Abstract
Scrub typhus is a neglected tropical disease that affects one-third of the world’s population. The disease is caused by Orientia tsutsugamushi (OT), an obligate intracellular Gram-negative bacterium. OT efficiently escapes from the endosomal pathway after entering the host cell and replicates inside cytosol. OT infection promotes cellular autophagy, the autonomous defense mechanism unlike other bacteria. This study has discussed the bacterial invasion process through the extracellular matrix and the immune response activated by the bacterium within the hosts. Furthermore, we have emphasized the importance of extracellular matrix and their cross-talk with the immune cells, such as, macrophages, neutrophils, and dendritic cells followed by their inflammatory response. We have also put an insight into the host factors associated with signaling pathways during scrub typhus disease with a special focus on the OT-induced stress response, autophagy, apoptosis, and innate immunity. Multiple cytokines and chemokines play a significant role in activating different immune-related signaling pathways. Due to the presence of high antigenic diversity among strains, the signaling pathways during the host–pathogen interplay of OT with its host is very complicated. Thus, it hinders to mitigate the severity of the pandemic occurred by the respective pathogen. Our investigation will provide a useful guide to better understand the virulence and physiology of this intracellular pathogen which will lead towards a better therapeutic diagnosis and vaccine development.
Keywords: Host–pathogen interaction, Orientia tsutsugamushi, Inflammatory response, Gene expression
The Bacterium
Orientia tsutsugamushi (OT) is an intracellular bacterium; the causative agent of scrub typhus that affects 1mllion people per year globally, especially in the Asia–pacific region (Watt et al. 2003; Xu et al. 2017). The trombiculid mites infect humans through larval bites, and responsible for spread of different genotype strains within human (Rajapakse et al. 2012; Bhate et al. 2017). Some cases from Chile and Northern Laos have been documented where the terrestrial leeches are the vector of scrub typhus-like illness (Balcellset al. 2011). The bite of leeches is not familiar in urban areas but might play a considerable role in rural places. Although global data on leech transmission are yet not available, parasitological research is required to conclude the role of leeches as a vector for infectious diseases (Slesaket al. 2015). The mite-borne disease’s clinical presentations and symptoms range from asymptomatic to fatal conditions, including headache, fever, rash, myalgia, and lymphadenopathy after 6 to 10 days of post-infection. If it remains untreated, patients leads to multiorgan dysfunction like respiratory involvement, myocarditis, renal impairment, leucocytosis, and thrombocytopenia and death.
India has experienced a significant increase in scrub typhus incidence and documented an expansion in geographic distribution throughout the country. From the observational, interventional, and population-based studies, it has been seen that the Northeast region of India is prone to scrub typhus disease. In the last decade, approximately 18,781 cases of scrub typhus were reported from all over India (Devasagayam et al. 2021). The highest cases were from the southern region such as Tamilnadu, Puducherry, during the cooler months, and the northern Himalayan region during the rainy season (Chakraborty and Sarma 2017). Previously, it was endemic to the Asia–pacific region known as the “tsutsugamushi triangle”, but many reports suggest that it is spreading all over the world including Dubai, United Arab Emirates, Chile, Peru, and Africa and cause a severe public health problem throughout the globe (Xu et al. 2017). Though scrub typhus is becoming a common life-threatening and re-emerging disease that accounts for one million cases every year with a high mortality rate, still, it is neglected and underdiagnosed (Luce-Fedrowet al. 2018; Swain et al. 2022).
OT penetrates the target cell, disrupting physiological cell functions and interrupting the host immune response. The survival strategy employed by the bacterium can be understood by studying the mechanisms by which OT enters the host cell. Host cell signaling subverts and provides new insight into drug discovery and therapeutics design (Seong et al. 2001). There is no effective vaccine available as the adaptive immunity appears short-lived against Orientia infection. However, the route of infection, infectivity, and protective mechanisms of the host immune response to OT is unclear.
Structure
The molecular systems of bacteria have been developed over a million years, and the evolutionary study remains limited compared to the diversity of the bacterial kingdom. To maintain correct cell shape and protect themselves from extreme environmental conditions, many bacteria synthesize a rigid cell wall that mainly forms from the macromolecule peptidoglycan. Almost all known bacteria have some form of peptidoglycan (Vollmer et al. 2008). The cell envelope stability is due to the presence of a peptidoglycan-like structure, also a network of cross-linked proteins in the outer membrane. Orientia was initially thought to be devoid of peptidoglycan, but Atwal et al. 2017 suggested the presence of a peptidoglycan-like structure and an outer membrane comprising a network of cross-linked proteins that together confers cell envelope stability (Atwal et al. 2017). Previously it was concluded that Orientia does not have both peptidoglycan and LPS, which leads to insensitive to penicillin (Amao et al. 1987). However, the genome sequence of the Orientia shows a lack of LPS biosynthesis gene, but it possesses peptidoglycan biosynthesis genes. Therefore, in response to Orientia infection, an intracellular host immune receptor Nod1 recognizes the peptidoglycan fragments and gets activated. However, the structure is challenging to detect as it is present at a low level (Cho et al. 2010a, b).
The OT cell shape is asymmetrical and more variable than other Rickettsiaceae, peptidoglycan-containing bacteria (Atwal et al. 2017). Low-requirement and minimal peptidoglycan in OT is similar to the cell wall of Chlamydiae, an unrelated group of bacteria. The genes responsible for peptidoglycan biosynthesis show similarities with Chlamydiae and suggest a few conserved gene set. MurA-G gene regulate the shape, elongation, division, and sporulation; and class B penicillin-binding proteins (PBPs) are conserved in both Orientia and Chlamydiae, and are responsible for peptidoglycan transpeptidase activity (Salje et al. 2017). In peptide side chains of peptidoglycan, the d-isomer amino acids (d-Glu and d-Ala) and the diamino acid meso-diaminopimelic acid (meso-DAP) are present. d-amino acids are seldom found in proteins. It is synthesized by a particular activity of amino acid Racemases, which is absent in the OT genome (Vollemer and Bertsche 2008; Vollemer et al. 2008). Even though OT does not encode the main l-alanine racemase, the DDL gene produces a protein that catalyzes the ligation of two d-Ala residues present in peptidoglycan side chains. In Chlamydia pneumonia, the GlyA enzyme may represent an alternate for d-Ala biosynthesis and considered as a homolog of Orientia (OTBS 0253) (Shostak et al. 1988; DeBenedetti et al. 2014).
OT has a highly repetitive single chromosomal genome of 2.1 megabases consisting of short repetitive sequences, transposable elements (miniature inverted-repeat transposable elements, Group II intron), and a massively amplified integrative and conjugative element (ICE) termed the rickettsial-amplified genetic element (RAGE) represent 42% of the whole genome. It also undergoes a lot of reshuffling. Type IV secretion systems and possible effector proteins like ankyrin repeat-containing proteins, histidine kinases, and tetratricopeptide repeat (TPR) domain-containing proteins are controlled by the integrase and transposase genes found in ICE. Due to numerous repetitions and mobile DNA elements, there is a bare connection between the positions of the genes in the two complete genome sequences of OT and other rickettsial genomes.
Intracellular bacterial pathogens use ankyrin repeat-containing protein (Anks) as a virulence factor. These proteins include one or more ankyrin repeats of 33-amino acid residue motifs, which induce protein–protein interaction. Within a protein, these domains generate a helix-turn-helix structure that mediates interaction with the target protein (Al-Khodoret al. 2010). The OT genome of strain Ikeda has 47 Ank open reading frames (ORFs), the most obligate intracellular bacterium (Nakayama et al. 2008; VieBrock et al. 2015). The whole-genome structure of Orientia shows the origin of replication, the repetition rate of each chromosomal region, transposable elements, RAGE sequences, and forward and reverse strands. OT, strain Ikeda has a circular chromosome that consists of 2008987 bp. It contains a higher number of protein-coding genes, i.e., 1967CDS. Of 1196 repeated CDSs, 64% (766 CDSs) are pseudogenes among the singleton genes (Nakayama et al. 2008) (Fig. 1). Eighteen types of a repetitive sequence having 933.8 kb have been identified, that contributes 46.5% of the genome. The repetitive sequence is characterized as a Orientia tsutsugamushi amplified genetic element (OtAGE). In the Boryong strain, there are 2179 protein-coding sequences, of which 963 are fragmented genes, revealing a minimal percentage (49.6%) of coding capacity. The genome size of Boryong is 2127051 bp which is longer than Ikeda. More than 200 bp, 4197 identical repeats are reported, which is the unique feature of OT, consisting of 37.1% of the total genome. The mean size is about 947 bp of the perfect repeat with a maximum length of 8909 bp, corresponding to approximately 40% of the total genome (Cho et al. 2007). These genome informations will be helpful in the study of OT molecular pathology, followed by genotype evaluation and effective vaccine development.
