Abstract
Background
Traumatic vascular injury in the extremities may be associated with a low mortality rate but can lead to limb loss that seriously affects patients’ functionality. Multiple scoring systems have been designed to evaluate the prognosis, but none are 100% predictive. The management of traumatic vascular injury remains challenging and depends mostly on the surgeon’s experience.
Objectives
We identified the risks associated with limb loss and further investigated the utility of current amputation indexes.
Methods
We retrospectively reviewed 53 cases of traumatic vascular injury in the extremities at a tertiary referral medical center over the past ten years (January 2011-December 2020). The mangled extremity severity score (MESS), limb salvage index (LSI), and predictive salvage index (PSI) were used to assess the traumatized limbs. The injury characteristics and outcomes were evaluated using regression analysis.
Results
The incidence of limb loss was 20.8% (n = 11), and open fractures were the most related factor. Extensive involvement of soft tissue, vascular injury combined with tibia or fibula fractures, initial shock status, and the amount of transfusion were associated with limb loss.
Conclusions
Our study identified the risk factors and clinical utility of MESS, PSI, and LSI. While both LSI and PSI had acceptable diagnostic accuracy, amputation should be decided based on a variety of criteria and clinical features. Salvaging any limb that has not become apparently futile seems logical, yet the presence of certain factors may suggest a worse outcome.
Keywords: Amputation index, Mangled extremity, Vascular trauma
INTRODUCTION
Traumatic vascular injury in the extremities can be caused by direct puncture wounds, lacerations, sharp fracture fragments, or, more commonly, indirect stretching and shear forces acting on a vessel, leading to intimal tear and occlusion.1-7 As tissue perfusion depends largely on collateral circulation, severe ischemia develops in areas lacking collaterals, such as the knee, contributing to limb loss.5-9
During World War II, the amputation rate of popliteal artery trauma reached 72.5% when vascular ligation was the standard practice. This declined to 30%-40% during the Korean War, as repairing large vessels became common.3,10-12
Due to technological advances, improved understanding of the mechanism of damage, superior damage control protocols, and intensive care, the mortality rate ranged between 1.5-4.5% in a recent civilian study. Nevertheless, the amputation rate was 14%-25%.4,5,7,9,13
Timely diagnosis and vascular interventions are imperative. Rescuing an insensate extremity may lead to sepsis or even mortality. Multiple scoring systems have been designed to evaluate the prognosis and identify which limbs should be amputated. However, none of them are precise and should not direct the treatment strategy independently.
The aim of this study was to investigate the risk factors from our experience and evaluate the utility of several scoring systems for mangled extremities.
METHODS
We retrospectively reviewed 53 cases of traumatic vascular injury in the extremities (eight upper and 45 lower limbs) at a tertiary referral medical center between January 2011 and December 2020. Forty-six patients were male, and seven were female, with a mean age of 44.5 years (range: 15-80 years). All of the patients underwent vascular repair. The mangled extremity severity score (MESS)14,15 was used for all injured extremities, along with the limb salvage index (LSI)16 and predictive salvage index (PSI)17 for lower limbs. We modified these amputation indexes by excluding the time factor, because the exact warm ischemia duration could not be obtained. Each traumatized limb was evaluated using the original and modified scoring systems.
Clinical and image assessment protocols
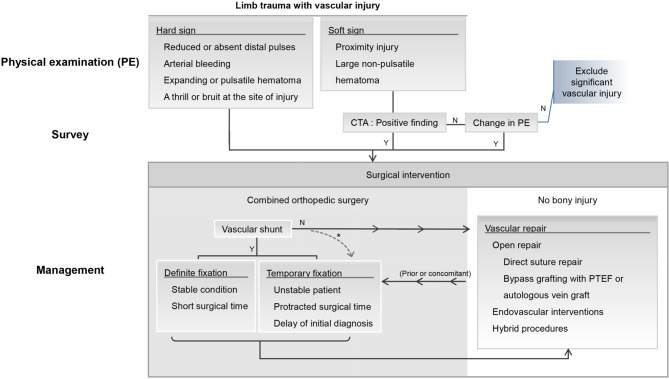
Patients presenting with shock status, defined as systolic blood pressure less than 90 mmHg, were resuscitated. Distal pulsations of the affected limbs were examined using a Doppler scan. For patients with hard signs of arterial injury (reduced or absent distal pulses, arterial bleeding, expanding hematoma, pulsatile hematoma, and presence of a thrill or bruit at the site of injury),5 surgery was performed immediately. Those with soft signs (proximity injury, large non-pulsatile hematoma)5 underwent angiography and vascular repair, while positive findings were documented. The treatment strategy is summarized in Figure 1.
Figure 1.
Diagnostic and treatment algorithm for vascular injury associated with extremity trauma. This figure summarizes the preoperative assessment and treatment protocol for patients with vascular injuries in the traumatized limb. A fasciotomy should be performed whenever a tense compartment is present. * Confronting an extremely bony dislocation, a damage control orthopedic surgery with temporary fixation may be performed prior to vascular repair to maintain stability at the anastomosis site.4 CTA, computed tomography angiography; PTEF, polytetrafluoroethylene.
