Abstract
We report the case of a patient with multiple myeloma who presented acutely with bilateral vitelliform-like macular lesions. This 85-year-old Caucasian lady was referred for treatment of presumed bilateral neovascular age-related macular degeneration (nAMD). Interestingly, this was a few months after starting on chemotherapy for multiple myeloma. We performed a clinical examination and multimodal imaging. This comprised colour fundus photography, fundus autofluorescence, near-infrared fundus photography, spectral-domain optical coherence tomography, and optical coherence tomography angiography. These tests excluded nAMD and demonstrated instead vitelliform-like macular lesions. Subfoveal vitelliform-like macular lesions, believed to be subretinal deposition of immunoglobulin, are one of the retinal signs of multiple myeloma. This can present acutely mimicking nAMD. These retinal lesions can be a presenting manifestation of the disease or may present later on during the course of the disease. Therefore, acute presentation of vitelliform macular lesions in an elderly patient should arouse suspicion. Serum protein electrophoresis is recommended to detect multiple myeloma at an early stage.
Keywords: Multiple myeloma, Vitelliform-like macular lesions, Neovascular age-related macular degeneration
Introduction
Multiple myeloma is the second most common haematological malignancy after B-cell chronic lymphocytic leukaemia. It is characterized by expansion of clonal bone marrow plasma cells of ≥10%, the presence of serum and or urinary M component, and end-organ damage that can be attributed to a plasma cells proliferative disorder [1]. Vitelliform-like macular lesions in multiple myeloma are uncommon with only eight cases previously reported [2, 3, 4, 5, 6].
Case Presentation
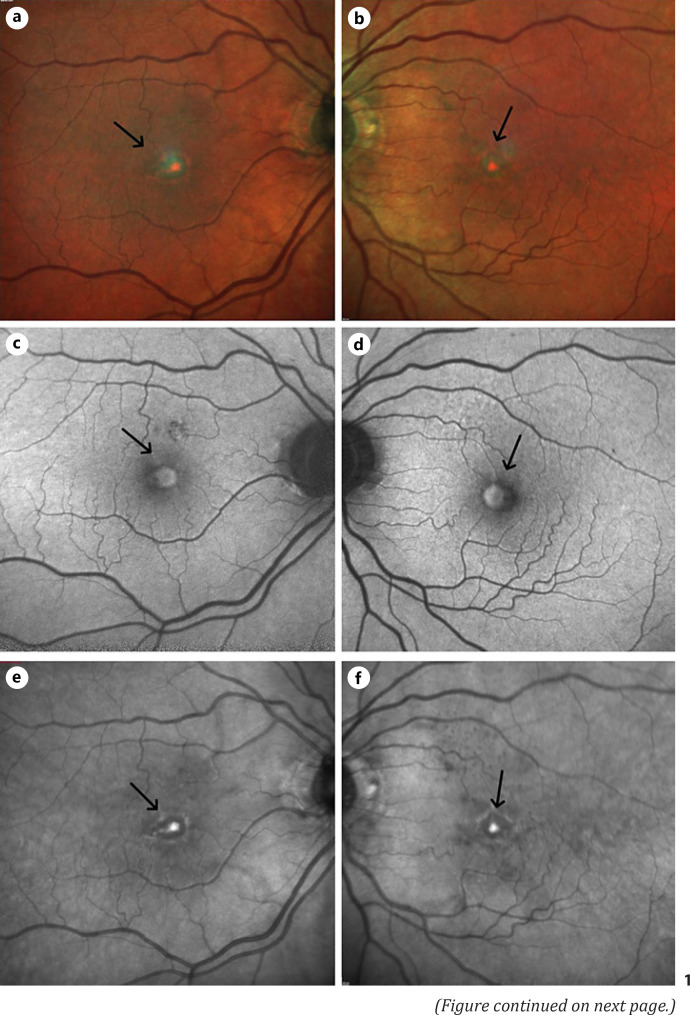
Our case is of an 85-year-old patient who was diagnosed with immunoglobulin G Kappa light chain myeloma with Multiple Myeloma International Staging System (ISS) stage III. Her optician referred her urgently to our hospital 4 months after her myeloma diagnosis, with bilateral macular drusen and a possible choroidal neovascular membrane (CNVM). He was concerned she had neovascular age-related macular degeneration (nAMD). She presented with a 2-month history of reduced central vision in both eyes. This began a few weeks after starting treatment for multiple myeloma. Her past ocular history was unremarkable. She was seen by her optician 18 months prior to their referral date and her visual acuity was 6/10 (20/30) OD and 6/7.5 (20/25) OS. She was hypertensive on medications, never smoked, and had a family history of glaucoma. On examination, her best corrected visual acuity was 6/24 (20/80) OD and 6/18 (20/60) OS. Intraocular pressure was 16 mm Hg OU. Anterior segment examination showed mild corneal guttata with insignificant cataracts OU. Fundus examination demonstrated a few small hard drusen at the posterior poles. There were bilateral elevated subretinal yellowish-orange foveal lesions (Fig. 1a, b) with matching central hyperautofluorescence surrounded by hypoautofluorescence on fundus autofluorescence imaging (Fig. 1c, d), and elevated near-infrared signals centrally (Fig. 1e, f). The macular optical coherence tomography (OCT) scan of both eyes (Fig. 1g, h) showed ovoid, subretinal, hyperreflective dome-shaped lesions centred at the fovea with no evidence of CNVM on optical coherence tomography angiography (Fig. 1i, j). There were no signs of hyperviscosity retinopathy in either eye, and both optic discs were healthy. She does not have any previous imaging to compare with. The finding was explained to the patient, she was registered as sight impaired, and referred to a low vision aid clinic. A follow-up appointment was made to monitor her ocular disease progression. Choroidal neovascularization due to AMD was ruled out by the multimodal imaging which did not show significant drusen or RPE changes to signify a diagnosis of AMD and the absence of subretinal/intraretinal fluid on OCT scans and the absence of any CNVM on OCTA.