Fig. 1.
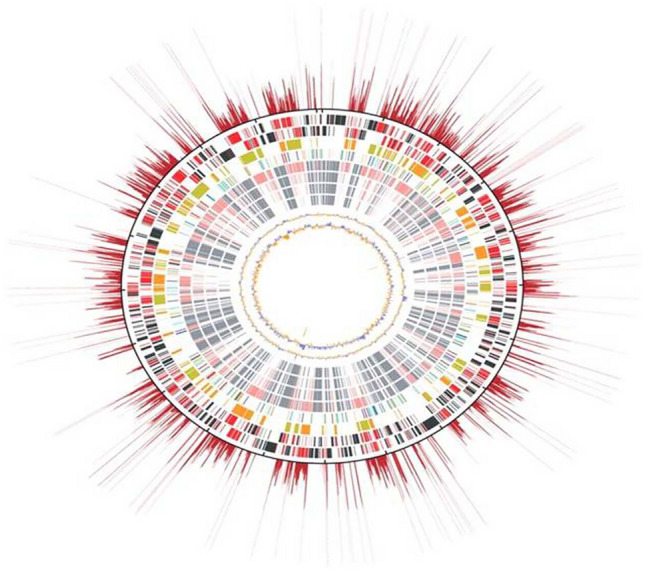
The genome structure of Orientia tsutsugamushi showing genome size, CDS, distribution of Simple Sequence Repeats (SSR) markers, and GC content. The outermost red rays show the replication rate of each chromosomal region. Origin of replication (Ori) is on the top. The feature of the genome moving inwards from the outermost region shows; Red: repeated genes, Black: singleton gene, Green: core gene, Blue: pseudogene, Orange: OtAGE forward sequence, Greenish yellow: OtAGE reverse sequence
OtAGE is a significant genetic element consisting of 33 kb, which encodes about 33–38 genes. Mainly it encodes an integrase gene (int gene) followed by a set of other genes regarded as integrative and conjugative element (ICE). Miniature inverted-repeat transposable element (MITES) and group II intron were also identified (Nakayama et al. 2008). Seven short repeat sequences (SRS) have been reported, but their functions and origins are still unknown. The genome backbone is highly conserved and similar, but for surface proteins, sequence variations were reported. Batty et al. 2018 reported a high-quality genome of OT of six strains such as Gilliam, Karp, Kato, UT76, UT176, and TA686 compared with the Boryong and Ikeda strains having the genes 2709, 2578, 2406, 2247, 2086, and 2546, respectively (Batty et al. 2018) (Table 1).
Table 1.
Orientia tsutsugamushi strain with protein-coding gene numbers
| Strain | Serotype | Genome length (bp) | Predicted gene numbers | References |
|---|---|---|---|---|
| Boryong | Boryong | 2,127,051 | 2443 | Chang et al. (1990) |
| Ikeda | Ikeda | 2,008,987 | 2186 | Tamura et al. (1984) |
| Gilliam | Gilliam | 2,465,012 | 2709 | Rights and Smadel (1948) |
| Karp | Karp | 2,469,803 | 2578 | Enatsu et al. (1999) |
| Kato | Kato | 2,319,449 | 2406 | Enatsu et al. (1999) |
| UT76 | Karp | 2,078,193 | 2247 | Blacksell et al. (2008) |
| UT176 | Karp | 1,932,116 | 2086 | Paris et al. (2009) |
| TA686 | TA686 | 2,254,485 | 2546 | Enatsu et al. (1999) |
Diagnosis strategies and treatment
Scrub typhus is associated with various clinical manifestations, and the key diagnostic indicator is the presence of eschar. However, in the absence of pathognomonic eschar, different serological diagnostic tests such as the Weil-Felix test (WF) followed by ELISA (IgM and IgG), rapid immunochromatographic test, Immunofluorescent assay (IFA), and molecular-based methods such as polymerase chain reaction (PCR) and nested PCR (nPCR) are commonly used. The serological test method ELISA for antibody detection, is most widely used due to its ease of availability and cost-effectiveness. However, PCR method is still considered as a gold standard method for identification and strain demarcation of the respective bacterium (Varghese et al. 2014). But still it requires an efficient tool like Variable Number of Tanden Repeats (VNTRs) multiplexing for genotype evaluation. For treatment, patients are well respond to antibiotics such as chloramphenicol, tetracycline, doxycycline and azithromycin (Mahajan et al. 2016). Due to the genotype complexity, evidence of antibiotic resistance strain that withstand to the commonly used antibiotic mentioned above as well as widespread other antibiotics like β-lactams, fluoroquinolones, and aminoglycosides (Watt et al. 1999; Takhar et al. 2017). The mortality rate ranged from 1 to 40% was observed depending on different endemic are as and encountered OT strain if the patients remained untreated (Luce-Fedrow et al. 2018). So, it is necessary to understand in depth regarding the bacterium biology to develop an effective treatment strategy.
Pathogenicity
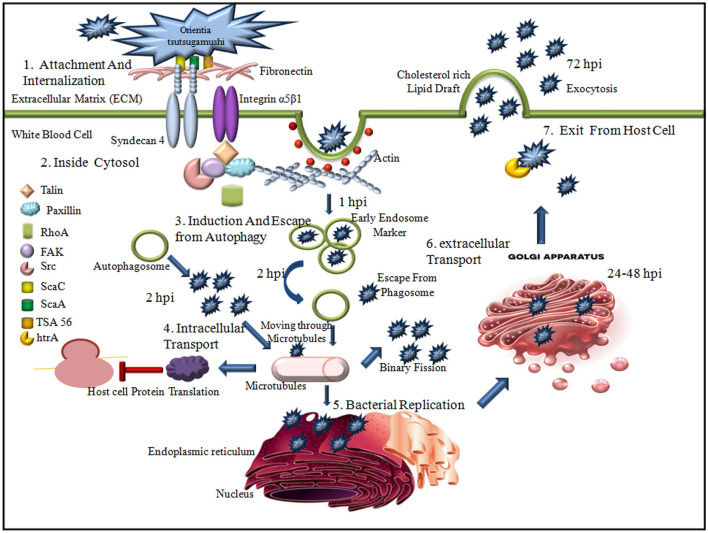
Orientia tsutsugamushi infects nonphagocytic cells such as fibroblasts, endothelial cells, and phagocytic cells, i.e., monocytes/macrophages, neutrophils, and dendritic cells (Jeong et al. 2007). Endothelial cells with dendritic and macrophages are the primary targets of infection in vivo, innate phagocytes of the dermal layer. The pathogen recognition receptors (PRR), macrophages, dendritic cells, and neutrophils sense the invading pathogens to activate the natural immune response and promote adaptive immune responses (Ting et al. 2008). At the chigger bite site, the bacteria infect leukocytes. Infected leukocytes travel to regional lymph nodes and spread throughout the body via the peripheral vascular system. The pathogen eventually egresses from leukocytes and infects the skin and significant organ endothelial cells (Paris et al. 2012, 2013). The bacteria escape their host cell-derived vacuole and replicate in the cytosol within the first hour after uptake into the host cell (Ge and Rikihisa 2011).
The bacterial replication occurs within a polysaccharide-enriched microcolony (Lee et al. 2009) when it traffics to the perinuclear region after evading autophagy in the cytoplasm (Ko et al. 2013; Choi et al. 2013). OT enters the host cells through a clathrin-mediated zipper-like mechanism and exits through late endosomes to avoid the endolysosomal route. The microtubule-mediated process is not standard for OT intracellular trafficking (Kim et al. 2001). The synthesis of polymerized microtubules and the minus–end-directed motor protein dynein is required for microtubule-mediated motility (Kim et al. 2001). It's still unclear whether OT surface proteins facilitate the linking to microtubule-binding proteins, how this is regulated, or why the perinuclear area is employed for bacterial reproduction (Salje et al. 2017).
