Abstract
We aimed to review the records of cancer and kidney transplant patients of out of 1135 COVID-19 patients, who were referred to our hospital (Valiasr) in Zanjan, from March 16th, 2020, to June 11th, 2020. This was single-center, historical cohort study. Patients were divided into different subgroups and compared of disease outcomes. The only predictor of death was lactate dehydrogenase (LDH). The rate of red cell distribution width (RDW) in patients with active cancer was higher than kidney transplant patients and was statistically significant. There was no statistically significant difference in mortality between active and non-active cancer groups. Female sex and low SpO2 has increased the chances of ICU admission. Patients with active cancer generally have severe and more complicated disease and RDW can be a predictable option.
Key Words: COVID-19, kidney transplantation, cancer, immunocompromised patient
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The pandemic of COVID-19 has a huge effect on public health and is still a major cause of death in Iran.1 During the COVID-19 pandemic, cancer patients are an especially vulnerable group. Because of their underlying condition and treatment complication, they are often immunosuppressed.2 Many individuals assume that cancer patients who receive systemic anticancer drugs are at a higher risk of developing the disease than patients who do not receive anticancer treatment.3 There is few information on the risk of developing COVID-19 in hematological cancer patients. Many people with hematological cancer take anti-cancer drugs that suppress bone marrow function, putting them at risk of acquiring infections in the community and hospitals.4 The epidemiology, clinical characteristics, and outcomes of COVID-19 among solid organ transplant (SOT) recipients are undefined. Few early descriptive case reports and case series of SOT recipients with COVID-19 suggest poor outcomes; but, difference is unclear between in the SOT and non-transplant population.5 Due to chronic immunosuppression and coexisting conditions, kidney transplant recipients are particularly vulnerable to COVID-19.6 Patients with COVID-19 have hematological abnormality, such as a lower lymphocyte and platelet count but a normal white blood cell (WBC).7 Red cell distribution width (RDW) conveys the degree of anisocytosis between red blood cells. Anisocytosis is a mechanism that is highly dependent on inflammation. Many of the proinflammatory cytokines like TNF-α and interleukin-1 decrease erythropoietin synthesis during cytokine storm.8 In addition, hypoxia causes erythropoietic disturbance in COVID-19. Super infections are prevalent in COVID-19, thus increasing sepsis. RDW plays a considerable alarm in sepsis.8 In particular, several previous studies have shown that increased RDW is correlated with mortality in non-specific acute respiratory distress syndrome (ARDS) patients.9 Adding RDW at diagnosis of ARDS increased discrimination in the model using 4 clinical factors to estimate ICU mortality.10 Since the beginning of the pandemic, there have been grave concerns over the risk of developing severe COVID-19 for individuals with immunodeficiency’s or those taking immunosuppressive therapies. Two main immunosuppressant diseases are cancer and SOT. There are conflicting data about increased risk of COVID-19 in patients with a history of immunosuppressant.11,12 Type and duration of immunosuppressant are important in evaluation of susceptibility to infection. Therefore, we aimed to review the records of cancer and kidney transplant patients of out of 1135 COVID-19 patients, who were referred to our hospital (Valiasr) in Zanjan, Iran.
Figure 1.
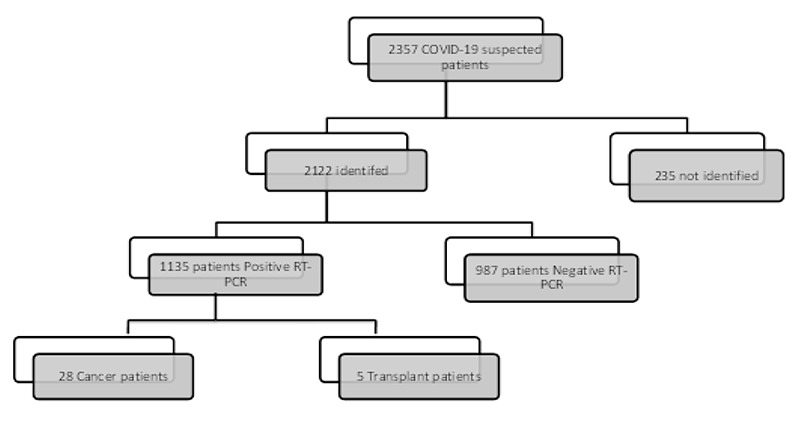
Statistics of patients suspected to COVID-19: Cancer and kidney transplant patients with positive COVID-19 RT-PCR were included.
