Abstract
Background and aims
Compulsive sexual behavior disorder (CSBD) is characterized by persistent patterns of failure to control sexual impulses resulting in repetitive sexual behavior, pursued despite adverse consequences. Despite previous indications of addiction-like mechanisms and the recent impulse-control disorder classification in the International Classification of Diseases (ICD-11), the neurobiological processes underlying CSBD are unknown.
Methods
We designed and applied a behavioral paradigm aimed at disentangling processes related to anticipation and viewing of erotic stimuli. In 22 male CSBD patients (age: M = 38.7, SD = 11.7) and 20 healthy male controls (HC, age: M = 37.6, SD = 8.5), we measured behavioral responses and neural activity during functional magnetic resonance imaging (fMRI). The main outcomes were response time differences between erotic and non-erotic trials and ventral striatum (VS) activity during anticipation of visual stimuli. We related these outcomes with each other, to CSBD diagnosis, and symptom severity.
Results
We found robust case-control differences on behavioral level, where CSBD patients showed larger response time differences between erotic and non-erotic trials than HC. The task induced reliable main activations within each group. While we did not observe significant group differences in VS activity, VS activity during anticipation correlated with response time differences and self-ratings for anticipation of erotic stimuli.
Discussion and Conclusions
Our results support the validity and applicability of the developed task and suggest that CSBD is associated with altered behavioral correlates of anticipation, which were associated with ventral striatum activity during anticipation of erotic stimuli. This supports the idea that addiction-like mechanisms play a role in CSBD.
Keywords: compulsive sexual behavior disorder, hypersexual disorder, sex addiction, functional MRI, anticipation, sexual stimuli
Introduction
Compulsive sexual behavior disorder (CSBD) has been included in the International Statistical Classification of Diseases and Related Health Problems (ICD-11) (World Health Organization, 2019), listed in the subcategory of impulse-control disorders. According to ICD-11, CSBD is characterized by a persistent pattern of failure to control intense sexual impulses or urges resulting in repetitive sexual behavior, which is pursued despite adverse medical, psychological, and social consequences. The prevalence of CSBD symptoms is estimated to 3–10% of the general population (Blum, Badgaiyan, & Gold, 2015; Carnes et al., 2012; Derbyshire et al., 2015; Dickenson, Gleason, Coleman, & Miner, 2018; Estellon et al., 2012; Kafka, 2010; Kingston et al., 2013; Kor, Fogel, Reid, & Potenza, 2013; Kuhn et al., 2016; Weinstein, Katz, Eberhardt, Cohen, & Lejoyeux, 2015). Although some treatment options are available (Briken, 2020; Hallberg et al., 2019; 2020; Savard et al., 2020), they still warrant improvement to assure better long-term outcomes with high effectiveness.
Despite CSBD's inclusion in ICD-11, the neurobiological mechanisms underlying CSBD are still unknown (Derbyshire et al., 2015). There are ongoing debates about the ICD-11 classification of CSBD based on limited neurobiological findings (Fuss et al., 2019). Previous research suggests that similar mechanisms as found in obsessive compulsive disorder, substance use disorders, and behavioral addictions may play a role in CSBD. Impairments of brain regions regulating sexual desire and arousal have also been proposed (Blum et al., 2015; Carnes et al., 2012; Derbyshire et al., 2015; Estellon et al., 2012; Kafka, 2010; Kingston et al., 2013; Kor et al., 2013; Kraus, Voon, & Potenza, 2016; Kuhn et al., 2016; Weinstein et al., 2015). Recent neuroimaging studies have revealed that CSBD is associated with altered processing of sexual stimuli (Stark, Klucken, Potenza, Brand, & Strahler, 2018). A recent review concludes that CSBD is associated with aberrant functioning in brain regions implicated in habituation, impulse control, and reward processing (Kowalewska et al., 2018). Brain regions involved include prefrontal and temporal cortices, amygdala, and the ventral striatum (VS) (Gola et al., 2018; Kowalewska et al., 2018; Voon et al., 2014). Hence, the brain reward system seems to play an important role in CSBD (Kowalewska et al., 2018; Politis et al., 2013; Schmidt et al., 2017; Voon et al., 2014), and there is growing evidence for that key mechanisms overlap with those in substance and behavioral addictions (Gola et al., 2018; Kowalewska et al., 2018; Mechelmans et al., 2014). Therefore, it is still under dispute whether CSBD may be better classified as addictive behavior.
A key aspect in addiction is the impairment of the brain reward system leading to “excessive incentive salience”, or in other words an extreme “wanting” or desire for a reward. This leads to an intense urge to seek the reward, e.g., consume a drug. In line with this, individuals with substance use disorders show abnormal brain activity in the context of reward anticipation (Balodis et al., 2015), most consistently in the VS, which is a long established key region in reward anticipation processes (Jauhar et al., 2021; Oldham et al., 2018). However, functional magnetic resonance imaging (fMRI) studies that targeted anticipation processes in CSBD are scarce (Gola et al., 2018), and many conclusions about potential mechanisms have been derived from studies that investigated neural response to the simple viewing of sexual stimuli, omitting the investigation of stimuli anticipation.
Other limitations of previous fMRI studies include that control images do not sufficiently control for the processing of human body parts and social interactions. Furthermore, brain activity observed during processing of sexual stimuli can be confounded by general emotional arousal if not controlled for (Walter et al., 2008). Feelings of shame and guilt or trying to control sexual arousal during the experiment can be confounding. Long stimuli durations and the usage of block designs or videos make it difficult to determine which phases of the sexual response cycle are measured (Georgiadis et al., 2012; Markert, Klein, Strahler, Kruse, & Stark, 2021), hindering data interpretation. Most importantly, previous studies could not distinguish between brain activity related to anticipation and viewing of sexual stimuli. This distinction is however crucial to make claims about ‘addiction-like’ phenomena in CSBD (Gola, Wordecha, Marchewka, & Sescousse, 2016).
A task frequently used to measure reward anticipation-related brain activity is the well-validated monetary incentive delay task, which disentangles reward anticipation from reward receipt processes (Balodis et al., 2015; Knutson, Westdorp, Kaiser, & Hommer, 2000; Lutz et al., 2014). This is done by means of visual cues that predict the nature of a future reward. One study has used an incentive delay task in combination with visual sexual stimuli (Sescousse, Redouté, & Dreher, 2010), and using this task researchers have shown that problematic pornography consumption is associated with altered activation of VS activity in response to cues predicting erotic pictures, but not for cues predicting monetary rewards (Gola et al., 2017). To the best of our knowledge, this was the first study that quantified brain activity related to the anticipation of sexual stimuli in subjects with symptoms related to CSBD. However, monetary rewards were used as control trials, instead of non-sexual bodily (emotional) images. The anticipation cues were suggestive and contained – albeit sketched - sexual content, which may already activate networks involved in the processing of sexual stimuli (Gola et al., 2017). Notably, any symbolic differences in anticipation cues, including color and shape, can be confounding. Furthermore, image rating performed after presentation of each stimulus as part of the task can induce judgment related cognitive processes and affect neural activity during stimuli presentation (Walter et al., 2008).
The aim of the present study was two-fold. First, we aimed at overcoming task design limitations of previous paradigms. Therefore, we developed an incentive delay task, where visual sexual stimuli and bodily control images were carefully matched on various image characteristics. The task and data collection procedures were designed to avoid effects of order, conditioning, and anticipation cue symbols. Second, we aimed at applying the task in an fMRI experiment to test if CSBD is associated with both altered behavioral response and altered ventral striatum (VS) activity related to the anticipation of sexual stimuli.
