Abstract
Introduction:
After COVID-19 vaccination, women of reproductive age reported changes in their menstrual cycle.
Materials and methods:
A retrospective study was carried out after a survey on social networks that included women aged 18–41 years with normal cycles according to International Federation of Gynecology and Obstetrics and who were vaccinated (complete schedule for two doses, except J&J/Janssen or incomplete with a single dose). Women with following conditions were excluded: pregnant or lactating women; history of diseases that cause menstrual irregularities or early menopause: anorexia, bulimia, polycystic ovary syndrome, hypothyroidism, obesity, or low weight; hysterectomized or oophorectomized patients; and high performance athletes.
Results:
Overall, 950 women completed the survey between July and September 2021. In total, 408 women met the inclusion criteria, and 184 reported the following characteristics: frequency (normal 43.47%, infrequent 25%, and frequent 31.53%), regularity (regular 51.08%, irregular 42.93%, and absent/amenorrhea 5.97%), duration (normal 65.21%, prolonged 26.08%, absent/amenorrhea 8.69%), and volume (heavy 41.84%, light 20.65%, and absent/amenorrhea 6.52%).
Conclusions:
SARS-CoV-2 infection and COVID-19 vaccination can influence the menstrual cycle and cause alterations.
Keywords: adverse effects, COVID-19, menstrual cycle, SARS-CoV-2, vaccines
Introduction
The pandemic caused by COVID-19 has drawn attention to numerous investigations and publications worldwide in order to understand its scope and consequences on the human body in the short- and long-term. 1 Women’s health is part of the different scenarios on which the virus has impacted. A prospective study revealed that, of 237 women of reproductive age, 177 had alterations in the menstrual cycle after the diagnosis of COVID-19. Overall, 25% were alterations in the bleeding volume, 28% changes in regularity, 20% decreased volume, and 19% prolongation of the cycle, among others; however, these changes were transitory, and the normalization of the cycle occurred quickly after recovery. 2
In a historical time, thanks to advances in science, the development of vaccines was possible, which provide protection against the SARS-CoV-2 virus and its potentially lethal complications. 3 Mass vaccinations are a measure that addresses the current health situation, to mitigate the significant burden of morbidity and mortality from the SARS-CoV-2 virus. 4 A meta-analysis revealed that the efficacy of COVID-19 vaccines ranges from 80.2% to 94.6% depending on the type (viral vector versus mRNA-based). 4 Side effects include general malaise, headache, fever, and gastrointestinal symptoms. As the vaccination process advances and includes younger population groups (women of reproductive age), questions and concerns arise regarding women’s health and its relationship with the menstrual cycle.
Menstrual bleeding has dynamic and cyclical characteristics, and its monthly variability is a clear sign of health and fertility. 5 The International Federation of Gynecology and Obstetrics (FIGO) offers a nomenclature for the evaluation of normal menstrual bleeding and the diagnosis of abnormalities, establishing four evaluation parameters: frequency, regularity, duration, and volume 6 (Table 1).
Table 1.
Normal menstrual cycle used for the inclusion criteria.
| Parameter | Normal | Abnormal |
|---|---|---|
| Frequency | Normal ⩾24 days to ⩽38 days | Absent (No periods or bleeding) Amenorrhea Infrequent > 38 days |
| Duration | Normal ⩽ up to 8 days | Prolonged > 8 days |
| Regularity | Regular variation (shortest to longest) ⩽7–9 days |
Irregular (shortest to longest ⩾10 days) |
| Volume | Normal | Light Heavy |
The first reports of changes in post-vaccination menstrual cycles arise through social networks. 7 Since June 2021, the Norwegian Medicines Agency received a considerable number of reports of menstrual disorders via its virtual platform. 7 For its part, the yellow card surveillance scheme, run by the UK Medicines and Healthcare products Regulatory Agency (MHRA), has been notified of a total of 48,488 reactions related to menstrual disorders after the vaccination with three doses by January 2022. 8 The United States Vaccine Adverse Event Reporting System (VAERS) also documented this kind of reports, which led to the first study evaluating 3959 women (2403 vaccinated versus 1555 unvaccinated) finding that the COVID-19 vaccine was associated with a change of less than 1 day in cycle length for both vaccine doses compared with pre-vaccine cycles (first dose 0.75 days-increment, 98.75% CI = [0.47, 0.94], second dose 0.91 days-increment, 98.75% CI = [0.63, 1.19]); the unvaccinated did not see any significant change compared to three basal cycles. 9
Unfortunately, the clinical trials that address adverse reactions from vaccination for COVID-19 do not provide specific data on the menstrual cycle after vaccination, 4 and even routine surveillance systems for adverse effects of medications and vaccines do not actively search for this alteration either, which has made it difficult to determine if the association exists. In the case of South America, no reports have been documented on menstruation in vaccinated women. Given the lack of this information, there is a need to investigate about menstrual disturbances in a cohort of women of reproductive age, aiming to describe the occurrence of alterations post COVID-19 vaccination.
