Abstract
Episodic memory involves the formation of relational structures that bind information about the stimuli people experience to the contexts in which they experience them. The ability to form and retain such structures may be at the core of the development of episodic memory. In the first experiment reported here, 4- and 7-year-olds were presented with paired-associate learning tasks requiring memory structures of different complexity. A multinomial-processing tree model was applied to estimate the use of different structures in the two age groups. The use of two-way list-context-to-target structures and three-way structures was found to increase between the ages of 4 and 7. Experiment 2 demonstrated that the ability to form increasingly complex relational memory structures develops between the ages of 4 and 7 years and that this development extends well into adulthood. These results have important implications for theories of memory development.
Keywords: episodic memory, memory development, paired-associate learning, cued recall, cognitive development, memory, statistical analysis
Episodic memory refers to the ability to remember what happened as well as where and when it happened (Tulving, 1972). Therefore, accurate recall requires one to bind the what, when, and where information into a coherent relational structure (Cohen & Eichenbaum, 1993; Schacter & Tulving, 1994). For example, suppose that a child has a gym class on Monday morning. To remember this correctly, the child needs to form a relational representation that binds [gym] with [Monday]. Such two-way binding is the simplest episodic memory structure (Humphreys, Bain, & Pike, 1989). However, in reality, children have multiple classes on different days. As a result, [gym] is linked to multiple days, and [Monday] is linked to multiple classes. In this case, a simple two-way binding is insufficient to correctly remember the event, so at least two two-way bindings are required: (a) a link between the class and the day ([Monday]-[gym]) and (b) a link between the class and the time of the day ([morning]-[gym]). By using both two-way bindings, one can correctly recall which class he or she took on Monday morning.
Although two two-way bindings are often sufficient, sometimes a more complex structure is required. Suppose that on Monday, the child has a gym class in the morning and a math class in the afternoon, whereas on Tuesday, he or she has a math class in the morning and a gym class in the afternoon. Now [Monday] is bound to both [gym] and [math], and [morning] is also bound to both [gym] and [math]. Therefore, both cues (i.e., [Monday] and [morning]) are independently linked to multiple items and do not uniquely identify a particular item (Humphreys, Wiles, & Dennis, 1994). The minimal structure required to correctly recall such episodes is a three-way binding (Humphreys et al., 1989). In a three-way binding structure, [Monday]-[morning]-[gym] are linked together. In this case, a compound cue (i.e., [Monday]-[morning]) would lead to an unambiguous and correct answer.
One way of examining the ability to form and use these memory structures is a paired-associate learning paradigm, in which participants study two lists of item pairs sequentially and are then tested on both lists. Different list types may require different relational structures for correct recall. Specifically, in ABCD lists, the pairs in List 1 (A-B) and in List 2 (C-D) are unique in that there is no overlap between the two lists (see Table 1 for examples). Therefore, when asked what item A was paired with in List 1, participants, at the minimum, need a two-way binding ([A]-[B]). In ABAC lists (Barnes & Underwood, 1959), pairs in List 1 (A-B) and List 2 (A-C) have a common element: “A.” Thus, at least two two-way bindings are required (e.g., [A]-[B] and [List 1]-[B]). Finally, in ABABr lists (Porter & Duncan, 1953), the paired items in List 1 (e.g., A-B and C-D) are rearranged to form List 2 (e.g., A-D and C-B). Therefore, a three-way binding ([A]-[List 1]-[B]) is the minimal requirement. Although the ABABr list is the most difficult to accurately remember, adults typically show robust performance (Postman, 1964).
Table 1.
Examples of List Types Used in Experiments 1 and 2
| List type | List 1 | List 2 |
|---|---|---|
| ABCD | ||
| tree-shoe | box-cat | |
| elephant-candy | turtle-chocolate | |
| bus-apple | dog-chair | |
| dresser-glasses | umbrella-football | |
| bike-toothbrush | backpack-fork | |
| piano-pencil | hat-goldfish | |
| ABAC | ||
| bike-cup | bike-fork | |
| couch-cat | couch-apple | |
| airplane-strawberry | airplane-chair | |
| dresser-chocolate | dresser-candy | |
| backpack-football | backpack-pencil | |
| dog-balloon | dog-orange | |
| ABABr | ||
| door-cup | door-balloon | |
| couch-spoon | couch-strawberry | |
| horse–cell phone | horse-cup | |
| airplane-orange | airplane-spoon | |
| flag-balloon | flag–cell phone | |
| car-strawberry | car-orange |
Note: See the introduction for an explanation of the list types. In Experiment 1, each list-context cue (i.e., the cue associated with all items on the list) was a colored house randomly selected from a set of four colored houses (blue, red, green, and yellow). In Experiment 2, each list-context cue was a cartoon character also randomly selected from a set of four (Winnie the Pooh, Elmo, SpongeBob SquarePants, and Dora the Explorer). The same list-context cue was maintained throughout the list but differed across the lists.
