This cohort study examines the association of intravascular left ventricular assist device or intra-aortic balloon pump use with clinical outcomes and cost in patients with acute myocardial infarction complicated by cardiogenic shock.
Key Points
Question
Is there a difference in short-term and long-term clinical outcomes and costs for patients who received an intravascular microaxial left ventricular assist device (LVAD) vs intra-aortic balloon pump (IABP) for acute myocardial infarction (AMI) complicated by cardiogenic shock?
Findings
In this propensity-matched cohort study including 817 matched patients undergoing percutaneous coronary intervention for AMI complicated by cardiogenic shock, use of an intravascular LVAD vs IABP was associated with significantly higher 30-day and 1-year mortality, bleeding, kidney replacement therapy, and total health care costs.
Meaning
Use of an intravascular LVAD vs IABP in patients with AMI complicated by cardiogenic shock was associated with worse clinical outcomes and higher costs up to 1 year.
Abstract
Importance
Intravascular microaxial left ventricular assist device (LVAD) compared with intra-aortic balloon pump (IABP) has been associated with increased risk of mortality and bleeding among patients with acute myocardial infarction (AMI) and cardiogenic shock (CS) undergoing percutaneous coronary intervention (PCI). However, evidence on the association of device therapy with a broader array of clinical outcomes, including data on long-term outcomes and cost, is limited.
Objective
To examine the association between intravascular LVAD or IABP use and clinical outcomes and cost in patients with AMI complicated by CS.
Design, Setting, and Participants
This retrospective propensity-matched cohort study used administrative claims data for commercially insured patients from 14 states across the US. Patients included in the analysis underwent PCI for AMI complicated by CS from January 1, 2015, to April 30, 2020. Data analysis was performed from April to November 2021.
Exposures
Use of either an intravascular LVAD or IABP.
Main Outcomes and Measures
The primary outcomes were mortality, stroke, severe bleeding, repeat revascularization, kidney replacement therapy (KRT), and total health care costs during the index admission. Clinical outcomes and cost were also assessed at 30 days and 1 year.
Results
Among 3077 patients undergoing PCI for AMI complicated by CS, the mean (SD) age was 65.2 (12.5) years, and 986 (32.0%) had cardiac arrest. Among 817 propensity-matched pairs, intravascular LVAD use was associated with significantly higher in-hospital (36.2% vs 25.8%; odds ratio [OR], 1.63; 95% CI, 1.32-2.02), 30-day (40.1% vs 28.3%; OR, 1.71; 95% CI, 1.37-2.13), and 1-year mortality (58.9% vs 45.0%; hazard ratio [HR], 1.44; 95% CI, 1.21-1.71) compared with IABP. At 30 days, intravascular LVAD use was associated with significantly higher bleeding (19.1% vs 14.5%; OR, 1.35; 95% CI, 1.04-1.76), KRT (12.2% vs 7.0%; OR, 1.88; 95% CI, 1.30-2.73), and mean cost (+$51 680; 95% CI, $31 488-$75 178). At 1 year, the association of intravascular LVAD use with bleeding (29.7% vs 24.3%; HR, 1.36; 95% CI, 1.05-1.75), KRT (18.1% vs 10.9%; HR, 1.95; 95% CI, 1.35-2.83), and mean cost (+$46 609; 95% CI, $22 126-$75 461) persisted.
Conclusions and Relevance
In this propensity-matched analysis of patients undergoing PCI for AMI complicated by CS, intravascular LVAD use was associated with increased short-term and 1-year risk of mortality, bleeding, KRT, and cost compared with IABP. There is an urgent need for additional evidence surrounding the optimal management of patients with AMI complicated by CS.
Introduction
Cardiogenic shock is the leading cause of death in acute myocardial infarction and has an estimated mortality between 30% and 50%.1,2 Other than early revascularization,3 treatment paradigms have revolved around maintaining hemodynamics and preventing multiorgan dsyfunction.1,2 Over the past several decades, the predominant form of mechanical circulatory support has been the intra-aortic balloon pump (IABP).2 Given the lack of benefit of routine IABP use for cardiogenic shock and greater improvement in hemodynamic parameters from ventricular assist devices,4,5 there has been a substantial increase in percutaneous circulatory support devices, such as intravascular microaxial left ventricular assist devices (LVADs).6,7 However, randomized clinical trials comparing IABP use with intravascular LVADs have been small, limited by short follow-up, and have not shown a difference in mortality.5,8
Recent retrospective, propensity-matched analyses in patients with cardiogenic shock undergoing percutaneous coronary intervention (PCI) have found an association with worse clinical outcomes with intravascular LVAD compared with IABP use.9,10 A study from 2 national registries found a significantly higher risk of in-hospital death and major bleeding in patients receiving an intravascular LVAD.9 Another using administrative data similarly found that intravascular LVAD use was associated with a higher risk of in-hospital death, bleeding, and stroke.10 However, substantial gaps remain because these outcomes were limited to the index admission. Furthermore, little is known regarding device selection and associations with kidney dysfunction or cost. Similarly, older reports in high-risk PCI have suggested fewer episodes of repeat revascularization with intravascular LVAD support, but findings in cardiogenic shock are limited. In addition, both outcomes have important implications for patient quality of life.
