Abstract
Among patients affected by the virus COVID-19, physicians have observed ventilation disorders. It is relevant to assess neurological involvement, including the role of diaphragmatic function. Its possible impairment could be related to the systemic inflammatory response and disease progression that both typify COVID-19 infection. We distinguished two groups (severe group (SG) and mild group (MG)) according to the severity of respiratory symptomatology. We performed neurophysiological and sonography studies to evaluate the diaphragmatic function. Regarding the sonography variables, we identified statistically significant differences in the right mean diaphragmatic thickness along with the expiration, showing 1.56 mm (SEM: 0.11) in the SG vs 1.92 mm (SEM: 0.19) in the MG (p = 0.042). The contractibility of both hemidiaphragms was 15% lower in the severe group, though this difference is not statistically significant. In our examination of the neurophysiological variables, in the amplitude responses, we observed a greater difference between responses from both phrenic nerves as follows: the raw differences in amplitude were 0.40 μV (SEM: 0.14) in the SG vs 0.35 μV (SEM: 0.19) in the MG and the percentage difference was 25.92% (SEM: 7.22) in the SG vs 16.28% (SEM: 4.38%) in the MG. Although diaphragmatic dysfunction is difficult to detect, our combined functional and morphological approach with phrenic electroneurograms and chest ultrasounds could improve diagnostic sensitivity. We suggest that diaphragmatic dysfunction could play a relevant role in respiratory disturbance in hospitalised patients with severe COVID-19.
Key Words: Coronavirus, diaphragm, electromyography, phrenic nerve, ultrasound
Ethical Publication Statements
We confirm that we have read the journal’s position on ethical issues involved in publication and affirm that this report is consistent with those guidelines.
Among patients affected by coronavirus disease 2019 (COVID-19), the disease resulting from the virus SARS-CoV-2, physicians have observed ventilation disorders related to pneumonia and lung injury.1 However, it is relevant to assess neurological involvement, both central and peripheral, in these disorders to highlight the role of diaphragmatic function. These impairments could also be related to the significant systemic inflammatory response and disease progression that both typify SARS-CoV-2 infection.2 In previous studies concerning other coronaviruses, such as SARS-CoV-1, the virus that causes severe acute respiratory syndrome (SARS), peripheral nerve injury has been described,3–8 due to either post-infection immunological reaction or direct action of the coronavirus responsible for SARS.2 Previous reports on SARS-CoV-2 infection have described peripheral neurological damage and injury in the central nervous system.9,10 The virus can spread to multiple areas of the brain, such as the brainstem, as shown in patients and animal models infected by other coronaviruses.3,11 Inpatients affected by COVID-19 could experience marked hypoventilation, being diaphragmatic tests crucial. In the cases where diaphragmatic paralysis is observed, it is possible to localise the injury in muscular fibres or the phrenic nerve.4 Other reports describe similar findings from analyses of conditions unrelated to COVID-19, including polyneuropathic syndromes, inflammatory diseases,5–8 or viral infections, such as West Nile or dengue,12,13 that may follow coronavirus infection.
To determine the cause of ventilation disturbance in patients affected by COVID-19 who experienced severe breathing problems, we have assessed diaphragmatic function via neurophysiological and sonography anslyses.
Table 1.
Demographic and clinical data comparison of mild and severe groups.
| Mild (n = 10) | Severe (n = 9) | p-value | |
|---|---|---|---|
| IBM | |||
| Underweight | 0 (0%) | 1 (11.1%) | |
| Normal weight | 4 (40%) | 1 (11.1%) | 0.90 |
| Overweight I | 2 (20%) | 3 (33.3%) | |
| Overweight II | 2 (20%) | 3 (33.3%) | |
| Obesity I | 2 (20%) | 1 (11.1%) | |
| HBP | |||
| Yes | 3 (30%) | 0 (0%) | 0.29 |
| No | 7 (70%) | 9 (100%) | |
| Diabetes Mellitus | |||
| Yes | 2 (20%) | 1 (11.1%) | 0.77 |
| No | 8 (80%) | 8 (88.8%) | |
| Dyslipidemia | |||
| Yes | 6 (60%) | 1 (11.1%) | 0.08 |
| No | 4 (40%) | 8 (88.8%) |
Table shows distribution of clinical characteristics, and statistical comparison between mild and severe groups. IBM=Index Body Mass, HBP = High Blood Pressure (>120/80mmHg).
Materials and methods
This study has been accepted by the Research Ethics Committee from La Princesa Hospital, with local reference number 4144, and it was carried out following the legislation in force regarding confidentiality. All included patients (or their representatives) were informed about the study and provided oral informed consent as proposed by the Agencia Española de Medicamentos y Productos Sanitarios because of the COVID-19 emergency.
