Abstract
Purpose
The purpose of this study was to investigate the effect of a cardiac rehabilitation program on the acute response on endothelial progenitor cells and circulating endothelial cells after maximal exercise in patients with chronic heart failure of different severity.
Methods
Forty-four chronic heart failure patients were enrolled in a 36-session cardiac rehabilitation program. All patients underwent an initial maximal cardiopulmonary exercise test before and a final maximal cardiopulmonary exercise test after the cardiac rehabilitation program. The patients were divided in two groups of severity according to the median value of peak VO2. Blood was collected at 4 time points; 2 time points at rest, and 2 time points after each cardiopulmonary exercise test. Five endothelial cellular populations were quantified by flow cytometry.
Results
Although there was a higher increase in the mobilization of subgroups of endothelial progenitor cells and circulating endothelial cells after the final cardiopulmonary exercise test compared to the initial test within each severity group (p < 0.05), no significant differences between severity groups were observed (p > 0.05).
Conclusions
A 36-session cardiac rehabilitation program had similar beneficial effects on the acute response of endothelial progenitor cells and circulating endothelial cells after maximal exercise in patients with chronic heart failure of different severity.
Keywords: Acute maximal exercise, Cardiac rehabilitation, Chronic heart failure, Endothelial progenitor cells, Exercise, Severity
INTRODUCTION
Chronic heart failure (CHF) is a multifactorial clinical syndrome with high incidence worldwide, and it is associated with a poor prognosis and poor quality of life for patients. Vascular endothelial dysfunction is strongly associated with the severity of the syndrome.1,2 Patients with CHF usually present with low levels of endothelial progenitor cells (EPCs) and circulating endothelial cells (CECs) at rest and after exercise compared with healthy populations. Low levels of EPCs and CECs have been associated with attenuated endothelial function,3 while EPCs have been linked to the repair mechanism of endothelial damage.4,5 Maximal exercise has been previously proven to increase the acute mobilization of EPCs and CECs in CHF patients,6 with the beneficial effect being similar in patients of different severity.7 However, the effect of exercise training on the acute response of EPCs and CECs after maximal exercise and the role of severity have not been studied. We hypothesized that an exercise training program would increase the acute response of EPCs and CECs after maximal exercise in patients with CHF of different severity. The aim of the study was to compare the acute mobilization of EPCs and CECs after a maximal cardiopulmonary exercise test (CPET) before and after a cardiac rehabilitation (CR) program in patients with CHF of different severity according to peak VO2.
MATERIALS AND METHODS
This is a post-hoc analysis of a recently published study from our Institute (approval number: 117/3-7-2017) which assessed EPC mobilization after exercise training in patients with CHF.8
Forty-four CHF patients with New York Heart Association (NYHA) class ≥ II and ejection fraction (EF) ≤ 49% signed an informed consent form to enroll in a 36-session CR program. The program included high intensity interval training (HIIT) or a combined HIIT and strength training protocol, as previously described.8 Moreover, all patients underwent an initial symptom-limited maximal CPET on a cycle ergometer (Ergoline 800; SensorMedics Corporation, Anaheim, California) before the CR program, and a final maximal CPET after the 36 sessions of the CR program.
We collected venous blood at 4 time points; 2 time points at rest before the initial and the final CPET, and 2 time points after maximal exercise after the 2 CPET. Monoclonal antibodies CD45-PerCP (BD Pharmingen, cat. no. 340665), CD34-APC (BD Pharmingen, cat. no. 340441), CD133-PE (Miltenyi Biotec, cat. no. 130-080-801) and VEGFR2 (KDR)-PE (R&D Systems, cat. no. FAB 3578) defined 5 endothelial populations; 3 EPC subgroups (CD34+/CD45-/CD133+, CD34+/CD45-/CD133+/VEGFR2 and CD34+/CD133+/VEGFR2), and 2 CEC subgroups (CD34+/CD45-/CD133- and CD34+/CD45-/CD133-/VEGFR2).9 These endothelial populations were quantified by flow cytometry and expressed as median (25th-75th percentiles) in cells/106 enucleated cells.9
The patients were divided in two groups of severity according to the median value of peak VO2 (18.3 ml/kg/min). Their main medications included diuretics, beta-blockers, aldosterone antagonists, and angiotensin-converting enzyme inhibitors (Table 1). Factorial analysis of variance (ANOVA) 2 × 2 × 2 (time × intervention × severity groups) was performed using IBM SPSS version 25. Unadjusted differences between severity groups were analyzed, and p < 0.05 was defined as being statistically significant.