Materials and Methods
Study design
This was a single-center, historical cohort study. We reviewed the records of cancer and kidney transplant patients with COVID-19 were referred to our hospital (Valiasr) in Zanjan, from March 16th, 2020, to June 11th, 2020 (Figure 1). The most prevalence between SOT patients that referred to our system was kidney transplant. Cancer (solid tumor and hematologic malignancy) and kidney transplant patients with positive COVID-19 RT-PCR (reverse-transcriptase polymerase chain reaction) were included.
This study was approved by the institutional ethics review boards of our university (approval number IR.ZUMS.REC.1399.265 date: Oct 15th, 2020). The Research Ethics Committee waived the requirement informed consent before the study started because of the urgent need to collect epidemiological and clinical data. We analyzed all the data anonymously.
Diagnostic methods
The method of diagnosis is RT-PCR assay test using throat swab specimens collected from upper respiratory tracts. All patient aged was more than 18. Patients with a radiological or clinical diagnosis of COVID-19, without a positive RT-PCR test were not included in this analysis. Patients with non-invasive cancers including non-melanomatous skin cancer, in-situ carcinoma, or precursor hematological neoplasms were excluded from this analysis. Patients with room air oxygen saturation (SpO2) < 90% were considered as severe COVID-19, and ≥90% were considered moderate COVID-19.13 Clinical data of each patient were collected, which included age, gender, and known comorbidities (diabetes mellitus (DM), hypertension (HTN)). Other underlying diseases were not included in the study due to their lower prevalence. Cancer stage was not chosen for the multivariable analysis as this variable was only collected in solid tumors. Patients with cancer were studied in two groups: active cancer (for which anticancer treatment (chemotherapy) had been administered in the past 6 months; or hematological cancer that is not in complete remission) and inactive cancer. Also cancer patients were studied in 3 groups: blood malignancy, gastrointestinal cancer and non-gastrointestinal cancers (Figure 2).
Figure 2.
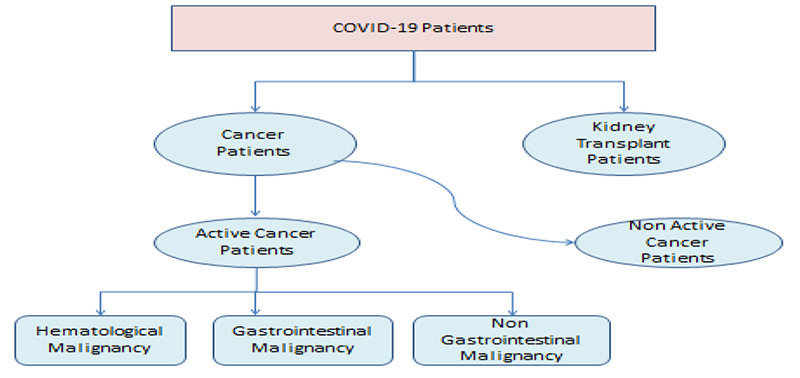
Division algorithm of patients with COVID-19, including two groups 1: Patients with cancer, 2: Patients with renal transplant. Patients with cancer were studied in two groups: active cancer and inactive cancer. Cancers were studied in 3 groups: blood malignancy, gastrointestinal cancer and non-gastrointestinal cancers.
Table 1.