We distinguished two groups (severe and mild) of patients affected by COVID-19 disease hospitalized at La Princesa University Hospital, according to the severity of respiratory symptoms. The severe group consisted of patients who experienced dyspnoea on mild exertion, pain of diaphragmatic origin or hiccup. The mild group consisted of patients who experienced only dyspnoea and only during severe exertion. We performed electroneurograms of both phrenic nerves and sonography of both hemidiaphragms, and we recorded the following sociodemographic variables and primary comorbidities among patients: age, sex, body mass index (BMI), presence of high blood pressure, diabetes mellitus, previous diagnosis of polyneuropathy or myopathy (clinically or electromyographically) and history of stroke or cerebral haemorrhage prior to the infection. The inclusion criteria were:
Table 2.
Demographic, clinical, respiratory variables at time of testing and clinical course of the mild group.
| Patient | Genre | Age | Days of symptoms | Symptoms suggesting phrenic nerve damage | Respiratory variables | Clinical course | |||
|---|---|---|---|---|---|---|---|---|---|
| Ventilation disturbances | Costal pain | Hiccups | Oxygen Saturation | Supplemental Oxygen | |||||
| 1 | M | 76 | 7 | No | No | No | 95% | No | Good |
| 2 | M | 85 | 9 | No | No | No | 97% | No | Good |
| 3 | M | 43 | 7 | No | No | No | 98% | No | Good |
| 4 | F | 69 | 8 | No | No | No | 96% | NC 4 L | Good |
| 5 | F | 48 | 11 | Yes | No | No | 99% | NC 2 L | Good |
| 6 | M | 54 | 11 | No | No | No | 96% | No | Good |
| 7 | M | 31 | 6 | No | No | No | 96% | No | Good |
| 8 | M | 68 | 17 | Yes | No | No | 92% | NC 4 L | ICU during 6 days -> Good at discharge |
| 9 | M | 41 | 6 | Yes | No | No | 95% | No | Good |
| 10 | F | 59 | 8 | No | No | No | 95% | NC 2 L | Good |
In one patient (number 9), we performed only nerve conduction of both phrenic nerves performing sonography during the hospital admission. MEP = Motor Evoked Potential, Dif = Difference, M = Male, F= Female, NC = Nasal Cannula, ICU = Intensive Care Unit.
Table 3.
Sonography variables from the mild group.
| Patient | Sonography variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Right diaphragmatic thickness on inspiration | Left diaphragmatic thickness on inspiration | Right diaphragmatic thickness on expiration | Left diaphragmatic thickness on expiration | Right contraction on inspiration (%) | Left contraction on inspiration (%) | Right expiratory fraction | Left expiratory fraction | |
| 1 | 3.2 | 3.7 | 1.8 | 1.8 | 77.0 | 105.0 | 0.8 | 1.0 |
| 2 | 6.0 | 5.0 | 3.3 | 2.6 | 81.0 | 94.0 | 0.8 | 0.9 |
| 3 | 2.1 | 2.3 | 1.4 | 1.3 | 43.8 | 69.1 | 0.4 | 0.7 |
| 4 | 2.4 | 2.1 | 1.5 | 1.4 | 56.0 | 47.0 | 0.6 | 0.5 |
| 5 | 3.7 | 3.1 | 2.1 | 1.9 | 79.0 | 62.2 | 0.8 | 0.6 |
| 6 | 3.1 | 3.2 | 2.0 | 2.0 | 52.7 | 60.6 | 0.5 | 0.6 |
| 7 | 2.1 | 2.9 | 1.4 | 1.5 | 43.0 | 91.0 | 0.4 | 0.9 |
| 8 | 2.7 | 1.8 | 1.8 | 1.2 | 46.8 | 51.2 | 0.5 | 0.5 |
| 9 | ||||||||
| 10 | 2.6 | 2.3 | 1.7 | 1.3 | 80.0 | 80.0 | 0.5 | 0.8 |
In one patient (number 9), we performed only nerve conduction of both phrenic nerves performing sonography during the hospital admission
Age > 18 years.
COVID-19 disease confirmed by a positive Polymerase Chain Reaction (PCR) from a nasopharyngeal swab.
Patients (or their legal guardians) had to consent and sign the informed consent document.
The exclusion criteria were:
History of polyneuropathy or myopathy.
History of stroke or cerebral haemorrhage prior to the COVID-19 disease.
To determine the presence of diaphragmatic injury, we performed electromyography of both phrenic nerves and diaphragmatic sonography. The neurophysiological variables collected were as follows: the latency of motor evoked potentials of both right and left phrenic nerves, the difference in the latency in each side as the percentage of this difference, the amplitude of the motor evoked potentials of both right and left phrenic nerves, and the difference in the amplitude in each side as the percentage of this difference, as Vincent et al. previously described.14 The sonography variables evaluated in each group were the right and left diaphragm thickness during inspiration and expiration, hemidiaphragm displacement, and expiratory fraction from both sides.
To evaluate the diaphragmatic function, we performed both techniques either in imaging tests (e.g. ultrasounds) or in functional tests (e.g. electroneurograms).