Many believe that episodic memory has a relatively late onset and a protracted development, especially compared with the rapid development of semantic memory (Drummey & Newcombe, 2002; Rubin, 2000; but see Bauer, Wenner, Dropik, & Wewerka, 2000). However, even those researchers who argue for an early onset acknowledge that throughout the preschool years, many aspects of episodic memory are fragile. Although sometimes young children exhibit excellent memory for individual items (Sloutsky & Fisher, 2004), they have difficulty remembering what happened, as well as where and when it happened (Bauer, 2007). Children’s testimonies in forensic contexts are often unreliable (Pipe & Salmon, 2009), and laboratory recall and recognition tasks demonstrate that episodic memory continues to improve through adolescence (Brainerd, Reyna, & Ceci, 2008; Ghetti & Lee, 2011).
If the ability to form episodic memory undergoes protracted development (Sluzenski, Newcombe, & Kovacs, 2006; Sluzenski, Newcombe, & Ottinger, 2004), it is reasonable to ask what changes in the course of development. Do people develop the ability to encode complex relational structures? Or do people develop the ability to retain more complex structures in long-term memory? The former possibility may reflect the difficulty of simultaneously attending to multiple components, forming a relational structure, or both (e.g., Doumas, Hummel, & Sandhofer, 2008; Halford, Wilson, & Phillips, 1998). The latter possibility may reflect the fact that more complex structures may decay faster, particularly early in development. For example, infancy research using simpler structures suggests that the ability to retain them could be a limiting factor early in development (Bauer, Wiebe, Carver, Waters, & Nelson, 2003; see also Richmond & Nelson, 2007, for a review). Answering these questions is fundamental for understanding memory development, and we attempt to provide such answers in the current research with respect to postinfancy memory development.
In Experiment 1, we presented 4- and 7-year-olds with tasks that required them to form relational structures of varying complexity (i.e., two-way, two two-way, and three-way bindings). To reduce the possibility of failure to retain these structures, we minimized the delay between training and testing. In Experiment 2, to increase attention to context, we increased the saliency of the list-context cue—the cue associated with all items on the list. In both experiments, a multinomial-processing tree (MPT) model was applied to estimate the use of different memory structures across development.
Experiment 1
In Experiment 1, we investigated the development of the ability to use increasingly complex memory structures. Because previous research has suggested that major changes in episodic memory occur when children are between 4 and 6 years of age (Drummey & Newcombe, 2002; Sluzenski et al., 2006), we recruited 4- and 7-year-olds.
Method
Participants.
Seventy-four 4-year-olds (38 girls; mean age = 4.67 years, SD = 0.31 years) and seventy-two 7-year-olds (37 girls; mean age = 7.35 years, SD = 0.33 years) participated. Participants were recruited from schools in middle-class suburbs of Columbus, Ohio. Each participant was randomly assigned to one of the three list conditions: ABCD (4-year-olds: n = 25, 7-year-olds: n = 25), ABAC (4-year-olds: n = 23, 7-year-olds: n = 24), or ABABr (4-year-olds: n = 26, 7-year-olds: n = 23). Twelve 4-year-olds were excluded from the analysis (9 did not complete the experiment, 2 did not meet the learning criterion, and 1 was excluded because of experimenter error), and three 7-year-olds were excluded (2 did not meet the learning criterion and 1 was excluded because of experimenter error).
Stimuli and design.
In each condition (i.e., ABCD, ABAC, and ABABr), two study lists and a cued-recall test were drawn from two sets of prerandomized study lists, with two prerandomized cued-recall tests in each set. Each study list contained six pairs of pictured objects and a colored house serving as a list-context cue. The same list-context cue was maintained throughout a given list but differed between the lists. The cued-recall test had six trials covering half of the items from List 1 and half from List 2. In each condition, the order of the study lists and tests was counterbalanced.
Procedure.
Participants were tested individually in their schools. In the study phase (see Fig. 1a), the participants were told that they were invited to a house of a given color. Pictures of six objects in the house appeared on a computer screen, and the experimenter told the participants that some other objects were hidden behind the shown objects. Then each member of the pair was introduced separately, and a reference to the second item in that pair was made (e.g., “Let’s see what’s hiding behind the bike in the red house”). An animated movement then revealed the second item on screen, after which the experimenter pointed to the two pictures and named both items and the list-context cue (e.g., “There is a cup hiding behind the bike in the red house”). Finally, the second item was covered again by the first item. All pairs were presented sequentially, one item at a time.
Fig. 1.