Materials and methods
Population
A retrospective study was carried out where information was collected. We made a survey (adnex 1) in Google Forms and delivered through social networks (Twitter, Instagram, Facebook, WhatsApp) to randomized women population. In this survey, we asked sociodemographic information and the characteristics of the menstrual cycle immediately before and immediately after COVID-19 vaccination. The survey was implemented between July and September 2021. All women voluntarily completed the survey and gave a written informed consent to participate in this study. Confidentiality of sensible data was assured.
The inclusion criteria were women between 18 and 41 years with normal cycles according to FIGO (Table 1) before vaccination against COVID-19 and who were vaccinated against COVID-19. In addition, women who despite the use of hormonal contraceptives (combined or progestin-only) have normal cycles and bleeding were included. The following were excluded: pregnant or lactating (in the last 6 months); history of diseases that per se produce menstrual irregularities or early menopause such as anorexia, bulimia, polycystic ovary syndrome, hypothyroidism, obesity (Body Mass Index (BMI) > 30), or low weight (BMI < 18); hysterectomized or oophorectomized patients; and high performance athletes. Also, women who had COVID-19 in the last year were ruled out.
Measures
Respondents were asked their age, weight, height, residence, medical history, planning method, if available, and vaccination status (received vaccine, complete schedule for two doses, except for J&J/Janssen or incomplete with a single dose for the rest of the vaccine brands). In all cases, questions were asked about the characteristics of the menstrual cycle: frequency (normal ⩾24 to ⩽38 days), regularity (variation 7–9 days), duration (⩽8 days), and volume (subjectively with alteration of the usual amount for the patient). Subsequently, they were asked the same list of questions for their cycles after the first and second doses of the vaccine, including impact on quality of life (QoL). The impact on QoL was based on a personal perception of the women enrolled in the survey asking about the following standard indicators: wealthiness, physical and mental health, and pain.
Statistical analysis
All statistical analyzes were performed using free Software R for Windows Version 4.0.2. Continuous variables were described as median (interquartile range (IQR) when not, and categorical variables were described as absolute and relative frequency (percentage).
Results
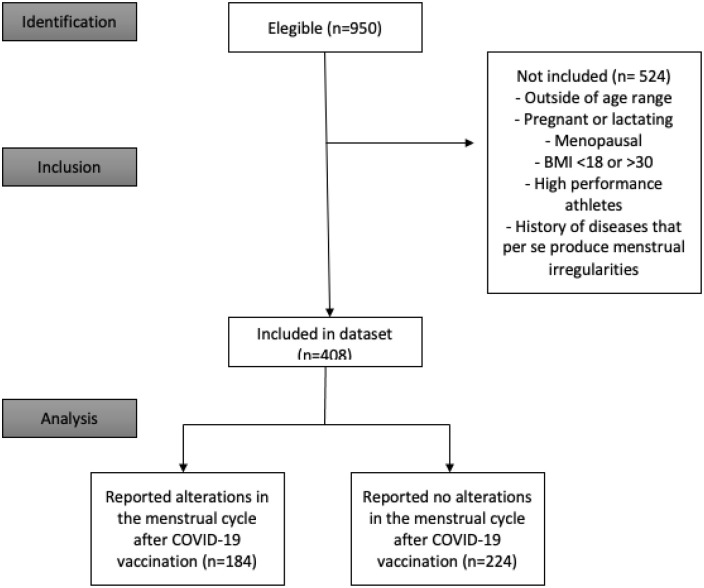
In total, 950 women completed the survey, and 408 women met the inclusion criteria of which 184 reported alterations in the menstrual cycle and were included in the study (Figure 1). The median age of the whole population that completed the survey was 28 years, where 89.36% live in South America and 25.68% use oral contraceptives (OCs). The clinical characteristics of the entire sample are found in Table 2. Almost half of the vaccinated cohort received Pfizer (43.57%; Sinovac 18%, J&J/Janssen 15.68%, Moderna 12.31%, AstraZeneca 6%, and others 4.42%). It is worth mentioning that the other category included clinical trials (cansino, clover), as well as the following vaccines: Sinopharm and Sputnik; they were grouped due to their low presentation.