Vascular repair
Most patients underwent open surgical repair under general anesthesia. An incision was made and extended slightly proximal to the presumed site of the injured artery to explore and control the in-flow, followed by an assessment of the involved intima, backflow, and in-flow. Distal embolectomy was performed using a Fogarty® thru-lumen embolectomy catheter passing through a .035-inch wire (Radiofocus® Guidewire M, Terumo Vietnam Co., Ltd) under fluoroscopy. Proximal embolectomy was performed when the in-flow was suboptimal. Any damaged arterial segment was resected.
Limited arterial defects were anastomosed end-to-end. Blunt trauma often compromises the longer segment of the artery and requires grafting. Contralateral long saphenous veins were harvested in preference to synthetic or ipsilateral vein grafts. For concomitant venous injury, we sutured the simple ruptures and ligated complex injured veins, as their repair requires bypass grafting, which is time-consuming.
The endovascular technique was performed when a short segmental occlusion was documented using preoperative computed tomography angiography (CTA). The patients received general anesthesia, and common femoral arteries were cannulated. Under fluoroscopic guidance, the wire was passed through the target arterial segment, followed by the deployment of an endoprosthesis (Viabahn®).
After vascular repair, angiography was performed by puncturing the site proximal to the anastomosis. Any situation that caused inadequate runoff was revised.
Evaluation of associated bony and soft tissue injuries
Open fractures were classified as Gustilo type I-IIIc, signifying the severity of the fracture and extent of soft tissue or vascular involvement.18 Substantial soft tissue deficits were defined as a subcutaneous break > 10 cm requiring flap coverage, extensive periosteal stripping, degloving injuries, or extensive muscle damage resulting from high energy or crush injuries, irrespective of the size of the wound. Massive contamination was initially defined as a severely contaminated open fracture caused by farm machinery according to the Gustilo classification.18 As livelihood activities have changed along with general societal changes, we extended this definition to a deep tissue injury (beneath the subcutaneous layer, fascia, muscle, or bone) impregnated with soil or other debris, following any mechanism of injury.
Concomitant orthopedic fixation
Since we did not have peripheral vascular shunts, we performed vascular repair before or concomitantly with orthopedic interventions when both surgeries were required. For extremely bony dislocations, we attempted external fixation prior to vascular repair to maintain stability at the anastomosis site. Otherwise, we harvested the venous graft concomitantly when two types of surgery were to be performed.
Fasciotomy
Four-compartment fasciotomy was performed whenever a clinically tense compartment was detected. Prophylactic fasciotomy was defined as a procedure performed before or immediately after the initial vascular repair during the same operation.
Post-operative management
Positive pedal or wrist Doppler signals were achieved in every patient after surgery. Distal pulses were assessed hourly in the first 24 h, and every 8 h thereafter. Absent Doppler signals with signs of limb ischemia indicated surgical failure. Re-exploration or conservative strategies were carried out according to the patient’s general status. Amputation was decided when gangrene, nonviable muscle in bulk, or signs of sepsis became apparent.
Statistical analysis
Risk factors were identified using Fisher’s exact test, the Mann-Whitney U test, and Cox regression analysis for amputation. Subsequent limb salvage was estimated using Kaplan-Meier survival rate analysis. The accuracy of the scoring systems was assessed using receiver operating characteristic (ROC) curves and areas under the ROC curve (AUC). A p value of ≤ 0.05 was considered to be significant.
Ethical standards
This study was approved by the Institutional Review Board of Changhua Christian Hospital. Medical records and patient information were deidentified prior to analysis.
RESULTS
Three of the 53 patients died of postoperative multiorgan failure during hospitalization. One patient had multiple injuries, and two had excessive hemorrhage on site. The incidence of limb loss was 20.8% (n = 11). All amputated limbs were lower extremities traumatized by a blunt mechanism. There were no primary amputations, and the median duration from vascular repair to amputation was 99 h (range: 28-569 h). The patients’ demographic data are presented in Table 1.
Table 1. Patient demographics and mechanisms of injuries.
| Total (N= 53) | |
| Age (mean ± SD) | 44.5 ± 19.8 |
| Male sex | 46 (86.8%) |
| Lower limb | 45 (84.9%) |
| Traumatic type | |
| Penetration | 5 (9.4%) |
| Blunt | 46 (86.8%) |
| Combined | 2 (3.8%) |
| Shock | 10 (18.9%) |
| Open fracture* | 25 (47.2%) |
| Type I | 2 (3.8%) |
| Type II | 5 (9.4%) |
| Type IIIa | 2 (3.8%) |
| Type IIIb | 3 (5.7%) |
| Type IIIc | 13 (24.5%) |
| Soft tissue | |
| Contamination | 8 (15.1%) |
| Tissue loss | 20 (37.7%) |
| Bony injury | |
| Femur | 18 (34%) |
| Knee dislocation | 8 (15.1%) |
| Tibia/fibula | 22 (41.5%) |
| Ankle/foot | 5 (9.4%) |
| Humerus | 3 (5.7%) |
| Ulna/radius | 3 (5.7%) |
| Elbow | 2 (3.8%) |
| Pelvis | 2 (3.8%) |
* Open fracture is classified by Gustilo type.