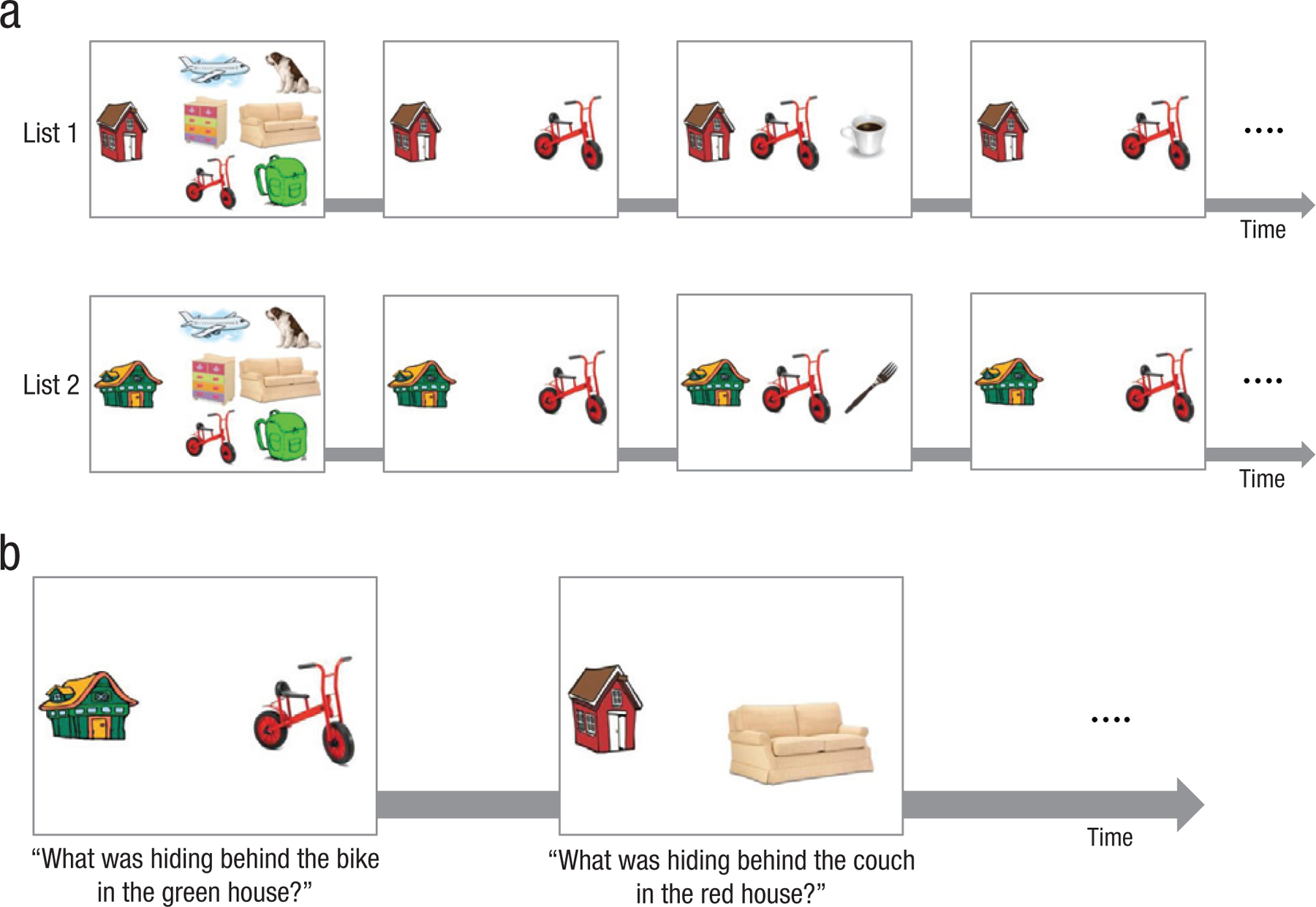
Sample study-phase trial sequence and cued-recall test in Experiment 1. In the study phase of Experiment 1 (a; the ABAC condition is shown here), participants were shown two lists one after another. Each list featured six pairs of pictured objects and one of four colored houses (which served as a list-context cue for the study items). The experimenter first presented six objects that were the first items of each pair and then introduced the individual items one at a time, accompanied by the list-context cue. Next, the second item was revealed from behind the first item by an animated movement. Finally, the second item was covered again by the first item. This process was repeated until all items in the list were introduced. After each list was studied, a 3- to 4-min retention interval followed. In the subsequent cued-recall test (b), participants saw the list-context cue from one of the lists and an item. They were then asked to recall the other item that had been presented with the given item and list context.