Table 4.
Neurophysiological variables of the mild group.
| Patient | Neurophysiological variables | |||||
|---|---|---|---|---|---|---|
| Right MEP Lat | Left MEP Lat | Right MEP Amp | Left MEP Amp | Lat Dif (%) | Amp Dif (%) | |
| 1 | 9.8 | 9.7 | 0.1 | 0.2 | 0.5 | 33.3 |
| 2 | 5.2 | 4.8 | 0.5 | 0.9 | 4.0 | 28.6 |
| 3 | 8.8 | 9.0 | 0.1 | 0.1 | 1.1 | 0.0 |
| 4 | 6.7 | 8.0 | 0.3 | 0.6 | 8.8 | 33.3 |
| 5 | 6.8 | 8.5 | 0.6 | 0.9 | 11.1 | 20.0 |
| 6 | 8.6 | 8.3 | 0.5 | 0.6 | 1.8 | 9.1 |
| 7 | 8.7 | 8.7 | 0.7 | 0.7 | 0.0 | 0.0 |
| 8 | 6.5 | 6.5 | 0.5 | 0.4 | 0.0 | 11.1 |
| 9 | 8.1 | 8.7 | 0.8 | 1.0 | 3.6 | 11.1 |
| 10 | 9.1 | 9.1 | 0.8 | 2.5 | 0.0 | 51.5 |
Table 5.
Demographic, clinical, respiratory variables at the time of testing and clinical course of the severe group.
| Patient | Genre | Age | Days of symptoms | Symptoms suggesting phrenic nerve damage | Respiratory variables | Clinical course | |||
|---|---|---|---|---|---|---|---|---|---|
| Ventilation disturbances | Costal pain | Hiccups | Oxygen Saturation | Supplemental Oxygen | |||||
| 1 | M | 65 | 93 | Yes | No | No | 92% | No | ICU -> Death |
| 2 | M | 51 | 10 | Yes | No | No | 96% | NC 1 L | Chronic course: oxygen therapy and corticosteroids at discharge |
| 3 | M | 58 | 7 | Yes | No | No | 97% | No | Good |
| 4 | F | 33 | 7 | Yes | No | No | 95% | NC 1.5L | Good |
| 5 | F | 61 | 6 | Yes | No | No | 92% | VMK 40% | Good |
| 6 7 | M | 39 | 9 | Yes | Yes | Yes | 90% | VMK 60% | ICU -> Death |
| F | 62 | 7 | Yes | Yes | No | 93% | VMK 35% | ICU (PTE) -> Good at discharge | |
| 8 | F | 59 | 9 | Yes | Yes | No | 94% | VMK 40% | Good |
| 9 | M | 42 | 6 | Yes | Yes | No | 94% | NC 4 L | Good |
Sonography technique
We positioned the patient in the supine position to evaluate the diaphragmatic movement in the respiratory effort. We used mode B (bidimensional mode) to assess the diaphragm’s thickness and mode M (movement mode) to evaluate the diaphragmatic displacement. We measured the diaphragmatic thickness of both the right and left sides along with the maximum inspiration and at the end of the expiratory effort. Similarly, we calculated dynamic changes, the percentage of contraction of each diaphragm and each side’s expiratory fraction.
Statistical analysis
Quantitative data are shown by the mean and the standard error of the mean (SEM). The differences in latency and hemidiaphragm amplitude are shown in percentages. To compare quantitative measures related to electromyographical and sonography studies, we studied their normality via the Kolmogorov–Smirnov test. In the case of fulfilling criteria of normality, we used a t-test. In the contrary case, we used the Mann–Whitney U test and the Wilcoxon signed-rank test.
The statistics were performed by the statistical software XLSTAT (Addinsoft®). We considered p ≤ 0.05 statistically significant.
Results
We recruited a total of 19 patients with confirmed diagnosis of both COVID-19 and dyspnoea: 10 with mild disease (the mild group, or MG) and 9 with severe disease (symptoms suggesting diaphragmatic disturbance; the severe group, or SG). The sample consisted of 12 men (63.2%) and 7 women (36.8%), as follows: the raw differences in amplitude were 0.40 μV (SEM: 0.14) in the SG vs 0.35 μV (SEM: 0.19) in the MG and the percentage difference was 25.92% (SEM: 7.22) in the SG vs 16.28% (SEM: 4.38%) in the MG (Table 8 and Figure 1). Sonography results of one patient from the mild group (patient number 9) were not available. Regarding the sonography variables in the remaining patients, we identified statistically significant differences in the right mean diaphragmatic thickness along with the expiration, showing 1.56 mm (SEM: 0.11) in the SG vs 1.92 mm (SEM: 0.19) in the MG (p = 0.042). In addition, a lower mean expiratory fraction and hemidiaphragmatic contraction were observed in the SG as follows: the right mean diaphragmatic contraction was 53.10% (SEM: 11.15) in the SG vs 62.15% (SEM: 5.58) in the MG, the left mean diaphragmatic contraction was 58.62% (SEM: 15.36) in the SG vs 73.34% (SEM: 6.74) in the MG, the right mean expiratory fraction was 0.53 (SEM: 0.11) in the SG vs 0.59 (SEM: 0.05) in the MG and the left mean expiratory fraction was 0.58 (SEM: 0.15) in the SG vs 0.73 (SEM: 0.07) in the MG (Table 8 ; Figures 2 and 3).