We applied the fMRI paradigm in 22 CSBD patients and 20 healthy controls (HC) and tested two hypotheses: 1) we expected CSBD patients to show higher anticipation-driven motivation to view erotic rather than non-erotic images, reflected in corresponding response time differences, after correcting for age. 2): While we expected higher VS involvement during anticipation of erotic images compared to non-erotic images (erotic > non-erotic) in both groups, we also tested whether CSBD patients showed a larger VS response than HC. In this context, we also expected an inverse relationship between behavioral measures and VS activity during anticipation.
In secondary tests, using neurocognitive testing, we assessed objective measures of risk taking, inhibitory control, and non-verbal intelligence, which were related to CSBD diagnosis, behavioral, and fMRI outcomes. We also tested for potential confounding effects by demographic, clinical variables, and ratings of emotions during the task. Finally, we explored how desire, liking, and arousal ratings relate to the study outcomes.
Methods
Participants
The study was performed at Karolinska Institutet and at ANOVA, Karolinska University Hospital, Stockholm, Sweden. CSBD patients were recruited through the Swedish phone helpline PrevenTell (Adebahr, Söderström, Arver, Jokinen, & Öberg, 2021). More recruitment details, in- and exclusion criteria are provided in the Supplemental Materials and elsewhere (Hallberg et al., 2020; Savard et al., 2020). In brief, male patients who met criteria for CSBD according to ICD-11 were invited to participate. Healthy age- and sex-matched controls from the Stockholm catchment area were recruited through public and multi-media advertisements. Controls showed no indication of CSBD.
We enrolled 20 HC and 23 CSBD patients, of which 22 patients provided MRI data. All data were collected between May 2018 and December 2020.
Clinical characteristics and questionnaires
Through online questionnaires, we assessed depression symptom levels (Montgomery Asberg Depression Rating Scale (MADRS-S) (Montgomery et al., 1979; Svanborg et al., 2001)), attention deficit levels (Adult ADHD Self-Report Scale (ASRS) (Kessler et al., 2005), alcohol and drug consumption (Alcohol Use Disorders Identification Test (AUDIT) (Bergman et al., 2002); Drug Use Disorders Identification Test (DUDIT) (Berman, Bergman, Palmstierna, & Schlyter, 2005)), hypersexual symptoms (Hypersexual Disorder Screening Inventory (HDSI) (Parsons et al., 2013), Hypersexual Behavior Inventory (HBI) (Reid, Garos, & Carpenter, 2011)), sexual compulsivity (Sexual Compulsivity Scale (SCS) (Kalichman et al., 1995)), sexual inhibition/excitation scales (SIS/SES) (Carpenter, Janssen, Graham, Vorst, & Wicherts, 2008), anxiety levels (State-Trait Anxiety Inventory - State (STAI-S) (Tluczek, Henriques, & Brown, 2009)), autism spectrum disorder symptoms (Ritvo Autism Asperger Diagnostic Scale (RAADS-14) (Eriksson, Andersen, & Bejerot, 2013)), sexual desire (Sexual Desire Inventory (SDI) (Spector, Carey, & Steinberg, 1996)), general impulsivity (Barratt Impulsiveness Scale (BIS-11) (Stanford et al., 2009)), and behavioral inhibition (Behavioral Inhibition/Activation System (BIS/BAS) (Carver et al., 1994)). We assessed the frequency of internet pornography consumption and sexual encounters within the last 6 months, as well as sexual orientation (7-point Kinsey scale) (Kinsey, Pomeroy, & Martin, 1948). The latter ranged from 0-6 with 0 defined as ‘exclusively heterosexual’ and 6 ‘exclusively homosexual’.
Neurocognitive testing
We administered neuropsychological tests to obtain objective estimates of impulsivity/risk taking (Balloon Analogue Risk Task, BART (Lejuez et al., 2002)), inhibitory/impulse control (Stop Signal Task, STOP-IT (Verbruggen, Logan, & Stevens, 2008)), and non-verbal intelligence (Ravens Standard Progressive Matrices, SPM (Raven et al., 2000)). SPM classifies a person's performance into grade I (lowest) to V (highest). Higher Stop-Signal Reaction Time (SSRT) obtained from the STOP-IT indicates lower inhibitory control. Measures for risk taking obtained from BART were the adjusted number of balloons and number of explosions (Lejuez et al., 2002), where higher scores indicate more risk-taking behavior.
fMRI paradigm and stimuli
A detailed description of the fMRI paradigm is presented in the Supplemental Materials. Figure 1 shows a scheme of the paradigm. In brief, the task design was based on the frequently used monetary incentive delay (MID) task (Knutson et al., 2000) and the incentive delay task used by Gola and colleagues (Gola et al., 2017). The total number of trials was n = 80 (40 erotic and 40 non-erotic trials). Picture stimuli were obtained from the International Affective Picture System (IAPS) (Lang, Bradley, & Cuthbert, 2008) and the Nencki Affective Picture System (NAPS) (Marchewka, Zurawski, Jednorog, & Grabowska, 2014; Wierzba et al., 2015). Stimuli from both databases have been validated and shown to induce significant levels of sexual arousal in various previous studies (Gola et al., 2016; Marchewka et al., 2014; Politis et al., 2013; Walter et al., 2008; Wierzba et al., 2015). Erotic and non-erotic control stimuli were carefully matched with respect to valence and arousal ratings, and other image features. Since participants were included regardless of their sexual orientation, we created two versions of the paradigm that erotic stimuli could be matched to participants' preference. More details on the stimuli characteristics are provided in the Supplemental Materials.
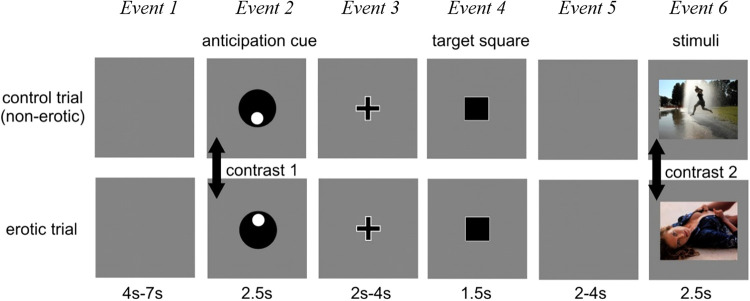
Fig. 1.
Schematic representation of the sexual incentive delay fMRI task. Two example trials of non-erotic control trials (top) and erotic trials (bottom) are shown. The total number of trials was n = 80 (40 for each trial type) acquired in two sessions. Each session contained 20 erotic and 20 non-erotic control trials. Total task duration was approximately 24 min. The trial order was pseudo-randomized. Event durations are indicated. Event 1 grey screen (determined the inter-trial interval): random duration between 4 and 7 s. Event 2 was the anticipation phase presenting a cue symbol that indicated the type of trial, i.e., the future presentation of either an “erotic” or a “non-erotic” image (main event of interest). The meaning of each symbol was explained to the participants outside the scanner, who also performed a short practice session prior to the experiment. Event 3 (fixation cross) indicated task preparation. Event 4 target square: task requires button press. Participants were instructed to press a button as quick as possible when the square appeared and if they would respond fast enough the outcome image will be presented. The button-press task was included to keep participants alert and to assess reaction times as a proxy measure for ‘motivation to win’. Fail rate was fixed to 20%, where an image of noise was presented instead as visual stimuli (see Supplemental Materials for more details on task design). Event 5 grey screen: waiting period (random duration). In event 6, the image corresponding to the trial type was presented, i.e., either an erotic or non-erotic visual stimulus (secondary event of interest). Acquisition procedure was designed to avoid potential order effects, effects induced by symbol rotation, and habituation/conditioning effects (see Supplemental Materials). Jittering (random presentation times) of inter-stimuli durations was applied to disentangle reward anticipation from receipt or button press-related brain activation.