To ensure that all pairs on each list were encoded, we gave participants a cued-recall test after they saw all six pairs. Regardless of their accuracy, corrective feedback was given on each trial. If any of the six items were recalled incorrectly, another cued-recall test was given in a different random order. If a participant failed to recall all six items after 10 attempts, the experiment stopped, and the data were excluded from analysis. At the end of studying each list, participants played a simple, engaging video game, which filled the 3- to 4-min-long retention interval. The procedure for List 2 was identical to that for List 1. In the test phase, participants were shown a colored house (i.e., list-context cue) and an item that was presented first in the study phase (i.e., item cue), and they were asked to recall the second item (i.e., target) in the pair (see Fig. 1b).
Before studying List 1, a practice session was administered that resembled the ABAC condition. There was only one pair in each list, and there was no retention interval. Objects and list-context cues used in the practice phase did not appear in the experiment proper.
Results and discussion
Behavioral results.
Initial analyses focused on proactive interference from List 1 to List 2 during study and recall accuracy during test. Proactive interference was calculated by subtracting the number of repetitions to learn List 1 from the number required to learn List 2, where a positive number indicated proactive interference. If participants formed a three-way binding, there should be no interference in any of the conditions. If they formed item-cue-to-target and list-context-to-target bindings, there should be no interference in the ABCD and ABAC conditions. Finally, if they formed only item-cue-to-target bindings, there should be no interference in the ABCD condition. Interference was inferred if the mean difference was above zero, and facilitation was inferred when it was below zero. As shown in Figure 2a, interference was observed in the ABAC and ABABr conditions (ps < .05, one-sample Bonferroni-adjusted t tests), but there was no interference in the ABCD condition (ps > .19; outliers whose average differed by ±2.5 standard deviations from the group mean were eliminated, which excluded one 7-year-old from the ABAC condition). Therefore, when presented with List 1, participants primarily formed item-cue-to-target bindings, whereas they were less likely to form list-context-to-target or three-way bindings: Forming such bindings should have prevented proactive interference.
Fig. 2.

Behavioral and multinomial-processing tree (MPT) model results from Experiment 1. The mean magnitude of proactive interference (a) and mean accuracy (b) are shown as a function of condition and age group. Proactive interference was calculated by subtracting the number of repetitions to learn List 1 from the number required to learn List 2. Positive numbers indicate interference effects. Accuracy was calculated as the number of items from each list that were correctly recalled. Estimated parameter values implied by the MPT model (c) are shown as a function of age group. The distribution frequency of the four parameters in the MPT model (d) is shown as a function of the values of each parameter in the model and of age group. The parameters in the model were e (experiment-to-target binding), i (item-cue-to-target binding), l (list-context-to-target binding), and b (three-way binding). Error bars represent ±1 SEM.
In terms of recall accuracy (see Fig. 2b), performance in the ABCD condition was better than performance in the ABAC and ABABr conditions, and 7-year-olds were more accurate than 4-year-olds. A 2 (age) ×3 (condition) between-subjects analysis of variance (ANOVA) revealed a main effect of age, F(1, 140) = 9.88, p < .005, ηp2 = .07, and condition, F(2, 140) = 20.32, p < .001, ηp2 = .23, with no interaction between the two. The ABCD condition elicited greater accuracy than the other two conditions (Tukey’s HSD, or honestly significant difference, test, ps < .001).
The analyses of interference implicated encoding differences in the three conditions, whereas the analyses of recall revealed a developmental increase in accuracy. However, the standard analyses of accuracy are some-what limited. First, accurate recall sets only a minimal requirement for the implied memory structure. For example, a participant can form a three-way structure in an ABCD condition, which requires only a two-way structure, and the standard analyses cannot detect this possibility. Second, the standard analyses used only correct responses, whereas error patterns could be highly informative. To overcome these limitations, we applied an MPT model, which utilized the entire pattern of responses.
MPT model.
MPT models attempt to infer the contributions of latent processes or structures to categorical data and have been successfully used in various domains (see Batchelder & Riefer, 1999, for a review). The current model assumed that the proportion of a specific response was determined by the availability of four types of latent structures: (a) the experiment-to-target links (which enable one to distinguish items presented during the experiment from all other items), (b) item-cue-to-target links (which enable one to preserve item-cue-to-target pairing), (c) list-context-to-target links (which enable one to distinguish target items in one list from the other), and (d) three-way binding (which enable one to use conjoint context-cue-target cues). Therefore, if participants do not form experiment-to-target links, they would fail to recall anything or would respond with items that were not presented in the experiment (resulting in a miss). If the item-cue-to-target link is absent, participants would not distinguish correct members of a pair from incorrect ones, producing any item that was presented as a target. If the list-context-to-target link is absent, the participant would confuse the lists. Therefore, list intrusions would occur in the ABAC condition. Finally, in the absence of the three-way binding, participants would fail to use a conjoint cue to retrieve the target in the ABABr condition and might produce the other item that is bound to the same item cue or to the same list-context cue. These four types of information were used in the model as the parameters e (experiment-to-target binding), i (item-cue-to-target binding), l (list-context-to-target binding), and b (three-way binding). Using the combination of parameters, three processing trees were constructed to represent each condition (see Fig. 3 for the ABABr tree and Tree Structures of the MPT Model in the Supplemental Material available online for all tree structures).