Leptotrombidium mite infection
The disease is transmitted to humans through the bite of infected Leptotrombidium mites' larval stage. They feed for 3–5 days utilizing a cyclostome to deliver salivary secretions that lyse host tissue into the skin through hair follicles and pores (Cho et al. 2007). Infection is spread by biting "chiggers" (the larvae of trombiculid mites, Leptotrombidium deliense). Chiggers eat a variety of vertebrates, but the disease's primary and possible reservoir is the vector itself, which maintains the pathogen via transovarial and transstadial transmission. The bacterium is detected in the cytosol of host cells in numerous organs, including the oocytes, in infected mites and is efficiently passed on to the offspring by transovarial transmission. Trombiculid mites only suck mammalian tissue fluid once throughout their life cycle, when the bacteria is introduced to the animal, it can causes scrub typhus.
Cell invasion (bacterial ligand and host cell receptor)
The pathogen internalization with the host cell is done in two ways: the first one is phagocytosis, where programmed phagocytic cells activate to internalize the pathogen within the cells. Another one is pinocytosis, where the pathogens are exploited to enable invasion. To initiate the infection, the intracellular bacterium first needs to attach to the host cell and the extracellular matrix (ECM). Before the invasion, OT attaches to the ECM through the receptor. The cell surface 56 kDa Type-specific antigen (TSA 56) with ScaC, the autotransporter protein of OT, interacts with glycoprotein fibronectin of ECM. Another transporter protein ScaA is also involved in the attachment process, although the bacterial counter-component with which it interacts is unclear (Ha et al. 2011, 2015). For interaction with Syndecans, fibronectin contains heparin-binding domains. The functional domains serve as motifs, and for interaction with integrins central cell-binding Arg-Gly-Asp (RGD) motif is required (Leiss et al. 2008). The cell-surface proteoglycans, i.e., heparin sulfate proteoglycans (HSPGs), act as a co-receptor for the initial attachment of the microbe, and secondary receptors mediate host cell entry (Chen et al. 2008). The core proteins present in HSPG attach covalently with other heparin sulfates (HS) glycosaminoglycan (GAG) chains. They are usually expressed on the adherent cell surface and the ECM (Chen et al. 2008). Further, cytoplasmic enzymes like focal adhesion kinases (FAK), Src kinases, and Rho GTPases work downstream to aid the attachment process (Aguilar et al. 2017).
Major transmembrane HSPGs such as Syndecans are expressed on the most mammalian cell surface, facilitating OT’s initial attachment to the host cells (Kim et al. 2004). In invertebrates, four Syndecan family members are found, among which syndecan-4 has the highest cell type distribution (Xian et al. 2010). The expression level of syndecan-4 affects Orientia infection (Kim et al. 2004). Therefore, the syndecan-4 core protein is also involved in the attachment process of OT host cells besides heparin/ heparan sulfate (Ihn et al. 2000). It can signal independently through the interaction with actin-associated proteins rather than working as co-receptors. In Orientia attachment, the interaction component of transmembrane molecules Syndecan 4 and Integrin α5β1 are still unknown.
The integrins form non-covalent heterodimers where extracellular domains interact with different ligands of ECM glycoprotein and other cell-surface proteins, and the actin cytoskeleton components interact with cytoplasmic domains (Morgan et al. 2007). It has been seen that the engagement of integrins may facilitate by the interaction between TSA56 antigen and fibronectin, by which downstream signaling molecules may be activated and influence the bacterial internalization with nonphagocytic cells (Cho et al. 2010a, b). Still, it is unclear whether fibronectin and Syndecan interaction involve in the attachment and cellular uptake of OT.
Orientia tsutsugamushi adopts host cellular modulation to survive
Activation of autophagy during infection
Autophagy is a regulated catabolic process that inhibits the growth of the pathogen in a cell by degrading the cytosolic components. During pathogenesis, autophagy is initiated in nucleation sites of the endoplasmic reticulum. When autophagosomes join with other endosomal compartments to engulf the target, the target is degraded. A part of the cytoplasm and different organelles are cut off in autophagosomes and fused with lysosomes to degrade (Yang and Klionsky 2010). ATGs, a highly conserved autophagy-related gene, regulate autophagy. In OT-infected polymorphonuclear leukocyte (PMNs), more autophagosomes are found, within 1 hour post-infection (hpi) (Ko et al. 2013). Rapamycin, a chemical inducer of autophagy, is present in the cell that induces autophagy as soon as 2hpi. The active evasion of OT from autophagy, which occurred solely in the presence of rapamycin, suggests that cellular autophagy evasion may play a key role in inhibiting rather than improving bacterial clearance (Ko et al. 2013). Autophagy kills the bacteria by lysosome-dependent degradation and presents the antigen to naive T cells by delivering cyanobacteria to MHC class II molecules. Autophagy is activated by IFN-gamma and tumor necrosis factor (TNF) and inhibited by Th2 cytokines IL-4 and IL-13 (Deretic 2009).
Cytotoxic activity and induction of apoptosis during infection
Apoptosis is a host defense mechanism that the pathogen replication can be limited by the apoptotic pathway inside the host cell. Histological studies of different tissues have shown apoptotic modulation with various OT strains. This process depends on other factors, such as, the type of infected cell, the rate of infection, and the strain of OT. The highly regulated process plays a significant role in the infected cell-pathogen interplay (Gao and Kwaik 2000; Sahni and Rydkina 2009). There are two pathways for activating apoptosis such as intrinsic and extrinsic pathways. In the intrinsic pathway, BAX and BAK, i.e., B cell lymphoma 2 (Bcl2) family proteins (proapoptotic) and Bcl2-like proteins (Bcl2, Bcl-xL, and Mcl-1) (anti-apoptotic) inhibit the process. The channel-forming proteins BAX and BAK increase the mitochondrial outer membrane permeability (MOMP). BH-only proteins are activated under a stressful condition to overcome the inhibitory effect of Bcl2-like proteins, resulting in an increased MOMP which secrete cytochrome c. In the extrinsic pathway, FasL and TNF-α, the death ligands bind to Fas and TNF receptor 1, the cell-surface death receptors. This helps to form a death-inducing signaling complex and activation of caspase 8. Therefore effector caspases either directly activated, or BH3-only protein Bid start to cross-talk with the intrinsic pathway (Taylor et al. 2008).
Different stages of Orientia infection may differentially modulate the apoptotic pathway. The mouse kidney endothelial cells demonstrated apoptosis with lethal Karp infection, whereas endothelial cell line ECV304 infected with Boryong (20:1) strain culture-induced apoptosis. The decreasing intracellular calcium level inhibits apoptosis in Boryong strain infection in the human monocyte cell line. A calcium-dependent signaling molecule, protein kinase C (PKC), plays an essential role in apoptosis. So the shortage of calcium may affect the regulation of PKC and also impact the apoptotic pathway.
Activation of endoplasmic reticulum (ER) degradation during infection
The endoplasmic reticulum is the transportation system with many functions in eukaryotic cells like protein synthesis, folding, and modification of secreted and transmembrane proteins. The amount of protein synthesized is affected by the change in extracellular environment or physiological changes. The Unfolded Protein Response (UPR), a signaling pathway that activates when misfolded proteins get in the ER lumen, triggers the endoplasmic reticulum-associated degradation (ERAD) pathway to maintain homeostasis. It consists of interconnected trails by the transmembrane sensors like RNA-activated ER protein kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). Misfolded proteins are translocated into the cytoplasm through this pathway, and the lysine residues bind covalently to ubiquitin. Within proteasome, multiple ubiquitins are linked together to create a chain-like structure that serves as a marker for the degradation of the misfolded proteins. The polyubiquitination proteins get degraded by proteolysis (Meusser et al. 2005). OT inhibits ERDA and modulates the UPR pathway. OT growth is low during 48 hpi; during this time, it uses ER stress for stable growth. ER stress is alleviated after 72 h, during the log phase of OT amplification, and protein breakdown in ERAD continues to produce amino acids to provide adequate nutrients. Ank 4 is produced by Orientia and interacts with Bat 3, a chaperone found in the host cell essential for ERAD (Rodino et al. 2017). As a result, Ank 4 is one of the OT virulence factors targeted to disrupt pathogenesis.