Description of patients on admission.
| Variables | Cancer patient’s N (%) | Graft patients N (%) | |
|---|---|---|---|
| Sex | Male | 16(57.1) | 4(80) |
| Female | 12(42.9) | 1(20) | |
| Age | M ± SD | M ± SD | |
| 62.54±14.78 | 48.60±15.14 | ||
| Severity | severe | 11(39.3) | 2(40) |
| Non-severe | 17(60.7) | 3(60) | |
| Comorbidity | DM | 3(10.7) | 1(20) |
| HTN | 6(21.4) | 4(80) | |
| Cancer activity | Active | 22(78.5) | |
| Non-active | 6(21.4) | ||
| Type of cancer | Hematologic | 5(17.9) | N/A |
| GI | 8(28.6) | ||
| Non-GI | 15(53.6) | ||
| Total | 28(100) | 5(100) |
GI: Gastrointestinal; Non GI: Non Gastrointestinal; HTN: Hypertension; DM: Diabetes Mellitus; N: Number; SD: standard deviation
Figure 3.
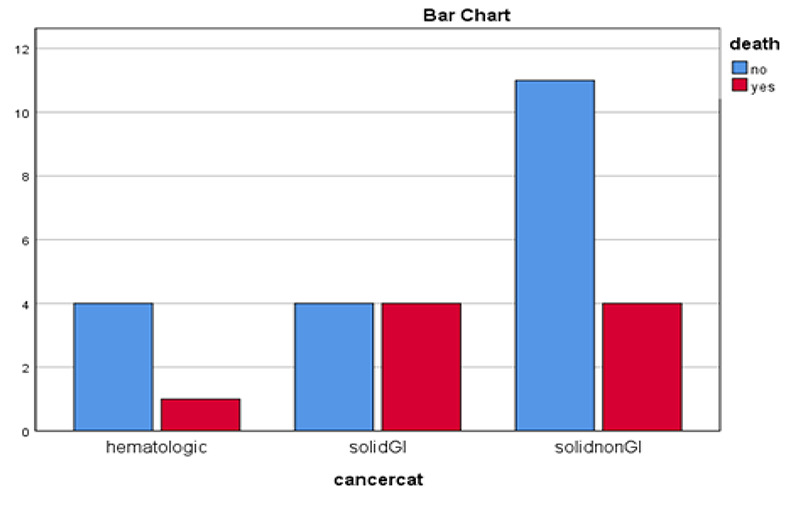
Comparison of cancer type with mortality. GI: Gastrointestinal, Non GI: Non Gastrointestinal
Table 2.
Patients’ laboratory tests and the type of cancer.
| Patient with Cancer type | WBC | Hb | PLT | RDW | LDH | Lymph count | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Breast Cancer | 2900 | 12.3 | 89000 | 13.2 | 403 | 41.6 | ||
| 2 | Lung Cancer | 15200 | 8.9 | 215000 | 15.3 | 612 | 6.4 | ||
| 3 | Esophageal Cancer | 3200 | 10.1 | 404000 | 13.4 | 259 | 9.9 | ||
| 4 | Oral SCC | 4200 | 12.3 | 252000 | 14.1 | 570 | 18.2 | ||
| 5 | Hypopharyngeal Cancer | 19400 | 7.3 | 276000 | 17.5 | 3.5 | |||
| 6 | Gastric Cancer | 10300 | 11.6 | 204000 | 16.2 | 587 | 17.1 | ||
| 7 | CLL | 116000 | 11.5 | 44000 | 15.2 | 786 | 0 | ||
| 8 | Metastatic Lung Cancer | 5300 | 10.5 | 256000 | 17.4 | 539 | 37.6 | ||
| 9 | Prostate cancer | 4300 | 13.4 | 126000 | 13.6 | 405 | 18.7 | ||
| 10 | Glioblastoma multiforme | ||||||||
| 11 | Prostate Cancer | 5000 | 16.6 | 171000 | 13.5 | 514 | 23.9 | ||
| 12 | Breast Cancer | 7500 | 12 | 191000 | 12.4 | 419 | 10.7 | ||
| 13 | Thyroid Cancer | 4800 | 13.8 | 160000 | 13.1 | 316 | 16.8 | ||
| 14 | Gastric Cancer | 7.9 | 9.7 | 317 | 14.9 | 316 | 25.3 | ||
| 15 | CLL | 79.6 | 8.8 | 31 | 15.3 | 887 | 87.8 | ||
| 16 | Ovarian Cancer | 15.6 | 12.2 | 473 | 15.8 | 298 | 22.3 | ||
| 17 | Non Hodgkin Lymphoma + Prostate | 2.5 | 8.3 | 30 | 21.6 | 548 | 11.8 | ||
| 18 | Multiple Myeloma | 0.6 | 7 | 65 | 15.1 | 194 | |||
| 19 | RCC | 4 | 10.4 | 114 | 15.1 | 659 | 21.3 | ||
| 20 | Laryngeal Carcinoma | 254 | |||||||
| 21 | Chollangiocarcinoma | 0.8 | 11.2 | 167 | 15.1 | 1338 | |||
| 22 | Hodgkin Lymphoma | 6.5 | 12.5 | 90 | 13.5 | 197 | 10.1 | ||