SD, standard deviation.
Ten patients presented with shock. Blunt trauma accounted for 90.6% (n = 48) of all injuries. Forty-five (84.9%) patients had associated bony injuries, with 22 involving the tibia or fibula. More than half of the fractures (55.6%, n = 25) were open-type, and 72% (n = 18) were Gustilo type III fractures. Twenty patients (37.7%) sustained massive soft tissue deficits, eight (15.1%) had extensive wound contamination, and four of five amputees with contaminated wounds lost their limbs due to sepsis.
To confirm the diagnosis, 75.5% (n = 40), 5.7% (n = 3), and 18.9% (n = 10) of the lesions were documented using CTA, intraoperative angiography, and surgical exposure, respectively. The intervals from injury to the initiation of vascular repair were less than 6 h, 6-9 h, 12-15 h, and more than 15 h in 66% (n = 35), 15.1% (n = 8), 5.7% (n = 3), and 13.2% (n = 7) of the cases, respectively. The distribution of ischemic severity was 3.8% (n = 2), 20.8% (n = 11), 58.5% (n = 31), and 17.0% (n = 9) in class I, IIA, IIB, and III,19 respectively. A higher ischemic class was correlated with worse outcomes (p = 0.021), whereas the diagnostic tool or warm ischemic duration was not. The distribution of injured vessels and the associated interventions are detailed in Table 2.
Table 2. Involved vessels and subsequent interventions and their association with the outcome.
| Amputation (N = 11) | No amputation (N = 42) | p value | |
| Traumatized vessels | |||
| Femoral a. | 3 (27.3%) | 12 (28.6%) | > 0.999 |
| Popliteal a. | 6 (54.5%) | 15 (35.7%) | 0.31 |
| Shank a. | 4 (36.4%) | 8 (19%). | 0.244 |
| Iliac a. | 1 (9.1%) | 1 (2.4%) | 0.375 |
| Ulna/radial a. | 0 | 1 (2.4%) | > 0.999 |
| Brachial a. | 0 | 5 (11.9%) | 0.571 |
| AXI/SCV a. | 0 | 3 (7.1%) | > 0.999 |
| Vein | 1 (9.1%) | 8 (19%). | 0.665 |
| Ischemic class | 0.021* | ||
| I | 0 | 2 (4.8%) | |
| IIA | 0 | 11 (26.2%) | |
| IIB | 6 (54.5%) | 25 (59.5%) | |
| III | 5 (45.5%) | 4 (9.5%) | |
| Vascular repair | 0.553 | ||
| Bypass (VG) | 8 (72.7%) | 24 (57.1%) | |
| Bypass (PTFE) | 1 (9.1%) | 2 (4.8%) | |
| Endovascular | 0 | 1 (2.4%) | |
| Hybrid | 2 (18.2%) | 15 (35.7%) | |
| Surgical sequence | 0.608 | ||
| VF | 3 (27.3%) | 12 (28.6%) | |
| Concomitant | 0 | 1 (2.4%) | |
| OF | 7 (63.6%) | 12 (28.6%) | |
| Additional procedures | |||
| Vein repair | 1 (9.1%) | 5 (11.9%) | > 0.999 |
| Fasciotomy (P) | 6 (54.5%) | 19 (45.2%) | 0.582 |
| Fasciotomy (T) | 3 (27.3%) | 5 (11.9%) | 0.34 |
p value by Fisher’s Exact Test.
* p value = 0.004 by Chi-square test for trend.
a., artery; AXI, axillary; OF, orthopedic fixation performed first; P, prophylactic; PTFE, polytetrafluoroethylene; SCV, subclavian; T, therapeutic; VF, vascular repair performed first; VG, autologous vein graft.
Of all vascular repairs, 35 used bypass grafting, with 32 (60.4%) requiring autologous vein grafts, three (5.7%) polytetrafluoroethylene (PTFE) grafts, 17 (32.1%) hybrid procedures, and one (1.9%) endovascular repair.
Concomitant venous injuries were present in nine limbs. Six were mended, while three received ligations. All injured venous segments were related to the corresponding artery sites.
The popliteal artery was the most frequently traumatized vessel (n = 21, 39.6%), followed by the femoral artery (n = 15, 28.3%) and shank arteries (n = 12, 22.6%), and it accounted for most of the amputees (6/11, 54.5%). However, the anatomical location did not reach statistical significance regarding the outcome.
The fasciotomy rate was 62.3% (n = 33), with 75.8% (n = 25) of these performed prophylactically. In the remaining cases (24.2%, n = 8), fasciotomy was performed therapeutically to relieve compartment syndrome after vascular repair.