Two contrasts were compared between CSBD patients and controls: Contrast 1 (main): Difference in brain activation between erotic and non-erotic trials during anticipation phase (event 2). Contrast 2 (secondary): Difference in brain activation between erotic and non-erotic trials during image presentation (event 6).
fMRI-experiment related questionnaires
Before and after MRI scan, participants were asked to rate their cravings/desires for different items (including sexual desire). Before the experiment, participants were asked how much they are looking forward to the viewing of non-erotic and erotic images. This was the primary rating of interest, as it directly relates to anticipation. After the experiment, participants were asked to provide ratings of valence and arousal induced by visual stimuli. Additional questions focused on factors that could potentially have confounding effects on brain activity during the experiment, such as the experienced feelings of shame, guilt, and how much a participant tried to control sexual arousal. See Supplemental Materials for more information on fMRI-related questionnaires.
Magnetic resonance imaging
Acquisition
MRI scans were performed on a 3T GE scanner (Discovery MR750) equipped with an eight-channel head coil. fMRI data was acquired with a 2D gradient-echo EPI sequence and T1-weighted images were acquired using a 3D-BRAVO sequence. In addition to the fMRI scan, a T1-weighted scan was performed and used for co-registration of fMRI data. Imaging parameters are provided in the Supplemental Materials.
Processing
Details on fMRI processing and analyses are provided in the Supplemental Materials. In brief, using the FSL 6.0.1 software suite, whole brain mean activation maps (Contrast Of Parameter Estimates: COPE) for the effect of interest (erotic > non-erotic events) were computed for both the anticipation (main contrast 1, Fig. 1) and viewing phase (contrast 2). These were used to investigate task-related mean activation within-groups and between-group differences (contrast of interest: CSBD > HC).
While whole-brain group comparisons were exploratory, our primary aim was to test for group differences in VS activity during anticipation. Therefore, we extracted the mean COPE values during the anticipation phase (and viewing phase as control) from the VS (Figure S7) (Tziortzi et al., 2011). These measures were analyzed in SPSS with respect to case-control differences, sensitivity analyses for potential confounding, and correlations with behavioral outcomes (ΔRT) and CSBD symptoms (see below).
Statistical analyses
Group characteristics (demographic, clinical, and cognitive data)
Group characteristics in demographic and clinical variables listed in Table 1 were compared using t-tests or Fisher's exact/Chi2. Group comparisons in risk taking and SSRT were conducted using a univariate test of covariance (ANCOVA), while correcting for age, in SPSS v26.
Table 1.
Demographics and clinical characteristics
| Measure | HC (n = 20) | CSBD (n = 23) | HC vs. CSBD (P-value) |
| Age, mean (SD) | 37.6 (8.5) | 38.7 (11.7) | 0.741 |
| BMI, mean (SD) | 23.1 (2.8) | 25.8 (4.5) | 0.026 |
| nicotine use (yes/no/sometimes), n | |||
| Moist snuff | 3/16/0* | 7/13/0* | 0.157 |
| Smoking | 0/16/4 | 0/21/0* | 0.048 |
| Handedness (R/L/M), n | 16/4/0 | 16/1/1* | 0.822 |
| Sexual orientation | |||
| Self-identified homosexual, n | 1 | 1 | 0.919 |
| Kinsey scale, mean (SD) | 0.6 (1.1) | 0.71 (1.3) | 0.778 |
| HDSI, mean (SD) | 1.9 (2.2) | 20.2 (3.8) | <0.001 |
| HBI, mean (SD) | 22.5 (4.1) | 69.4 (13.4) | <0.001 |
| SDI, mean (SD) | 55.2 (12.6) | 80.6 (17.1) | <0.001 |
| SCS, mean (SD) | 11.2 (0.9) | 29.4 (6.3) | <0.001 |
| Pornography consumption | |||
| times per week, mean (SD) | 2.2 (2.3) | 13.0 (20.7) | 0.033 |
| hours per week, mean (SD) | 0.7 (0.7) | 9.2 (8.0) | <0.001 |
| age at first consumption, mean (SD) | 14.2 (3.4) | 13.2 (4.9) | 0.424 |
| MADRS, mean (SD) | 3.9 (4.9) | 18.3 (7.8) | <0.001 |
| AUDIT, mean (SD) | 4.1 (3.8) | 6.3 (3.8) | 0.059 |
| DUDIT, mean (SD) | 2.7 (4.5) | 2.1 (3.0) | 0.582 |
| RAADS, mean (SD) | 6.1 (6.0) | 11.1 (7.7) | 0.025 |
| ASRS, mean (SD) | 14.7 (10.6) | 34.2 (11.7) | <0.001 |
| BIS-11, mean (SD) | 53.1 (7.3) | 66.7 (10.8) | <0.001 |
| BIS/BAS | |||
| BAS drive, mean (SD) | 7.4 (2.3) | 9.0 (2.7) | 0.048 |
| BAS fun seeking, mean (SD) | 10.5 (2.5) | 11.9 (1.7) | 0.037 |
| BAS reward response, mean (SD) | 16.3 (2.1) | 16.5 (1.6) | 0.726 |
| BIS, mean (SD) | 17.9 (5.1) | 20.7 (3.1) | 0.033 |
| STAI-S, mean (SD) | 9.3 (2.0) | 12.6 (2.5) | <0.001 |
Demographic and clinical characteristics (mean (SD) or number of participants n) of both groups and corresponding results (P-values) of group comparisons are presented. Note, data reported for all patients enrolled. Sexual orientation was measured through self-identification and on a 7-point Kinsey scale. * indicates variables with missing data.
Incentive delay reaction times from fMRI task
Differences between mean reaction times during erotic (RTE) and non-erotic trials (RTN) - the behavioral equivalent of fMRI contrasts - was expected to differ between CSBD patients and controls, as we hypothesized faster RTE in CSBD patients. Using a repeated measures ANCOVA, we tested for the effects trial type (erotic vs. non-erotic), group (CSBD vs HC), and trial type-by-group interaction on RT, while correcting for age. Age correction was performed to account for potential age-related variance in the data given that adult human response times slow with age. We followed up by computing ΔRT = RTE–RTN for each participant and comparing ΔRT between groups using ANCOVA, while correcting for age. We further explored whether ΔRT correlated with CSBD symptom scores, including pornography consumption measures. Given the small sample size and the fact that symptom scores are commonly skewed, we computed non-parametric Spearman rank correlations.
VS activation analyses
VS mean activation during anticipation was compared between groups using ANCOVA, while correcting for age (SPSS). We further tested whether VS activity during anticipation correlated with its behavioral equivalent ΔRT, and explored its relationship with CSBD symptom severity and pornography consumption measures (Spearman correlations) in the combined cohort. The rationale was to identify genuine associations between VS and ΔRT/CSBD symptoms regardless of categorical diagnostic label and to increase both score variance and statistical power. VS activation for contrast 2 was analyzed similarly for interpretational purpose. In further secondary regression analyses, we investigated the relationship between VS activation during anticipation and the main pre-fMRI rating of interest ‘looking forward to viewing erotic images’ rating scores (Supplemental Materials).
Sensitivity analyses
For both, VS activity and ΔRT we repeated group comparisons to test for potential confounding by demographic, clinical, desire/image rating, and neurocognitive variables. Detailed methodology, list of variables tested, and results of these tests are provided in the Supplemental Materials (Table S8).
Ethics
The study procedures were carried out in accordance with the Declaration of Helsinki. The study was approved by the regional Ethical Review Board, Stockholm, Sweden. All participants provided written informed consent.
Results
Participants
Cohort characteristics are presented in Table 1. Groups matched on age (CSBD: M = 38.7, SD = 11.7, HC: M = 37.6, SD = 8.5) and sexual orientation (one self-identified homosexual in each group). CSBD patients had higher BMI than HC (CSBD: M = 25.8, SD = 4.5, HC: M = 23.1, SD = 2.8), albeit still in the normal range. HC contained four occasional smokers. There were no group differences in medication use or psychiatric comorbidity (Table S1). Compared with HC, CSBD patients scored significantly higher on scales assessing hypersexuality symptoms, sexual compulsivity and desire (HDSI, HBI, SDI, SCS), depression levels (MADRS), attention deficits (ASRS), autism symptoms (RAADS), anxiety (STAI-S), impulsivity and behavioral inhibition (BIS-11, BIS), but not reward response (BAS). CSBD patients consumed more pornography than HC. There were no group differences in drug and alcohol consumption or number of sexual encounters or partners (Table S2).