Figure 1.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) flow diagram.
Table 2.
Clinical characteristics and demographics of people who complete the survey.
| Parameter | No, N = 458 a | Yes, N = 492 a |
|---|---|---|
| Age (years) | 28 (25, 33) | 27 (23, 34) |
| Weight (kg) | 60 (55, 67) | 61 (55, 70) |
| Height (m) | 1.62 (1.58, 1.65) | 1.62 (1.58, 1.67) |
| BMI | 22.9 (21.1, 24.9) | 23.4 (21.1, 26.3) |
| BMI category | ||
| Low weight (BMI < 18) | 13 (2.8%) | 20 (4.1%) |
| Normal | 335 (73%) | 307 (62%) |
| Obesity (BMI > 30) | 31 (6.8%) | 56 (11%) |
| Overweight (BMI >25 and <29.9) | 79 (17%) | 109 (22%) |
| Continent | ||
| North America | 14 (3.1%) | 37 (7.5%) |
| South America | 427 (93%) | 422 (86%) |
| Australia | 0 (0%) | 1 (0.2%) |
| Europe | 17 (3.7%) | 32 (6.5%) |
| Planning | ||
| No | 82 (18%) | 119 (24%) |
| Yes | 376 (82%) | 373 (76%) |
| Contraceptive method | ||
| Periodic abstinence | 1 (0.2%) | 5 (1.0%) |
| Combined vaginal ring | 10 (2.2%) | 2 (0.4%) |
| Combined injectable contraceptives | 12 (2.6%) | 19 (3.9%) |
| Combined oral contraceptives (OCs) | 150 (33%) | 94 (19%) |
| Progestin-only oral contraceptives | 5 (1.1%) | 12 (2.4%) |
| Condom | 96 (21%) | 143 (29%) |
| Intrauterine devices | 43 (9.4%) | 33 (6.7%) |
| Progestin-only implants | 25 (5.5%) | 25 (5.1%) |
| Tubal ligation | 21 (4.6%) | 29 (5.9%) |
| Symptothermal method | 2 (0.4%) | 1 (0.2%) |
| I don’t use contraceptive methods | 82 (18%) | 119 (24%) |
| Combined contraceptive patch | 0 (0%) | 1 (0.2%) |
| Vasectomy | 11 (2.4%) | 9 (1.8%) |
| Pregnancy | ||
| No | 453 (99%) | 486 (99%) |
| Yes | 5 (1.1%) | 6 (1.2%) |
| Pre-COVID-19 vaccination menstruation frequency | ||
| Amenorrhea | 25 (5.5%) | 7 (1.4%) |
| Infrequent | 29 (6.3%) | 44 (8.9%) |
| Normal | 387 (84%) | 417 (85%) |
| Frequent | 17 (3.7%) | 24 (4.9%) |
| Pre-COVID-19 vaccination menstruation regularity | ||
| Amenorrhea | 21 (4.6%) | 4 (0.8%) |
| Irregular | 66 (14%) | 76 (15%) |
| Regular | 371 (81%) | 412 (84%) |
| Pre-COVID-19 vaccination menstruation duration | ||
| Amenorrhea | 19 (4.1%) | 4 (0.8%) |
| Normal | 412 (90%) | 449 (91%) |
| Prolonged | 27 (5.9%) | 39 (7.9%) |
| Pre-COVID-19 vaccination menstrual volume | ||
| Amenorrhea | 14 (3.1%) | 5 (1.0%) |
| Light volume | 40 (8.7%) | 37 (7.5%) |
| Normal | 328 (72%) | 335 (68%) |
| Heavy volume | 76 (17%) | 115 (23%) |
| Comorbidities | ||
| Anorexy | 2 (0.4%) | 1 (0.2%) |
| Bulimia | 1 (0.2%) | 1 (0.2%) |
| Polycystic ovary syndrome | 56 (12%) | 63 (13%) |
| None of the above | 399 (87%) | 427 (87%) |
| Weight loss | ||
| No | 406 (89%) | 428 (87%) |
| Yes | 52 (11%) | 64 (13%) |