Active escape
The mechanism by which Orientia escapes from the phagosome is still unclear. When OT was cultured in L929 cells, a hemolysin gene, tlyC, and a gene that might encode phospholipase D were expressed (Suttinont et al. 2006; Kasper et al. 2012). The protein may disrupt the phagosomal membrane and allow the pathogen to discharge into the host cell’s cytoplasm (Bourgeois et al. 1982; Chattopadhyay and Richards 2007). OT release into the cytoplasm travels via microtubules to the microtubule-organizing center as a naked cytosolic bacterium (Mendell et al. 2017). Microtubules mediate OT migration from the cell periphery to the perinucleus in ECV304 cells (Mendell et al. 2017). OT reproduces via binary fission (Soong et al. 2014) that takes approximately 9 h to complete (Fuxelius et al. 2007). After proliferation, it is liberated from the host cell to envelop viral budding (Mendell et al. 2017; Soong et al. 2014). In mouse peritoneal mesothelial cells, the host cell membrane covers bacteria, which may start a new infection cycle through a phagocytic mechanism (Soong et al. 2014). Before the bacterium infects a new host cell, the host cell membrane encapsulates budding OT may be lysed. If the host cells break, intracellular OT may be discharged straight from highly infected cells (Fig. 2).
Fig. 2.
The repetitive cellular cycle of Orientia tsutsugamushi in nonphagocytic cells. It represents the detailed overview of the invasion and internalization process of OT as well as the active escape process. Different proteins involved in this process were mentioned inside. Invasion of the host through primary barriers, bacterial invasion of host defense, bacterial replication within-host, and host immunological competence to control/eliminate the pathogens are the four phages of host–pathogen interaction represented in this figure
Host defense mechanism against Orientia tsutsugamushi survival
Intracellular pathogens are well organized to hide away in the host cells. So, they are mainly elicited by T cells and can evade the antibodies. Because T cells have an automatic killing mechanism, they do not allow the infection to spread to the surrounding cells.
Innate immunity and pro-inflammatory response against Orientia infection
Bacteria mainly target endothelial cells and retard intracellular calcium releasing that induces apoptosis in different host cells (Watt et al. 2003). OT primarily targets the macrophages in human skin, but monocytes and neutrophils can also be infected. TSA56, an outer membrane protein, is the leading cause of adhesion and invasion into the mammalian cell. It takes 2 h to enter the host cell and escape from the endosome. Membrane-bound endosome uptakes the initial phagocytosis, but OT easily escapes and replicates in the host cytosol. In the dermis, endothelial cells and macrophages initiate inflammation, and through monocytes, pathogens spread into the circulatory system.
Role of dendritic cell
Dendritic cells are the antigen processing and presenting cells (APCs) that initiate and orchestrate the initial antigen-specific immune responses when the OT-infected trombiculid mite bites the host (Kadosaka and Kimura 2003) and also presents specific antigenic molecules to T cells. The maturational changes occur when foreign antigens are captured. It includes migratory activity, co-stimulatory molecule expression, major histocompatibility complex (MHC) increased expression, cytokine and chemokines secretion such as CD40, CD80, and CD86. In vitro studies show evidence of upregulation of MHC class II molecules and CD40, CD80, CD86, and CD83 co-stimulatory molecules after 24hpi, which indicates DC activation (Chu et al. 2013; Gorvel et al.2014). Eventually, the dendritic cells activate T cells (Ho et al. 2004; Lebre et al. 2005). Once the DCs get started, they move towards the lymph nodes to prime naive T cells to develop adaptive immunity (Randolph et al. 2005). The pro-inflammatory molecules like tumor necrosis factor α (TNF- α), Interleukin-6 (IL-6), IL-8, and IL-12p70 are also upregulated within Orientia infected dendritic cells (Choi et al. 2013; Chu et al. 2013).
CCL19 and CCL21, expressed in lymphatic endothelium and the T-cell region of lymph nodes, are the primary chemokines that drive DC movement (Vivekanandan et al. 2010). In addition, antigen intake by DC causes maturational changes, including decreased expression of the chemokines receptors CCR1, CCR2, and CCR5, which keep DC in peripheral tissue, and increased expression of CCR7, which helps antigen-bearing DC migrate to lymphatic tissue. However, there is currently little understanding of the function of DCs during OT infection.
Role of macrophage/monocytes
Macrophages are the primary cellular target of bacterial replication during the OT infection and act as an inflammatory response initiator. It also triggers an inflammatory signaling cascade and produces chemokines for recruiting T cells, neutrophils, and monocytes (Cho et al. 2000). In the dermal tissues, monocytes are either present or draft to the site of inoculation and instigate an early inflammatory response. The macrophages also act as a place for bacterial replication. The innate immunity can detect pathogen recognition receptors (PRRs), and a high level of TLR expressed on macrophages (Kawai and Akira 2010). In macrophages, OT induces an M1-type genetic program. Human monocytes stimulated by the inflammatory response and interferon-stimulated genes (ISGs) during OT infection. In addition, circulating leukocyte from scrub typhus patients shows various hallmarks of M1 polarization and survival of OT in macrophages (Tantibhedhyangkul et al. 2011, 2013).
Role of endothelial cells
Cytokine signals mainly influence the endothelial cells that control pathological processes. Pathogen-associated membrane protein (PAMP) and pathogen replications directly activate endothelial cells whereas damage-associated molecular patterns (DAMP) indirectly activate ECs. The development of self-proinflammatory and permeable conditions of EC activation helps in the clearance of pathogens. It mainly helps to promote leukocyte adhesion and antigen presentation that leads to the excessive production of macrophages and neutrophils. Vascular permeability increases due to the rise of macrophages and neutrophils that causes edema, hypoxia, and ARDS.
In vivo studies from a mouse model with the Boryong strain have revealed the involvement of Interleukin-32 (IL-32) in response to ECs during Orientia infection. After activating the upstream NOD1–IL-32 pathway, the production and secretion of ICAM-1, IL-1, IL-6, and IL-8 occurs (Cho et al. 2010a, b). In intravascular inoculation of Karp strain in a mouse model, early activation and deregulation of endothelial cells with two regulators, Angiopoieti-1 or Ang-1 and Ang-2, were observed. Ang-1 acts as a regulator of activation, and Ang-2 is usually kept in endothelial cells but triggers the action of ECs that releases after inflammation (Chousterman et al. 2017).
Role of neutrophils
In humans, neutrophils are present at the site of infection and also occur during acute infection. During tissue injury or any disease, neutrophils are considered the 'First responder' and early control of human pathogens (Summers et al. 2010). Its main functions are neutrophil extracellular trap, phagocytosis, and also releases antimicrobial granules. Neutrophils not only kill the bacteria but also damage host tissues. Nearly 40 years ago, it was unclear whether neutrophils could kill the engulfed pathogen or provide another place for bacteria growth. Neutrophils can be seen at the site of chigger bites in humans, and neutrophilia can occur during acute illness (Cho et al. 2012; Paris et al. 2012). Pariset al. 2015, discovered that, the indicators of neutrophil activation and potential neutrophil extracellular trap (NET) formation were considerably increased in the plasma of severe scrub typhus patients compared to with less severe scrub typhus (Paris et al. 2015). More research into OT role within neutrophils is essential; this information will help to clarify bacterial invasion and its dispersion.
Characterization of chemokines and cytokine network and expression of genes
OT is a potent inducer of chemokines and observes inflammation by attracting and activating phagocytic leukocytes. For both innate and acquired immunity, OT stimulates the production of pro-inflammatory cytokines and chemokines. The pathogen and endothelial cell interaction trigger many inflammatory responses, including several cytokines. On the invasion of OT, monocytes and macrophages primarily release the pro-inflammatory cytokine such as Tumor necrosis factor-alpha (TNF-α) (Cho et al. 2000). The inflammatory cytokines such as TNF and interleukin (IL)-6 were produced by OT in murine macrophages. The production of IL-10 inhibits TNF production (Watt and Kantipong 2007). In scrub typhus patients, an increased level of TNF-α, IL-1β, IL-6, IFN-γ, and IL-10 have been observed. The bacterial DNA concentration is positively related to IL-10 secretion levels (Paris et al. 2013). The inflammatory response causes cytokine storm that leads to respiratory distress syndrome or multiorgan dysfunction.