Table 1. Baseline demographic characteristics and maximal cardiopulmonary exercise testing indices of patients with chronic heart failure of different severity according to peak VO2.
| Demographic characteristics | Group 1: ≤ 18.3 ml/kg/min | Group 2: > 18.3 ml/kg/min |
| Number of patients (N) | 23 | 21 |
| Gender (males/females) | 17/6 | 18/3 |
| Age (years)a | 57 ± 11 | 54 ± 9 |
| NYHA stage (class II/III) | 17/6 | 17/4 |
| Ejection fraction (%) | 30 (25-40) | 35 (28-38) |
| Medication | ||
| Diuretics [n (%)] | 19 (83%) | 10 (48%)* |
| ACE inhibitors [n (%)] | 11 (48%) | 11 (52%) |
| beta-blockers [n (%)] | 23 (100%) | 20 (95%) |
| Aldosterone antagonists [n (%)] | 17 (74%) | 15 (71%) |
| Baseline cardiopulmonary exercise testing indices | ||
| Peak VO2 (ml/kg/min)a | 15.1 ± 2.8 | 22.1 ± 2.3* |
| Predicted peak VO2 (%)a | 55 ± 14 | 74 ± 11* |
| Peak WR (watts)a | 82 ± 33 | 122 ± 33* |
ACE, angiotensin-converting-enzyme; NYHA, New York Heart Association; VO2, oxygen uptake; WR, work rate.
Values are expressed as a mean ± SD, b median (25th-75th percentiles).
Difference between the 2 severity groups for demographic characteristics and CPET parameters (* p < 0.05).
RESULTS
Patients with a lower peak VO2 were more likely to receive diuretics compared to those with a higher peak VO2 (p < 0.05, Table 1). No other significant differences regarding demographics, EF or medications were observed between the 2 groups. CPET indices, including peak VO2, predicted peak VO2, and peak work rate were significantly higher in the patients with better functional capacity status (Table 1).
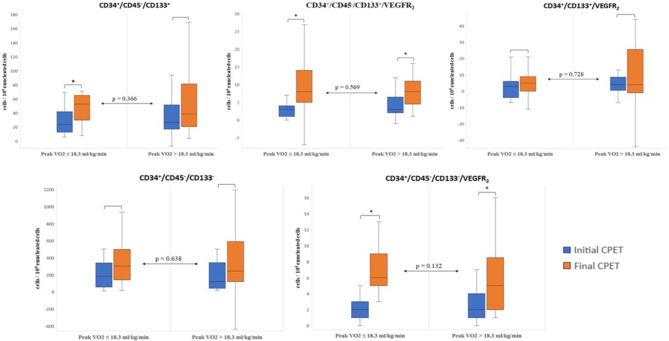
The severity group with higher peak VO2 had increased acute responses of CD34+/CD45-/CD133+/VEGFR2 EPCs (p < 0.001) and CD34+/CD45-/CD133-/VEGFR2 CECs (p = 0.003) after the final maximal CPET compared with the initial CPET before the CR program, while the severity group with lower peak VO2 had a greater increase in the acute response of CD34+/CD45-/CD133+ EPCs (p = 0.004) and CD34+/CD45-/CD133+/VEGFR2 EPCs (p = 0.001) and CD34+/CD45-/CD133-/VEGFR2 CECs (p = 0.003) after the CR program (Figure 1, Supplementary Tables 1 and 2). No significant differences in the acute responses of EPCs and CECs after maximal exercise before and after the CR program were observed between the severity groups (p > 0.05, Figure 1). Moreover, there were no significant correlations between the initial peak VO2 of patients and the numeric or percentage differences of the acute responses after maximal exercise in these endothelial cellular populations (p > 0.05).
Figure 1.
Boxplots representing the acute response of the mobilization of each endothelial cellular population after the initial and the final cardiopulmonary exercise training within and between patients with chronic heart failure of high and low severity, based on peak VO2. The asterisk (*) indicates statistically significant difference within each severity group (p < 0.05). No differences were observed between patients of high and low severity in each endothelial cellular population (p > 0.05). CPET, cardiopulmonary exercise test.