Incentive delay reaction times obtained from fMRI task
Repeated measures ANCOVA revealed a significant effect of trial type (P = 0.005, F 1, 39 = 9.0) and trial-by-group interaction (P = 0.009, F 1, 39 = 7.5). Main effects of age and group were not significant, (P = 0.737 and P = 0.867). Follow-up tests of the main effect of trial type revealed that in the combined group participants reacted significantly faster during erotic compared with non-erotic trials (RTE < RTN). Paired t-test comparing RTE and RTN within each group showed that this was the case both in patients (P < 0.001) and controls (P = 0.004). ΔRT (RTE–RTN) was negative in both groups and significantly differed between CSDB and HC (P = 0.009, d = 0.84), where CSBD patients showed larger ΔRT, confirming the observed trial-by-group interaction (displayed in Fig. 2). This difference may have been driven by slightly lower RTE and larger RTN means in CSBD compared with HC (Fig. 2, Table 2).
Fig. 2.
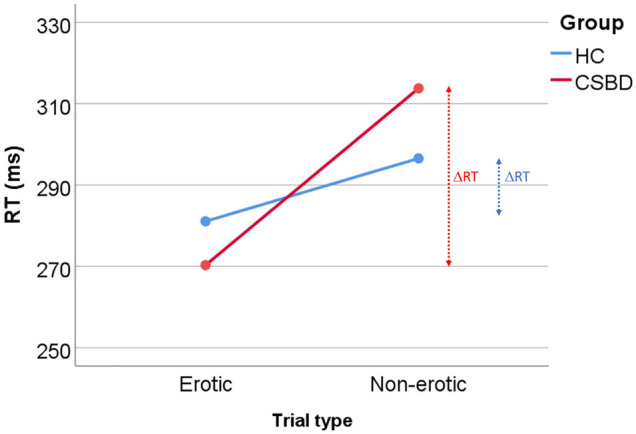
Behavioral results from the sexual incentive delay task performed during fMRI. The scheme demonstrated the observed trial-by-group interaction and corresponding ΔRT differences. Mean reaction time for each trial type (erotic vs. non-erotic) and group (HC vs. CSBD) are shown. ΔRT for each group is indicated (vertical arrows). Numerical values are listed in Table 2
Table 2.
Neurocognitive test results
| Cognitive Tests | HC (n = 20) | CSBD (n = 23) | HC vs. CSBD; P |
| Sexual incentive delay task (fMRI) in ms* | |||
| RT E , mean (SD) | 281 (65) | 270 (46) | 0.544 |
| RT N , mean (SD) | 297 (72) | 314 (68) | 0.434 |
| ΔRT, mean (SD) | −15 (22) | −43 (42) | 0.009 |
| SSRT in ms, mean (SD) | 285 (30) | 300 (59) | 0.324 |
| BART | |||
| Adj. pumps, mean (SD) | 10.1 (5) | 11.1 (4.8) | 0.486 |
| Nr. explosions, mean (SD) | 13.6 (4.8) | 14.3 (4.4) | 0.664 |
| Raven SPM | |||
| Mean (SD) | 2.3 (1.0) | 2.9 (0.8) | 0.041 |
| Grade I, n | 4 | 1 | 0.042 |
| Grade II, n | 9 | 6 | |
| Grade III (average), n | 4 | 11 | |
| Grade IV, n | 1 | 5 | |
| Grade V, n | 1 | 0 | |
Results obtained from cognitive testing are shown. Means and standard deviations (SD) of each group are listed. Results of group comparisons (P-values) are provided. BART: Balloon Analogue Risk Task, SSRT: Stop-Signal Reaction Time (inhibitory/impulse control), Raven SPM: Raven standard progressive matrices (non-verbal intelligence). Outcome measures from the sexual incentive delay task performed during fMRI are listed as well: RTE: average reaction time during erotic trials, RTN: average reaction time during non-erotic trials. ΔRT = RTE−RTN. *one CSBD patient did not perform the fMRI task.
ΔRT correlated negatively with hypersexuality symptoms and sexual compulsivity (HDSI, HBI, SCS) (Table S9), and with drive and reward response items of BIS/BAS (Table S14).
Exploratory tests revealed that the CSBD group displayed larger RT variability (standard deviation) during non-erotic trials (SDN) than in erotic trials (SDE), which was not observed in HC (Supplemental Materials; Table S3), indicating that the group differences in ΔRT were likely influenced by CSBD patients performing worse (or less consistent) during non-erotic trials than HC, rather than performing better during erotic trials.
Neurocognitive testing
There were no group differences in performance on the BART (risk taking) or STOP-IT (SSRT, inhibitory/impulse control). HC performed better on the Raven SPM test (non-verbal intelligence) than CSBD patients. However, CSBD patients showed an average performance, while HC performed above average (Table 2).
Task-related activity (fMRI)
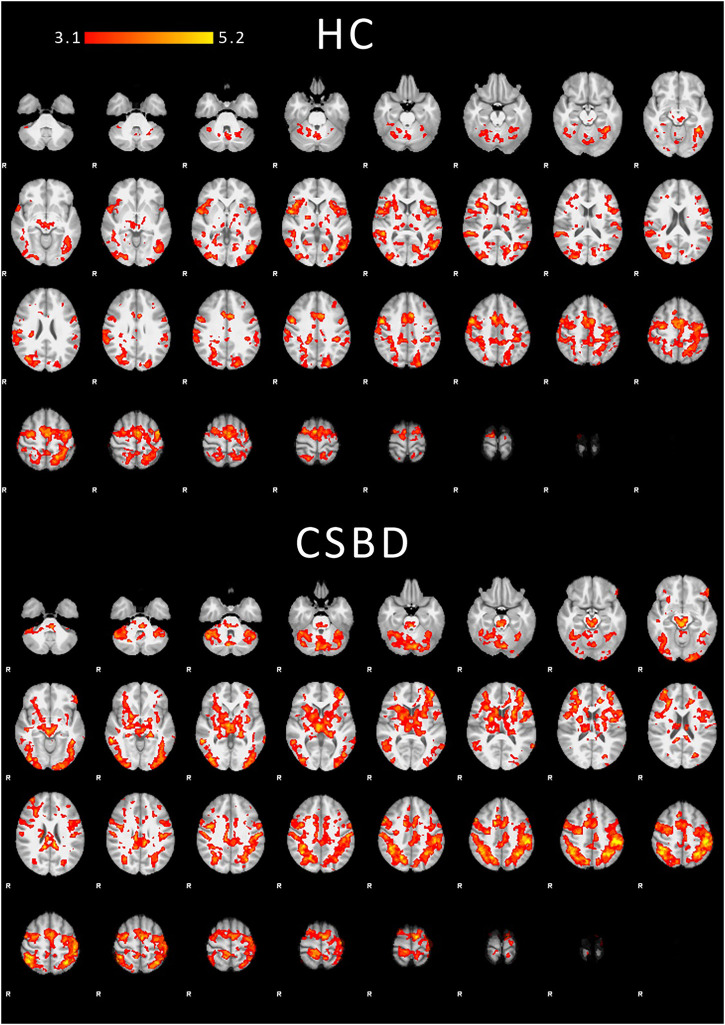
Within-group task-related mean activations during anticipation are shown in Fig. 3. Results for the viewing phase are shown in the Supplemental Materials (Figures S4–S5). Corresponding activations comprised regions previously reported during anticipation and processing of visual sexual stimuli, respectively, including VS, anterior cingulate cortex, orbitofrontal cortex, insula, (pre)motor, visual, and occipitotemporal regions (Georgiadis et al., 2012; Jauhar et al., 2021; Oldham et al., 2018). On whole-brain level (exploratory), no group differences were observed after correction. See Figure S3 and S6 for uncorrected results.