Fig. 1.
Multimodal imaging of both eyes of an 85-year-old patient with multiple myeloma presenting with bilateral sudden drop of her central vision and bilateral vitelliform macular lesions. Multicolour fundus photos showed bilateral elevated subretinal yellowish-orange foveal lesions (a, b, arrow) with matching central hyperautofluorescence surrounded by hypoautofluorescence on FAF imaging (c, d, arrow), and elevated near-infrared signals centrally (e, f, arrow). Her macular OCT scan of both eyes (g, h, arrow) displayed ovoid, subretinal, hyper-reflective dome-shaped lesions centred at the fovea with no evidence of CNVM on OCTA in either eye (i, j, arrow). FAF, fundus autofluorescence; OCTA, optical coherence tomography angiography.
Discussion
Retinal manifestations of multiple myeloma are variable [2, 3, 4, 5, 6, 7, 8]. The eye can be affected by direct infiltration by myeloma cells or via an extramedullary plasmacytoma, hypergammaglobulinemic state, and immunoglobulin deposition in ocular structures. This can be an initial manifestation of myeloma [2] or a sign of relapse and insufficient therapy [9].
Myeloma-associated vitelliform lesions can mimic an acute presentation of nAMD. nAMD is the commonest cause of blindness in people over 50 years of age, characterized by drusen, lipid exudation, subretinal and/or intraretinal fluid, macular haemorrhage, and CNVM. In our case, vitelliform macular lesions developed acutely 4 months after the initial diagnosis of myeloma and were initially thought by her treating physicians to be related to her chemotherapy, in particular her dexamethasone medication. This is unlike other cases reported, where the macular vitelliform lesions preceded the diagnosis of multiple myeloma and disappeared on commencement of therapy [2, 4]. Vitelliform macular detachment has been reported to resolve in multiple myeloma patients after chemotherapy [3].
Myeloma-associated vitelliform macular lesions should also be differentiated from adult vitelliform macular dystrophy (AVMD). This is a slowly progressive condition, associated with mutation of the PRPH2 gene. In our case, genetic testing to rule out AVMD was not carried out, as the latter typically presents in 40- to 60-year olds with a milder, gradual reduction in central vision which is usually discovered incidentally on routine check-ups.
In a previous case report, subretinal lesions disappeared nearly 2 years after the initial presentation and were replaced with mild retinal pigment epithelial changes and a few retinal haemorrhages. At this stage, serum protein electrophoresis detected immunoglobulin G and a bone marrow biopsy revealed 25% plasma cells. The patient was then started on chemotherapy, which did not appear to change the course of her retinal lesions [2], a subfoveal neurosensory retinal detachment developed 28 months after the initial presentation [2]. Macular detachment has also been observed in other cases of multiple myeloma, macroglobulinemia, and monoclonal gammopathy of undetermined significance [3, 4, 5, 6, 7].
In our case, the vitelliform lesions were not associated with macular detachment and demonstrated increased hyperautofluorescence on fundus autofluorescence at presentation. This is consistent with a previous case where macular lesions did not demonstrate increased autofluorescence at presentation, but hyperautofluorescence developed 18 months later when myeloma was diagnosed [2]. No leakage on fluorescein angiography was observed in other case reports. We used optical coherence tomography angiography, which is a noninvasive method to evaluate if CNVM was present.