Supplementary Table 1. Acute response of the mobilization of endothelial cellular populations after maximal exercise in patients with chronic heart failure of high severity before and after a cardiac rehabilitation program.
| Endothelial cellular populationsa | Before rehabilitation | After rehabilitation | p value of the acute response between CPETs | ||
| Before CPET | After CPET | Before CPET | After CPET | ||
| CD34+/CD45-/CD133+ | 54 (24-74) | 84 (52-102)# | 98 (76-131) | 154 (102-209)# | 0.004 |
| CD34+/CD45-/CD133+/VEGFR2 | 2 (1-4) | 5 (3-8)# | 7 (4-9) | 15 (10-20)# | 0.001 |
| CD34+/CD133+/VEGFR2 | 13 (9-16) | 13 (9-19) | 22 (17-36) | 27 (19-38)* | 0.165 |
| CD34+/CD45-/CD133- | 186 (131-287) | 402 (204-544)# | 431 (301-618) | 738 (496-931)# | 0.073 |
| CD34+/CD45-/CD133-/VEGFR2 | 1 (1-3) | 3 (3-5)# | 4 (3-8) | 10 (9-15)# | 0.003 |
High severity group: Peak VO2 ≤ 18.3 ml/kg/min; CPET, cardiopulmonary exercise testing.
a Values are expressed as “cells/106 enucleated cells” in median (25th-75th percentiles).
Significant difference in the acute mobilization of endothelial cellular populations after a symptom-limited maximal CPET (* p < 0.05; # p < 0.001). CPET, cardiopulmonary exercise test.
Supplementary Table 2. Acute response of the mobilization of endothelial cellular populations after maximal exercise in patients with chronic heart failure of low severity before and after a cardiac rehabilitation program.
| Endothelial cellular populationsa | Before rehabilitation | After rehabilitation | p value of the acute response between CPETs | ||
| Before CPET | After CPET | Before CPET | After CPET | ||
| CD34+/CD45-/CD133+ | 42 (20-71) | 90 (40-119)# | 85 (50-112) | 127 (95-179)# | 0.123 |
| CD34+/CD45-/CD133+/VEGFR2 | 2 (1-3) | 5 (3-9)# | 5 (3-7) | 14 (9-17)# | < 0.001 |
| CD34+/CD133+/VEGFR2 | 10 (7-19) | 14 (10-19)* | 23 (14-54) | 22 (16-73) | 0.836 |
| CD34+/CD45-/CD133- | 234 (164-259) | 314 (263-637)# | 520 (297-866) | 740 (526-1194)* | 0.231 |
| CD34+/CD45-/CD133-/VEGFR2 | 1 (1-2) | 4 (2-6)# | 5 (3-8) | 10 (8-12)# | 0.003 |
Low severity group: Peak VO2 > 18.3 ml/kg/min; CPET, cardiopulmonary exercise testing.
a Values are expressed as “cells/106 enucleated cells” in median (25th-75th percentiles).
Significant difference in the acute mobilization of endothelial cellular populations after a symptom-limited maximal CPET (* p < 0.05; # p < 0.001). CPET, cardiopulmonary exercise test.
Finally, although the CR program increased EF in both Group 1 [from 30 (25-40) to 35 (30-45), p = 0.01] and Group 2 [from 35 (28-38) to 39 (30-43), p = 0.02], there was no significant difference between the 2 groups (p > 0.05).
DISCUSSION
The present study demonstrated that a CR program consisting of 36 sessions enhanced the acute responses of EPCs and CECs after maximal exercise in a similar way in patients with CHF of different severity according to peak VO2. The beneficial effects of exercise training were similar in the patients with either lower or higher peak VO2. This is the first study to investigate the effect of a structured exercise training intervention on cellular level, and most specifically on the acute response of EPC and CEC mobilization after maximal exercise in patients with different CHF severity based on indexes of functional capacity. In our previous study,6 we showed that maximal exercise increased the acute mobilization of these endothelial cellular populations in CHF patients. In the present study, we extend our previous findings by showing that not only maximal exercise but also exercise training seemed to be similarly effective for the acute response of EPCs, and therefore for vascular endothelial function of CHF patients of different severity. Increases in the absolute and percentage numbers of EPCs after exercise have been proven to reverse endothelial damage.4,5
There are some similarities between this and our previous study.7 The patients initially underwent a symptom-limited maximal CPET as a single bout of maximal exercise before participating in a structured exercise training program in both studies, and the acute mobilization of different endothelial cellular populations was quantified after this single exercise bout. Moreover, in both studies, the patients were divided into groups of different CHF severity according to CPET indices. The main difference in the present study, and the main novelty compared to our previous study7 and other studies, is the fact that we performed a symptom-limited maximal CPET not only before a rehabilitation program, but also after 36 sessions of regular aerobic exercise training. As a result, we could compare the effect of the rehabilitation program on the acute mobilization of EPCs and CECs after maximal exercise, and whether regular aerobic exercise acts collaboratively with regards to the acute effect of maximal exercise on microcirculation and vascular endothelial function.