Immune-related signaling and pathway activation
Integrin signaling
Primarily, the bacterial invasion is mediated by the interaction between the host receptor and the ligand of the bacterial surface compound by which host signal transduction pathways are stimulated for bacterial entry (Lee et al. 2008). The bacteria enter the host by binding TSA56 protein with fibronectin via interaction with integrins. The integrins signaling effector is activated when OT localizes with integrins α5β1 and activates focal adhesion kinase (FAK), Src kinase, and Rho GTPase. OT utilizes the integrin-mediated signaling pathway to invade the eukaryotic host cell. The signaling adaptors such as talin and paxillin recruit to the site of infection (Cho et al. 2010a, b).
MAPK signaling
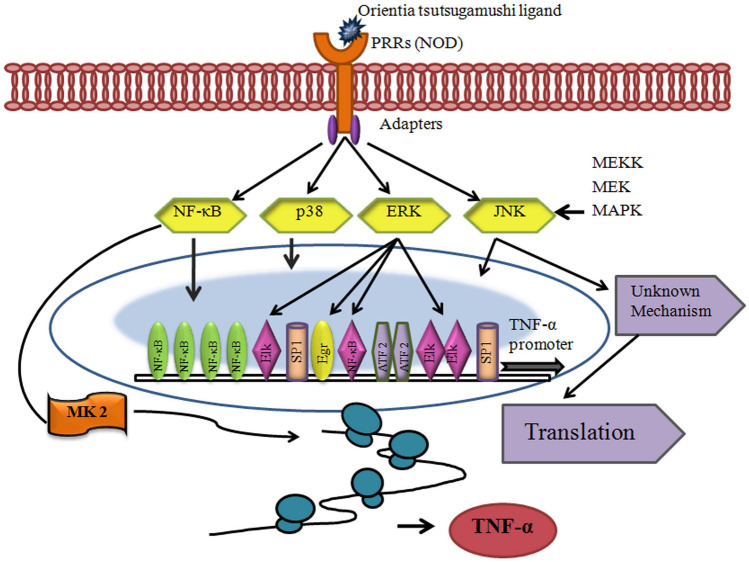
During infection, OT within the dendritic cell in the cytosolic compartment enhances the phosphorylation of p38 mitogen-activated protein kinase (MAPK), followed by the production of IFN-β and upregulation of the TNF (Type 1 interferon) pathway. Previously it has been studied that the CCL19 ligand of CCR7 activates MAPK members and the chemotaxis of dendritic cells and are subsequently regulated by these signaling pathways (Koo et al. 2009).
MAPK signaling is involved in the induction of TNF-α expression and maximal IFN-β gene expression in macrophages during OT infection. The post-transcriptional level through the p38 and JNK pathway regulates TNF-α expression. On the other hand, the transcriptional expression of TNF-α is controlled by the ERK pathway. J774A.1 cells, when infected with OT, the three MAPKs were phosphorylated where ERK1/2 and JNK1/2 phosphorylation was seen after 15 and 30 min, respectively, whereas p38 phosphorylation was detected at 15 min after OT induction. Within 1hpi, the MAPK signaling pathway gets activated. It suggested that MAPK activation in de novo bacterial protein synthesis is not required (Yun et al. 2009). However, it remains to be explored in detail (Fig. 3).
Fig. 3.
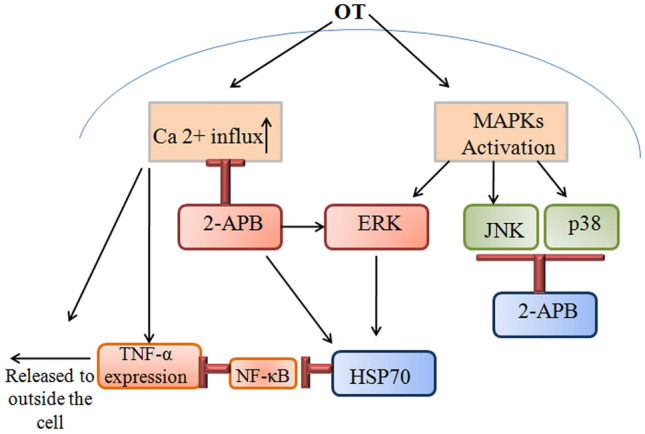
The participation of 2-ABP can block the increased in Orientia tsutsugamushi induced TNF-α and Ca2 + signaling activity. Ca2 + metabolism can also decrease TNF-α release and expression. TNF-α production can be regulated by the MAPK pathway. 2-ABP inhibits the JNK and p38 signaling pathways while activating ERK to enhance HSP70 over expression. HSP70 inhibits the translocation of NF-κB and suppresses TNF-α expression
Data from different studies revealed that TNF-α in monocytes might vary due to factors like the nature of the cell line, tissue, or animal model during OT infection. In several studies, a high level of IL-10 expression was observed in low dose infections, which promotes bacterial replication. In high-dose infection, diminished IL-10 production and upregulation of NF-κB/TNF-α were characterized. Kim et al. 2021 predicted the protein–protein interaction of 224 genes with candidate genes of scrub typhus (Kim et al. 2021). The candidate genes were classified according to the signaling pathways. PRMT6, DTWD2, BATF, JDP2, ONCUT1, KLK4, MAP3K7, and TGFBR2 were the eight candidate genes that belong to the same signaling pathways. Over 10 genes actively engaged in 15 signaling pathways like angiogenesis, apoptosis signaling pathway, CCKR signaling map, EGF receptor signaling pathway, FGF signaling pathway, gonadotropin-releasing hormone receptor pathway, inflammation mediated by chemokines and cytokine signaling pathway, integrin signaling pathway, PDGF signaling pathway, Ras pathway, TGF-beta signaling pathway, Toll receptor signaling pathway, Wnt signaling pathway, and p53 pathway (Kim et al. 2021). The OT surface antigen activated the nuclear factor-kB and p53 MAPK pathway, which invasion mediated by integrins signaling pathway (Jan et al. 2018) (Fig. 4). Toll-like receptors (TLRs) are the main PRRs (pathogen recognition receptors) that (TLR2, TLR4, TLR5) can recognize the PAMP (pathogen-associated molecular pattern) of bacteria at the cell surface or the intracellular endosomal compartment (TLR3 and TLR7-9) (Blasius and Beutler 2010).
Fig. 4.
Model of signaling and gene regulatory mechanisms of TNF-α biosynthesis during Orientia tsutsugamushi infection. Orientia tsutsugamushi stimulates receptor(s) and activates host MAPK and NF-κB signaling pathways. Signals from NF-κB and ERK pathways are required to promote strong transcription. Formation of enhanceosome was followed. NF-κB and other transcription factors interact with DNA, co-activators such as CBP/p300, and the RNA polymerase II holoenzyme. Transport of TNF-α mRNA is facilitated by ERK pathway
Gene expressions
OT has a significant effect on human immune responses. In different in-vitro studies, it has been seen the induction of MCP-1 (CCL2), RANTES (CCL5), and IL-8 (CXCL8) genes encoding for chemokines in human endothelial cells after OT infection (Cho et al. 2001, 2002). In protein–protein interaction, Kim et al. 2021 showed five genes that play an essential role in immune-related functions which interacts with the candidate genes of scrub typhus, such as interleukin enhancer-binding factor 2 (ILF2), 2′-5′-oligoadenylate synthetase 1 (OAS1), BATF, interferon-induced protein 35 (IFI35) and major histocompatibility complex, class II, DR alpha (HLA-DRA) genes (Kim et al. 2021). The pro-inflammatory cytokine serum level, i.e., TNF, IL-12p40, IL-15, IL-18, and IFN-γ, were increased in scrub typhus patients (Chierakul et al. 2004). The Cytotoxic T cell, NK cells, and Th1 attracted by the CXCL9 (MIG) and CXCL10 (IP-10) cytokines were also increased (de Fost et al. 2005). In human monocytes, the gene transcription changes were induced by OT (Tantibhedhyangkul et al. 2011).
In microarray analysis, the upregulated inflammation-related genes were involved. After 8 and 18 hpi of OT, CCL5, CCL20, CXCL10, and CXCL11, chemotaxis associated genes as well as TNF, IL-1b, IL-16, IL-12p40, IL-23p19, and IL-15 inflammatory cytokine encoding genes were upregulated, but upregulation was slowed down for the gene encoding for IL-1b. It can be due to the robust modulation of inflammatory genes associated with the M1 polarization of macrophages. The genes involved and modulation with their mode of activation with immune cells has given in Table 2. In bacterial mechanism, several genes were involved. In macrophages, atrC1 and proP amino acid transporter genes; for phospholipid biosynthesis uppS, fabI, pccB, coq7 and ubiA genes were upregulated (Soballe and Poolle 1999). Within macrophage, four genes i.e. xerD, nth, dnaB, and a holiday junction resolvase gene involved in DNA repair. It protects the genome from oxidative damage in macrophages by increasing the bacterial repair system (Cho et al. 2010a, b).