| High performance athlete | ||
| No | 438 (96%) | 482 (98%) |
| Yes | 20 (4.4%) | 10 (2.0%) |
| Vaccine | ||
| AstraZeneca | 22 (4.8%) | 35 (7.1%) |
| Janssen-Johnson & Johnson | 61 (13%) | 88 (18%) |
| Modern | 47 (10%) | 70 (14%) |
| Others | 9 (2.0%) | 33 (6.7%) |
| Pfizer-BioNTech1 | 256 (56%) | 158 (32%) |
| Sinovac | 63 (14%) | 108 (22%) |
| Complete scheme | ||
| No | 75 (16%) | 130 (26%) |
| Yes | 383 (84%) | 362 (74%) |
| Post-vaccination menstrual frequency | ||
| Amenorrhea | 5 (1.1%) | 48 (9.8%) |
| Infrequent | 2 (0.4%) | 128 (26%) |
| Normal | 449 (98%) | 199 (40%) |
| Frequent | 2 (0.4%) | 117 (24%) |
| Post-vaccination menstrual regularity | ||
| Amenorrhea | 5 (1.1%) | 36 (7.3%) |
| Irregular | 4 (0.9%) | 226 (46%) |
| Normal or regular | 449 (98%) | 230 (47%) |
| Post-vaccination menstrual duration | ||
| Amenorrhea | 5 (1.1%) | 50 (10.1%) |
| Normal | 451 (98%) | 284 (58%) |
| Prolonged | 2 (0.4%) | 158 (32%) |
| Post-vaccination menstrual volume | ||
| Amenorrhea | 6 (1.3%) | 39 (7.9%) |
| Light volume | 4 (0.9%) | 105 (21%) |
| Normal | 444 (97%) | 127 (26%) |
| Heavy volume | 4 (0.9%) | 221 (45%) |
| Change in quality of life | ||
| No | 438 (96%) | 214 (43%) |
| Yes | 20 (4.4%) | 278 (57%) |
BMI: Body Mass Index; IQR: interquartile range.
Median (IQR); No. (%).
Characteristics of the population with alterations in the menstrual cycle
Of the 184 women who reported alterations in the menstrual cycle, the average age was 27 years, with a predominantly normal weight according to BMI (74.46%, overweight 25.54%). Overall, 150 (81.5%) women had a complete vaccination schedule, mostly with Sinovac (n = 53; Pfizer (n = 51), J&J/Janssen (n = 33), others (n = 19), Modern (n = 15), AstraZeneca (n = 13)).
In the geographical distribution, 158 (85.86%) live in South America, 13 (7.06%) in Europe, and 13 (7.06%) in North America—Central America. No alterations were reported in Oceania, nor were data obtained from other continents. Overall, 43% of the sample did not use contraceptive methods; Of those who used contraceptive methods, 106 were (57.60%) hormonal methods (OCs 33 (17.93%), intrauterine devices 10 (5.43%), combined injectable contraceptives 8 (4.34%), progestogen-only contraceptives 5 (2.71%), progestogen-only implants 5 (2.71%), combined vaginal ring 1 (0.54%), combined contraceptive patch 1 (0.54%)), and 78 were (42.39%) non-hormonal methods (condom 65 (35.32%), tubal ligation 9 (4.89%), vasectomy 2 (1.08%), symptothermal 1 (0.54%), periodic abstinence 1 (0.54%); Table 3).
Table 3.
Characteristics of the patients with alterations on the menstrual cycle, discriminated by biological applied.