| 23 | Anal Cancer | 3.2 | 13.6 | 70 | 14.2 | 408 | 13.4 | ||
| 24 | Astrocytoma | 3.7 | 13.2 | 149 | 15.3 | 753 | 35.6 | ||
| 25 | Lung Cancer | 9 | 10.3 | 211 | 17.8 | 351 | 14.7 | ||
| 26 | Breast Cancer | 7.9 | 12.1 | 279 | 13.3 | 416 | 21.8 | ||
| 27 | Esophageal Cancer | 0.3 | 10.1 | 33 | 16.8 | 445 | |||
| 28 | Gastric Cancer | 2.6 | 10.3 | 172 | 13.2 | 461 | 37.2 | ||
| Graft patient | Sex | Age | Underlying disease HTN | SPO2 | WBC | Hb 16.2 | PLT | RDW | LDH |
| Patient 1 | Male | 41 | 93 | 6100 | 133000 | 12.1 | 319 | ||
| Patient 2 | Female | 27 | HTN | 89 | 6500 | 8.4 | 234000 | 12.8 | 769 |
| Patient 3 | Male | 50 | DM HTN | 82 | 7000 | 13.2 | 177 | 13.5 | 1145 |
| Patient 4 | Female | 61 | HTN | 93 | 4800 | 9.8 | 130 | 12.4 | 477 |
| Patient 5 | Male | 64 | 97 | 11300 | 11.8 | 166 | 14.4 | 209 | |
SCC: Squamous Cell Carcinoma; CLL: Chronic Lymphocytic Leukemia; GBM: Glioblastoma Multiform; RCC: Renal Cell Carcinoma; WBC: White Blood Cell; Hb: Hemoglobin; PLT: Platelets; RDW: Red Distribution Width; LDH: Lactate Dehydrogenase; SPO2: Oxygen Saturation; HTN: Hypertension; DM: Diabetes Mellitus
Table 3.
Cancer and Graft patients
| Cancer patients | Graft patients | ||||||
|---|---|---|---|---|---|---|---|
| ICU admission N (%) | Days of hospitalization (Med ±IQR) | Mortality N (%) | ICU admission N (%) | Days of hospitalization (Med ±IQR) | Mortality N (%) | ||
| Sex | Male | 4(25) | 7±5 | 5(31.3) | 1(25) | 8±11 | 1(25) |
| Female | 5(41) | 5.5±5 | 4(33.3%) | 0 | 4 | 0 | |
| Severity | Severe | 4(36.4%) | 6±6 | 4(36.4%) | 1(50) | 18 | 1(50) |
| Non severe | 5(29.4%) | 7±7 | 5(29.4%) | 0 | 5.5±4.5 | 0 | |
| Cancer | Active | 7(31.8%) | 6±5 | 8(36.4%) | N/A | N/A | N/A |
| activity | Non active | 2(33.3%) | 7±13 | 1(16.7%) | N/A | N/A | N/A |
| Total | 9(32) | 6.50±6 | 9(32.1) | 1(20) | 7±10 | 1(20) | |
IQR: Inter Quartile Range, N: Number, Med: Medium
Indicators measurements and analysis
The main outcome was patient survival during hospitalization. Measurements included RDW (elevated RDW defined as greater than 14.5%), Lymphocyte count (ALC < 1,000 cells/mm3 was defined as lymphopenia) and Platelet (PLT < 150,000 platelets/mm3 was defined as thrombocytopenia) at first day admission in hospital. Secondary outcomes were: a composite of severe illness (death, severe illness, admission to an intensive care unit (ICU), or a combination of these). Statistical analysis carried out using SPSS version 22. Significance level considered 0.05.