Given these gaps in the literature, we sought to use contemporary data collected from a large, commercially insured population to assess associations with clinical outcomes between intravascular LVAD vs IABP use in patients undergoing PCI for acute myocardial infarction complicated by cardiogenic shock. Outcomes of interest included mortality, bleeding, stroke, kidney replacement therapy, repeat revascularization, and cost during the index admission, at 30 days, and up to 1 year.
Methods
Study Participants
We used administrative claims data from a large, geographically diverse commercially insured cohort of patients from 14 states across the US. The data come from the largest health insurer by market share and are representative of the commercially insured population in the US. The data contain fully integrated and identifiable longitudinal databases combining medical and pharmacy claims and laboratory results drawn from nearly 60 million unique individuals from 2006 to present. In 2020, the data covered 13.0% of the US population. Data are complete except for zip code–level data, which have less than 5.0% missingness.
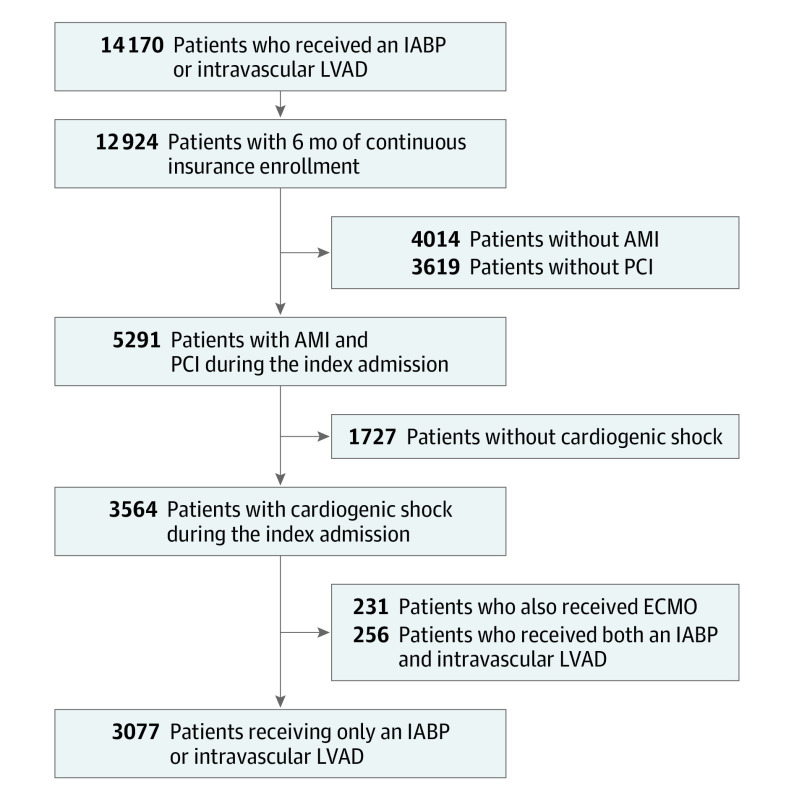
Overall, there were 14 169 patients who received an IABP or intravascular LVAD during an inpatient hospitalization from January 1, 2015, to April 30, 2020. All individuals were required to have 6 months of continuous health insurance enrollment prior to the index procedure. Additionally, participants were restricted to those who had a hospitalization with a first or second diagnosis of acute myocardial infarction complicated by cardiogenic shock, underwent PCI, were not receiving extracorporeal membrane oxygenation (ECMO), and did not receive both an IABP and intravascular LVAD device during their index hospitalization. After these criteria were applied, data from 3101 patients were available for analysis (Figure 1). This study did not require institutional review board review and patient informed consent because it was not considered human participant research, and only deidentified data were used.
Figure 1. Flow Diagram.
AMI indicates acute myocardial infarction; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; PCI, percutaneous coronary intervention.
Patient demographics, area-level socioeconomic status, medical history, health care utilization, and costs were observed for 6 months prior to the index hospitalization (defined as the baseline period). Demographic characteristics included age, sex, region, health plan type, and year of index hospitalization. Area-level race and ethnicity classification and socioeconomic status were estimated at the block-group level from the American Community Survey. Comorbidity was assessed by the Deyo-Charlson Comorbidity Index score, a validated comorbidity assessment consisting of a weighted sum of 19 comorbidities, with a higher score indicating more comorbidity.11,12
Outcomes
The International Classification of Diseases, Ninth Revision (ICD-9), International Classification of Diseases, Tenth Revision (ICD-10), and Current Procedural Terminology codes used to identify procedures, outcomes, and diagnoses are listed in eTable 1 in the Supplement. Primary outcomes during the index hospitalization included severe bleeding (inpatient hospitalization with bleeding or bleeding requiring a transfusion),10 length of hospital stay, intensive care unit (ICU) length of stay, mortality, and health care cost. We also assessed outcomes at 30 days and 1 year after the index date. Outcomes measured during these follow-up periods included bleeding, stroke/transient ischemic attack, repeat revascularization, kidney replacement therapy, mortality, and health care cost. For the 1-year outcomes, patients were followed up until the end of the study period (April 30, 2020), end of insurance enrollment, or death, whichever event occurred first. Total health care costs include the entire allowed amount incurred by all treatment provided during the specified time span (eg, index, 30 days, 1 year). Costs reflect the total paid by both the insurer and patient (as opposed to charges or projected cost).