Table 6.
Sonography variables of the severe group
| Patient | Sonography variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Right diaphragmatic thickness on inspiration | Left diaphragmatic thickness on inspiration | Right diaphragmatic thickness on expiration | Left diaphragmatic thickness on expiration | Right contraction on inspiration (%) | Left contraction on inspiration (%) | Right expiratory fraction | Left expiratory fraction | |
| 1 | 1.9 | 1.0 | 1.9 | 0.9 | 0.0 | 16.3 | 0.0 | 0.1 |
| 2 | 2.7 | 2.7 | 1.9 | 1.6 | 42.1 | 68.8 | 0.4 | 0.7 |
| 3 | 2.2 | 3.5 | 1.2 | 1.3 | 83.3 | 169.2 | 0.8 | 1.7 |
| 4 | 2.7 | 3.0 | 1.4 | 2.3 | 96.4 | 27.7 | 1.0 | 0.3 |
| 5 | 2.6 | 2.5 | 1.8 | 1.6 | 39.9 | 60.3 | 0.4 | 0.6 |
| 6 | 1.5 | 1.8 | 1.2 | 1.5 | 28.3 | 21.5 | 0.3 | 0.2 |
| 7 | 1.8 | 1.4 | 1.3 | 0.8 | 38.3 | 70.2 | 0.3 | 0.7 |
| 8 | 2.6 | 2.2 | 1.3 | 1.5 | 100.0 | 45.2 | 1 | 0.4 |
| 9 | 2.9 | 3.1 | 2.0 | 2.1 | 49.5 | 48.5 | 0.5 | 0.5 |
Table 7.
Neurophysiological variables of the severe group
| Patient | Neurophysiological variables | |||||
|---|---|---|---|---|---|---|
| Right MEP Lat | Left MEP Lat | Right MEP Amp | Left MEP Amp | Lat Dif (%) | Amp Dif (%) | |
| 1 | 7.4 | 7.2 | 1.7 | 0.7 | 1.4 | 41.7 |
| 2 | 8.7 | 8.3 | 0.3 | 0.1 | 2.3 | 50.0 |
| 3 | 6.9 | 6.5 | 0.3 | 1.2 | 3.0 | 60.0 |
| 4 | 7.8 | 8.3 | 0.4 | 0.2 | 3.1 | 33.3 |
| 5 | 7.8 | 8.1 | 0.6 | 0.4 | 1.9 | 20.0 |
| 6 | 9.3 | 9.2 | 1.9 | 2.8 | 0.5 | 19.1 |
| 7 | 9.8 | 9.9 | 1.7 | 1.7 | 0.5 | 0.0 |
| 8 | 7.1 | 7 | 0.4 | 0.4 | 0.7 | 0.0 |
| 9 | 5.2 | 5.2 | 1 | 1.2 | 0.0 | 9.1 |
Table 8.
Nerve conduction study and variables of severe and mild groups
| Mild group (mean ± SEM) | Severe group (mean ± SEM) | p-value | |
|---|---|---|---|
| Right Latency (ms) | 7.83 ± 0.46 | 7.78 ± 0.46 | 0.759 |
| Left Latency (ms) | 8.13 ± 0.46 | 7.74 ± 0.48 | 0.843 |
| Right Amplitude (mV) | 0.46 ± 0.08 | 0.92 ± 0.22 | 0.703 |
| Left Amplitude (mV) | 0.60 ± 0.11 | 0.97 ± 0.29 | 0.716 |
| Differences in Latency (%) | 3.09 ± 1.24 | 1.50 ± 0.38 | 0.231 |
| Differences in Amplitude (%) | 16.28 ± 4.38 | 25.92 ± 7.22 | 0.711 |
| Right thickness during inspiration (mm) | 3.11±0.40 | 2.34±0.16 | 0.945 |
| Left thickness during inspiration (mm) | 2.97±0.33 | 2.36±0.27 | 0.547 |
| Right thickness during expiration (mm) | 1.92±0.19 | 1.56±0.11 | 0.042 |
| Left thickness during expiration (mm) | 1.69±0.15 | 1.51±0.16 | 0.742 |
| Right contractibility (%) | 62.15±5.58 | 53.10±11.15 | 0.742 |
| Left contractibility (%) | 73.34±6.74 | 58.62±15.36 | 0.195 |
| Right expiratory fraction | 0.59±0.05 | 0.53±0.11 | 0.674 |
| Left expiratory fraction | 0.73±0.07 | 0.58±0.15 | 0.195 |
The table shows bilateral amplitude and latency results of both phrenic nerves and their difference and thickness of each hemidiaphragm along with inspiration and expiration, contractibility, and expiratory fraction from each hemidiaphragm
Discussion
This study has generated preliminary findings of neurophysiological and sonography data through diaphragmatic assessment. Concerning nerve conduction studies from both phrenic nerves, we observe a greater difference (25.92%) between phrenic nerves in each patient in the SG than in the MG (16.28%). However, these results are not statistically significant. Conversely, we do not observe relevant differences in the latency because of the variability in the length of the thorax among the patients included in the study. The amplitude of the motor evoked potentials evinces individual variability driven by BMI and diaphragmatic fat storage, and thus, the main parameters assessed stem from the percentage of variation between both phrenic nerves.