Results
We retrospectively enrolled 28 cancer (2.4%) and 5 kidney transplant patients of the 1135 patients admitted to Valiasr hospital for treatment of COVID-19. Demographic, clinical feature and underlying diseases of the patients are shown in Table 1. The mean age was 62 for cancer and 48 for kidney transplant patients (Mann-Whitney sig=0.053). The sex distribution in patients was not significantly different between cancer and kidney transplant patients (Exact sig=0.625). The most types of cancer patients were Gastric (3 patients), lung (3 patients), Breast (3 patients). Gastrointestinal cancer was the most frequent type of cancer (28.6%). The patient with Glioblastoma Multiform (GBM) died on the day of referral and no blood test was recorded for the patient, but the RT-PCR test came back positive later. The result of CBC taken in first day of case with laryngeal carcinoma was laboratory's missing, in which the results of the patient's tests were not entered in the system.
Table 4.
Main Laboratory Findings according to clinical situation
| Cancer patients | Graft patients | ||||
|---|---|---|---|---|---|
| Sex | Male Female | PLT count<150.000 N (%) 11(68%) 7(63.6%) | RDW>14.5 % N (%) 11(68.8%) 6(50%) | PLT count<150.000 N (%) 3(75) 1(100) | RDW>14.5% N (%) 0 0 |
| Exact sig Severity | Severe Non-severe | 1.00 8(72.7%) 10(62.5%) | 0.441 8(72.7%) 9(52.9%) | 0.78 1(50) 3(100) | N/A 0 0 |
| Exact sig Cancer activity | Active Non-active Exact sig | 0.692 15(71.4%) 3(50%) 0.305 | 0.435 15(68.2%) 2(33.3%) 0.174 | 0.04 | N/A |
PLT: Platelets, RDW: Red Distribution Width, N: Number
Table 5.
Comparison of active cancer patients with kidney transplant patients
| Active Cancer | Kidney Transplant | p-value | |
|---|---|---|---|
| ICU admission | 7(31.8%) | 1(20%) | 0.52 |
| Mortality | 8(36.4%) | 1(20%) | 0.44 |
| RDW>14.5% | 15(68.2%) | 0 | 0.01 |
ICU: Intensive Care Unit; RDW: Red Distribution Width.
In comparison between cancers type, gastrointestinal had higher mortality, but there was no statistically significant difference (P-Value= 0.54) (Figure 3). Among cancer patients, 9 (32.1%) patients had at least one or more underlying diseases whereas 80% kidney transplant patients had chronic comorbidity (Exact sig=0.041). In the severe cancer group, 6 of the 9 patients with the underlying disease had severe COVID-19. The patients' laboratory tests and the type of cancer in Table 2 are shown. Twenty-two (78.5%) cancer patients had active and six (21.4%) had inactive disease. Eight patients (36.3%) of active cancer and one (16.6%) inactive cancer died. Mortality of active versus inactive cancer patients was higher, but the differences was not statistically significant (Exact sig=0.63). Comparing mortality rate of cancer (32%) and graft patients (20%), the difference was not significant (Exact sig=1.000). Frequency of ICU admission was not statistically different between graft (20%) and cancer patients (32%) (Exact sig=1.000), also duration of hospitalization was not different between groups of patients (Mann-Whitney p=0.88) (Table 3). Nine (32.1%) of cancer patients needed invasive mechanical ventilation. In this study, among age, sex, diabetes mellitus, hypertension and baseline laboratory values, the only predictor of mortality was LDH level. The prevalence of thrombocytopenia (PLT<150000) and RDW> 14.5% were higher in severe patients but the difference was not statistically significant (Table 4). With each unit increase in LDH, the patient's chance of death increased by 0.5%. Patients were assessed for risk of mortality using LDH. ROC analysis with AUC = 0.750 and sig = 0.038 revealed the cut-off values of 404 with a sensitivity of 0.87 and a specificity of 0.64. To predict the need for ICU based on clinical conditions and laboratory findings, two variables of sex and O2 saturation were entered the Logistic regression model.