When combining orthopedic and vascular surgery (n = 35), 19 patients underwent orthopedic procedures prior to vascular repair. The overall median duration of bone fixation before vascular repair was 70 min, including 70 min and 71 min for the amputation (n = 7) and salvage (n = 12) subgroups, respectively. Table 3 lists the variables associated with outcomes during the treatment course.
Table 3. Hospital course and factors in association with the outcome.
| Amputation (N = 11) | No amputation (N = 42) | p value | |||
| n | Median (IQR) | n | Median (IQR) | ||
| Age (year) | 11 | 46 (21-60) | 42 | 39 (29-62) | 0.622 |
| Time-amp (hr) | 11 | 99 (56-170) | -- | -- | |
| ICU stay (day) | 11 | 13 (7-25) | 42 | 1.5 (0-9) | 0.001 |
| Hospital stay (day) | 11 | 48 (18-68) | 42 | 23 (11-35) | 0.025 |
| IA duration (day) | 11 | 0 (0-2) | 42 | 0 (0-0) | 0.039 |
| pRBC-T (unit) | 11 | 34 (8-50) | 42 | 18 (6-22) | 0.086 |
| Readmission | 11 | 0 (0-0) | 39 | 1 (0-2) | 0.016 |
| LSI* | 11 | 7 (6-8) | 34 | 4 (2-6) | 0.001 |
| MESS | 11 | 8 (5-10) | 42 | 6 (5-8) | 0.092 |
| PSI* | 11 | 9 (8-10) | 34 | 6 (5-8) | 0.004 |
| M-LSI* | 11 | 6 (5-7) | 34 | 3 (2-5) | 0.001 |
| M-MESS | 11 | 7 (5-9) | 42 | 6 (5-7) | 0.048 |
| M-PSI* | 11 | 8 (7-9) | 34 | 5 (4-7) | 0.003 |
| P-OS (min) | 7 | 70 (55-89) | 12 | 71 (56-119) | 0.735 |
| Later operation# | 11 | 4 (1-7) | 42 | 2 (1-5) | 0.246 |
| Time-VR (min) | 11 | 258 (194-317) | 42 | 221.5 (155-307) | 0.263 |
p value by Mann-Whitney U Test.
* (M-)LSI and (M-)PSI are used to assess lower limb trauma. # Number of additional surgical interventions after the initial vascular repair.
hr, hour; IA, inotropic agent; ICU, intensive care unit; IQR, interquartile range represents the distance between the 25th and 75th percentiles; LSI, limb salvage index; M-, modified amputation index (excluding the time factor); MESS, mangled extremity severity score; P-OS, delay from orthopedic surgery prior to the vascular repair; pRBC-T, amounts of pack red blood cell transfusion; PSI, predictive salvage index; Time-amp, duration between initial vascular repair and amputation; Time-VR, surgical time of vascular repair.
The median MESS score was 8 in the amputation group (n = 11) and 6 (n = 42) in the salvage group (p = 0.092). The median LSI score was 7 in the amputation group and 4 in the salvage group (n = 34, p = 0.001). The median PSI score was 9 in the amputation group and 6 in the salvage group (n = 34; p = 0.004).
Compared to the limb salvage group, the amputation group had higher amputation scores, more subsequent operations, greater packed red blood cell (pRBC) transfusion and inotropic agent (IA) requirements, longer hospital stay, and fewer readmissions.
DISCUSSION
The overall incidence of amputation in this study was 20.8%. This incidence was higher (24.4%) after excluding upper limb injury, compared to a 14 to 25% amputation rate reported in prior investigations.4,5,7,9,16,21,22 This may be due to a higher proportion of blunt trauma, which usually causes soft tissue damage, interrupts the main and collateral circulation, and affects multilevel arterial supplies.4,23 Stab wounds cause minimal tissue damage and are associated with an incidence of primary amputation of about 1%.5,7 Even though previous studies have reported that different mechanisms affect the outcomes and none of our patients with penetrating injuries underwent amputation, we found no significant association between injury mechanisms and outcomes. This may be explained by our limited sample size.
The risks of amputation identified in this study were open fractures, tibia or fibula fractures, wound contamination or extensive soft tissue deficits, initial shock status, longer duration of IA treatment, and more pRBC transfusions.