Sonography data demonstrate a statistically relevant outcome in comparing the right thickness during expiration between both groups, which shows a thicker diaphragm in MG. The other differences in sonography variables between both groups are not statistically significant, probably related to the small sample size. Moreover, thickness in both right and left hemidiaphragms, along with inspiration and expiration, is lesser in the SG. We should consider other sonography variables as sources of the differences in the contractibility of both hemidiaphragms because they present 10-15% lower contraction in the SG, though this difference is not statistically significant. The complexity of the symptoms and the clinical variability produced by COVID-19 require study of these symptoms’ physiopathological repercussions, one of the most relevant of which is damage in respiratory function, given their importance in the deleterious progression of the disease.15-17 Previous analyses have documented cases that feature peripheral nerve damage, namely, via demyelination and axonal injury, which constitute the most frequent damage in the severest cases. 10,11,18 These conditions also result from other viral infections, such as the varicella-zoster virus,19 Cytomegalovirus,20 human immunodeficiency virus (HIV),21,22 hepatitis E,23 or secondary infection with the arbovirus dengue.24 Based on the information described in prior studies of these viral infections, we could infer the similarity of neurological symptoms that can appear in patients infected with SARS-CoV-2. Just as generalised nerve damage may occur along the progression of COVID-19 disease, focal neuropathies are another attested consequence. Phrenic nerve damage is an example of immune-mediated focal neuropathies, which carry focal demyelination or axonal degeneration.25,26 The injury of the peripheral nerves is described in several viral infections, as the infection by the herpes-zoster virus,27 Lyme disease,28 or HIV.29 As examined in patients and animal models infected with other coronaviruses, such as those that cause SARS and Middle East respiratory syndrome,3,11,18 the brain stem is one of the primarily infected nervous structures. The more typical entry of SARS-COV-2 is intranasal,9,30,31 hence its propagation to the central nervous system. 9,10 The brain stem’s direct damage, where the pneumotaxic, apneustic and bulbar respiratory centres deteriorate, could explain the ventilatory disturbance. Diaphragm paralysis involves a loss of muscle strength of the primary muscle implied in the inspiration. We could locate the damage at the muscle fibres’ level or phrenic nerve damage.4 It has been described several neurological causes: polyneuropathic Guillain–Barré syndrome,32 inflammatory causes, neuralgic amyotrophy or Parsonage–Turner syndrome.5–8 An infection can also cause dysfunction akin to the symptoms of West Nile or dengue.12,13 We could not dismiss this possible injury in patients affected with COVID-19.2,33 The main imaging test performed in patients with suspected COVID-19 and symptoms suggesting damage in respiratory function is thorax radiography to confirm possible viral pneumonia. However, this test has a low positive predictive value in detecting the diaphragmatic damage, 33%, to evaluate its dysfunction.34 Given these findings, the phrenic nerve’s electroneurography helps us confirm the appropriate conduction of these nerves.14,15 Other authors have observed a high diagnostic correlation between the diaphragm’s thickness observed in sonography and the amplitude of the motor evoked potential in electroneurography.35,36 Therefore, we have performed both techniques to improve this test’s diagnostic sensibility in detecting the diaphragmatic dysfunction and the phrenic nerve damage. The patients with more severe respiratory symptoms have dyspnoea, cough, thoracic pain and hiccups.37,38 We observe a particular trend in the presentation of dyspnoea and thoracic pain among patients with diaphragmatic dysfunction, all of whom have dyspnoea at low effort (100%) and of whom have thorax pain in this group (44.4%). In the MG, only three patients exhibit dyspnoea, always at high effort (30%), and without symptoms suggesting diaphragmatic-origin pain in any case. As described in other reports, we note the possibility of disturbance of the nervous system in the disease’s progression. In our data series, we observe an amplitude of differences between both conduction studies from phrenic nerves, which could be explained by axonal degeneration in the acute phase of this viral infection, as happened with other viruses.27–29 Moreover, we identify a greater difference in the contraction of both hemidiaphragms in patients affected with a moderate degree of the disease and a lesser thickness in the SG, as described in neuromuscular damage from other viral infections.4,13
Figure 1.