Female sex and SpO2 <90% increased the chances of admission in ICU. None of the variables could estimate the number of days a patient will spend in the hospital based on clinical conditions and laboratory results at the time of patient admission using linear regression. Comparing active cancer and kidney transplant patients, interesting results were obtained that are shown in Table 5. Mortality and the need for hospitalization in ICU were higher in patients with active cancer, although the difference was not statistically significant (exact sig>0.05). RDW in patients with active cancer was higher than kidney transplant patients (exact sig=0.01).
Discussion
It was surprising for us that mortality and the need for ICU care were not significantly difference between active and inactive cancer patients. Liu study showed that the anti-tumor treatment did not lead to poorer prognosis in patients with solid tumors diagnosed with COVID-19.14 Lee study showed that chemotherapy in the past 4 weeks had no significant effect on COVID-19 mortality.15
In our study, although the rate of mortality and admission in the ICU were higher in patients with active cancer, but there were not statistically significant.
Active hematologic malignancies with COVID-19 had a similar risk of death versus non active hematologic patients.16 In Shoumariyeh study no significant difference was observed between solid tumor and hematological malignancy in overall survival.17 Our study shows same result (between GI cancer, non-GI cancer and hematologic cancer) but mortality was higher in GI malignancy without statistical significance. In this study among the cancer patients, gastrointestinal was the most frequent type of cancer.
It is noteworthy that in the Ma study; the most common cancer was colorectal (29.7%), some studies indicated that lung cancer patients were the most common to be infected.18,19 Elevated LDH have been observed in the blood of patients with COVID-19, and levels of this enzyme correlate with disease severity. The findings of this study also confirmed this point.20 Men have a much greater risk of severe acute COVID-19 than women.21 While in our study, woman had increased risk of admitted to the ICU.
COVID-19 is an immunosuppressant disease. An important question that has not yet been properly answered is: which patient with immunosuppression is more sensitive to COVID-19? Compared with active cancer and kidney transplant patients, interestingly high RDW was significant between the two groups, although the mortality rate was not statistically different, but it was higher in the active cancer group. In Sharma et al. study RDW in COVID-19 patients, was found to be higher than normal patients; however, it had no significant association with disease severity.22 In our study, the proportion of severe COVID-19 with active cancer was 31.8% which was also significantly higher than that of the Iranian general population with severe COVID-19 (11%).23 It seems cancer patients were more likely to be immunosuppressed than kidney transplant patients included in our study and are more susceptible to COVID-19, but why there isn’t statistical difference between mortality in active cancer and kidney transplant patients? One of the reasons is the presence of associated underlying disease (hypertension and diabetes) that more predispose patients to COVID-19 in most kidney transplant patients. However, we cannot ignore the limitations of our study, the most important of which is the small number of immunosuppressed patients in each group and don’t enrolled other immunocompromised condition.
In conclusion, our data suggest that patients with active cancer generally have severe and more complicated disease. But in our study, there was no higher mortality among patients with active versus inactive cancer in COVID-19. Therefore, it seems logical not to deprive cancer patients who need chemotherapy as basic treatment. The severity of COVID-19 varies in different types of immunosuppressed patients. RDW can be a predictor in these patients, but for clearer results, studies with larger statistical populations should be evaluated.