Statistical Analyses
Baseline characteristics were compared between patients receiving an intravascular LVAD or IABP. A 1:1 propensity score–matched study sample consisting of patients who received an intravascular LVAD or IABP was calculated and used for all analyses. The propensity score match was performed using multivariate logistic regression to estimate the association of intravascular LVAD use with baseline characteristics, including age, sex, race and ethnicity, region, Deyo-Charlson Comorbidity Index score,12 comorbid conditions, and preceding health care utilization and costs. The IABP and intravascular LVAD groups were selected using a greedy algorithm using an 8-to-1 digit match.13 In total, 20 patients with intravascular LVADs had missing zip code data, and 21 were not matched based on our algorithm.
To analyze binary outcomes associated with intravascular LVAD compared with IABP users during the index hospitalization and at 30 days, we used conditional logistic regression models to calculate odds ratios (ORs). Poisson regression models were used to calculate the rate ratios (RRs) for length of stay and health care utilization variables. Gamma regression models were used to calculate the mean difference in cost variables associated with device groups. Time-to-event outcomes were analyzed using stratified Cox proportional hazard regression models to calculate the association between clinical outcomes and device groups up to 1 year. Patients were censored if they were no longer enrolled with our health plans or reached the end of the study period. Cox models were assessed for violation of the proportional hazards assumption by including a time-dependent covariate for the interaction between intravascular LVAD and time to event. Lastly, we constructed Kaplan-Meier survival curves to compare mechanical circulatory support devices and compared the groups using log-rank tests. Use of IABP was the referent category in all regression analyses.
To confirm the robustness of our findings, we performed several sensitivity analyses. First, to include all patients in the analysis, including those dropped owing to matching, we used our propensity score as an adjustment variable with the entire cohort. Assumptions of linearity for use of the propensity score as a covariate were assessed by scatter plots and the Box-Tidwell test. Second, given that ECMO is often combined with other support devices to treat cardiogenic shock,14 we performed a sensitivity analysis including patients who received ECMO in addition to an IABP or intravascular LVAD during the index hospitalization (n = 231). Third, given the potential effect of cardiac arrest on outcomes, we repeated our analyses excluding patients with cardiac arrest. Fourth, we conducted a landmark analysis for patients that survived their index hospitalization up to 1 year. Finally, we performed analyses on falsification end points to assess for residual bias. Falsification end points included pneumonia and the composite outcome of diarrhea, cellulitis, deep vein thrombosis, intestinal obstruction, and osteomyelitis consistent with previous analyses.10 P values were 2-tailed, and values less than .05 were considered statistically significant. All statistical analyses were performed using SAS Enterprise Guide, version 9.3 (SAS Institute Inc).
Results
Study Cohort
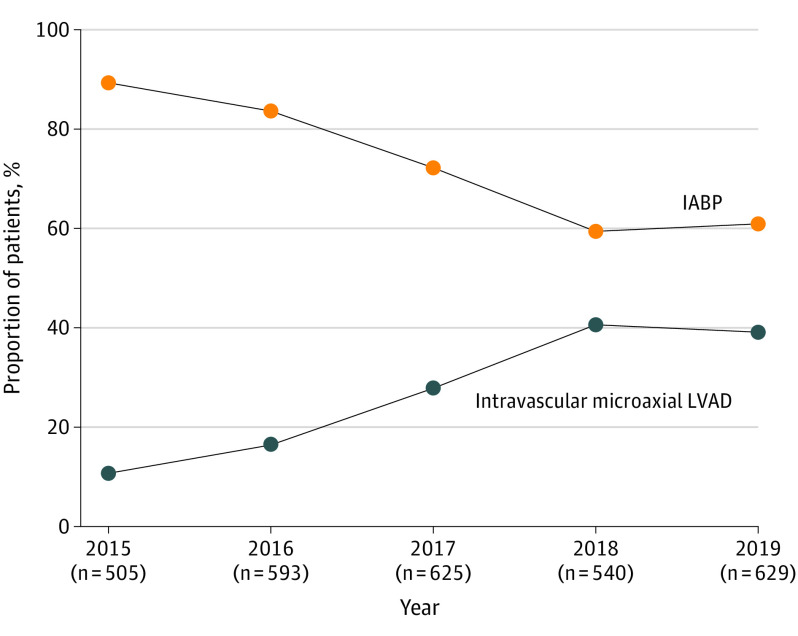
Over the study period, we identified 3077 patients across 14 states who received an intravascular LVAD or IABP for acute myocardial infarction complicated by cardiogenic shock undergoing PCI. Of these patients, 858 (27.9%) received an intravascular LVAD, and 2219 (72.1%) received an IABP (Table). The mean (SD) age was 65.2 (12.5) years, 28.9% (n = 890) were women, and 32.0% (n = 986) had cardiac arrest between first medical contact and the day of device implantation (eTable 2 in the Supplement). Beginning in 2015, the proportion of patients per year receiving an intravascular LVAD increased from 10.7% (n = 54) to 39.1% (n = 246), and IABP use decreased from 89.3% (n = 451) to 60.9% (n = 383) in 2019 (Figure 2). Patients receiving an intravascular LVAD were more likely than those receiving an IABP to have hypertension, diabetes mellitus, heart failure, atrial fibrillation, peripheral vascular disease, chronic lung disease, and chronic kidney disease and to develop cardiac arrest before the date of device implantation (all, P < .05; Table). Sex and age were similar between groups (both, P > .05; Table). Health care utilization in the preceding 6 months before the index hospitalization was higher in patients receiving an intravascular LVAD compared with IABP (P > .05; eTable 3 in the Supplement).