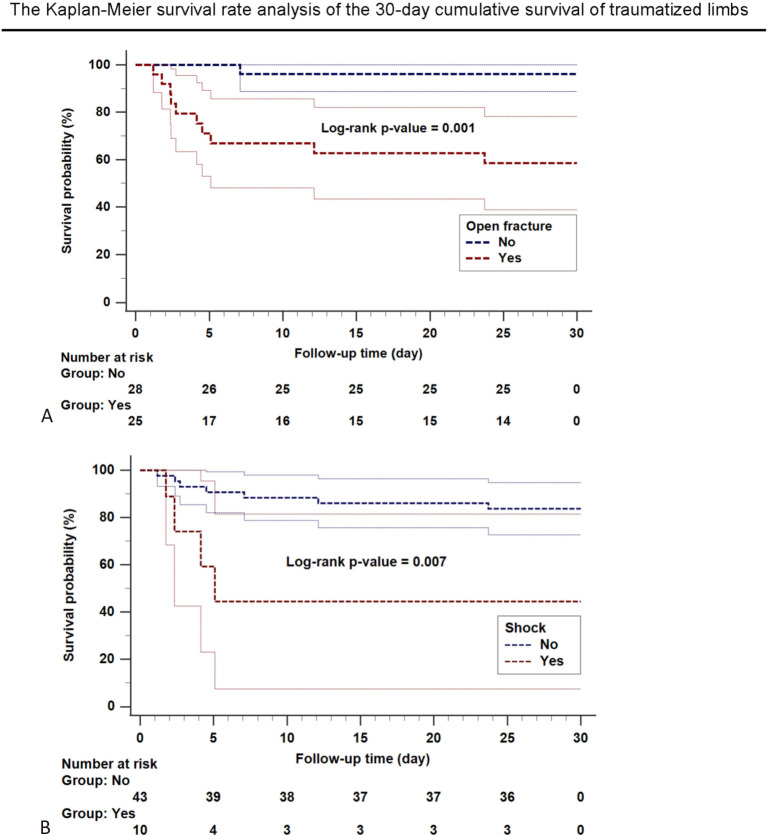
Compound fractures, when associated with extensive soft tissue damage, are known to be a significant risk factor for limb loss.4,5,23 Overall, 47% (n = 25) of our patients had open fractures, and it was significantly associated with 30-day limb survival (Figure 2A). Tibia or fibula fractures accounted for 72.7% (n = 8) of the amputees, and they have been associated with an increased risk of limb loss and occasional mortality from sepsis.24,25 In the present study, extensive soft tissue damage and contamination had hazard ratios (HRs) of 5.3 [confidence interval (CI): 1.4-20.1; p = 0.014] and 5.8 (CI: 1.8-19.1; p = 0.004), respectively. Limbs with substantial soft tissue involvement can barely recover and tend to be sacrificed due to muscle loss, nonunion, and infection.4,7,16,23,25-27
Figure 2.
The Kaplan-Meier survival rate analysis of the 30-day cumulative survival of traumatized limbs associated with open fractures and shock. (A) Significantly lower incidence of limb salvage in extremities with an open fracture (red dashed line) than in those without an open fracture (blue dashed line) is demonstrated. (B) Patients with an initial shock status (red dashed line) lose their limbs more rapidly than those without (blue dashed line). The associated confidence interval (CI) is plotted in a continuous line with the same color as the risk factor.
Amputees with an initial shock status were associated with losing their limbs more rapidly (Figure 2B), and they required a significantly higher number of blood transfusions during their treatment process. This is consistent with previous studies, and reflects an overall higher level of injury.8,9 Factors associated with limb loss are listed in Table 4.
Table 4. Factors associated with amputation.
| Amputation (N = 11) | No amputation (N = 42) | Bivariable analysis | Multivariable analysis | |||
| HR (95% CI) | p | HR (95% CI) | p | |||
| Fibula/tibia fx | 8 | 14 | 4.11 (1.09-15.49) | 0.037 | ||
| Open fx | 10 | 15 | 13.73 (1.76-107.44) | 0.013 | 20.89 (1.27-343.96) | 0.033 |
| Contamination | 5 | 3 | 5.78 (1.75-19.07) | 0.004 | ||
| Soft tissue | 8 | 12 | 5.31 (1.41-20.05) | 0.014 | ||
| Shock | 4 | 6 | 4.80 (1.38-16.66) | 0.014 | 9.11 (1.709-48.96) | 0.01 |
| IA duration (day) | 0 (0-2)* | 0 (0-0)* | 1.32 (1.06-1.65) | 0.013 | ||
| pRBC-T (unit) | 34 (8-50)* | 18 (6-22)* | 1.03 (1.01-1.06) | 0.01 | 1.05 (1.01-1.10) | 0.01 |
Cox proportional-hazards regression analysis of amputation.
* The data were expressed as median (IQR).
CI, confidence interval; fx, fracture; HR, hazard ratio; IA, inotropic agent; IQR, interquartile range represents the distance between the 25th and 75th percentiles; pRBC-T, amounts of pack red blood cell transfusion.
After multivariable analysis, open fractures were most strongly correlated with amputation (HR: 20.9; CI: 1.3-344; p = 0.033), followed by shock (HR: 9.1; CI: 1.7-49; p = 0.01), and pRBC transfusion (HR: 1.05; CI: 1.0-1.1; p = 0.01). We found no significant impact of ischemic duration on the outcomes, although a delay in restoring arterial continuity for more than 8 h has been associated with an 86% amputation rate.3,4,21 Temporary arterial shunting has been advocated to maintain limb perfusion in patients with multiple injuries.5,16,28 Since we endeavored to minimize the warm ischemia time to less than 6 h (66%, n = 35) and none of our patients had profound shock stemming from critical remote injuries, the use of arterial shunting was not required. Recent evidence suggests that there is no statistical difference in the overall amputation rate associated with the surgical sequence.4,5,9,21,29 Surgeons should decide whether to restore arterial continuity or obtain bony stability preceding vessel repair.