Acknowledgments
None
List of acronyms
- ALC
Lymphocyte count
- ARDS
acute respiratory distress syndrome
- CBC
complete blood count
- CLL
Chronic Lymphocytic Leukemia
- DM
diabetes mellitus
- GBM
Glioblastoma Multiform
- GI
Gastrointestinal;
- Hb
Hemoglobin
- HTN
hypertension
- ICU
intensive care unit
- LDH
lactate dehydrogenase
- Non GI
Non Gastrointestinal
- PLT
platelet
- RCC
Renal Cell Carcinoma
- RDW
red cell distribution width
- ROC
receiver operating characteristic
- RT-PCR
Reverse transcription polymerase chain reaction
- SCC
Squamous Cell Carcinoma
- SOT
solid organ transplant
- SpO2
oxygen saturation
- WBC
white blood cell
Funding Statement
Funding None
Contributor Information
Minoosh Moghimi, Email: mmoghimi2000@yahoo.com.
Manizheh Jozpanahi, Email: dr.panahi48@gmail.com.
Seyede Pegah Saeed, Email: Zmed1996@gmail.com.
Seyede Vanoushe Azimi Pirsaraie, Email: Venosheh.a@gmail.com.
Nooshin Jalili, Email: dr.nooshinjalili@zums.ac.ir.
References
- 1.Moradzadeh R, Jamalian SM, Nazari J, Kamali A, Sadeghi B, Hosseinkhani Z, Sofian M, Zamanian M. Age-standardized mortality rate and predictors of mortality among COVID-19 patients in Iran. J Educ Health Promot. 2021. May 31;10(1):169. doi: 10.4103/jehp.jehp_946_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y, Pan D, Shu C, Li J, Wei J, Huang Y, Peng L, Wu M, Zhang R, Wu B, Li Y, Cai L, Li G, Zhang T, Wu G. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020. Jul;21(7):904-913. doi: 10.1016/S1470-2045(20)30310-7. Epub 2020 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Li Z, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020. Jun;10(6):783-791. doi: 10.1158/2159-8290.CD-20-0422. Epub 2020 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maschmeyer G, De Greef J, Mellinghoff SC, Nosari A, Thiebaut-Bertrand A, Bergeron A, Franquet T, Blijlevens NMA, Maertens JA; European Conference on Infections in Leukemia (ECIL). Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia. 2019. Apr;33(4):844-862. doi: 10.1038/s41375-019-0388-x. Epub 2019 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, Kinkhabwala M. Covid-19 and Kidney Transplantation. N Engl J Med. 2020. Jun 18;382(25):2475-2477. doi: 10.1056/NEJMc2011117. Epub 2020 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Zambetti N, Moscato M, Venturini M, Affatato S, Gaggiotti M, Bossini N, Scolari F. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020. Jun;97(6):1083-1088. doi: 10.1016/j.kint.2020.04.002. Epub 2020 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020. Jun;99(6):1205-1208. doi: 10.1007/s00277-020-04019-0. Epub 2020 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Henry BM, Sanchis-Gomar F. Red Blood Cell Distribution Is a Significant Predictor of Severe Illness in Coronavirus Disease 2019. Acta Haematol. 2021;144(4):360-364. doi: 10.1159/000510914. Epub 2020 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Gong Y, Ying B, Cheng B. Relation between Red Cell Distribution Width and Mortality in Critically Ill Patients with Acute Respiratory Distress Syndrome. Biomed Res Int. 2019. Mar 21;2019:1942078. doi: 10.1155/2019/1942078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkhatib A, Price LL, Esteitie R, LaCamera P. A Predictive Model for Acute Respiratory Distress Syndrome Mortality Using Red Cell Distribution Width. Crit Care Res Pract. 2020. Jan 4;2020:3832683. doi: 10.1155/2020/3832683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. J Infect. 2020. Aug;81(2):e93-e95. doi: 10.1016/j.jinf.2020.05.017. Epub 2020 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung M, Babik JM. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin Infect Dis. 2021. Jan 27;72(2):340-350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Güçlü E, Kocayiğit H, Okan HD, Erkorkmaz U, Yürümez Y, Yaylacı S, Koroglu M, Uzun C, Karabay O. Effect of COVID-19 on platelet count and its indices. Rev Assoc Med Bras (1992). 2020. Aug;66(8):1122-1127. doi: 10.1590/1806-9282.66.8.1122. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Yang D, Chen X, Sun Z, Zou Y, Chen C, Sun S. The effect of anticancer treatment on cancer patients with COVID-19: A systematic review and meta-analysis. Cancer Med. 2021. Feb;10(3):1043-1056. doi: 10.1002/cam4.3692. Epub 2020 Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, Freeman-Mills L, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJ, Lee RJ, McGrath SE, Middleton CP, Murugaesu N, Newsom-Davis T, Okines AF, Olsson-Brown AC, Palles C, Pan Y, Pettengell R, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Starkey T, Turnbull CD, Várnai C, Yousaf N; UK Coronavirus Monitoring Project Team, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020. Jun 20;395(10241):1919-1926. doi: 10.1016/S0140-6736(20)31173-9. Epub 2020 May 28. Erratum in: Lancet. 2020 Aug 22;396(10250): 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, Martín-Moro F, Razanamahery J, Riches JC, Zwicker J, Patell R, Vekemans MC, Scarfò L, Chatzikonstantinou T, Yildiz H, Lattenist R, Mantzaris I, Wood WA, Hicks LK. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020. Dec 17;136(25):2881-2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoumariyeh K, Biavasco F, Ihorst G, Rieg S, Nieters A, Kern WV, Miething C, Duyster J, Engelhardt M, Bertz H. Covid-19 in patients with hematological and solid cancers at a Comprehensive Cancer Center in Germany. Cancer Med. 2020. Nov;9(22):8412-8422. doi: 10.1002/cam4.3460. Epub 2020 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: A single center's retrospective study. J Infect. 2020. Aug;81(2):318-356. doi: 10.1016/j.jinf.2020.04. 006. Epub 2020 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavanna L, Citterio C, Di Nunzio C, Biasini C, Palladino MA, Ambroggi M, Madaro S, Bidin L, Porzio R, Proietto M. Prevalence of COVID-19 Infection in Asymptomatic Cancer Patients in a District With High Prevalence of SARS-CoV-2 in Italy. Cureus. 2021. Mar 9;13(3):e13774. doi: 10.7759/cureus.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020. Feb 15;395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, Crooks M, Gabbay M, Brady M, Hishmeh L, Attree E, Heightman M, Banerjee R, Banerjee A; COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021 Mar 30;11(3):e048391. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma D, Dayama A, Banerjee S, Bhandhari S, Chatterjee A, Chatterjee D. To Study the Role of Absolute Lymphocyte Count and RDW in COVID 19 Patients and their Association with Appearance of Symptoms and Severity. J Assoc Physicians India. 2020. Aug;68(8):39-42. [PubMed] [Google Scholar]
- 23.Allameh SF, Nemati S, Ghalehtaki R, Mohammadnejad E, Aghili SM, Khajavirad N, Beigmohammadi MT, Salehi M, Mirfazaelian H, Edalatifard M, Kazemizadeh H, Dehghan Manshadi SA, Hasannezhad M, Amoozadeh L, Radnia M, Khatami SR, Nahvijou A, Seyyedsalehi MS, Rashidian L, Ayoobi Yazdi N, Nasiri Toosi M, Sadeghniiat-Haghighi K, Jafarian A, Yunesian M, Zendehdel K. Clinical Characteristics and Outcomes of 905 COVID-19 Patients Admitted to Imam Khomeini Hospital Complex in the Capital City of Tehran, Iran. Arch Iran Med. 2020. Nov 1;23(11):766-775. doi: 10.34172/aim.2020.102. [DOI] [PubMed] [Google Scholar]