Fig. 3.
Within-group task-related fMRI mean activations. Corrected COPE mean activations (erotic > non-erotic) for contrast 1 (anticipation) are displayed for both healthy controls (HC, top) and CSBD patients (bottom). Z values are indicated by color (heat map). Although there are visual regional differences in activation patterns between HC and CSBD, direct group comparisons were not significant after correction (same applied to the reversed contrast HC > CSBD). Note that whole brain analyses were exploratory. Results for contrast 2 (viewing phase) and uncorrected group comparisons at a threshold of P = 0.01 are shown in the Supplemental Materials (Figure S3–S6). Cluster statistics, MNI coordinates of activation maxima, and regional labels are provided in the Supplemental Materials Table S10 and S12
VS activation and correlations with ΔRT and CSBD symptoms
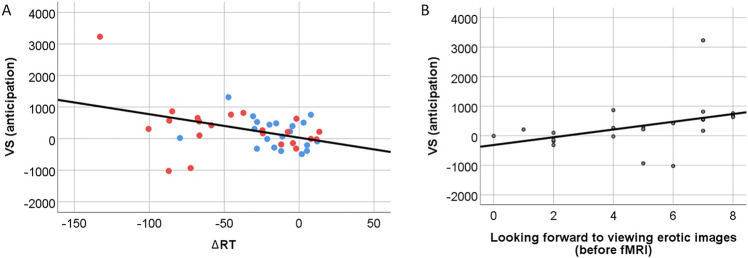
There were no significant group differences in VS mean activation during anticipation (or viewing phase, Table 3). However, VS activity during anticipation correlated negatively with ΔRT (r = −0.33, P = 0.031), whereas ΔRT did not correlate with VS activation during the viewing phase (r = 0.18, P = 0.250). There was one visual outlier with low ΔRT and high VS activity during anticipation (Fig. 4). The correlations between ΔRT and VS activity during anticipation was still suggestive (P = 0.072) after removing this outlier (Figure S2, Table S10), and the directionality and effect strength remained (r = −0.28). Note that we could not identify reasons that justified removing the outlier from analyses (no erroneous data). Among all participant, this subject scored the highest on all CSBD symptoms scores (indicated by multivariate outlier analyses; Supplemental Materials). Further, a non-parametric Spearman rank correlation was applied, which is, compared to a conventional Pearson correlation, less sensitive to outliers. Hence, all performed tests deem the results including the outlier reliable.
Table 3.
Group comparisons in VS mean activation
| HC (n = 20) | CSBD (n = 22) | HC vs. CSBD; P | Cohen's d | |
| VS activity (contrast 1: anticipation) | 173 (471) | 329 (819) | 0.457 | 0.20 |
| VS activity (contrast 2: viewing) | 181 (481) | 69 (700) | 0.54 | 0.19 |
Mean (SD) of COPE activation extracted for VS during contrast 1 (anticipation) and 2 (viewing phase) are listed for each group. Results (P-values) and effect size (Cohen's d) of group comparisons are provided (HC vs. CSBD).
Fig. 4.
A: Correlation between VS activation during anticipation and ΔRT. Patient data is plotted in red, HC data in blue. Supplementary Figure S2 shows the regression plot when excluding the outlier with highest VS and lowest ΔRT. Note that we deem results including the outlier reliable (see main text and Supplemental Materials for reasoning). B: Correlation between VS activity during anticipation phase and rating of how much CSBD patients reported to look forward to viewing erotic images (asked before fMRI experiment) (r = 0.61, P = 0.002). Such correlation was not observed in controls (r = −0.221, P = 0.362; see Supplemental Materials for more details)
Finally, VS activation during anticipation, but not VS activation during viewing phase, correlated with pornography consumption measures (Table S9), but not with other CSBD symptom scores.
Desire, liking, and other emotional responses during the fMRI task
Detailed results of the fMRI experiment-related questionnaires can be found in the Supplemental Materials (Table S4–S6). In brief, CSBD patients desired to engage in sexual activity more than HC, and this desire increased after the experiment in both groups. Although there were no group differences with respect how much participants liked the stimuli, CSBD patients looked significantly more forward to viewing erotic images than non-erotic images. This was not observed in HC. In CSBD patients, not in HC, VS activity during anticipation correlated positively with the ‘looking forward to erotic images’ rating (r = 0.61, P = 0.002; Fig. 4). Such correlations with ΔRT were suggestive (Supplemental Materials).
Sensitivity analyses
The results remained robust when controlling for potential confounders (Table S8) with the exception that group differences in ΔRT were not significant when controlling for depression ratings (MDRS). This result should, however, be interpreted with caution, as depression relates to CSBD, the phenotype of interest (Ballester-Arnal, Castro-Calvo, Giménez-García, Gil-Juliá, & Gil-Llario, 2020; Hyatt et al., 2020).
Discussion
In this study, we applied a new experimental fMRI paradigm aimed at separating processes related to anticipation from those related to processing of visual sexual stimuli. The task was used to investigate behavioral and neural correlates of CSBD with a focus on VS activity during anticipation. We further tested how CSBD symptoms and objective measures of risk taking, inhibitory control, and non-verbal intelligence related to our outcomes.
Behavioral differences between HC and CSBD
In line with our hypothesis, CSDB patients showed larger differences between reaction times measured during erotic and non-erotic trials (ΔRT) than HC. The effect size was large (d = 0.84). The results remained robust when correcting for potential confounder variables and indicate potential differences in motivational drive – and potentially desire - to view erotic or non-erotic images. The differences seemed to be driven by CSBD patients showing slower mean reaction times and larger performance variability during non-erotic trials, indicating less motivation/desire to view non-erotic images compared with HC. Note that this does not exclude the possibility for higher motivational drive or desire in CSBD patients towards viewing erotic images (indicated by lower mean RTE) compared to HC, as there are physical limitations to motor response speed. Importantly, these behavioral differences suggest that processes involving the anticipation of erotic and non-erotic stimuli may be altered in CSBD and support the idea that reward anticipation-related mechanisms similar to those in substance use disorders and behavioral addictions may play an important role in CSBD, as previously suggested (Chatzittofis et al., 2016; Gola et al., 2018; Jokinen et al., 2017; Kowalewska et al., 2018; Mechelmans et al., 2014; Politis et al., 2013; Schmidt et al., 2017; Sinke et al., 2020; Voon et al., 2014). This was further supported by the fact that we did not observe differences in other cognitive tasks measuring risk taking and impulse control, opposing the idea that general compulsivity-related mechanisms are at play (Norman et al., 2019; Mar, Townes, Pechlivanoglou, Arnold, & Schachar, 2022). Intriguingly, the behavioral measure ΔRT correlated negatively with hypersexuality symptoms and sexual compulsivity, indicating that anticipation-related behavioral alterations increase along with CSBD symptom severity.
Sexual incentive delay task-related brain activity
Within each group, the task induced explicit region-specific activations both during anticipation and viewing phases (Fig. 3). Mean activations comprised regions previously reported during both anticipation and processing of visual sexual stimuli, including activations in VS, anterior cingulate cortex, orbitofrontal cortex, insula, (pre)motor, visual, and occipitotemporal regions (Georgiadis et al., 2012; Jauhar et al., 2021; Oldham et al., 2018), supporting the specificity, validity, and applicability of the task. This was further supported by the fact that performing the task increased sexual desire, while desires for other assessed items did not increase after the experiment, indicating that the task specifically targeted sexual desire.