Table. Baseline Characteristics of Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction Complicated by Cardiogenic Shock and Propensity-Matched Patients Receiving an IABP or Intravascular LVAD.
| Characteristic | No. (%) | P value | Propensity-matched cohort | |||
|---|---|---|---|---|---|---|
| IABP (n = 2219) | Intravascular LVAD (n = 858) | No. (%) | Standardized mean difference | |||
| IABP (n = 817) | Intravascular LVAD (n = 817) | |||||
| Demographics | ||||||
| Age, mean (SD), y | 65.0 (12.5) | 65.6 (12.6) | .23 | 66.1 (12.7) | 65.7 (125) | −0.03 |
| Sex | ||||||
| Female | 643 (29) | 242 (28) | .68 | 218 (27) | 231 (28) | 0.04 |
| Male | 1576 (71) | 616 (72) | 599 (73) | 586 (72) | ||
| Ratio of race and ethnicity at zip code level, mean (SD) | ||||||
| African American/Black | 0.09 (0.2) | 0.11 (0.2) | <.001 | 0.10 (0.2) | 0.11 (0.2) | 0.03 |
| Hispanic | 0.12 (0.2) | 0.12 (0.2) | .99 | 0.12 (0.2) | 0.12 (0.2) | 0.01 |
| White | 0.80 (0.2) | 0.77 (0.2) | <.001 | 0.78 (0.2) | 0.78 (0.2) | 0.02 |
| Health plan type | ||||||
| HMO | 394 (18) | 152 (18) | .92 | 144 (18) | 149 (18) | 0.00 |
| PPO | 1592 (72) | 620 (72) | 591 (72) | 587 (72) | ||
| Other | 232 (11) | 86 (10) | 82 (10) | 81 (10) | ||
| Geographic region | ||||||
| Northeast | 368 (17) | 100 (12) | <.001 | 96 (12) | 98 (12) | 0.03 |
| Midwest | 678 (31) | 212 (25) | 202 (25) | 208 (25) | ||
| South | 604 (27) | 301 (35) | 280 (34) | 284 (35) | ||
| West | 497 (22) | 219 (26) | 223 (27) | 212 (26) | ||
| Mean socioeconomic status index, %a | 54.4 (5.8) | 53.9 (5.8) | .05 | 54.2 (5.8) | 54.0 (5.8) | 0.007 |
| Year of index admission | ||||||
| 2015 | 451 (20) | 54 (6) | <.001 | 58 (7) | 53 (6) | 0.06 |
| 2016 | 496 (22) | 97 (12) | 90 (11) | 96 (12) | ||
| 2017 | 451 (20) | 174 (20) | 178 (22) | 171 (21) | ||
| 2018 | 321 (14) | 219 (25) | 201 (25) | 201 (24) | ||
| 2019 | 383 (17) | 246 (29) | 224 (27) | 230 (28) | ||
| 2020 (Up to April 30) | 117 (5) | 68 (8) | 66 (8) | 66 (8) | ||
| Cardiac arrestb | 685 (31) | 301 (35) | .022 | 285 (35) | 288 (35) | 0.007 |
| Comorbidities | ||||||
| Charlson Comorbidity Index score, mean (SD) | 1.5 (2.2) | 1.9 (2.5) | <.001 | 1.8 (2.3) | 1.8 (2.4) | 0.02 |
| Hypertension | 1157 (52) | 485 (57) | .02 | 457 (56) | 459 (56) | 0.00 |
| Dyslipidemia | 911 (41) | 379 (44) | .08 | 354 (43) | 354 (43) | 0.00 |
| Diabetes mellitus | 615 (28) | 272 (32) | .02 | 249 (30) | 253 (31) | 0.01 |
| Tobacco use | 303 (14) | 131 (15) | .26 | 119 (15) | 121 (15) | 0.01 |
| Obesity | 177 (8) | 80 (9) | .18 | 78 (10) | 75 (9) | −0.01 |
| Previous PCI | 44 (2) | 21 (2) | .10 | 21 (3) | 18 (2) | −0.02 |
| Previous CABG | <10 (<1)c | <10 (1)c | .15 | <10 (<1)c | <10 (<1)c | 0.00 |
| Heart failure | 264 (12) | 163 (19) | <.001 | 147 (18) | 143 (18) | −0.01 |
| Atrial fibrillation | 154 (7) | 88 (10) | .002 | 82 (10) | 79(10) | 0.01 |
| Cerebrovascular disease | 189 (9) | 83 (10) | .36 | 84 (10) | 78 (10) | −0.02 |
| Peripheral vascular disease | 235 (11) | 146 (17) | <.001 | 123 (15) | 125 (15) | 0.01 |
| Chronic lung disease | 222 (10) | 116 (14) | .006 | 97 (12) | 104 (13) | 0.03 |
| Cancer | 189 (9) | 66 (8) | .65 | 60 (7) | 63 (8) | 0.01 |
| Chronic kidney disease | 271 (12) | 154 (18) | <.001 | 136 (17) | 137 (17) | 0.00 |
Abbreviations: CABG, coronary artery bypass grafting; HMO, health maintenance organization; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; PCI, percutaneous coronary intervention; PPO, preferred provider organization.