Fig. 3.

Structure of the ABABr condition in the multinomial-processing tree model. The model shows the probability of a correct response in the cued-recall test using four parameters: e (experiment-to-target binding), i (item-cue-to-target binding), l (list-context-to-target binding), and b (three-way binding). If there is more than one response at the end of a branch, those responses have an equal chance of being produced. Examples of the responses are based on the list on the left of the tree structure when the cued-recall test question, “What was hiding under the bike in the green house?” was given.
Figure 3 depicts the tree structure for the ABABr condition. How do the four parameters affect the probability of a correct response (e.g., “cup”)? The correct response could (a) stem from any branch that has a b parameter (e.g., the left-most branch, with the probability e × i × l × b) or (b) be derived by chance from a branch that does not have a b parameter (e.g., second right branch next to “miss,” with the probability e × (1 – i) × (1 – l) × (1 – b) × 1/6, where 1/6 is the probability of getting a correct response out of 6 possible responses). The sum of all these probabilities would be the probability of correct responses in the ABABr condition. Similar trees were drawn for the other conditions, and the four probability parameter values were estimated from data (code used for parameter estimation is available at http://cogdev.osu.edu/mpt/code.php). In addition, distributions of parameter values were estimated by using nonparametric bootstrapping (100,000 samples).
Model results and discussion.
The proposed model was evaluated against the saturated model using the Schwarz weights (wBIC; Moshagen, 2010; Wagenmakers & Farrell, 2004). The wBIC calculates the relative likelihood ratio between the current model (log-likelihood = −69.15, Bayesian information criterion, or BIC = 192.53) and the saturated model (log-likelihood = −55.94, BIC = 247.46). If the current model is favored, the values will be closer to 1, and if the saturated model is favored, the values will be closer to 0. The wBIC for Experiment 1 was approximately 1.0.
Figure 2c presents estimated parameter values, and Figure 2d presents their distributions. A randomization test (Lunneborg, 1999) showed a significant difference in e (experiment-to-target binding) and b (three-way binding), ps < .02, with values of 7-year-olds exceeding those of 4-year-olds. At the same time, neither l (list-context-to-target binding) nor i (item-cue-to-target binding), ps > .18, exhibited developmental differences.
The results indicate that older children exhibited greater ability to use experiment-to-target binding and three-way bindings than younger children did. The increase of the experiment-to-target parameter (e) between 4 and 7 years of age is consistent with the results of previous research demonstrating an increase in performance on source-monitoring tasks across similar age groups (cf. Newcombe, Lloyd, & Ratliff, 2007). However, the developmental increase in three-way binding (b) is a novel finding.
Experiment 1 found novel evidence (i.e., proactive-interference data) that young children fail to encode complex structures. However, it remains unclear whether their failure stemmed from a lack of attention to context information, an inability to form a relational memory structure, or a combination of both. To address this issue directly, we increased the saliency of the list-context cue in Experiment 2, which was expected to increase attention to the list context. Additionally, adults were included to further examine the development of episodic memory.
Experiment 2
Saliency of the list-context cue was increased by using cartoon characters, which had a greater effect on 4-year-olds than on 7-year-olds (see Evidence for Differential Saliency of Context Across Experiments in the Supplemental Material).
Method
Participants.
Seventy-three 4-year-olds (38 girls; mean age = 4.68 years, SD = 0.24 years), sixty-nine 7-year-olds (35 girls; mean age = 7.47 years, SD = 0.37 years), and 127 undergraduates at The Ohio State University (68 females; mean age = 19.44 years, SD = 2.50 years) participated in the experiment. As in Experiment 1, children were recruited from schools in middle-class suburbs of Columbus, Ohio. Each participant was randomly assigned to one of three conditions: ABCD (4-year-olds: n = 25, 7-year-olds: n = 21, adults: n = 45), ABAC (4-year-olds: n = 25, 7-year-olds: n = 26, adults: n = 41), or ABABr (4-year-olds: n = 23, 7-year-olds: n = 22, adults: n = 41). Sixteen 4-year-olds were excluded from the analyses (10 did not complete the experiment, 4 did not meet the learning criterion, and 2 were excluded because of experimenter error). Two 7-year-olds were excluded because of experimenter error.
Stimuli, design, and procedure.