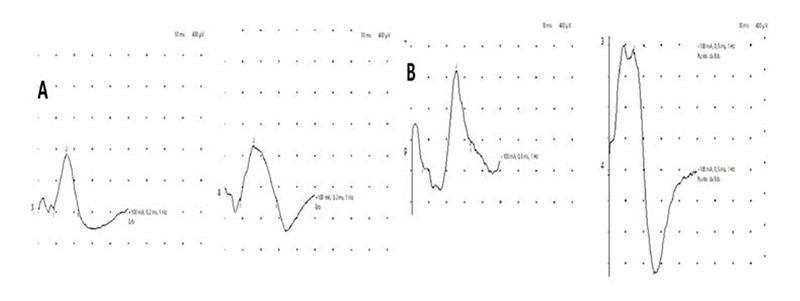
A) Example of a mild group patient. Recordings of left and right phrenic nerves are similar. B) Example of a patient from the severe group. Different amplitude of left and right phrenic nerve recordings.
Figure 2.
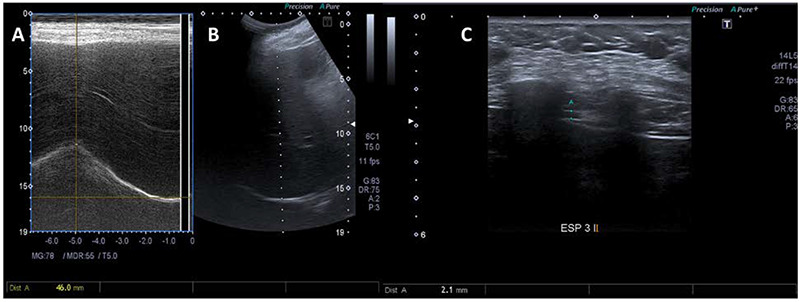
Ultrasound images of a patient from the mild group. Right hemidiaphragm displacement measured during deep inspiration, using M-mode (A) and B-Mode (B) ultrasonography. Diaphragm thickness measured at end-expiration using B-mode (C).
Figure 3.
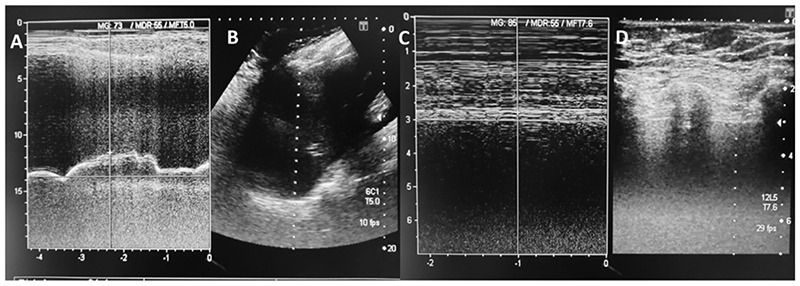
Ultrasound images of a patient from the severe group. Right hemidiaphragm displacement measured during deep inspiration, using M-mode (A) and B-Mode (B) ultrasonography. There is a shorter displacement compared to the patient from Figure 2, suggesting phrenic dysfunction. Diaphragm thickness, measured at end-inspiration using M-mode (C) and B-mode ultrasound (D).
Because this study features a small sample size, data observed have insufficient power to detect a statistical significance between mild and severe groups. Nevertheless, these results allow us to point out the importance of the diaphragm’s role in patients with more respiratory dysfunction, which should engender stronger rehabilitation measures to improve the progression of patients infected with COVID-19. This report aims to raise awareness about the role of diaphragmatic damage in patients affected by COVID-19. During treatment of these patients, it is important to bear in mind the possible diaphragmatic injury that may necessitate rehabilitation therapies. We should assess this disturbance’s progression and each patient’s evolution in the long term as a respiratory sequel.
In conclusion, diaphragmatic dysfunction could play a relevant role in respiratory disturbance in hospitalised patients with severe COVID-19. Although diaphragmatic dysfunction is difficult to detect, the combined functional and morphological approach with phrenic electroneurograms and chest ultrasounds could improve diagnostic sensitivity. If the implication of diaphragmatic dysfunction in COVID-19 disease is confirmed, the ventilatory rehabilitation approach will allow an additional therapeutic option in treatment of this serious disease.
Acknowledgments
None
List of acronyms
- BMI
Body Mass Index
- COVID-19
coronavirus disease 2019
- HIV
Human Immunodeficiency Virus
- MG
Mild Group
- SARS
Severe Acute Respiratory Syndrome
- SEM
Standard Error of the Mean
- SG
Severe Group
Funding Statement
Funding: The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Juan Vega-Villar, Email: juanvegavillar@gmail.com.
Esmeralda Rocío-Martín, Email: erociomartin@hotmail.es.
Patricia García-García, Email: patriciagarc@hotmail.com.