Table 2.
Genes involved in host–pathogen interaction and modulation with their mode of activation in other immune cells and function
| Gene | Gene full name | Receptor | Chemokine | Mode of activation | Function |
|---|---|---|---|---|---|
| MCP-1 | Monocytes chemo-attractant protein 1 | CCR-2 | CCL-2 | Endothelial cell | Regulate migration and infiltration of monocytes and macrophages |
| RANETS | Regulated on activation, normal T cell expressed & secreted | CCR-3 | CCL-5 | Endothelial cell | Play role in recruiting leukocytes in inflammatory sites also activates G-protein coupled receptor (GPR-75) |
| IL-8 | Interleukin 8 | CXCR1 and CXCR2 | CXCL-8 | Endothelial cell | Attracts and activates neutrophils in inflammatory region |
| IRF7 | Interferon regulatory factor 7 | IRF3 | CCL5 | Monocytes |
Play role in transcriptional activation of virus inducible cellular gene Promotes macrophage differentiation |
| ISG15 | Interferon stimulated exonuclease gene | LFA1integrin receptor | C-terminal domain (CTD) | Monocytes | Modulates host signaling pathway, host damage and repair response |
| IFI35 | Interferon induced protein 35 | TLR4 | – | Macrophage | Involved in interferon γ signaling and innate immune system |
| STAT2 | Signal transducer and activator of transcription 2 | IFNAR1and IFNAR2 | IFN α/β and IFN γ | – | Mediates anti-viral and anti proliferative signaling |
| AIM2 | Interferon inducible protein AIM2 or absent in melanoma2 | – | IL-1β and IL-18 | – | Activated AIM2 recruit apoptosis associated speck like protein containing CARD and forming inflammasomes |
| MCAM | Melanoma cell adhesion molecule | Laminin α1 | CD146 | Endothelial cell | Part of endothelial junction associated with actin cytoskeleton |
| ILF2 | Interleukin enhancer-binding factor2 | CDC5L | – | – | Transcription factor required for T-cell expression of IL2 gene |
| CTSC | Cathepsin C or dipeptidyl peptidase 1 | – | – | – |
Degradation of ECM component Bacterial properties Cleavage of inflammatory mediator |
| OAS1 | 2′-5′-oligodenylate synthetase1 | – | – | – | Interferon induced, dsRNA activated anti-viral enzyme plays role in cellular innate immune system |
| HLA-DRA | Major histocompatibility complex, class II, DRα | – | – | – | Presents peptides derived from extracellular protein |
| BATF | Basic leucine zipper ATF-like transcription factor | – | – | – | AP-1 family transcription factor that controls the differentiation of lineage specific cell in immune system |
Conclusion
OT cause life-threatening disease that can invade and replicate inside various host cell types. The host–pathogen interaction has been revealed, but the exact molecular mechanism of scrub typhus progression has not been fully elucidated yet. Under specific conditions, different kinds of host and bacterial protein are involved to regulate host–pathogen interaction. All pathogens have adopted to hijack host components to promote their replication. Meanwhile, host cells have developed complex signaling networks to detect, regulate, and destroy invading pathogens. However, diverse pathogenic counter mechanisms frequently resist, inhibit or subvert these antibacterial pathways. The current study describes the signaling pathways involved during Orientia infection along with the involvement of various genes that modulate immune cells. This information would elucidate the important scientific information with regard to host–pathogen interaction. Due to the genotype complexity and lack of enough genomic resources, act as abarrier to mitigate the pathogenesis of the respective bacterium. Thus, it is hindering to develop an effective diagnostic tool as well as eradication strategy for this neglected pathogen. So, we are continuously doing surveillance of the desease outbreak and planning to decipher several complete genome of this bacterium derived from India. Hope the present review corroborated with our plan to get an indepth insight into the OT biology to address the raised concerns.
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
References
- Aguilar BJ, Zhu Y, Lu Q. Rho GTPases as therapeutic targets in Alzheimer’s disease. Alzheimer's Res Ther. 2017;9:1. doi: 10.1186/s13195-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khodor S, Price CT, Kalia A, Kwaik YA. Functional diversity of ankyrin repeats in microbial proteins. Trends Microbiol. 2010;18:132–139. doi: 10.1016/j.tim.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K, Tamura A, Ohashi N, Urakami H, Kaya S, Fukushi K. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect Immun. 1987;55:2290–2292. doi: 10.1128/iai.55.9.2290-2292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal S, Giengkam S, Chaemchuen S, Dorling J, Kosaisawe N, VanNieuwenhze M, Sampattavanich S, Schumann P, Salje J. Evidence for a peptidoglycan-like structure in Orientia tsutsugamushi. Mol Microbiol. 2017;105:440–452. doi: 10.1111/mmi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells ME, Rabagliati R, García P, Poggi H, Oddó D, Concha M, Abarca K, Jiang J, Kelly DJ, Richards AL, Fuerst PA. Endemic scrub typhus–like illness, Chile. Emerg Infect Dis. 2011;17:1659. doi: 10.3201/eid1709.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty EM, Chaemchuen S, Blacksell S, Richards AL, Paris D, Bowden R, Chan C, Lachumanan R, Day N, Donnelly P, Chen S. Long-read whole genome sequencing and comparative analysis of six strains of the human pathogen Orientia tsutsugamushi. PLoS Negl Trop Dis. 2018;12:e0006566. doi: 10.1371/journal.pntd.0006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhate R, Pansare N, Chaudhari SP, Barbuddhe SB, Choudhary VK, Kurkure NV, Kolte SW. Prevalence and phylogenetic analysis of Orientia tsutsugamushi in rodents and mites from central India. Vector-Borne Zoonotic Dis. 2017;17:749–754. doi: 10.1089/vbz.2017.2159. [DOI] [PubMed] [Google Scholar]
- Blacksell SD, Luksameetanasan R, Kalambaheti T, Aukkanit N, Paris DH, McGready R, Nosten F, Peacock SJ, Day NP. Genetic typing of the 56-kDa type-specific antigen gene of contemporary Orientia tsutsugamushi isolates causing human scrub typhus at two sites in north-eastern and western Thailand. FEMS Immunol Med Microbiol. 2008;52:335–342. doi: 10.1111/j.1574-695X.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Bourgeois AL, Olson JG, Fang RC. Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1982;31:532–340. doi: 10.4269/ajtmh.1982.31.532. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Sarma N. Scrub typhus: an emerging threat. Indian J Dermatol. 2017;62:478. doi: 10.4103/ijd.IJD_388_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Kang JS, Lee WK, Choi MS, Lee JH. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990;28:685–688. doi: 10.1128/jcm.28.4.685-688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Richards AL. Scrub typhus vaccines: past history and recent developments. Hum Vaccin. 2007;3:73–80. doi: 10.4161/hv.3.3.4009. [DOI] [PubMed] [Google Scholar]
- Chen Y, Götte M, Liu J, Park PW. Microbial subversion of heparan sulfate proteoglycans. Mol Cells. 2008;26:415–426. [PubMed] [Google Scholar]