The stimuli, design, and procedure were identical to those in Experiment 1, except that cartoon characters (i.e., Winnie the Pooh, Elmo, Dora the Explorer, and SpongeBob SquarePants) were used as list contexts instead of colored houses.
Results and discussion
Behavioral results.
Proactive interference was analyzed as in Experiment 1. As shown in Figure 4a, none of the groups exhibited interference in the ABCD condition, all Bonferroni-adjusted ps > .14, whereas in the ABAC and ABABr conditions, children, but not adults, exhibited interference, all Bonferroni-adjusted ps < .05 (outliers, whose average differed by ±2.5 standard deviations from the group mean were eliminated, which excluded one 4-year-old in the ABAC condition, one 7-year-old in each condition, and one adult in the ABCD condition).
Fig. 4.

Behavioral and multinomial-processing tree (MPT) model results from Experiment 2. The mean magnitude of proactive interference (a) and mean accuracy (b) are shown as a function of condition and age group. Proactive interference was calculated by subtracting the number of repetitions to learn List 1 from the number required to learn List 2. Positive numbers indicate interference effects. Accuracy was calculated as the number of items from each list that were correctly recalled. Estimated parameter values implied by the MPT model (c) are shown as a function of age group. The distribution frequency of the four parameters in the MPT model (d) is shown as a function of the values of each parameter in the model and of age group. The parameters in the model were e (experiment-to-target binding), i (item-cue-to-target binding), l (list-context-to-target binding), and b (three-way binding). Error bars represent ±1 SEM.
Similar to Experiment 1, when studying List 1, children primarily formed item-cue-to-target bindings while failing to form list-context-to-target or three-way bindings. At the same time, a lack of interference in adults, coupled with the fact that they did not know which condition they were in and what would be presented in List 2, suggests that adults spontaneously formed three-way bindings across the conditions when studying List 1.
Recall accuracy differed across conditions (see Fig. 4b), with adults exhibiting greater accuracy than children. A 3 (age) × 3 (condition) between-subjects ANOVA revealed a main effect of age, F(2, 260) = 14.58, p < .001, ηp2 = .101 and condition, F(2, 260) = 33.95, p < .001, ηp2 = .207, with no significant interaction between the two. The ABCD condition elicited the best performance, followed by the ABAC and ABABr (Tukey’s HSD test, ps < .001); in all three conditions, adults showed the highest accuracy, followed by 7-year-olds and 4-year-olds (Tukey’s HSD test, ps < .001).
Both patterns of interference and recall accuracy indicate that the difference between the 7-year-olds and adults was at least as great as that between the 4-year-olds and 7-year-olds. Therefore, episodic memory continues to develop between the age of seven years and adulthood. To further estimate how memory parameters change with age, the MPT model was used as in Experiment 1.
MPT model results and discussion.
The wBIC between the current model (log-likelihood = −118.93, BIC = 326.51) and the saturated model (log-likelihood = −82.57, BIC = 386.72) for Experiment 2 was approximately 1.0.
Figure 4c presents estimated parameter values, and Figure 4d presents their distributions. First, when parameters were compared across experiments using a randomization test, only the 4-year-olds’ l parameter exhibited a significant increase compared with the same age group in Experiment 1 (p < .01). At the same time, the b parameter exhibited only a numerical increase. Therefore, lack of attention to context may play a role in young children’s failure to encode episodic information. However, the fact that significantly increased attention to context resulted in only a small increase in the b parameter suggests that failure to form a complex relational structure is also responsible for young children’s failure to encode episodic information.
To compare parameters within Experiment 2, we performed a randomization test with Sidak adjustment. The test indicated that, in contrast to Experiment 1 (in which the e and b parameters differed between the 4- and 7-year-olds), neither of the four parameters differed between the age groups in Experiment 2, Sidak-adjusted ps > .16.
Adults had higher values than 4-year-olds for all parameters (Sidak-adjusted ps < .03), except for the l parameter. Adults also had higher values than 7-year-olds of the l and b parameter (Sidak-adjusted ps < .03) and lower values of the i parameter (Sidak-adjusted p = .003), with no differences in e (Sidak-adjusted p = .71). The i parameter decreased with age: Three-way binding may have obviated the need for the cue-to-target information.
The results of Experiment 2 replicated and substantially extended the results of Experiment 1. First, relational memory continues to develop between the age of 7 years and adulthood: There is a substantial increase in the use of list-context-to-target bindings (l) and three-way bindings (b). Second, the results of Experiment 2 indicate that the development of episodic memory involves the ability (a) to attend to context and (b) to form progressively complex memory structures.