| Parameter | Pfizer, N = 51 a | Sinovac, N = 53 a | Janssen, N = 33 a | Moderna, N = 15 a | AstraZeneca, N = 13 a | Others, N = 19 a |
|---|---|---|---|---|---|---|
| Age (years) | 29 (26, 35) | 27 (24, 38) | 32 (26, 36) | 27 (26, 28) | 29 (24, 34) | 22 (18, 25) |
| Weight (kg) | 59 (55, 66) | 62 (56, 68) | 62 (58, 69) | 55 (50, 62) | 65 (61, 68) | 60 (53, 62) |
| Height (m) | 1.60 (1.58, 1.65) | 1.62 (1.57, 1.67) | 1.62 (1.59, 1.69) | 1.59 (1.55, 1.68) | 1.64 (1.60, 1.66) | 1.63 (1.60, 1.65) |
| BMI | 23.19 (21.48, 24.99) | 23.50 (22.04, 25.30) | 23.73 (20.70, 25.32) | 20.81 (20.21, 23.79) | 24.17 (22.27, 26.30) | 21.78 (20.73, 24.57) |
| BMI Category | ||||||
| Normal | 39 (76%) | 38 (72%) | 23 (70%) | 14 (93%) | 9 (69%) | 14 (74%) |
| Overweight | 12 (24%) | 15 (28%) | 10 (30%) | 1 (6.7%) | 4 (31%) | 5 (26%) |
| Continent | ||||||
| South America | 43 (84%) | 48 (91%) | 30 (91%) | 11 (73%) | 8 (62%) | 18 (95%) |
| Europe | 7 (14%) | 2 (3.8%) | 1 (3.0%) | 1 (6.7%) | 2 (15%) | 0 (0%) |
| North and Central America | 1 (2.0%) | 3 (5.7%) | 2 (6.1%) | 3 (20%) | 3 (23%) | 1 (5.3%) |
| Planning | ||||||
| No | 13 (25%) | 16 (30%) | 8 (24%) | 2 (13%) | 2 (15%) | 2 (11%) |
| Yes | 38 (75%) | 37 (70%) | 25 (76%) | 13 (87%) | 11 (85%) | 17 (89%) |
| Contraceptive method | ||||||
| Periodic abstinence | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Combined vaginal ring | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6.7%) | 0 (0%) | 0 (0%) |
| Combined injectable contraceptives | 5 (9.8%) | 3 (5.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Combined oral contraceptives (OCs) | 6 (12%) | 11 (21%) | 4 (12%) | 4 (27%) | 2 (15%) | 6 (32%) |
| Progestin-only oral contraceptives | 2 (3.9%) | 1 (1.9%) | 1 (3.0%) | 0 (0%) | 0 (0%) | 1 (5.3%) |
| Condom | 15 (29%) | 16 (30%) | 13 (39%) | 6 (40%) | 7 (54%) | 8 (42%) |
| Intrauterine devices | 5 (9.8%) | 3 (5.7%) | 1 (3.0%) | 1 (6.7%) | 0 (0%) | 0 (0%) |
| Progestin-only implants | 2 (3.9%) | 0 (0%) | 1 (3.0%) | 0 (0%) | 1 (7.7%) | 1 (5.3%) |
| Tubal ligation | 2 (3.9%) | 2 (3.8%) | 4 (12%) | 1 (6.7%) | 0 (0%) | 0 (0%) |
| Symptothermal method | 0 (0%) | 0 (0%) | 1 (3.0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| I don’t use contraceptive methods | 13 (25%) | 16 (30%) | 8 (24%) | 2 (13%) | 2 (15%) | 2 (11%) |
| Combined contraceptive patch | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (5.3%) |
| Vasectomy | 0 (0%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 1 (7.7%) | 0 (0%) |
| Complete scheme | ||||||
| No | 2 (3.9%) | 6 (11%) | 0 (0%) | 10 (67%) | 8 (62%) | 8 (42%) |
| Yes | 49 (96%) | 47 (89%) | 33 (100%) | 5 (33%) | 5 (38%) | 11 (58%) |
| Post-vaccination menstrual frequency | ||||||
| Amenorrhea | 5 (9.8%) | 7 (13%) | 1 (3.0%) | 1 (6.7%) | 1 (7.7%) | 2 (11%) |
| Infrequent | 16 (31%) | 13 (25%) | 9 (27%) | 2 (13%) | 2 (15%) | 4 (21%) |
| Normal | 19 (37%) | 28 (53%) | 13 (39%) | 7 (47%) | 6 (46%) | 7 (37%) |
| Frequent | 11 (22%) | 5 (9.4%) | 10 (30%) | 5 (33%) | 4 (31%) | 6 (32%) |
| Post-vaccination menstrual regularity | ||||||
| Amenorrhea | 3 (5.9%) | 4 (7.5%) | 0 (0%) | 1 (6.7%) | 1 (7.7%) | 2 (11%) |