Although clear regional activation differences were observed in HC and CSBD patients during the anticipation phase (Fig. 3), where, compared with HC, CSBD patients showed more pronounced activations in prefrontal cortex and subcortical regions, including VS, we did not find significant group differences on whole-brain level. Note that whole-brain analyses were exploratory, and larger samples may be required to identify small effects. Hence, from these findings it should not be concluded that CSBD is not associated with functional brain abnormalities during anticipation, especially, since correlational analyses as discussed below point to the opposite.
Main analysis on VS activity during anticipation
Although numerical differences were as expected (CSBD > HC), the effect size was small and there were no significant group differences in VS mean activation during anticipation. Also here, larger samples may be required to capture task-based case-control differences in VS activation. However, VS activity during anticipation correlated negatively with ΔRT (moderate correlation), whereas ΔRT did not correlate with VS activation during the viewing phase. Hence, the larger the behavioral differences between erotic and non-erotic trials, the larger the VS mean activity during anticipation (note that also here erotic vs. non-erotic trials were contrasted). Since the behavioral response could directly be linked to VS activity during anticipation, but not viewing, of images, we suggest that differential neural responses related to anticipation may in fact explain the behavioral abnormalities observed in CSBD. In line with this notion, compared with HC, CSBD patients looked significantly more forward to viewing erotic than non-erotic images, and VS activity during anticipation correlated with ratings on how much patients were looking forward to the viewing of erotic images prior to the experiment.
In summary, the observed behavioral group differences and the fact that VS activity during anticipation related to both objective (ΔRT) and self-rated measures of anticipation were in line with our hypothesis that excessive incentive salience and related neural processes of reward anticipation play a role in CSBD.
Limitations
First, conclusions about causality cannot be drawn, as this study was cross-sectional. Second, since group differences in neural activity during anticipation may be of small effect size (here d = 0.2), or potentially non-existent, larger study samples may be required to detect this. Third, there are scientific debates around whether CSBD symptoms may result from coping mechanisms compensating for unpleasant affective states (e.g., depression) or if depressive mood states result from distress caused by CSBD. While both mechanisms may contribute, they cannot be disentangled in this study. However, it is well-known that depression and CSBD are highly correlated (Antons et al., 2021), thus, our study cohort represented an ecologically valid clinical sample of patients with CSBD. Fourth, the frequency of sexual encounters did not differ between groups. CSBD patients, however, showed more frequent pornography consumption often observed in CSBD (Antons et al., 2021). In addition, we found a correlation between VS activity during anticipation and pornography consumption measures. While a previous study by Markert et al. did not find such correlations in healthy individuals, the authors stated that such associations may be observed in samples with increased levels of pornography use (Markert et al., 2021), which may explain why we were able to detect these relationships in the present study. Hence, our findings are in line with studies suggesting that problematic pornography consumption is associated with altered VS activity during visual cues predicting erotic pictures (Gola et al., 2017). Although sexual behavior outcomes may have been different if some participants would not have been recruited during the COVID-19 pandemic, it remains to be investigated whether our results are more generalizable to CSBD subgroups with high-frequency pornography use. Notably, the identification of clinical subgroups was not the aim of the present study, but we suggest that it should be considered in future research. Finally, we have used a low and fixed fail rate in the fMRI task to maximize anticipatory effects and enhance data homogeneity. Although we provided explanations for unexpected outcomes and there was no indication that participants suspected predetermined fails, it remains unknown how participants would have performed using an adaptive paradigm.
Conclusion
The developed fMRI paradigm overcomes several limitations of previous paradigms, and our results support its applicability in healthy and in clinical cohorts. Our findings suggest that CSBD is associated with altered behavioral correlates of anticipation, which further related to VS activity during anticipation of erotic stimuli. The findings support the idea that mechanisms similar as in substance and behavioral addictions play a role in CSBD and suggest that the classification of CSBD as an impulse-control disorder may be arguable on the basis of neurobiological findings.
Funding sources
This work was supported by Karolinska Institutet's Research Foundation Grants (2016 and 2017; CA) and the Swedish Research Council (Dnr: 2020-01183; JJ, CA).
Authors’ contribution
CA was principal investigator, designed the study and developed the fMRI paradigm. CA collected fMRI and behavioral data, performed behavioral analyses and wrote the first draft of the manuscript. BL performed fMRI processing and fMRI analyses. KJÖ, SA, CD, and MI contributed to study design and with clinical advice. BL, KJÖ, JJ, JS, and JF provided important intellectual input and contributed to manuscript writing. JS recruited and screened patients for eligibility and contributed to data collection. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have reviewed the manuscript, provided intellectual input, and approved the submission of the manuscript.
Conflict of interest
CA is employed by Quantify Research (consultancy work unrelated to the present work). The authors report no financial or other relationship relevant to the subject of this article.
Acknowledgements
We thank the study nurses, medical, and administrative staff at ANOVA for their support in data collection and study organization, Christoffer Rahm for discussions during the study design phase, and Christian Mannfolk for his help in the recruitment of HC participants.
fMRI task availability statement
The fMRI task can be made available upon reasonable request.
Supplementary Materials
Contributor Information
Benny Liberg, Email: benny.liberg@ki.se.
Katarina Görts-Öberg, Email: katarina.gorts-oberg@regionstockholm.se.
Jussi Jokinen, Email: jussi.jokinen@ki.se.
Josephine Savard, Email: josephine.savard@regionstockholm.se.
Cecilia Dhejne, Email: cecilia.dhejne@regionstockholm.se.
Stefan Arver, Email: stefan.arver@ki.se.
Johannes Fuss, Email: Johannes.fuss@uni-due.de.
Martin Ingvar, Email: martin.ingvar@ki.se.
Christoph Abé, Email: christoph.abe@ki.se.