Based on zip code.
From first medical contact to the day of device implantation.
Groups with fewer than 10 patients are shown as “<10” to preserve patient confidentiality.
Figure 2. Proportion of Patients Receiving an Intravascular Left Ventricular Assist Device (LVAD) vs Intra-aortic Balloon Pump (IABP) Over Time.
Outcomes of Intravascular LVAD vs IABP
Our 1:1 propensity-matched algorithm including patients who received an intravascular LVAD or IABP yielded 817 matched pairs and accounted for 95.2% (817 of 858) of the patients who received an intravascular LVAD. The standardized mean differences for 36 of 36 characteristics of the propensity-matched cohorts were below 0.10.
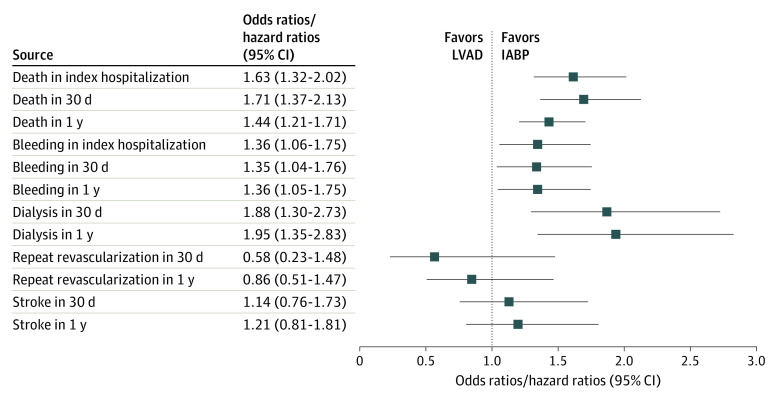
In the propensity-matched cohort, use of an intravascular LVAD was associated with a significantly higher risk of in-hospital mortality compared with patients receiving an IABP (36.2% vs 25.8%; OR, 1.63; 95% CI, 1.32-2.02; P < .001) (Figure 3). Severe bleeding during the index hospitalization was also higher in patients who received an intravascular LVAD (20.3% vs 15.5%; OR, 1.36; 95% CI, 1.06-1.75; P = .02). The mean hospital length of stay (11.8 vs 11.7 days; P = .53) was similar, but the ICU length of stay was shorter in the IABP group (10.3 vs 9.7 days; P = .001).
Figure 3. Clinical Outcomes Among Propensity-Matched Patients Receiving an Intravascular Left Ventricular Assist Device (LVAD) vs Intra-aortic Balloon Pump (IABP).
Odds ratios are for outcomes during the index hospitalization and at 30 days; hazard ratios are for outcomes at 1 year.
At 30 days, 7.4% of patients in both groups remained in the hospital (P = .84). Intravascular LVAD use was associated with a higher risk of mortality (40.1% vs 28.3%; OR, 1.71; 95% CI, 1.37-2.13; P < .001), severe bleeding (19.1% vs 14.5%; OR, 1.35; 95% CI, 1.04-1.76; P = .03), and kidney replacement therapy (12.2% vs 7.0%; OR, 1.88; 95% CI, 1.30-2.73; P = .001) compared with IABP use (eTable 4 in the Supplement). We did not find a statistically significant difference in incident stroke/transient ischemic attack or repeat revascularization between device groups.