| Irregular | 20 (39%) | 20 (38%) | 19 (58%) | 4 (27%) | 5 (38%) | 11 (58%) |
| Normal or regular | 28 (55%) | 29 (55%) | 14 (42%) | 10 (67%) | 7 (54%) | 6 (32%) |
| Post-vaccination menstrual duration | ||||||
| Amenorrhea | 5 (9.8%) | 5 (9.4%) | 1 (3.0%) | 1 (6.7%) | 2 (15%) | 2 (11%) |
| Normal | 33 (65%) | 38 (72%) | 22 (67%) | 10 (67%) | 7 (54%) | 10 (53%) |
| Prolonged | 13 (25%) | 10 (19%) | 10 (30%) | 4 (27%) | 4 (31%) | 7 (37%) |
| Post-vaccination menstrual volume | ||||||
| Amenorrhea | 3 (5.9%) | 4 (7.5%) | 0 (0%) | 1 (6.7%) | 2 (15%) | 2 (11%) |
| Light | 17 (33%) | 11 (21%) | 4 (12%) | 1 (6.7%) | 1 (7.7%) | 4 (21%) |
| Normal | 15 (29%) | 17 (32%) | 11 (33%) | 4 (27%) | 4 (31%) | 6 (32%) |
| Heavy | 16 (31%) | 21 (40%) | 18 (55%) | 9 (60%) | 6 (46%) | 7 (37%) |
| Change in quality of life | ||||||
| No | 27 (53%) | 24 (45%) | 10 (30%) | 5 (33%) | 7 (54%) | 8 (42%) |
| Yes | 24 (47%) | 29 (55%) | 23 (70%) | 10 (67%) | 6 (46%) | 11 (58%) |
BMI: Body Mass Index; IQR: interquartile range.
Median (IQR); No. (%).
Post-vaccination disorders: frequency
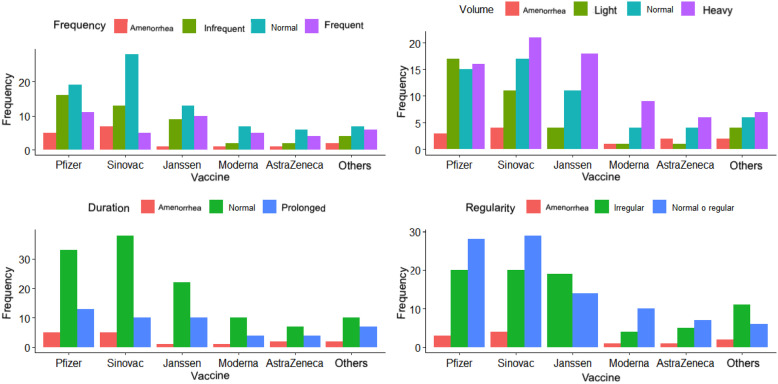
Post-vaccination menstrual cycle frequency was normal in 43.47% (n = 80) of the women. Overall, 25% (n = 46) reported the cycle became infrequent (>38 days); while 41 women (22.28%) indicated that it was frequent (<24 days), 11 of them vaccinated with Pfizer. And, 17 women (9.23%) reported amenorrhea (Figure 2).
Figure 2.
Alterations of the menstrual cycle discriminated by vaccine.
Post-vaccination changes: regularity
Overall, 94 women (51.08%) reported regular cycles after vaccination, 79 (42.93%) noted irregular cycles after vaccination (variation > 9 days), and 11 (5.97%) of them reported having amenorrhea after vaccination. The vaccine that most frequently affected cycle regularity was Pfizer and Sinovac; however, for the J&J/Janssen vaccine more women reported irregular cycles (n = 19) than normal (58% versus 42%).
Post-vaccination changes: duration
In relation to the duration of the menstrual cycle, 120 women (65.21%) mentioned a duration in normal ranges (<8 days), 26.08% (48) commented a prolongation of the cycle (>9 days), and only 16 women (8.69%) reported amenorrhea/absent.
Post-vaccination changes: volume
The volume variable was reported as abnormal by 127 women (69.02%), being heavy in 41.84% (n = 77), light in 20.65% (n = 38), and absent in 6.52% (n = 12). Overall, 30.97% (n = 57) of the women described the volume as normal. When discriminating by biologics, 17 women (9.23%) vaccinated with Pfizer reported light volume, while the others reported predominantly heavy cycles (J&J/Janssen n = 18, Sinovac n = 21, Moderna n = 9, others n = 7, AstraZeneca n = 6).
Affectation in the quality of life
Of the 950 women who answered the survey, 31.36% (n = 298) commented that their QoL was affected after vaccination. Likewise, of the 184 who reported alterations in their menstrual cycle, 55.97% (103) stated a deterioration in their QoL after having been vaccinated in all the standard indicators evaluated perception of wealthiness, physical and mental health, pain.