- Chierakul W, de Fost M, Suputtamongkol Y, Limpaiboon R, Dondorp A. Differential expression of interferon-γ and interferon-γ-inducing cytokines in Thai patients with scrub typhus or leptospirosis. Clin Immunol. 2004;113:140–144. doi: 10.1016/j.clim.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Cho NH, Seong SY, Huh MS, Han TH, Koh YS, Choi MS, Kim IS. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun. 2000;68:594–602. doi: 10.1128/iai.68.2.594-602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Seong SY, Choi MS, Kim IS. Expression of chemokine genes in human dermal microvascular endothelial cell lines infected with Orientia tsutsugamushi. Infect Immun. 2001;69:1265–1272. doi: 10.1128/IAI.69.3.1265-1272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Seong SY, Huh MS, Kim NH, Choi MS. Induction of the gene encoding macrophage chemoattractant protein 1 by Orientia tsutsugamushi in human endothelial cells involves activation of transcription factor activator protein 1. Infect Immun. 2002;70:4841–4850. doi: 10.1128/IAI.70.9.4841-4850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Kim HR, Lee JH, Kim SY, Kim J, Cha S, Kim SY, Darby AC, Fuxelius HH, Yin J, Kim JH. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci. 2007;104:7981–7986. doi: 10.1073/pnas.0611553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BA, Cho NH, Seong SY, Choi MS, Kim IS. Intracellular invasion by Orientia tsutsugamushi is mediated by integrin signaling and actin cytoskeleton rearrangements. Infect Immun. 2010;78:1915–1923. doi: 10.1128/IAI.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KA, Jun YH, Suh JW, Kang JS, Choi HJ, Woo SY. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb Pathog. 2010;49:95–104. doi: 10.1016/j.micpath.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Cho BA, Ko Y, Kim YS, Kim S, Choi MS, Kim IS, Kim HR, Cho NH. Phenotypic characterization of peripheral T cells and their dynamics in scrub typhus patients. PLoS Negl Trop Dis. 2012;6:e1789. doi: 10.1371/journal.pntd.0001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Cheong TC, Ha NY, Ko Y, Cho CH, Jeon JH, So I, Kim IK, Choi MS, Kim IS, Cho NH. Orientia tsutsugamushi subverts dendritic cell functions by escaping from autophagy and impairing their migration. PLoS Negl Trop. 2013;7:e1981. doi: 10.1371/journal.pntd.0001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Chu H, Park SM, Cheon IS, Park MY, Shim BS, Gil BC, Jeung WH, Hwang KJ, Song KD, Hong KJ, Song M. Orientia tsutsugamushi infection induces CD4+ T cell activation via human dendritic cell activity. J Microbiol Biotechnol. 2013;23:1159–1166. doi: 10.4014/jmb.1303.03019. [DOI] [PubMed] [Google Scholar]
- De Benedetti S, Bühl H, Gaballah A, Klöckner A, Otten C, Schneider T, Sahl HG, Henrichfreise B. Characterization of serine hydroxymethyltransferaseGlyA as a potential source of D-alanine in Chlamydia pneumoniae. Front Cell Infect Microbiol. 2014;4:19. doi: 10.3389/fcimb.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fost M, Chierakul W, Pimda K, Dondorp AM, White NJ. Activation of cytotoxic lymphocytes in patients with scrub typhus. Am J Trop Med Hyg. 2005;72:465–467. [PubMed] [Google Scholar]
- Deretic V. Multiple regulatory and effector roles of autophagy in immunity. Curr Opin Immunol. 2009;21:53–62. doi: 10.1016/j.coi.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasagayam E, Dayanand D, Kundu D, Kamath MS, Kirubakaran R, Varghese GM. The burden of scrub typhus in India: a systematic review. PLoS Negl Trop Dis. 2021;15:e0009619. doi: 10.1371/journal.pntd.0009619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu T, Urakami H, Tamura A. Phylogenetic analysis of Orientia tsutsugamushi strains based on the sequence homologies of 56-kDa type-specific antigen genes. FEMS Microbiol Lett. 1999;180:163–169. doi: 10.1111/j.1574-6968.1999.tb08791.x. [DOI] [PubMed] [Google Scholar]
- Fuxelius HH, Darby A, Min CK, Cho NH, Andersson SG. The genomic and metabolic diversity of Rickettsia. Res Microbiol. 2007;158:745–753. doi: 10.1016/j.resmic.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Gao LY, Kwaik YA. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 2000;8:306–313. doi: 10.1016/s0966-842x(00)01784-4. [DOI] [PubMed] [Google Scholar]
- Ge Y, Rikihisa Y. Subversion of host cell signaling by Orientia tsutsugamushi. Microbes Infect. 2011;13:638–648. doi: 10.1016/j.micinf.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Gorvel L, Textoris J, Banchereau R, Ben Amara A, Tantibhedhyangkul W, von Bargen K, Ka MB, Capo C, Ghigo E, Gorvel JP, Mege JL. Intracellular bacteria interfere with dendritic cell functions: role of the type I interferon pathway. PLoS ONE. 2014;9:e99420. doi: 10.1371/journal.pone.0099420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha NY, Cho NH, Kim YS, Choi MS, Kim IS. An autotransporter protein from Orientia tsutsugamushi mediates adherence to nonphagocytic host cells. Infect Immun. 2011;79:1718–1727. doi: 10.1128/IAI.01239-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha NY, Sharma P, Kim G, Kim Y, Min CK, Choi MS, Kim IS, Cho NH. Immunization with an autotransporter protein of Orientia tsutsugamushi provides protective immunity against scrub typhus. PLoS Negl Trop Dis. 2015;9:e0003585. doi: 10.1371/journal.pntd.0003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho LJ, Shaio MF, Chang DM, Liao CL, Lai JH. Infection of human dendritic cells by dengue virus activates and primes T cells towards Th0-like phenotype producing both Th1 and Th2 cytokines. Immunol Invest. 2004;33:423–437. doi: 10.1081/imm-200038680. [DOI] [PubMed] [Google Scholar]
- Ihn KS, Han SH, Kim HR, Huh MS, Seong SY, Kang JS, Han TH, Kim IS, Choi MS. Cellular invasion of Orientia tsutsugamushi requires initial interaction with cell surface heparan sulfate. Microb Pathog. 2000;28:227–233. doi: 10.1006/mpat.1999.0344. [DOI] [PubMed] [Google Scholar]
- Jan RH, Chen CJ, Hong YR, Lin YL, Chen LK. A surface antigen of Orientia tsutsugamushi activates human monocyte-derived dendritic cells via nuclear factor-kB & p38 mitogen-activated protein kinase pathways. Indian J Med Res. 2018;148:215. doi: 10.4103/ijmr.IJMR_1417_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, Lee SH. Scrub typhus: clinical, pathologic, and imaging findings. Radiographics. 2007;27:161–172. doi: 10.1148/rg.271065074. [DOI] [PubMed] [Google Scholar]
- Jordan JM, Woods ME, Soong L, Walker DH. Rickettsiae stimulate dendritic cells through toll-Like receptor 4, leading To enhanced NK cell activation in vivo. J Infect Dis. 2009;199:236–242. doi: 10.1086/595833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosaka T, Kimura E. Electron microscopic observations of Orientia tsutsugamushi in salivary gland cells of naturally infected Leptotrombidium pallidum larvae during feeding. Microbiol Immunol. 2003;47:727–733. doi: 10.1111/j.1348-0421.2003.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Kasper MR, Blair PJ, Touch S, Sokhal B, Yasuda CY, Williams M, Richards AL, Burgess TH, Wierzba TF, Putnam SD. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am J Trop Med Hyg. 2012;86:246. doi: 10.4269/ajtmh.2012.11-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kim SW, Ihn KS, Han SH, Seong SY, Kim IS, Choi MS. Microtubule-and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect Immun. 2001;69:494–500. doi: 10.1128/IAI.69.1.494-500.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Choi MS, Kim IS. Role of Syndecan-4 in the cellular invasion of Orientia tsutsugamushi. Microb Pathog. 2004;36:219–225. doi: 10.1016/j.micpath.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Kim YC, Kim S, Kim HK, Lee Y, Shin C, Lee CS, Jeong BH. Genome-wide association study identifies eight novel loci for susceptibility of scrub typhus and highlights immune-related signaling pathways in its pathogenesis. Cells. 2021;10:570. doi: 10.3390/cells10030570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y, Choi JH, Ha NY, Kim IS, Cho NH, Choi MS. Active escape of Orientia tsutsugamushi from cellular autophagy. Infect Immun. 2013;81:552–559. doi: 10.1128/IAI.00861-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JE, Yun JH, Lee KH, Hyun JW, Kang HK, Jang WJ, Park KH, Koh YS. Activation of mitogen-activated protein kinases is involved in the induction of interferon β gene in macrophages infected with Orientia tsutsugamushi. Microbiol Immunol. 2009;53:123–129. doi: 10.1111/j.1348-0421.2008.00098.x. [DOI] [PubMed] [Google Scholar]
- Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, Clausen BE, De Jong EC. Differential expression of inflammatory chemokines by Th1-and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol. 2005;83:525–535. doi: 10.1111/j.1440-1711.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Cho NH, Kim SY, Bang SY, Chu H, Choi MS, Kim IS. Fibronectin facilitates the invasion of Orientia tsutsugamushi into host cells through interaction with a 56-kDa type-specific antigen. J Infect Dis. 2008;198:250–257. doi: 10.1086/589284. [DOI] [PubMed] [Google Scholar]
