Huang, Xiaomei Li, Bohan Luo, Zhengyu Wang, Jie Yuan, Wengang Guo, Kai Li, Bing Li, Ruijun Li, Zhanxin Yin, Jielai Xia, and Guohong Han. Statistical analysis: Dongdong Xia, Jielai Xia, and Guohong Han. Manuscript drafting and revision: Dongdong Xia, Wei Bai, Enxin Wang, Jiaping Li, Xiaoming Chen, Zhexuan Wang, Mingsheng Huang, Ming Huang, Junhui Sun, Weizhu Yang, Zhengyu Lin, Jianbing Wu, Zixiang Li, Shufa Yang, Xu Zhu, Zaizhong Chen, Yanfang Zhang, Wenzhe Fan, Qicong Mai, Rong Ding, Chunhui Nie, Long Feng, Xueda Li, Wukui Huang, Qiaoyi Yang, Jun Sun, Qiuhe Wang, Yong Lv, Xiaomei Li, Bohan Luo, Zhengyu Wang, Jie Yuan, Wengang Guo, Kai Li, Bing Li, Ruijun Li, Zhanxin Yin, Jielai Xia, and Guohong Han.
Data Availability Statement
Research data are not publicly available on legal or ethical grounds. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge Xia Wu (Oncology Global Medical Affairs HCC North team of MSD China Holding Co., Ltd.) for her support in literature retrieval and proofreading.
Dongdong Xia, Wei Bai, Enxin Wang, Jiaping Li, Xiaoming Chen, and Zhexuan Wang contributed equally to this work.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380((15)):1450–62. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7((1)):6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69((1)):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD Consensus Conference. Hepatology. 2021;73 Suppl 1:158–91. doi: 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2((8)):565–75. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 9.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64((5)):1090–8. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69((8)):1492–501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47((14)):2117–27. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70((4)):684–91. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60((5)):1697–707. doi: 10.1002/hep.27290. [DOI] [PubMed] [Google Scholar]
- 14.Kudo M, Cheng AL, Park JW, Park JH, Liang PC, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol. 2018;3((1)):37–46. doi: 10.1016/S2468-1253(17)30290-X. [DOI] [PubMed] [Google Scholar]
- 15.Haber PK, Puigvehí M, Castet F, Lourdusamy V, Montal R, Tabrizian P, et al. Evidence-based management of HCC: systematic review and meta-analysis of randomized controlled trials (2002–2020) Gastroenterology. 2021 Sep;161((3)):879–98. doi: 10.1053/j.gastro.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7((6)):2641–53. doi: 10.1002/cam4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and Child-Pugh a liver function: a proof-of-concept study. Cancers. 2019;11((8)):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9((3)):245–60. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10((3)):181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8((5)):299–311. doi: 10.1159/000502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67((1)):358–80. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 22.Tamai T, Hayato S, Hojo S, Suzuki T, Okusaka T, Ikeda K, et al. Dose finding of lenvatinib in subjects with advanced hepatocellular carcinoma based on population pharmacokinetic and exposure-response analyses. J Clin Pharmacol. 2017;57((9)):1138–47. doi: 10.1002/jcph.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda M, Okusaka T, Mitsunaga S, Ueno H, Tamai T, Suzuki T, et al. Safety and pharmacokinetics of lenvatinib in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2016;22((6)):1385–94. doi: 10.1158/1078-0432.CCR-15-1354. [DOI] [PubMed] [Google Scholar]
- 24.Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014;37((2)):381–7. doi: 10.1007/s00270-013-0654-7. [DOI] [PubMed] [Google Scholar]
- 25.Prajapati HJ, Dhanasekaran R, El-Rayes BF, Kauh JS, Maithel SK, Chen Z, et al. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24((3)):307–15. doi: 10.1016/j.jvir.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33((1)):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30((01)):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72((2)):288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reig M, Rimola J, Torres F, Darnell A, Rodriguez-Lope C, Forner A, et al. Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology. 2013;58((6)):2023–31. doi: 10.1002/hep.26586. [DOI] [PubMed] [Google Scholar]
- 30.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60((8)):646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33((6)):550–8. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27((3)):446–52. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 33.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41((2)):514–20. doi: 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheon J, Chon HJ, Bang Y, Park NH, Shin JW, Kim KM, et al. Real-world efficacy and safety of lenvatinib in korean patients with advanced hepatocellular carcinoma: a multicenter retrospective analysis. Liver Cancer. 2020;9((5)):613–24. doi: 10.1159/000508901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruta S, Ogasawara S, Ooka Y, Obu M, Inoue M, Itokawa N, et al. Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer. 2020;9((4)):382–96. doi: 10.1159/000507022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goh MJ, Oh JH, Park Y, Kim J, Kang W, Sinn DH, et al. Efficacy and safety of lenvatinib therapy for unresectable hepatocellular carcinoma in a real-world practice in Korea. Liver Cancer. 2021;10((1)):52–62. doi: 10.1159/000512239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-multicenter analysis. Cancer Med. 2019;8((8)):3719–28. doi: 10.1002/cam4.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127((20)):3782–93. doi: 10.1002/cncr.33677. [DOI] [PubMed] [Google Scholar]
- 39.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382((20)):1894–905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 40.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38((36)):4317–45. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 41.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389((10064)):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 42.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379((1)):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20((2)):282–96. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 44.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38((3)):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 45.Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21((4)):571–80. doi: 10.1016/S1470-2045(20)30011-5. [DOI] [PubMed] [Google Scholar]
- 46.Hiraoka A, Kumada T, Tada T, Kariyama K, Tani J, Fukunishi S, et al. What can be done to solve the unmet clinical need of hepatocellular carcinoma patients following lenvatinib failure? Liver Cancer. 2021;10((2)):115–25. doi: 10.1159/000513355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorodetski B, Chapiro J, Schernthaner R, Duran R, Lin M, Lee H, et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2017;27((2)):526–35. doi: 10.1007/s00330-016-4445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib − a retrospective controlled study. Radiology. 2014;272((1)):284–93. doi: 10.1148/radiol.14131946. [DOI] [PubMed] [Google Scholar]
- 49.Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology. 2012;263((2)):590–9. doi: 10.1148/radiol.12111550. [DOI] [PubMed] [Google Scholar]
- 50.Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258((2)):627–34. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 51.Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, et al. Efficacy and safety of lenvatinib-transcatheter arterial chemoembolization sequential therapy for patients with intermediate-stage hepatocellular carcinoma. Oncology. 2021;99((8)):507–17. doi: 10.1159/000515865. [DOI] [PubMed] [Google Scholar]
- 52.Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Okubo S, Tominaga L, et al. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer. 2020;9((6)):756–70. doi: 10.1159/000510299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirino S, Tsuchiya K, Kurosaki M, Kaneko S, Inada K, Yamashita K, et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS One. 2020;15((4)):e0231828. doi: 10.1371/journal.pone.0231828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not publicly available on legal or ethical grounds. Further inquiries can be directed to the corresponding author.