General Discussion
The current experiments, in which we examined the development of episodic memory, had two innovative aspects, one pertaining to the mechanism of memory development and another to the methodology of studying memory development. In terms of memory development, the current experiments generated three important findings. First, episodic memory undergoes substantial development between the age of 7 years and adulthood. Second, encoding is an important factor in the development of episodic memory. The fact that children, but not adults, showed proactive interference in the ABAC and ABABr conditions suggests that many children did not spontaneously encode complex structures. The fact that increasing the saliency of the list-context cue for 4-year-olds increased their ability to bind list contexts with items (i.e., l parameter) suggests that the failure of encoding could reflect a failure to attend to context information. At the same time, the fact that a significant increase in attention to list context in 4-year-olds resulted in only a small increase in the b parameter suggests that failure of encoding also reflects the difficulty to form a complex three-way relational structure. Therefore, the third finding stemming from this research is that the development of encoding reflects the increasing ability to attend to context information and to form complex relational structures.
The second innovative aspect of the present research is the use of the MPT model to estimate the contributions of the underlying structures from error patterns and correct responses. Whereas most methods rely exclusively on accuracy and do not utilize the whole response pattern, we believe the method is a promising approach in understanding the development of episodic memory. Error patterns may reveal which components of episodic memory are missing, whereas changes in these patterns may reveal when these components come on-line. MPT models have been successful in explaining latent processes of behavioral patterns outside of memory development (Erdfelder et al., 2009; Smith & Batchelder, 2008), and they appear to be a productive tool for studying mechanisms of memory development.
The present results have important implications for theories of episodic memory because they implicate encoding in memory development. Especially, the results point to the role of attention to context and the ability to form complex relational structures in the development of episodic memory. These results may also have implications for the understanding of neural mechanisms of episodic memory and its development. Two major brain structures have been implicated in episodic memory, the medial temporal lobe (MTL) and the prefrontal cortex (PFC; e.g., Zeithamova & Preston, 2010). The functional role of these structures is a matter of considerable debate. According to one view, the MTL (including the hippocampus) is involved in the retention of memory traces (Bauer, 2008; Newcombe et al., 2007), whereas the PFC is linked to encoding multiple sources of information into an episodic memory trace (Newcombe et al., 2007; Ofen et al., 2007). At the same time, other researchers argue that both the MTL and PFC subserve encoding and that their respective roles change with development (Ghetti, DeMaster, Yonelinas, & Bunge, 2010).
Furthermore, neural changes subserving episodic memory development are a matter of debate as well. Some researchers argue that there is significant maturation of the PFC even after adolescence (Gogtay et al., 2006; Sowell et al., 2004), whereas there are relatively small changes in the hippocampus after the age of 4 years, which suggests that the PFC is the primary source of change past infancy. Alternatively, studies focusing on the hippocampus report that structures such as the posterior parahippocampal gyrus play a substantial role in episodic memory development (Ghetti et al., 2010).
The current behavioral findings are consistent with either position and cannot resolve these debates. However, the modeling approach, which successfully decomposes memory performance into components, may be productively used to make model-based neural predictions, thus allowing a focused examination of the involvement of different brain structures in the development of episodic memory.
In sum, the research reported here yielded novel findings demonstrating that episodic memory undergoes substantial development between 7 years of age and adulthood, and that the ability to form complex three-way binding structures develops during this period. It also introduces a novel memory-modeling method revealing that this development involves the ability to form increasingly complex memory structures.
Supplementary Material
Funding
This research was supported by a National Science Foundation grant to V. M. Sloutsky (BCS-0720135).