Exercise can activate two different mechanisms for the mobilization of EPCs and CECs and their action on the endothelium. A mechanism related to shear stress stimulates the increase of endothelial nitric oxide and matrix metalloproteinases (MMPs). MMPs cut the bonds between EPCs and bone marrow, and facilitate their entry into the circulation.4,10 Another mechanism is hypoxic stimulation of angiogenetic factors such as vascular endothelial growth factor, which guides EPCs and their mature form, CECs, to the point of endothelial damage.4,10
A study limitation is that our results cannot be generalized to all CHF populations, including patients with unstable and decompensated CHF.
Our findings indicate that the 36-session CR program had similar beneficial effects on the acute responses of EPCs and CECs after maximal exercise in patients with CHF of different severity. Further research on the potential mechanisms and in different CHF populations is required.
Acknowledgments
The authors would like to thank the staff of Clinical Ergospirometry, Exercise & Rehabilitation Laboratory of "Evaggelismos" General Hospital of Athens.
FUNDING
This research was co-financed by national resources and the European Union (European Social Fund) through the Operational Program «Human Resources Development, Education and Lifelong Learning» in the context of the project "Strengthening Human Resources Research Potential via Doctorate Research" (MIS-5000432), implemented by the State Scholarships Foundation (IKY).
DECLARATION OF CONFLICT OF INTEREST
All authors contributed equally to this work. The authors declare that there is no conflict of interest.
REFERENCES
- 1.Manetos C, Dimopoulos S, Tzanis G, et al. Skeletal muscle microcirculatory abnormalities are associated with exercise intolerance, ventilatory inefficiency, and impaired autonomic control in heart failure. J Heart Lung Transplant. 2011;30:1403–1408. doi: 10.1016/j.healun.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Kovacic S, Plazonic Z, Batinac T, et al. Endothelial dysfunction as assessed with magnetic resonance imaging - a major determinant in chronic heart failure. Med Hypotheses. 2016;90:76–78. doi: 10.1016/j.mehy.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Hu DJ, Yan H, et al. Attenuated endothelial function is associated with decreased endothelial progenitor cells and nitric oxide in premenopausal diabetic women. Mol Med Rep. 2018;18:4666–4674. doi: 10.3892/mmr.2018.9451. [DOI] [PubMed] [Google Scholar]
- 4.Morrone D, Felice F, Scatena C, et al. Role of circulating endothelial progenitor cells in the reparative mechanisms of stable ischemic myocardium. Int J Cardiol. 2018;257:243–246. doi: 10.1016/j.ijcard.2017.05.070. [DOI] [PubMed] [Google Scholar]
- 5.Chan KH, Simpson PJ, Yong AS, et al. The relationship between endothelial progenitor cell populations and epicardial and microvascular coronary disease-a cellular, angiographic and physiologic study. PLoS One. 2014;9:e93980. doi: 10.1371/journal.pone.0093980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kourek C, Karatzanos E, Psarra K, et al. Endothelial progenitor cells mobilization after maximal exercise in patients with chronic heart failure. Hellenic J Cardiol. 2021;62:70–72. doi: 10.1016/j.hjc.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Kourek C, Karatzanos E, Psarra K, et al. Endothelial progenitor cells mobilization after maximal exercise according to heart failure severity. World J Cardiol. 2020;12:526–539. doi: 10.4330/wjc.v12.i11.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kourek C, Alshamari M, Mitsiou G, et al. The acute and long-term effects of a cardiac rehabilitation program on endothelial progenitor cells in chronic heart failure patients: comparing two different exercise training protocols. Int J Cardiol Heart Vasc. 2020;32:100702. doi: 10.1016/j.ijcha.2020.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2:805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Craenenbroeck EM, Bruyndonckx L, Van Berckelaer C, et al. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur J Appl Physiol. 2011;111:2375–2379. doi: 10.1007/s00421-011-1843-1. [DOI] [PubMed] [Google Scholar]