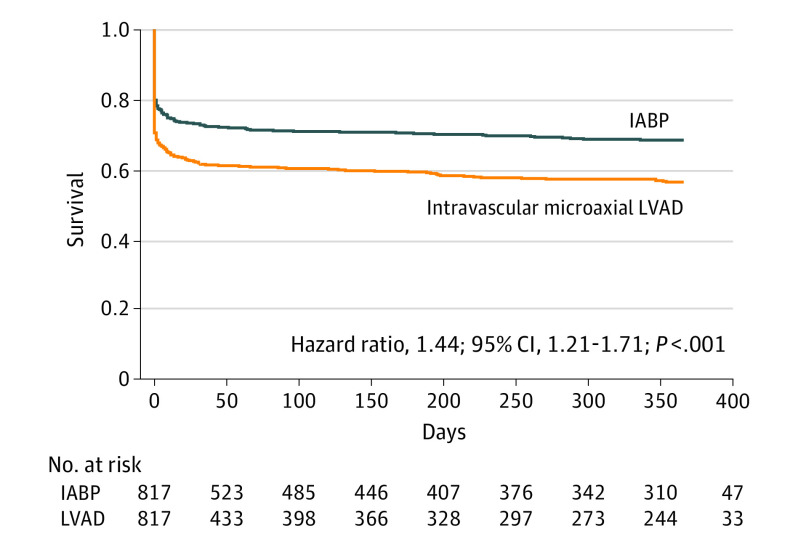
At 1 year, mortality (58.9% vs 45.0%; hazard ratio [HR], 1.44; 95% CI, 1.21-1.71; P < .001) (Figure 4), severe bleeding (29.7% vs 24.3%; HR, 1.36; 95% CI, 1.05-1.75; P = .02), and kidney replacement therapy (18.1% vs 10.9%; HR, 1.95; 95% CI, 1.35-2.83; P < .001) remained higher in the intravascular LVAD group compared with the IABP group. There were no differences in incident stroke/transient ischemic attack or repeat revascularization between device groups (both P > .05; eFigure in the Supplement).
Figure 4. Kaplan-Meier Curves for 1-Year Mortality Among Propensity-Matched Patients Receiving an Intravascular Left Ventricular Assist Device (LVAD) vs Intra-aortic Balloon Pump (IABP).
Cost of Intravascular LVAD vs IABP
During the index hospitalization, intravascular LVAD use was associated with a significantly higher mean cost of +$60 279 (95% CI, $43 245-$79 328; P < .001) compared with IABP recipients (eTable 5 in the Supplement). Similarly, intravascular LVAD use was associated with an increased mean cost of +$51 680 (95% CI, $31 488-$75 178; P < .001) at 30 days and +$46 609 (95% CI, $22 126-$75 461; P = .004) at 1 year.
Sensitivity Analyses
After including all 3077 patients in the cohort with the propensity score as an adjustment variable, our primary findings were unchanged (eTable 6 in the Supplement). Including patients who also received ECMO, we identified 893 propensity-matched pairs. Use of an intravascular LVAD was associated with a significantly higher risk of severe bleeding (OR, 1.39; 95% CI, 1.11-1.75; P = .005) and in-hospital mortality (OR, 1.76; 95% CI, 1.43-2.69; P < .001) compared with patients receiving an IABP. With follow-up to 1 year, risk of severe bleeding (HR, 1.31; 95% CI, 1.05-1.64; P = .02), kidney replacement therapy (HR, 1.86; 95% CI, 1.35-2.56; P = .001), and mortality (HR, 1.50; 95% CI, 1.27-1.77; P < .001) remained higher in patients who received an intravascular LVAD compared with IABP. After excluding patients with cardiac arrest, kidney replacement therapy and mortality remained higher in those receiving an intravascular LVAD, but severe bleeding was no longer statistically significant. Finally, in a landmark analysis including patients who survived the index hospitalization, 1-year bleeding, kidney replacement therapy, and mortality remained higher in the intravascular LVAD group (P < .05 for all; eTable 7 in the Supplement).
Falsification End Points
In the propensity-matched cohort, we did not find a statistically significant difference between intravascular LVAD compared with IABP use with community-acquired pneumonia or the composite outcome of diarrhea, cellulitis, deep vein thrombosis, intestinal obstruction, or osteomyelitis (eTable 8 in the Supplement).
Discussion
In this commercially insured population, we found that the use of an intravascular LVAD was associated with a significantly higher risk of in-hospital, 30-day, and 1-year mortality compared with patients who received an IABP. Similarly, intravascular LVAD use was associated with a higher risk of bleeding and kidney replacement therapy at both 30 days and 1 year. We did not find a statistically significant difference in incident stroke/transient ischemic attack or repeat revascularization. In addition to higher index admission costs, intravascular LVAD use was associated with a mean increase in cost of +$51 680 at 30 days and +$46 609 at 1 year.
These findings build on and extend prior analyses that have examined the association of various devices and clinical outcomes among patients with acute myocardial infarction complicated by cardiogenic shock undergoing PCI. Our results showing an association with higher in-hospital mortality for patients receiving an intravascular LVAD compared with IABP are consistent with 2 recent analyses.9,10 However, these studies restricted outcomes to the index hospitalization. Our findings have extended the follow-up period to 30 days and 1 year, both of which showed an association with higher mortality in patients receiving an intravascular LVAD. In contrast, another recent propensity-matched analysis compared patients from the IABP-SHOCK II (Intra-aortic Balloon Pump in Cardiogenic Shock) trial (both IABP and medical therapy patients) to patients receiving an intravascular LVAD from the EUROSHOCK registry and did not find a difference in 30-day mortality.15 In addition, it must be noted that, albeit small (fewer than 100 patients combined),8 available randomized clinical trials did not show a difference in mortality. However, previous trials were not powered to detect a mortality difference and may not reflect clinical practice. In addition, discrepant findings with this study may represent differences in inclusion criteria and practice patterns in Europe compared with the US.