Our patient's blood result at the time of diagnosis showed fluorescence in situ hybridization gain at 11q13 and low-level loss of 17p13 (tumour-suppressor protein TP53) consistent with a single poor prognostic marker. Her bone marrow aspirate showed overall approximately 60% plasma cells. Therefore, it is not clear whether her vitelliform macular lesions can be considered as a sign of relapse of her condition, as she was in an advanced stage at the time of diagnosis. Our patient did not have a serous retinal detachment at the time of this report. After confirming the diagnosis, the patient was treated with chemotherapy in the form of tablets. She was prescribed lenalidomide days 1–21; dexamethasone days 1, 8, 15, and 22; and cyclophosphamide days 1, 8, and 15. Her latest clinical note showed a current medication of daratumumab, Velcade, and dexamethasone and that she continues to respond well to treatment and her blood counts are stable.
In summary, we report the clinical presentation and multimodal imaging of a patient with vitelliform macular lesions, who was on chemotherapy for multiple myeloma and was referred by her optician to rule out nAMD. We are not aware of a definitive method to differentiate myeloma-caused vitelliform-like lesions and AVMD from the ocular examination. Although in this case, interestingly, the vitelliform lesions appeared to have a central core within the vitelliform deposit seen on OCT scans. This possibly represents focal immunoprotein deposition between the retina and RPE. In our experience, AVMD has more homogeneous vitelliform deposits. It would be interesting to see if this is a common feature of myeloma-related deposits if further cases are reported. Ophthalmologists should consider myeloma-associated vitelliform lesions if a patient has a medical history of myeloma and/or there is a history of acute development of such lesions, which would be unusual for AVMD. It is important that ophthalmologists are aware that vitelliform-like macular deposits, particularly in the elderly, may represent a masquerade syndrome and can be the initial presentation of multiple myeloma.
Statement of Ethics
An informed written consent for publication of this case report and any accompanying photographs was obtained from the patient. This retrospective review of patient data did not require ethical approval in accordance with local guidelines by our institution − University Hospital Southampton NHS Foundation Trust.
Conflict of Interest Statement
The authors declare no financial/conflict of interests related to this work to disclose.
Funding Sources
The authors report that no funding was received
Author Contributions
The authors declare that they meet the current ICMJE criteria for authorship. Abeir Baltmr and Andrew Lotery both contributed to the clinical care of the patient and collected the clinical data. Both authors contributed to drafting and editing the manuscript, approved the final version to be submitted, and agree to be responsible for all aspects of this work.
Data Availability Statement
All data that support the findings of this case report are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95((5)):548–67. doi: 10.1002/ajh.25791. [DOI] [PubMed] [Google Scholar]
- 2.Khan JM, McBain V, Santiago C, Lois N. Bilateral “vitelliform-like” macular lesions in a patient with multiple myeloma. BMJ Case Rep. 2010;2010:bcr0520103049. doi: 10.1136/bcr.05.2010.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusu IM, Mrejen S, Engelbert M, Gallego-Pinazo R, Ober MD, Johnson MW, et al. Immunogammopathies and acquired vitelliform detachments: a report of four cases. Am J Ophthalmol. 2014;157((3)):648.e1–57.e1. doi: 10.1016/j.ajo.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 4.El Ameen A, Mrejen-Uretsky S, Abraham J, Souied EH, Cohen SY. Acquired vitelliform detachment in multiple myeloma. Retin Cases Brief Rep. 2017;11((1)):27–9. doi: 10.1097/ICB.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 5.Brody JM, Butrus SI, Ashraf MF, Rabinowitz AI, Whitmore PV. Multiple myeloma presenting with bilateral exudative macular detachments. Acta Ophthalmol Scand. 1995;73((1)):81–2. doi: 10.1111/j.1600-0420.1995.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith SJ, Johnson MW, Ober MD, Comer GM, Smith BD. Maculopathy in patients with monoclonal gammopathy of undetermined significance. Ophthalmol Retina. 2020;4((3)):300–9. doi: 10.1016/j.oret.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Leys A, Vandenberghe P. Serous macular detachments in a patient with IgM paraproteinemia: an optical coherence tomography study. Arch Ophthalmol. 2001;119((6)):911–3. [PubMed] [Google Scholar]
- 8.Knapp AJ, Gartner S, Henkind P. Multiple myeloma and its ocular manifestations. Surv Ophthalmol. 1987;31((5)):343–51. doi: 10.1016/0039-6257(87)90119-6. [DOI] [PubMed] [Google Scholar]
- 9.Kottler UB, Cursiefen C, Holbach LM. Orbital involvement in multiple myeloma: first sign of insufficient chemotherapy. Ophthalmologica. 2003;217((1)):76–8. doi: 10.1159/000068251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this case report are included in this article. Further enquiries can be directed to the corresponding author.