Discussion
In the present study, 184 women reported changes in their menstrual cycle. Results documented that at least one of the four parameters indicated by FIGO to describe the menstrual cycle was outside the normal range after application of the vaccine and, therefore, determined an alteration in the menstrual cycle.
The menstrual cycle involves complex interactions between various tissues, hormones, and organ systems and can be altered by multiple physiological and pathological variables, including viral infections and lifestyle changes.10,11 Most physiological processes in the female reproductive tract involve inflammatory elements. 12 Both cytokines and chemokines become regulators of the uterine environment, playing roles throughout the cycle. 12 The inflammatory response is involved in tissue repair, angiogenesis, degradation, remodeling, and proliferation of the endometrium. 12
COVID-19 is considered a pro-inflammatory disease, which generates a cytokine storm and the consequent immune exhaustion. 13 SARS-CoV-2 infection, regardless of vaccination status, has been reported to alter the menstrual cycle. 14
To achieve immunization against a disease, the body must mount an immune response, which is clinically manifested in minor post-vaccination side effects (malaise, headache, fever, and gastrointestinal symptoms), whose final result is the appearance of immunological memory. 15 The COVID-19 vaccine generates an activation of T lymphocytes capable of inducing SARS-CoV-2 neutralizing antibodies. 15 In the present study, after vaccination, the characteristic with the highest percentage of alteration was volume, presenting in 127 women (heavy 41.84% (n = 77), light 20.65% (n = 38), and amenorrhea/absent 6.52% (n = 12)). A plausible theory for this alteration is extrapolated from the pathophysiology of patients with abnormal uterine bleeding due to increased volume. 12 In these patients, a marked expression of macrophages and endometrial leukocytes capable of secreting powerful vasodilators is observed, explaining the increased volume of bleeding in patients. 12
Post-vaccination amenorrhea could be explained by the physiology of functional hypothalamic amenorrhea. 16 This consists in the absence of menstruation resulting from abrupt weight loss, excessive exercise, and/or stress, conditions that lead to negative energy expenditure, where menstruation, not being a vital function, becomes absent. 16 In response to vaccination, energy expenditure favors processes such as leukocyte activation of T lymphocytes to produce antibodies against COVID-19. This situation of stress favors alterations at the level of the hypothalamic-pituitary-gonadal axis, leading woman to a state of amenorrhea.13,15,16
The hypothalamic-pituitary-gonadal axis has the function of controlling the regularity of the cycle, 10 any stressful event or an exaggerated immune response, such as that triggered by immunization, can affect it temporarily. 9 The cycle length results from events leading to recruitment and maturation of the dominant follicle during the follicular phase, processes known to be affected by stress.9,10 Although the present study did not show an important alteration for all the parameters, the theories are applicable for the cases in which it did occur.
The findings of the research carried out are consistent with what is currently described in the literature.2,7,9,17 COVID-19 vaccination is associated with changes in the menstrual cycle. In any case, the alterations mentioned by different women imply an impact on their quality of life, so it is pertinent to delve into future studies focused on the subject.
Although the sample analyzed is small, this is the first study that includes participants from South America and other continents. The results were collected by self-registration, representing strengths and limitations. Within the limitations, there is the possibility of inaccuracies in the veracity of the information due to information and selection bias. Only women with normal basal cycles were analyzed to describe changes in the menstrual cycle after vaccination; this limits the scope of the information in other population groups. Just the cycle immediately before and after vaccination was investigated, so it is not possible to establish whether the referred alterations in the cycle are reversible based on this study. The most prevalent vaccine in our study was Pfizer, and this introduces additional potential bias and makes it hard to reach any specific conclusion about any vaccine; nevertheless, we described our results.
As strengths, the simplicity of the questionnaires with self-registration allows a greater scope for the measurement and description of this phenomenon. In addition, repetition bias was limited by filtering the number of surveys by identity document. Using digital surveys is a safe way to conduct research during the pandemic.
From this study, it is possible to generate the hypothesis that SARS-CoV-2 infection, the COVID-19 vaccine, and/or pandemic-related stress could influence the menstrual cycle.