All 3 of these recent studies found an association with increased bleeding in the intravascular LVAD group. However, bleeding was variably defined and differed substantially between hospitals, ranging from 3.0% to 16.0% in the IABP group and 8.5% to 33.0% in the intravascular LVAD group.9,10,15,16 Similarly, we found a statistically significant increase in bleeding for patients who received an intravascular LVAD compared with IABP, which may be 1 potential mechanism for the increased mortality.
Among numerous potential complications of cardiogenic shock,17 kidney failure often represents severe end-organ hypoperfusion and portends a particularly poor prognosis.18,19,20 Importantly, studies assessing associations of device selection and subsequent kidney failure are limited and often have mixed results depending on the included population. In a group of patients with acute myocardial infarction complicated by cardiogenic shock, intravascular LVAD compared with IABP use showed a borderline but not significant association (OR, 1.08; 95% CI, 1.00-1.17; P = .05) with acute kidney injury as assessed by administrative billing codes.10 Alternatively, smaller single-center or registry data have suggested a potentially protected effect of intravascular LVAD use in patients undergoing high-risk PCI.21,22 In our cohort of patients with cardiogenic shock, we found an association with intravascular LVAD use and kidney replacement therapy at 30 days and 1 year, which may partly explain the association with higher mortality in the intravascular LVAD group. Of note, the higher cardiac output ascribed to percutaneous circulatory support devices did not appear to provide kidney protection in our analysis; rather, use of the devices was associated with increased rates of kidney replacement therapy.
Using administrative data, Amin et al10 compared costs between the intravascular LVAD era, defined as 2008 to 2016, to the pre–intravascular LVAD era, defined as 2004 to 2007, in patients undergoing PCI for acute myocardial infarction. While not a direct comparison to IABP, they found that the mean per-patient hospitalization costs increased by $1775 despite similar ICU and hospital length of stays. In a more contemporary cohort directly comparing an intravascular LVAD with IABP, the current study found that in-hospital costs were associated with a mean increase of $60 279 despite a similar ICU and modestly shorter (<1 day) hospital length of stay in the intravascular LVAD group. Furthermore, this trend of higher costs with intravascular LVAD use continued at 30 days and 1 year.
Limitations
In addition to being a retrospective analysis, the results should be interpreted with several limitations. First, we used administrative billing and claims data, which lack important variables in cardiogenic shock, including laboratory values, vital signs, medications, coronary anatomy, timing of device implantation relative to PCI, and hemodynamics. However, the cohort included a relatively large, well-described patient population that required 6 months of preceding health care enrollment before the index hospitalization. Second, although we included a robust propensity-matched cohort and conducted several sensitivity and falsification end point analyses, we cannot rule out residual confounding or selection bias. Third, we were not able to identify which type of intravascular LVAD (eg, 2.5, CP, 5.0, 5.5, RP) was used and therefore cannot assess the level of hemodynamic support. Fourth, we included only commercially insured patients, who likely represent a unique, potentially healthier patient population, which may explain our reported mortality (approximately 30%) being on the lower end expected for cardiogenic shock. However, our cohort included a geographically diverse group of patients with demographics, including age and sex as well as the proportion experiencing cardiac arrest, similar to previous studies.9,10 In addition, because our insurance database does not include all patients presenting to an institution, we were unable to assess for hospital-level variation in care. Our contemporary analysis suggests that potential improvements in clinician experience using intravascular LVADs have not improved outcomes.7,9,10
Conclusions
In this cohort study among patients undergoing PCI for acute myocardial infarction complicated by cardiogenic shock, intravascular LVAD use was associated with significantly higher mortality, bleeding, kidney replacement therapy, and cost both during the hospitalization and up to 1 year after discharge. Given poorer short-term and long-term outcomes and higher associated health care costs, there is an urgent need for properly powered randomized clinical trials to better inform clinical decisions in this critically ill patient population, as well as to properly understand mechanistic differences between device selection and outcomes.