- Lee SM, Kim MK, Kim MJ, Kang JS. Novel polysaccharide antigen of Orientia tsutsugamushi revealed by a monoclonal antibody. FEMS Microbiol Lett. 2009;297:95–100. doi: 10.1111/j.1574-6968.2009.01663.x. [DOI] [PubMed] [Google Scholar]
- Leiss M, Beckmann K, Girós A, Costell M, Fässler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol. 2008;20:502–507. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Luce-Fedrow A, Lehman ML, Kelly DJ, Mullins K, Maina AN, Stewart RL. A review of scrub typhus (Orientia tsutsugamushi and related organisms): then, now, and tomorrow. Trop Med Inf Dis. 2018;3:8. doi: 10.3390/tropicalmed3010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SK, Sharma S, Kaushik M, Raina R, Thakur P, Taneja GP. Scrub typhus presenting as acute cerebellitis. J Assoc Phys India. 2016;64:69–70. [PubMed] [Google Scholar]
- Mendell NL, Bouyer DH, Walker DH. Murine models of scrub typhus associated with host control of Orientia tsutsugamushi infection. PLoS Negl Trop Dis. 2017;11:e0005453. doi: 10.1371/journal.pntd.0005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Yamashita A, Kurokawa K, Morimoto T, Ogawa M, Fukuhara M, Urakami H, Ohnishi M, Uchiyama I, Ogura Y, Ooka T. The whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res. 2008;15:185–199. doi: 10.1093/dnares/dsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Aukkanit N, Jenjaroen K, Blacksell SD, Day NP. A highly sensitive quantitative real-time PCR assay based on the groEL gene of contemporary Thai strains of Orientia tsutsugamushi. Clin Microbiol Infect. 2009;15:488–495. doi: 10.1111/j.1469-0691.2008.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M, Ferguson DP, Blacksell SD, Newton PN, Day NP, Turner GD. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:e1466. doi: 10.1371/journal.pntd.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Stephan F, Bulder I, Wouters D, Van der Poll T, Newton PN, Day NP, Zeerleder S. Increased nucleosomes and neutrophil activation link to disease progression in patients with scrub typhus but not murine typhus in Laos. PLoS Negl Trop Dis. 2015;9:e0003990. doi: 10.1371/journal.pntd.0003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo-Diaz MA, Song C, Xiong Y, Chen H, Wahl LM, Radulovic S, Medvedev AE. Involvement of TLR2 and TLR4 in cell responses to Rickettsia akari. J Leukoc Biol. 2010;88:675–685. doi: 10.1189/jlb.1009674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse S, Rodrigo C, Fernando D. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pac J Trop Med. 2012;5:261–264. doi: 10.1016/S1995-7645(12)60036-4. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Rights FL, Smadel JE. Studies on scrub typhus (tsutsugamushi Disease): III. Heterogenicity of strains of R. tsutsugamushi as demonstrated by cross-vaccination studies. J Exp Med. 1948;87:339. doi: 10.1084/jem.87.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino KG, VieBrock L, Evans SM, Ge H, Richards AL, Carlyon JA. Orientia tsutsugamushi modulates endoplasmic reticulum-associated degradation to benefit its growth. Infect Immun. 2017;86:e00596–e617. doi: 10.1128/IAI.00596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni SK, Rydkina E. Host-cell interactions with pathogenic Rickettsia species. Fut Microbiol. 2009;4:323–339. doi: 10.2217/FMB.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657. doi: 10.1371/journal.ppat.1006657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001;3:11–21. doi: 10.1016/s1286-4579(00)01352-6. [DOI] [PubMed] [Google Scholar]
- Shostak K, Schirch V. Serine hydroxymethyltransferase: mechanism of the racemization and transamination of D-and L-alanine. Biochemistry. 1988;27:8007–8014. doi: 10.1021/bi00421a006. [DOI] [PubMed] [Google Scholar]
- Slesak G, Inthalath S, Dittrich S, Paris DH, Newton PN. Leeches as further potential vectors for rickettsial infections. Proc Natl Acad Sci. 2015;112:6593–6594. doi: 10.1073/pnas.1515229112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soballe R, Poolle RK. Microbial ubiquinones: multiple roles in respiration, gene regulation and oxidative stress management. Microbiol Read. 1999;145:1817–1830. doi: 10.1099/13500872-145-8-1817. [DOI] [PubMed] [Google Scholar]
- Soong L, Wang H, Shelite TR, Liang Y, Mendell NL, Sun J, Gong B, Valbuena GA, Bouyer DH, Walker DH. Strong type 1, but impaired type 2, immune responses contribute to Orientia tsutsugamushi-induced pathology in mice. PLoS Negl Trop Dis. 2014;8:e3191. doi: 10.1371/journal.pntd.0003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, Suwancharoen D, Panlar P, Saisongkorh W, Rolain JM, Raoult D. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–370. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- Swain SK, Sahu BP, Panda S, Sarangi R. Molecular characterization and evolutionary analysis of Orientia tsutsugamushi in eastern Indian population. Arch Microbiol. 2022;204:1–7. doi: 10.1007/s00203-022-02823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takhar R, Bunkar M, Arya S, Mirdha N, Mohd A. Scrub typhus: a prospective, observational study during an outbreak in Rajasthan, India. Natl Med J India. 2017;30:69. [PubMed] [Google Scholar]
- Tamura A, Takahashi K, Tsuruhara T, Urakami H, Miyamura S, Sekikawa H. Isolation of Rickettsia tsutsugamushi antigenically different from Kato, Karp, and Gilliam strains from patients. Microbiol Immunol. 1984;28:873–882. doi: 10.1111/j.1348-0421.1984.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Tantibhedhyangkul W, Prachason T, Waywa D, El Filali A, Ghigo E, Thongnoppakhun W, Raoult D, Suputtamongkol Y, Capo C, Limwongse C, Mege JL. Orientia tsutsugamushi stimulates an original gene expression program in monocytes: relationship with gene expression in patients with scrub typhus. PLoS Negl Trop Dis. 2011;5:e1028. doi: 10.1371/journal.pntd.0001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantibhedhyangkul W, Amara AB, Textoris J, Gorvel L, Ghigo E, Capo C, Mege JL. Orientia tsutsugamushi, the causative agent of scrub typhus, induces an inflammatory program in human macrophages. Microb Pathog. 2013;55:55–63. doi: 10.1016/j.micpath.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- Varghese GM, Trowbridge P, Janardhanan J, Thomas K, Peter JV, Mathews P. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39–43. doi: 10.1016/j.ijid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- VieBrock L, Evans SM, Beyer AR, Larson CL, Beare PA, Ge H, Singh S, Rodino KG, Heinzen RA, Richards AL, Carlyon JA. Orientia tsutsugamushi ankyrin repeat-containing protein family members are Type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum. Front Cell Infect Microbiol. 2015;4:186. doi: 10.3389/fcimb.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India. 2010;58:24–28. [PubMed] [Google Scholar]
- Vollmer W, Bertsche U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochimica Et Biophysica Acta (BBA)-Biomembranes. 2008;1778:1714–1734. doi: 10.1016/j.bbamem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Vollmer W, Blanot D, De Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D. Azithromycin activities against Orientia tsutsugamushi strains isolated in cases of scrub typhus in Northern Thailand. Antimicrob Agents Chemother. 1999;43:2817–2818. doi: 10.1128/aac.43.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G, Kantipong P (2007) Orientia tsutsugamushi and Scrub Typhus. Rickettsial Dis 237
- Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010;339:31–46. doi: 10.1007/s00441-009-0829-3. [DOI] [PubMed] [Google Scholar]
- Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis. 2017;11:e0006062. doi: 10.1371/journal.pntd.0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JH, Koo JE, Koh YS. Mitogen-activated protein kinases are involved in tumor necrosis factor alpha production in macrophages infected with Orientia tsutsugamushi. Microbiol Immunol. 2009;53:349–355. doi: 10.1111/j.1348-0421.2009.00127.x. [DOI] [PubMed] [Google Scholar]