Elena de la Rosa Santiago, Email: elenarosa.santiago@gmail.com.
Jose María Galván-Román, Email: jm.galvanroman@gmail.com.
Rybel Wix-Ramos, Email: rybelwix@hotmail.com.
References
- 1.Brosnahan SB, Jonkman AH, Kugler MC, Munger JS, Kaufman DA. COVID-19 and Respiratory System Disorders: Current Knowledge, Future Clinical and Translational Research Questions. Arterioscler Thromb Vasc Biol. 2020. Nov;40(11):2586-2597. doi: 10.1161/ATVBAHA.120.314515. Epub 2020 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hund E. Critical illness polyneuropathy. Curr Opin Neurol. 2001. Oct;14(5):649-53. doi: 10.1097/00019052-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005. Aug 1;202(3):415-24. doi: 10.1084/jem.20050828. Epub 2005 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kokatnur L, Rudrappa M. Diaphragmatic Palsy. Diseases. 2018. Feb 13;6(1):16. doi: 10.3390/diseases6010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao BE, Ostrovskiy DA, Wilbourn AJ, Shields RW Jr. Phrenic neuropathy due to neuralgic amyotrophy. Neurology. 2006. May 23;66(10):1582-4. doi: 10.1212/01.wnl.0000216140.25497.40. Erratum in: Neurology. 2006 Jul 25;67(2):299. [DOI] [PubMed] [Google Scholar]
- 6.Nardone R, Bernhart H, Pozzera A, Taddei M, Tezzon F. Respiratory weakness in neuralgic amyotrophy: report of two cases with phrenic nerve involvement. Neurol Sci. 2000. Jun;21(3):177-81. doi: 10.1007/s100720070094. [DOI] [PubMed] [Google Scholar]
- 7.Jeon JS, Park JS. An early treated neuralgic amyotrophy with bilateral phrenic nerve involvement with a favorable outcome. Neurol India. 2016. May-Jun;64(3):566-9. doi: 10.4103/0028-3886.181578. [DOI] [PubMed] [Google Scholar]
- 8.Lahrmann H, Grisold W, Authier FJ, Zifko UA. Neuralgic amyotrophy with phrenic nerve involvement. Muscle Nerve. 1999. Apr;22(4):437-42. doi: 10.1002/(sici)1097-4598(199904)22:4<437::aid-mus2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci. 2020. Jun 15;413:116832. doi: 10.1016/j.jns.2020.116832. Epub 2020 Apr 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020. Jul;92(7):699-702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB Jr. Middle East Respiratory Syndrome Coronavirus Causes Multiple Organ Damage and Lethal Disease in Mice Transgenic for Human Dipeptidyl Peptidase 4. J Infect Dis. 2016. Mar 1;213(5):712-22. doi: 10.1093/infdis/jiv499. Epub 2015 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudrappa M, Kokatnur L, Chernyshev O. Neurological Respiratory Failure. Diseases. 2018. Jan 10;6(1):7. doi: 10.3390/diseases6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, Toubes ME, Riveiro V, Valdés L. Diaphragmatic dysfunction. Pulmonology. 2019. Jul-Aug;25(4):223-235. doi: 10.1016/j.pulmoe.2018.10.008. Epub 2018 Dec 1. [DOI] [PubMed] [Google Scholar]
- 14.Vincent M, Court-Fortune I, Costes F, Antoine JC, Camdessanché JP. Phrenic Nerve Conduction in Healthy Subjects. Muscle Nerve. 2019. Apr;59(4):451-456. doi: 10.1002/mus.26414. Epub 2019 Jan 24. [DOI] [PubMed] [Google Scholar]
- 15.Maranhão AA, Carvalho SRDS, Caetano MR, Alamy AH, Peixoto EM, Filgueiras PDEP. Phrenic nerve conduction studies: normative data and technical aspects. Arq Neuropsiquiatr. 2017. Dec;75(12):869-874. doi: 10.1590/0004-282X20170153. [DOI] [PubMed] [Google Scholar]
- 16.Resman-Gaspersc A, Podnar S. Phrenic nerve conduction studies: technical aspects and normative data. Muscle Nerve. 2008. Jan;37(1):36-41. doi: 10.1002/mus.20887. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020. Apr;8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18. Erratum in: Lancet Respir Med. 2020 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004. Jun;203(2):622-30. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox RJ, Galetta SL, Mahalingam R, Wellish M, Forghani B, Gilden DH. Acute, chronic, and recurrent varicella zoster virus neuropathy without zoster rash. Neurology. 2001. Jul 24;57(2):351-4. doi: 10.1212/wnl.57.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Kolson DL, Gonzalez-Scarano F. HIV-associated neuropathies: role of HIV-1, CMV, and other viruses. J Peripher Nerv Syst. 2001. Mar;6(1):2-7. doi: 10.1046/j.1529-8027.2001.006001002.x. [DOI] [PubMed] [Google Scholar]