Regarding vascular management, neither surgical procedure nor vascular graft material was associated with limb loss (p = 0.553) or with the coexistence of venous injury and manner of venous repair. The link between concomitant venous injury and limb loss is controversial.30-32 Adverse effects of venous ligation on limb salvage have been reported to be minimal, while venous continuity restored using a vein graft has been associated with a 39% failure rate within days, and failure of such a repair has been associated with a 22% incidence of pulmonary embolization.5,33
While these factors can help surgeons better understand the general prognosis, they seldom alter the ultimate outcome. Primary amputation is warranted when confronting an evidently nonviable limb with vast muscle rigidity, fixed skin staining, major nerve injury, or nonviable muscles involving major muscle compartments.5 It is challenging for surgeons and patients to make decisions following partial signs. The loss of functionality and body image concerns often preclude patients from choosing primary amputation. In addition, misgivings about removing a potentially salvageable limb can lead surgeons to repair vessels in almost every mangled limb. Salvaging a limb that is eventually amputated can lengthen the hospital stay, increase additional interventions, and may lead to sepsis. Multiple evaluation scores for mangled extremities have therefore been developed.14-17,34
In our analysis of the accuracy of the MESS, LSI, and PSI, sensitivity was defined as the proportion of amputees with a score above a given value, and specificity as the probability of patients in the limb preservation group with a score below the set point of the index. The cut-off scores obtained by Youden index analysis were 8, 7, and 6 for the MESS, PSI, and LSI, respectively, which is slightly different from that recommended (7 for the MESS, 8 for the PSI, and 6 for the LSI).14-17,34 Figure 3 illustrates statistical analysis of the accuracy of the diagnostic tests. The ROC curves for each amputation index indicated that the MESS had less discrimination (AUC: 0.64) than the LSI and PSI, which had AUC values of 0.84 and 0.79, respectively. Although excluding the time factor enhanced the accuracy of the diagnosis, the overall value for choosing an amputation remained unsatisfactory owing to the mediocre specificity. The outcome may be biased by the small sample size. However, aggressive revascularization of a traumatized limb with a lower score is justified. In contrast, primary amputation should be evaluated clinically, taking into account the risk variables identified in this study, not merely based on a higher score.5,34,35
Figure 3.
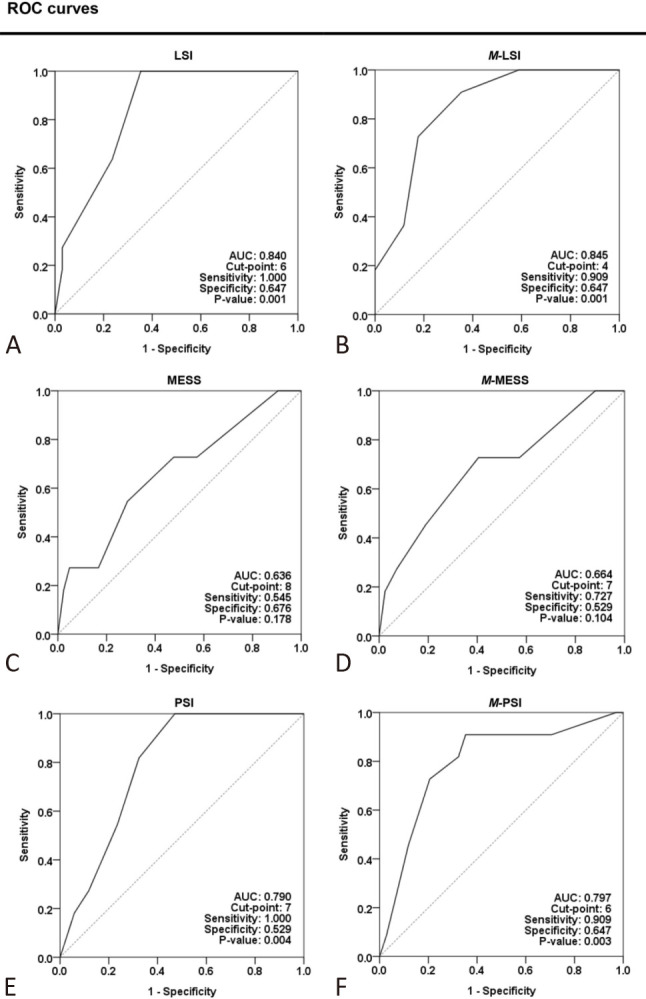
Receiver operating characteristic (ROC) curves of the accuracy concerning amputation indexes. The ROC curves of LSI (A), M-LSI (B), MESS (C), M-MESS (D), PSI (E), and M-PSI (F) in discriminating amputation by a cut-off value are plotted in this figure. The traumatized limb was evaluated with both original and modified (M-, the time factor was excluded) amputation indexes. The cut-off point was retrieved using Youden index analysis of an individual amputation index. Sensitivity is defined as the proportion of amputees with a score above a given value, whereas specificity is the percentage of patients in the limb salvage group having a score below the index’s set point. The AUC increased with the modified amputation index. Both the original (A) and modified (B) LSIs can better discriminate if a limb should be amputated or salvaged. AUC, area under the receiver operating characteristic curve; LSI, limb salvage index; MESS, mangled extremity severity score; PSI, predictive salvage index.