References
- Adebahr, R. , Söderström, E. Z. , Arver, S. , Jokinen, J. , & Öberg, K. G. (2021). Reaching men and women at risk of committing sexual offences - findings from the National Swedish telephone helpline PrevenTell. The Journal of Sexual Medicine , 18(9), 1571–1581. 10.1016/j.jsxm.2021.06.008. [DOI] [PubMed] [Google Scholar]
- Antons, S. , & Brand, M. (2021). Diagnostic and classification considerations related to compulsive sexual behavior disorder and problematic pornography use. Current Addiction Reports , 8(3), 452–457. 10.1007/s40429-021-00383-7. [DOI] [Google Scholar]
- Ballester-Arnal, R. , Castro-Calvo, J. , Giménez-García, C. , Gil-Juliá, B. , & Gil-Llario, M. D. (2020). Psychiatric comorbidity in compulsive sexual behavior disorder (CSBD). Addictive Behaviors , 107, 106384. 10.1016/j.addbeh.2020.106384. [DOI] [PubMed] [Google Scholar]
- Balodis, I. M. , & Potenza, M. N. (2015). Anticipatory reward processing in addicted populations: A focus on the monetary incentive delay task. Biological Psychiatry , 77(5), 434–444. 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman, H. , & Källmén, H. (2002). Alcohol use among Swedes and a psychometric evaluation of the alcohol use disorders identification test. Alcohol and Alcoholism , 37(3), 245–251. 10.1093/alcalc/37.3.245. [DOI] [PubMed] [Google Scholar]
- Berman, A. H. , Bergman, H. , Palmstierna, T. , & Schlyter, F. (2005). Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. European Addiction Research , 11(1), 22–31. 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- Blum, K. , Badgaiyan, R. D. , & Gold, M. S. (2015). Hypersexuality addiction and withdrawal: Phenomenology, neurogenetics and epigenetics. Cureus , 7(10), e348. 10.7759/cureus.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briken, P. (2020). An integrated model to assess and treat compulsive sexual behaviour disorder. Nature Reviews. Urology , 17(7), 391–406. 10.1038/s41585-020-0343-7. [DOI] [PubMed] [Google Scholar]
- Carnes, P. J. , Green, B. A. , Merlo, L. J. , Polles, A. , Carnes, S. , & Gold, M. S. (2012). Pathos: A brief screening application for assessing sexual addiction. Journal of Addiction Medicine , 6(1), 29–34. 10.1097/ADM.0b013e3182251a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, D. , Janssen, E. , Graham, C. , Vorst, H. , & Wicherts, J. (2008). Women's scores on the sexual inhibition/sexual excitation scales (SIS/SES): Gender similarities and differences. Journal of Sex Research , 45(1), 36–48. 10.1080/00224490701808076. [DOI] [PubMed] [Google Scholar]
- Carver, C. S. , & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology , 67(2), 319–333. 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Chatzittofis, A. , Arver, S. , Oberg, K. , Hallberg, J. , Nordstrom, P. , & Jokinen, J. (2016). HPA axis dysregulation in men with hypersexual disorder. Psychoneuroendocrinology , 63, 247–253. 10.1016/j.psyneuen.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Derbyshire, K. L. , & Grant, J. E. (2015). Compulsive sexual behavior: A review of the literature. Journal of Behavioral Addictions , 4(2), 37–43. 10.1556/2006.4.2015.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson, J. A. , Gleason, N. , Coleman, E. , & Miner, M. H. (2018). Prevalence of distress associated with difficulty controlling sexual urges, feelings, and behaviors in the United States. JAMA Network Open , 1(7), e184468. 10.1001/jamanetworkopen.2018.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, J. M. , Andersen, L. M. , & Bejerot, S. (2013). RAADS-14 screen: Validity of a screening tool for autism spectrum disorder in an adult psychiatric population. Molecular Autism , 4(1), 49. 10.1186/2040-2392-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estellon, V. , & Mouras, H. (2012). Sexual addiction: Insights from psychoanalysis and functional neuroimaging. Socioaffective Neuroscience & Psychology , 2, 11814. 10.3402/snp.v2i0.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss, J. , Lemay, K. , Stein, D. J. , Briken, P. , Jakob, R. , Reed, G. M. , & Kogan, C. S. (2019). Public stakeholders' comments on ICD-11 chapters related to mental and sexual health. World Psychiatry , 18(2), 233–235. 10.1002/wps.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis, J. R. , & Kringelbach, M. L. (2012). The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Progress in Neurobiology , 98(1), 49–81. 10.1016/j.pneurobio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Gola, M. , & Draps, M. (2018). Ventral striatal reactivity in compulsive sexual behaviors. Frontiers in Psychiatry , 9, 546–546. 10.3389/fpsyt.2018.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola, M. , Wordecha, M. , Marchewka, A. , & Sescousse, G. (2016). Visual sexual stimuli-cue or reward? A perspective for interpreting brain imaging findings on human sexual behaviors. Frontiers in Human Neuroscience , 10, 402. 10.3389/fnhum.2016.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola, M. , Wordecha, M. , Sescousse, G. , Lew-Starowicz, M. , Kossowski, B. , Wypych, M. , … Marchewka, A. (2017). Can pornography be addictive? An fMRI study of men seeking treatment for problematic pornography use. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology , 42(10), 2021–2031. 10.1038/npp.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg, J. , Kaldo, V. , Arver, S. , Dhejne, C. , Jokinen, J. , & Öberg, K. G. (2019). A randomized controlled study of group-administered cognitive behavioral therapy for hypersexual disorder in men. The Journal of Sexual Medicine , 16(5), 733–745. 10.1016/j.jsxm.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Hallberg, J. , Kaldo, V. , Arver, S. , Dhejne, C. , Piwowar, M. , Jokinen, J. , & Öberg, K. G. (2020). Internet-administered cognitive behavioral therapy for hypersexual disorder, with or without paraphilia(s) or paraphilic disorder(s) in men: A pilot study. The Journal of Sexual Medicine , 17(10), 2039–2054. 10.1016/j.jsxm.2020.07.018. [DOI] [PubMed] [Google Scholar]
- Hyatt, C. S. , Owens, M. M. , Crowe, M. L. , Carter, N. T. , Lynam, D. R. , & Miller, J. D. (2020). The quandary of covarying: A brief review and empirical examination of covariate use in structural neuroimaging studies on psychological variables. Neuroimage , 205, 116225. 10.1016/j.neuroimage.2019.116225. [DOI] [PubMed] [Google Scholar]
- Jauhar, S. , & Fortea, L. (2021). Brain activations associated with anticipation and delivery of monetary reward: A systematic review and meta-analysis of fMRI studies. Plos One , 16(8), e0255292. 10.1371/journal.pone.0255292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen, J. , Boström, A. E. , Chatzittofis, A. , Ciuculete, D. M. , Öberg, K. G. , Flanagan, J. N. , … Schiöth, H. B. (2017). Methylation of HPA axis related genes in men with hypersexual disorder. Psychoneuroendocrinology , 80, 67–73. 10.1016/j.psyneuen.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Kafka, M. P. (2010). Hypersexual disorder: A proposed diagnosis for DSM-V. Archives of Sexual Behavior , 39(2), 377–400. 10.1007/s10508-009-9574-7. [DOI] [PubMed] [Google Scholar]
- Kalichman, S. C. , & Rompa, D. (1995). Sexual sensation seeking and sexual compulsivity scales: Reliability, validity, and predicting HIV risk behavior. Journal of Personality Assessment , 65(3), 586–601. 10.1207/s15327752jpa6503_16. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , Adler, L. , Ames, M. , Demler, O. , Faraone, S. , Hiripi, E. , … Walters, E. E. (2005). The world health organization adult ADHD self-report scale (ASRS): A short screening scale for use in the general population. Psychological Medicine , 35(2), 245–256. 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Kingston, D. A. , & Bradford, J. M. (2013). Hypersexuality and recidivism among sexual offenders. Sexual Addiction & Compulsivity , 20(1–2), 91–105. [Google Scholar]
- Kinsey, A. C. , Pomeroy, W. R. , & Martin, C. (1948). Sexual behavior in the human male . Philadelphia: Indiana University Press. [Google Scholar]
- Knutson, B. , Westdorp, A. , Kaiser, E. , & Hommer, D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage , 12(1), 20–27. 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kor, A. , Fogel, Y. , Reid, R. C. , & Potenza, M. N. (2013). Should hypersexual disorder be classified as an addiction? Sexual Addiction & Compulsivity , 20(1–2). 10.1080/10720162.2013.768132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewska, E. , Grubbs, J. , Potenza, M. , Gola, M. , Draps, M. , & Kraus, S. (2018). Neurocognitive mechanisms in compulsive sexual behavior disorder. Current Sexual Health Reports . 10.1007/s11930-018-0176-z. [DOI] [Google Scholar]