eTable 1. Administrative coding used to identify procedures, outcomes, and diagnoses
eTable 2. Baseline characteristics of the overall population of patients undergoing percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock receiving an intra-aortic balloon pump or intravascular left ventricular assist device
eTable 3. Healthcare utilization preceding index hospitalization for acute myocardial infraction complicated by cardiogenic shock
eTable 4. Clinical outcomes at 30-days and 1-year in the propensity matched cohort
eTable 5. Mean total medical costs for patients undergoing percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock in the propensity-matched cohort
eTable 6. Sensitivity analyses including our propensity score as a covariate, including patients also receiving extracorporeal membrane oxygenation, and exclusion of patients who had cardiac arrest
eTable 7. Landmark analysis for patients who survived their index admission up to 1-year in the propensity matched cohort
eTable 8. Falsification endpoints among propensity-matched patients receiving an intravascular left ventricular assist device versus intra-aortic balloon pump
eFigure. Kaplan-Meier curves for 1-year bleeding (A), renal replacement therapy (B), stroke (C) and repeat revascularization (D) among propensity-matched patients receiving an intravascular left ventricular assist device versus intra-aortic balloon pump
References
- 1.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671-2683. doi: 10.1093/eurheartj/ehz363 [DOI] [PubMed] [Google Scholar]
- 2.van Diepen S, Katz JN, Albert NM, et al. ; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232-e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 3.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341(9):625-634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 4.Thiele H, Zeymer U, Neumann FJ, et al. ; IABP-SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287-1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 5.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584-1588. doi: 10.1016/j.jacc.2008.05.065 [DOI] [PubMed] [Google Scholar]
- 6.Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175(6):941-950. doi: 10.1001/jamainternmed.2014.7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhruva SS, Ross JS, Mortazavi BJ, et al. Use of mechanical circulatory support devices among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA Netw Open. 2021;4(2):e2037748. doi: 10.1001/jamanetworkopen.2020.37748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouweneel DM, Eriksen E, Seyfarth M, Henriques JP. Percutaneous mechanical circulatory support versus intra-aortic balloon pump for treating cardiogenic shock: meta-analysis. J Am Coll Cardiol. 2017;69(3):358-360. doi: 10.1016/j.jacc.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 9.Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2020;323(8):734-745. doi: 10.1001/jama.2020.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141(4):273-284. doi: 10.1161/CIRCULATIONAHA.119.044007 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 13.Parsons LS. Performing a 1:N case-control match on propensity score. In: Proceedings of the 29th Annual SAS Users Group International Conference SAS Institute. 2004:Abstract 165-29. Accessed June 14, 2022. https://support.sas.com/resources/papers/proceedings/proceedings/sugi29/165-29.pdf
- 14.Miller PE, Solomon MA, McAreavey D. Advanced percutaneous mechanical circulatory support devices for cardiogenic shock. Crit Care Med. 2017;45(11):1922-1929. doi: 10.1097/CCM.0000000000002676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrage B, Ibrahim K, Loehn T, et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139(10):1249-1258. doi: 10.1161/CIRCULATIONAHA.118.036614 [DOI] [PubMed] [Google Scholar]
- 16.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(3):278-287. doi: 10.1016/j.jacc.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 17.Rubini Giménez M, Miller PE, Alviar CL, et al. Outcomes associated with respiratory failure for patients with cardiogenic shock and acute myocardial infarction: a substudy of the CULPRIT-SHOCK trial. J Clin Med. 2020;9(3):E860. doi: 10.3390/jcm9030860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauridsen MD, Gammelager H, Schmidt M, et al. Acute kidney injury treated with renal replacement therapy and 5-year mortality after myocardial infarction-related cardiogenic shock: a nationwide population-based cohort study. Crit Care. 2015;19:452. doi: 10.1186/s13054-015-1170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padkins M, Breen T, Van Diepen S, Barsness G, Kashani K, Jentzer JC. Incidence and outcomes of acute kidney injury stratified by cardiogenic shock severity. Catheter Cardiovasc Interv. 2021;98(2):330-340. doi: 10.1002/ccd.29692 [DOI] [PubMed] [Google Scholar]
- 20.Pöss J, Köster J, Fuernau G, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(15):1913-1920. doi: 10.1016/j.jacc.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 21.Flaherty MP, Moses JW, Westenfeld R, et al. Impella support and acute kidney injury during high-risk percutaneous coronary intervention: the Global cVAD Renal Protection Study. Catheter Cardiovasc Interv. 2020;95(6):1111-1121. doi: 10.1002/ccd.28400 [DOI] [PubMed] [Google Scholar]
- 22.Flaherty MP, Pant S, Patel SV, et al. Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ Res. 2017;120(4):692-700. doi: 10.1161/CIRCRESAHA.116.309738 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Administrative coding used to identify procedures, outcomes, and diagnoses
eTable 2. Baseline characteristics of the overall population of patients undergoing percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock receiving an intra-aortic balloon pump or intravascular left ventricular assist device
eTable 3. Healthcare utilization preceding index hospitalization for acute myocardial infraction complicated by cardiogenic shock
eTable 4. Clinical outcomes at 30-days and 1-year in the propensity matched cohort
eTable 5. Mean total medical costs for patients undergoing percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock in the propensity-matched cohort
eTable 6. Sensitivity analyses including our propensity score as a covariate, including patients also receiving extracorporeal membrane oxygenation, and exclusion of patients who had cardiac arrest
eTable 7. Landmark analysis for patients who survived their index admission up to 1-year in the propensity matched cohort
eTable 8. Falsification endpoints among propensity-matched patients receiving an intravascular left ventricular assist device versus intra-aortic balloon pump
eFigure. Kaplan-Meier curves for 1-year bleeding (A), renal replacement therapy (B), stroke (C) and repeat revascularization (D) among propensity-matched patients receiving an intravascular left ventricular assist device versus intra-aortic balloon pump