Limb salvage remains worthwhile in most patients, although the presence of certain variables indicates a higher risk of adverse outcomes.36-38 Amputation itself is considered a part of the therapeutic approach for a mangled extremity instead of treatment failure.
Our study is limited by its small sample size and retrospective design. However, the results may provide an insight into the management of patients with peripheral vascular trauma.
CONCLUSIONS
Our results suggest that open fractures, tibia or fibula fractures, extent of soft tissue involvement, initial shock status, and amount of pRBC transfusions can adversely affect outcomes, and the existing amputation indexes may be acceptable in terms of accuracy.
Although overall mortality is minimal, trying to save a mangled extremity that is later amputated may extend the treatment course. Re-vascularizing a severely mangled limb should be individualized and expeditious in the light of overall risk evaluation.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare that they have no conflict of interest.
REFERENCES
- 1.Ouriel K, Veith FJ. Acute lower limb ischemia: determinants of outcome. Surgery. 1998;124:336–341. [PubMed] [Google Scholar]
- 2.Popescu GI, Lupescu O, Nagea M, Patru C. Diagnosis and treatment of limb fractures associated with acute peripheral ischemia. Chirurgia (Bucur) 2013;108:700–705. [PubMed] [Google Scholar]
- 3.Green NE, Allen BL. Vascular injuries associated with dislocation of the knee. J Bone Joint Surg Am. 1977;59:236–239. [PubMed] [Google Scholar]
- 4.Halvorson JJ, Anz A, Langfitt M, et al. Vascular injury associated with extremity trauma: initial diagnosis and management. J Am Acad Orthop Surg. 2011;19:495–504. doi: 10.5435/00124635-201108000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Hafez HM, Woolgar J, Robbs JV. Lower extremity arterial injury: results of 550 cases and review of risk factors associated with limb loss. J Vasc Surg. 2001;33:1212–1219. doi: 10.1067/mva.2001.113982. [DOI] [PubMed] [Google Scholar]
- 6.Fainzilber G, Roy-Shapira A, Wall MJ, Jr., Mattox KL. Predictors of amputation for popliteal artery injuries. Am J Surg. 1995;170:568–570. doi: 10.1016/s0002-9610(99)80017-8. [DOI] [PubMed] [Google Scholar]
- 7.Mullenix PS, Steele SR, Andersen CA, et al. Limb salvage and outcomes among patients with traumatic popliteal vascular injury: an analysis of the national trauma data bank. J Vasc Surg. 2006;44:94–100. doi: 10.1016/j.jvs.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 8.Dua A, Desai SS, Shah JO, et al. Outcome predictors of limb salvage in traumatic popliteal artery injury. Ann Vasc Surg. 2014;28:108–114. doi: 10.1016/j.avsg.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Jessica K, Matthew K, Huan Y, et al. Factors associated with amputation after popliteal vascular injuries. Ann Vasc Surg. 2016;33:83–87. doi: 10.1016/j.avsg.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 10.DeBakey ME, Simeone FA. Battle injuries of the arteries in World War II; an analysis of 2,471 cases. Ann Surg. 1946;123:534–579. [PubMed] [Google Scholar]
- 11.Hughes CW. Acute vascular trauma in Korean War casualties: an analysis of 180 cases. Surg Gynecol Obstet. 1954;99:91–100. [PubMed] [Google Scholar]
- 12.Rich NM, Baugh JH, Hughes CW. Popliteal artery injuries in Vietnam. Am J Surg. 1969;118:531–534. doi: 10.1016/0002-9610(69)90176-7. [DOI] [PubMed] [Google Scholar]
- 13.Dua A, Patel B, Kragh JF Jr, et al. Long-term follow-up and amputation-free survival in 497 casualties with combat related vascular injuries and damage-control resuscitation. J Trauma Acute Care Surg. 2012;73:1517–1524. doi: 10.1097/TA.0b013e31827826b7. [DOI] [PubMed] [Google Scholar]
- 14.Helfet DL, Howey T, Sanders R, Johansen K. Limb salvage versus amputation: preliminary results of the Mangled Extremity Severity Score. Clin Orthop. 1990;256:80–86. [PubMed] [Google Scholar]
- 15.Johansen K, Daines M, Howey T, et al. Objective criteria accurately predict amputation following lower extremity trauma. J Trauma. 1990;30:568–573. doi: 10.1097/00005373-199005000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Russell WL, Sailors DM, Whittle TB, et al. Limb salvage versus traumatic amputation. A decision based on a seven-part predictive index. Ann Surg. 1991;213:473–480. doi: 10.1097/00000658-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe HR, Jr., Poole GV, Jr., Hansen KJ, et al. Salvage of lower extremities following combined orthopedic and vascular trauma. A predictive salvage index. Am Surg. 1987;53:205–208. [PubMed] [Google Scholar]