- 21.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000. Jun;59(6):1251-60. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 22.Mahadevan A, Gayathri N, Taly AB, Santosh V, Yasha TC, Shankar SK. Vasculitic neuropathy in HIV infection: a clinicopathological study. Neurol India. 2001. Sep;49(3):277-83. [PubMed] [Google Scholar]
- 23.Martínez Rodríguez L, Carvajal P, Morís G. Neuralgia amiotrófica en relación con infección por el virus de la hepatitis E [Neuralgic amyotrophy associated to hepatitis E virus infection]. Med Clin (Barc). 2015. Nov 20;145(10):462-3. Spanish. doi: 10.1016/j.medcli.2015.01.021. Epub 2015 Mar 24. [DOI] [PubMed] [Google Scholar]
- 24.Verma R, Sahu R, Holla V. Neurological manifestations of dengue infection: a review. J Neurol Sci. 2014. Nov 15;346(1-2):26-34. doi: 10.1016/j.jns.2014.08.044. Epub 2014 Sep 6. [DOI] [PubMed] [Google Scholar]
- 25.Podnar S. Nosology of idiopathic phrenic neuropathies. J Neurol. 2015. Mar;262(3):558-62. doi: 10.1007/s00415-014-7596-0. Epub 2014 Dec 6. [DOI] [PubMed] [Google Scholar]
- 26.Sierra A, Prat J, Bas J, Romeu A, Montero J, Matos JA, Bella R, Ferrer I, Buendia E. Blood lymphocytes are sensitized to branchial plexus nerves in patients with neuralgic amyotrophy. Acta Neurol Scand. 1991. Mar;83(3):183-6. doi: 10.1111/j.1600-0404.1991.tb04674.x. [DOI] [PubMed] [Google Scholar]
- 27.Waki Y, Nobeyama Y, Fukuchi O, Mukai T, Takagi M, Asahina A. Case of herpes zoster complicated by diaphragmatic paralysis. J Dermatol. 2019. Sep;46(9):e322-e324. doi: 10.1111/1346-8138.14878. Epub 2019 Apr 2. [DOI] [PubMed] [Google Scholar]
- 28.Djukic M, Larsen J, Lingor P, Nau R. Unilateral phrenic nerve lesion in Lyme neuroborreliosis. BMC Pulm Med. 2013. Jan 18;13:4. doi: 10.1186/1471-2466-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melero MJ, Mazzei ME, Bergroth B, Cantardo DM, Duarte JM, Corti M. Bilateral diaphragmatic paralysis in an HIV patient: Second reported case and literature review. Lung India. 2014. Apr;31(2):149-51. doi: 10.4103/0970-2113.129846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008. Aug;82(15):7264-75. doi: 10.1128/JVI.00737-08. Epub 2008 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, Li Z, Deng P, Zhang J, Zhong N, Ding Y, Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005. Oct 15;41(8):1089-96. doi: 10.1086/444461. Epub 2005 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008. Oct;7(10):939-50. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 33.Brosnahan SB, Jonkman AH, Kugler MC, Munger JS, Kaufman DA. COVID-19 and Respiratory System Disorders: Current Knowledge, Future Clinical and Translational Research Questions. Arterioscler Thromb Vasc Biol. 2020. Nov;40(11):2586-2597. doi: 10.1161/ATVBAHA. 120.314515. Epub 2020 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chetta A, Rehman AK, Moxham J, Carr DH, Polkey MI. Chest radiography cannot predict diaphragm function. Respir Med. 2005. Jan;99(1):39-44. doi: 10.1016/j.rmed.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Noda Y, Sekiguchi K, Kohara N, Kanda F, Toda T. Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve. 2016. Apr;53(4):522-7. doi: 10.1002/mus.24902. Epub 2015 Oct 10. [DOI] [PubMed] [Google Scholar]
- 36.Pinto S, Alves P, Pimentel B, Swash M, de Carvalho M. Ultrasound for assessment of diaphragm in ALS. Clin Neurophysiol. 2016. Jan;127(1):892-897. doi: 10.1016/j.clinph.2015.03.024. Epub 2015 Apr 25. [DOI] [PubMed] [Google Scholar]
- 37.Lisboa C, Paré PD, Pertuzé J, Contreras G, Moreno R, Guillemi S, Cruz E. Inspiratory muscle function in unilateral diaphragmatic paralysis. Am Rev Respir Dis. 1986. Sep;134(3):488-92. doi: 10.1164/arrd.1986.134.3.488. [DOI] [PubMed] [Google Scholar]
- 38.Hart N, Nickol AH, Cramer D, Ward SP, Lofaso F, Pride NB, Moxham J, Polkey MI. Effect of severe isolated unilateral and bilateral diaphragm weakness on exercise performance. Am J Respir Crit Care Med. 2002. May 1;165(9):1265-70. doi: 10.1164/rccm.2110016. [DOI] [PubMed] [Google Scholar]