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Supplemental Material
Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Barnes JM, & Underwood BJ (1959). “Fate” of first-list associations in transfer theory. Journal of Experimental Psychology, 58, 97–105. [DOI] [PubMed] [Google Scholar]
- Batchelder WH, & Riefer DM (1999). Theoretical and empirical review of multinomial process tree modeling. Psychological Bulletin & Review, 6, 57–86. [DOI] [PubMed] [Google Scholar]
- Bauer PJ (2007). Remembering the times of our lives: Memory in infancy and beyond. Mahwah, NJ: Erlbaum. [Google Scholar]
- Bauer PJ (2008). Toward a neuro-developmental account of the development of declarative memory. Developmental Psychobiology, 50, 19–31. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Wenner JA, Dropik PL, & Wewerka SS (2000). Parameters of remembering and forgetting in the transition from infancy to early childhood. Monographs of the Society for Research in Child Development, 65(4), 1–204. [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Waters JM, & Nelson CA (2003). Developments in long-term explicit memory late in the first year of life: Behavioral and electrophysiological indices. Psychological Science, 14, 629–635. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, & Ceci SJ (2008). Developmental reversals in false memory: A review of data and theory. Psychological Bulletin, 134, 343–382. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, & Eichenbaum H (1993). Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press. [Google Scholar]
- Doumas LAA, Hummel JE, & Sandhofer CM (2008). A theory of the discovery and predication of relational concepts. Psychological Review, 115, 1–43. [DOI] [PubMed] [Google Scholar]
- Drummey AB, & Newcombe NS (2002). Developmental changes in source memory. Developmental Science, 5, 502–513. [Google Scholar]
- Erdfelder E, Auer T-S, Hilbig BE, Aßfalg A, Moshagen M, & Nadarevic L (2009). Multinomial processing tree models: A review of the literature. Journal of Psychology, 217, 108–124. [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, & Bunge SA (2010). Developmental differences in medial temporal lobe function during memory encoding. Journal of Neuroscience, 30, 9548–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, & Lee J (2011). Children’s episodic memory. Wiley Interdisciplinary Reviews: Cognitive Science, 2, 365–373. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, … Thompson PM (2006). Dynamic mapping of normal human hippocampal development. Hippocampus, 16, 664–672. [DOI] [PubMed] [Google Scholar]
- Halford GS, Wilson WH, & Phillips S (1998). Processing capacity defined by relational complexity: Implications for comparative, developmental, and cognitive psychology. Behavioral and Brain Sciences, 21, 803–865. [DOI] [PubMed] [Google Scholar]
- Humphreys MS, Bain JD, & Pike R (1989). Different ways to cue a coherent memory system: A theory for episodic, semantic, and procedural tasks. Psychological Review, 96, 208–233. [Google Scholar]
- Humphreys MS, Wiles J, & Dennis S (1994). Toward a theory of human memory: Data structures and access processes. Behavioral and Brain Sciences, 17, 655–666. [Google Scholar]
- Lunneborg CE (1999). Data analysis by resampling: Concepts and applications. Pacific Grove, CA: Duxbury Press. [Google Scholar]
- Moshagen M (2010). multiTree: A computer program for the analysis of multinomial processing tree models. Behavior Research Methods, 42, 42–54. [DOI] [PubMed] [Google Scholar]
- Newcombe NS, Lloyd ME, & Ratliff KR (2007). Development of episodic and autobiographical memory: A cognitive neuroscience perspective. In Kail RV (Ed.), Advances in child development and behavior (Vol. 35, pp. 37–85). San Diego, CA: Elsevier. [DOI] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, & Gabrieli JDE (2007). Development of the declarative memory system in the human brain. Nature Neuroscience, 10, 1198–1205. [DOI] [PubMed] [Google Scholar]
- Pipe M-E, & Salmon K (2009). Memory development and the forensic context. In Courage ML & Cowan N (Eds.), The development of memory in infancy and childhood (2nd ed., pp. 241–282). Hove, England: Psychology Press. [Google Scholar]
- Porter LW, & Duncan CP (1953). Negative transfer in verbal learning. Journal of Experimental Psychology, 46, 61–64. [DOI] [PubMed] [Google Scholar]
- Postman L (1964). Studies of learning to learn: II. Changes in transfer as a function of practice. Journal of Verbal Learning and Verbal Behavior, 3, 437–447. [Google Scholar]
- Richmond J, & Nelson CA (2007). Accounting for change in declarative memory: A cognitive neuroscience perspective. Developmental Review, 27, 349–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC (2000). The distribution of early childhood memories. Memory, 8, 265–269. [DOI] [PubMed] [Google Scholar]
- Schacter DL, & Tulving E (1994). Memory systems 1994 (1st ed.). Cambridge, MA: MIT Press. [Google Scholar]
- Sloutsky VM, & Fisher AV (2004). When development and learning decrease memory: Evidence against category-based induction in children. Psychological Science, 15, 553–558. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, & Kovacs SL (2006). Binding, relational memory, and recall of naturalistic events: A developmental perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition, 32, 89–100. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, & Ottinger W (2004). Changes in reality monitoring and episodic memory in early childhood. Developmental Science, 7, 225–245. [DOI] [PubMed] [Google Scholar]
- Smith JB, & Batchelder WH (2008). Assessing individual differences in categorical data. Psychonomic Bulletin & Review, 15, 713–731. [DOI] [PubMed] [Google Scholar]
- Sowell E, Thompson P, Leonard CM, Welcome S, Kan E, & Toga A (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24, 8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1972). Episodic and semantic memory. In Tulving E & Donaldson W (Eds.), Organization of memory (pp. 381–403). New York, NY: Academic Press. [Google Scholar]
- Wagenmakers E-J, & Farrell S (2004). AIC model selection using Akaike weights. Psychonomic Bulletin & Review, 11, 192–196. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, & Preston AR (2010). Flexible memories: Differential roles for medial temporal lobe and prefrontal cortex in cross-episode binding. Journal of Neuroscience, 30, 14676–14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