- 18.Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24:742–746. doi: 10.1097/00005373-198408000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366:2198–2206. doi: 10.1056/NEJMcp1006054. [DOI] [PubMed] [Google Scholar]
- 20.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 21.Vielgut I, Gregori M, Holzer LA, et al. Limb salvage and functional outcomes among patients with traumatic popliteal artery injury: a review of 64 cases. Wien Klin Wochenschr. 2015;127:561–566. doi: 10.1007/s00508-015-0715-9. [DOI] [PubMed] [Google Scholar]
- 22.de Mestral C, Sharma S, Haas B, et al. Contemporary analysis of the management of the mangled lower extremity. J Trauma Acute Care Surg. 2013;74:597–603. doi: 10.1097/TA.0b013e31827a05e3. [DOI] [PubMed] [Google Scholar]
- 23.Patterson BM, Agel J, Swiontkowski MF, et al. Knee dislocations with vascular injury: outcomes in the lower extremity assessment project (LEAP). J Trauma. 2007;63:855–858. doi: 10.1097/TA.0b013e31806915a7. [DOI] [PubMed] [Google Scholar]
- 24.Roessler MS, Wisner DH, Holcroft JW. The mangled extremity. When to amputate? Arch Surg. 1991;126:1243–1248. doi: 10.1001/archsurg.1991.01410340085012. [DOI] [PubMed] [Google Scholar]
- 25.Dagum AB, Best AK, Schemitsch EH, et al. Salvage after severe lower-extremity trauma: are the outcomes worth the means? Plast Reconstr Surg. 1999;103:1212–1220. doi: 10.1097/00006534-199904040-00017. [DOI] [PubMed] [Google Scholar]
- 26.Rozycki GS, Tremblay LN, Feliciano DV, McClelland WB. Blunt vascular trauma in the extremity: diagnosis, management, and outcome. J Trauma. 2003;55:814–824. doi: 10.1097/01.TA.0000087807.44105.AE. [DOI] [PubMed] [Google Scholar]
- 27.MacKenzie EJ, Bosse MJ, Kellam JF, et al. Factors influencing the decision to amputate or reconstruct after high-energy lower extremity trauma. J Trauma. 2002;52:641–649. doi: 10.1097/00005373-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Johansen K, Bandyk D, Thiele B, Hansen ST. Temporary intra-luminal shunts: resolution of a management dilemma in complex vascular injuries. J Trauma. 1982;22:395–402. doi: 10.1097/00005373-198205000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Fowler J, Macintyre N, Rehman S, et al. The importance of surgical sequence in the treatment of lower extremity injuries with concomitant vascular injury: a meta-analysis. Injury. 2009;40:72–76. doi: 10.1016/j.injury.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 30.Pappas PJ, Haser PB, Teehan EP, et al. Outcome of complex venous reconstructions in patients with trauma. J Vasc Surg. 1997;25:398–404. doi: 10.1016/s0741-5214(97)70362-8. [DOI] [PubMed] [Google Scholar]
- 31.Meyer J, Walsh J, Schuler J, et al. The early fate of venous repair after civilian vascular trauma: a clinical, hemodynamic, and venographic assessment. Ann Surg. 1987;206:458–464. doi: 10.1097/00000658-198710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yelon JA, Scalea TM. Venous injuries of the lower extremities and pelvis: repair versus ligation. J Trauma. 1992;33:532–536. doi: 10.1097/00005373-199210000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Cargile JS, III, Hunt JL, Purdue GF. Acute trauma of the femoral artery vein. J Trauma. 1992;32:364–370. doi: 10.1097/00005373-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Bosse MJ, MacKenzie EJ, Kellam JF, et al. A prospective evaluation of the clinical utility of the lower-extremity injury-severity scores. J Bone Joint Surg Am. 2001;83:3–14. doi: 10.2106/00004623-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Higgins TF, Klatt JB, Beals TC. Lower extremity assessment project (LEAP)--the best available evidence on limb-threatening lower extremity trauma. Orthop Clin North Am. 2010;41:233–239. doi: 10.1016/j.ocl.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Nawijn F, Westenberg RF, Langhammer CG, et al. Factors associated with primary and secondary amputation following limb-threatening upper extremity trauma. Plast Reconstr Surg. 2020;145:987–999. doi: 10.1097/PRS.0000000000006644. [DOI] [PubMed] [Google Scholar]
- 37.Loja MN, Sammann A, DuBose J, et al. The mangled extremity score and amputation: time for a revision. J Trauma Acute Care Surg. 2017;82:518–523. doi: 10.1097/TA.0000000000001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fodor L, Sobec R, Sita-Alb L, et al. Mangled lower extremity: can we trust the amputation scores? Int J Burns Trauma. 2012;2:51–58. [PMC free article] [PubMed] [Google Scholar]