- Kraus, S. W. , Voon, V. , & Potenza, M. N. (2016). Neurobiology of compulsive sexual behavior: Emerging science. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology , 41(1), 385–386. 10.1038/npp.2015.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, S. , & Gallinat, J. (2016). Neurobiological basis of hypersexuality. International Review of Neurobiology , 129, 67–83. 10.1016/bs.irn.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Lang, P. J. , Bradley, M. M. , & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual technical report A–8 . University of Florida. [Google Scholar]
- Lejuez, C. W. , Read, J. P. , Kahler, C. W. , Richards, J. B. , Ramsey, S. E. , Stuart, G. L. , … Brown, R. A. (2002). Evaluation of a behavioral measure of risk taking: The balloon Analogue risk task (BART). Journal of Experimental Psychology. Applied , 8(2), 75–84. 10.1037//1076-898x.8.2.75 [DOI] [PubMed] [Google Scholar]
- Lutz, K. , & Widmer, M. (2014). What can the monetary incentive delay task tell us about the neural processing of reward and punishment? Neuroscience and Neuroeconomics , 3, 33–45. 10.2147/NAN.S38864. [DOI] [Google Scholar]
- Marchewka, A. , Zurawski, L. , Jednorog, K. , & Grabowska, A. (2014). The Nencki affective picture system (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behavior Research Methods , 46(2), 596–610. 10.3758/s13428-013-0379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert, C. , Klein, S. , Strahler, J. , Kruse, O. , & Stark, R. (2021). Sexual incentive delay in the scanner: Sexual cue and reward processing, and links to problematic porn consumption and sexual motivation. Journal of Behavioral Addictions , 10(1), 65–76. 10.1556/2006.2021.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar, K. , Townes, P. , Pechlivanoglou, P. , Arnold, P. , & Schachar, R. (2022). Obsessive compulsive disorder and response inhibition: Meta-analysis of the stop-signal task. Journal of Psychopathology and Clinical Science , 131(2), 152–161. 10.1037/abn0000732. [DOI] [PubMed] [Google Scholar]
- Mechelmans, D. J. , Irvine, M. , Banca, P. , Porter, L. , Mitchell, S. , Mole, T. B. , … Voon, V. (2014). Enhanced attentional bias towards sexually explicit cues in individuals with and without compulsive sexual behaviours. Plos One , 9(8), e105476. 10.1371/journal.pone.0105476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery, S. A. , & Asberg, M. (1979). A new depression scale designed to be sensitive to change. British Journal Of Psychiatry , 134, 382–389. doi: 10.2147/NAN.S38864. [DOI] [PubMed] [Google Scholar]
- Norman, L. J. , Taylor, S. F. , Liu, Y. , Radua, J. , Chye, Y. , De Wit, S. J. , … Fitzgerald, K. (2019). Error processing and inhibitory control in obsessive-compulsive disorder: A meta-analysis using statistical parametric maps. Biological Psychiatry , 85(9), 713–725. 10.1016/j.biopsych.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham, S. , Murawski, C. , Fornito, A. , Youssef, G. , Yücel, M. , & Lorenzetti, V. (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping , 39(8), 3398–3418. 10.1002/hbm.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, J. T. , Rendina, H. J. , Ventuneac, A. , Cook, K. F. , Grov, C. , & Mustanski, B. (2013). A psychometric investigation of the hypersexual disorder screening inventory among highly sexually active gay and bisexual men: An item response theory analysis. The Journal of Sexual Medicine , 10(12), 3088–3101. 10.1111/jsm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis, M. , Loane, C. , Wu, K. , O'Sullivan, S. S. , Woodhead, Z. , Kiferle, L. , … Piccini, P. (2013). Neural response to visual sexual cues in dopamine treatment-linked hypersexuality in Parkinson's disease. Brain , 136(Pt 2), 400–411. 10.1093/brain/aws326. [DOI] [PubMed] [Google Scholar]
- Raven, J. , & Court, J. (2000). Manual for raven’s progressive matrices and vocabulary scales . Section 3, The Standard Progressive Matrices. Oxford Psychologists Press. [Google Scholar]
- Reid, R. C. , Garos, S. , & Carpenter, B. N. (2011). Reliability, validity, and psychometric development of the Hypersexual Behavior Inventory in an outpatient sample of men. Sexual Addiction & Compulsivity , 18(1), 30–51. 10.1080/10720162.2011.555709. [DOI] [Google Scholar]
- Savard, J. , Öberg, K. G. , Chatzittofis, A. , Dhejne, C. , Arver, S. , & Jokinen, J. (2020). Naltrexone in compulsive sexual behavior disorder: A feasibility study of twenty men. The Journal of Sexual Medicine , 17(8), 1544–1552. 10.1016/j.jsxm.2020.04.318. [DOI] [PubMed] [Google Scholar]
- Schmidt, C. , Morris, L. S. , Kvamme, T. L. , Hall, P. , Birchard, T. , & Voon, V. (2017). Compulsive sexual behavior: Prefrontal and limbic volume and interactions. Human Brain Mapping , 38(3), 1182–1190. 10.1002/hbm.23447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse, G. , Redouté, J. , & Dreher, J.-C. (2010). The architecture of reward value coding in the human orbitofrontal cortex. The Journal of Neuroscience , 30(39), 13095–13104. 10.1523/jneurosci.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinke, C. , Engel, J. , Veit, M. , Hartmann, U. , Hillemacher, T. , Kneer, J. , & Kruger, T. H. C. (2020). Sexual cues alter working memory performance and brain processing in men with compulsive sexual behavior. NeuroImage: Clinical , 27, 102308. 10.1016/j.nicl.2020.102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector, I. P. , Carey, M. P. , & Steinberg, L. (1996). The sexual desire inventory: Development, factor structure, and evidence of reliability. Journal of Sex & Marital Therapy , 22(3), 175–190. 10.1080/00926239608414655. [DOI] [PubMed] [Google Scholar]
- Stanford, M. S. , Mathias, C. W. , Dougherty, D. M. , Lake, S. L. , Anderson, N. E. , & Patton, J. H. (2009). Fifty years of the Barratt impulsiveness scale: An update and review. Personality and Individual Differences , 47(5), 385–395. 10.1016/j.paid.2009.04.008. [DOI] [Google Scholar]
- Stark, R. , Klucken, T. , Potenza, M. N. , Brand, M. , & Strahler, J. (2018). A current understanding of the behavioral neuroscience of compulsive sexual behavior disorder and problematic pornography use. Current Behavioral Neuroscience Reports , 5(4), 218–231. 10.1007/s40473-018-0162-9. [DOI] [Google Scholar]
- Svanborg, P. , & Asberg, M. (2001). A comparison between the beck depression inventory (BDI) and the self-rating version of the Montgomery Asberg depression rating scale (MADRS). Journal of Affective Disorders , 64(2–3), 203–216. 10.1016/s0165-0327(00)00242-1. [DOI] [PubMed] [Google Scholar]
- Tluczek, A. , Henriques, J. B. , & Brown, R. L. (2009). Support for the reliability and validity of a six-item state anxiety scale derived from the State-Trait Anxiety Inventory. Journal of Nursing Measurement , 17(1), 19–28. 10.1891/1061-3749.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi, A. C. , Searle, G. E. , Tzimopoulou, S. , Salinas, C. , Beaver, J. D. , Jenkinson, M. , … Gunn, R. N. (2011). Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. Neuroimage , 54(1), 264–277. 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Verbruggen, F. , Logan, G. D. , & Stevens, M. A. (2008). STOP-IT: Windows executable software for the stop-signal paradigm. Behavior Research Methods , 40(2), 479–483. doi: 10.3758/brm.40.2.479. [DOI] [PubMed] [Google Scholar]
- Voon, V. , Mole, T. B. , Banca, P. , Porter, L. , Morris, L. , Mitchell, S. , … Irvine, M. (2014). Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. Plos One , 9(7), e102419. 10.1371/journal.pone.0102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, M. , Bermpohl, F. , Mouras, H. , Schiltz, K. , Tempelmann, C. , Rotte, M. , … Northoff, G. (2008). Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. Neuroimage , 40(4), 1482–1494. 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Weinstein, A. , Katz, L. , Eberhardt, H. , Cohen, K. , & Lejoyeux, M. (2015). Sexual compulsion--relationship with sex, attachment and sexual orientation. Journal of Behavioral Addictions , 4(1), 22–26. 10.1556/jba.4.2015.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba, M. , Riegel, M. , Pucz, A. , Lesniewska, Z. , Dragan, W. L. , Gola, M. , … Marchewka, A. (2015). Erotic subset for the Nencki affective picture system (NAPS ERO): Cross-sexual comparison study. Frontiers in Psychology , 6, 1336. 10.3389/fpsyg.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2019). International statistical classification of diseases and related health problems (11th ed.). https://icd.who.int/. [Google Scholar]