Conclusion
Vaccination is the most effective public health measure available for the control of contagious infectious diseases such as COVID-19, regardless of its effect on the menstrual cycle. Considering that more than half of the world’s population is women, 18 it is important to know and state the side effects generated by vaccination and SARS-CoV-2 infection in women’s health. More studies are needed that include information on other parameters related to menstrual health (dysmenorrhea, constipation, premenstrual tension syndrome, emotional state, among others): as well as the evaluation of larger cohorts to determine the proportion of women with long-term alterations.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057221109375 for Menstrual cycle disturbances after COVID-19 vaccination by Luisa Rodríguez Quejada, María Fernanda Toro Wills, María Cristina Martínez-Ávila and Andrés Felipe Patiño-Aldana in Women’s Health
Footnotes
Author contribution(s): Luisa Rodriguez Quejada: Conceptualization; Methodology; Writing—original draft.
Maria Fernanda Toro Wills: Conceptualization; Methodology; Writing—original draft.
Maria Cristina Martínez-Ávila: Conceptualization; Data curation; Formal analysis; Methodology; Writing—review & editing.
Andrés Felipe Patiño-Aldana: Data curation; Formal analysis; Writing—review & editing.
Consent for publication: All authors provide full consent for publication.
Consent to participate: Informed consent was received from each participant surveyed in either English or Spanish.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethics approval and review were performed according to the Declaration of Helsinki and were obtained from the institutional review board (Reference Number: CMNR-01105.084W).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: María Cristina Martínez-Ávila  https://orcid.org/0000-0002-1542-0249
https://orcid.org/0000-0002-1542-0249
Supplemental material: Supplemental material for this article is available online.
References
- 1. Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiol Genomics 2020; 52: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li K, Chen G, Hou H, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online 2021; 42(1): 260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ElBagoury M, Tolba MM, Nasser HA, et al. The find of COVID-19 vaccine: challenges and opportunities. J Infect Public Health 2021; 14(3): 389–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines 2021; 9(5): 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Critchley HOD, Babayev E, Bulun SE, et al. Menstruation: science and society. Am J Obstet Gynecol 2020; 223(5): 624–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munro MG, Critchley HOD, Fraser IS. Corrigendum to “The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions” [Int J Gynecol Obstet 143(2018) 393–408]. Int J Gynaecol Obstet 2019; 144(2): 237. [DOI] [PubMed] [Google Scholar]
- 7. Trogstad L. Increased occurrence of menstrual disturbances in 18- to 30-year-old women after COVID-19 vaccination. SSRN Electron J. Epub ahead of print 1 January 2022. DOI: 10.2139/ssrn.3998180. [DOI] [Google Scholar]
- 8. GOV.UK. Coronavirus vaccine—summary of Yellow Card reporting, https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting
- 9. Edelman A, Boniface E, Benhar E, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a U.S. cohort. Obstet Gynecol 2022; 139: 940–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor H, Pal L, Seli E. Speroff’s clinical gynecologic endocrinology and infertility. Philadelphia, PA: Lippincott Williams & Wilkins, 2020. [Google Scholar]
- 11. Valsamakis G, Chrousos G, Mastorakos G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology 2019; 100: 48–57. [DOI] [PubMed] [Google Scholar]
- 12. Berbic M, Ng CHM, Fraser IS. Inflammation and endometrial bleeding. Climacteric 2014; 17(Suppl. 2): 47–53. [DOI] [PubMed] [Google Scholar]
- 13. Paces J, Strizova Z, Smrz D, et al. COVID-19 and the immune system. Physiolog Res 2020; 69(3): 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol 2022; 226(2): 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molaei S, Dadkhah M, Asghariazar V, et al. The immune response and immune evasion characteristics in SARS-CoV, MERS-CoV, and SARS-CoV-2: vaccine design strategies. Int Immunopharmacol 2021; 92: 107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huhmann K. Menses requires energy: a review of how disordered eating, excessive exercise, and high stress lead to menstrual irregularities. Clin Ther 2020; 42(3): 401–407. [DOI] [PubMed] [Google Scholar]
- 17. Male V. Menstruation and COVID-19 vaccination. BMJ 2022; 376: o142. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen MW, Stefanick ML, Peragine D, et al. Gender-related variables for health research. Biol Sex Differ 2021; 12(1): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057221109375 for Menstrual cycle disturbances after COVID-19 vaccination by Luisa Rodríguez Quejada, María Fernanda Toro Wills, María Cristina Martínez-Ávila and Andrés Felipe Patiño-Aldana in Women’s Health