Abstract
The effects of proline and caffeic acid on the survival of Shiga toxin-producing Escherichia coli (STEC) O157:H7 strain ATCC 43895 in a model apple juice medium were studied. It is hypothesized that the inhibitory effect of caffeic acid may explain why almost all outbreaks of STEC O157:H7 infections linked to apple juice or cider have occurred in October or November.
Infections with Shiga toxin-producing Escherichia coli (STEC) serotype O157:H7 have been associated with a variety of sources, such as minced beef, dairy products, surface water, and drinking water (29). Moreover, direct transmission from shedding animals to humans or from person to person has been described frequently (29). Also, some low-pH foods have been involved in STEC O157:H7 infections, including, for example, salami (10) and yogurt (30). In addition, several outbreaks of STEC O157:H7 in North America in the 1990s have been linked to apple juice or apple cider (6, 11, 12, 22, 38). Although STEC O157:H7 was not recognized as a human pathogen until 1982, it is likely that an apple juice-associated outbreak of hemorrhagic colitis and hemolytic-uremic syndrome caused by STEC had already occurred in 1980 (33, 37).
In a recently published survey, E. coli was found in 11 of 314 apple cider samples produced between mid-August and March in Connecticut (14). In that study, the presence of E. coli in the juice and cider was associated with time. All contaminated juice was produced from apples harvested between mid-October and mid-November (14). Interestingly, most outbreaks of gastroenteritis in North America caused by STEC O157:H7, Salmonella enterica serovar Typhimurium, or Cryptosporidium parvum, which have been associated with the consumption of unpasteurized apple juice or apple cider, have occurred in the same period of the year (6, 9, 11, 12, 22, 38).
Although unpasteurized apple cider is a traditional product consumed in the fall, the time frame in which the outbreaks occurred is much narrower than the period of consumption. Besides, the period of October and November is not in agreement with the incidence of most STEC O157:H7 infections in humans (regardless of the source) (29) and the prevalence of STEC O157:H7 in livestock, both of which are highest in the summer months (40). It is not clear why the presence of E. coli in apple juice and cider is associated with time. Many sources of contamination, both pre- and postharvest, have been suggested (7), including, for example, manured orchards (6, 12, 21, 33), recycled flume water used in processing (21), and insects that contaminate bruised apple tissue (24). A recent study involving the hazard analysis of critical control point method suggested that E. coli was introduced into cider during in-plant processing, because the organism was not detected in incoming apples, but was found in 4 of 32 in-line samples and 3 of 17 bottled fresh cider samples (35). These observations, however, cannot explain the association between E. coli contamination and time found by Dingman (14).
Recently, Dingman (15) observed that the growth of STEC O157:H7 in bruised tissue of fresh-picked apples of the McIntosh cultivar was inhibited, in contrast to findings in the tissue of other cultivars. However, apples of this cultivar occasionally supported growth after 2 days of incubation and also promoted growth after prolonged storage of the fruit at 4°C prior to testing. These observations suggest that a combination of intrinsic factors in apple juice or cider influence the growth and survival of STEC O157:H7 and other E. coli strains. Acidity is an obvious intrinsic factor in apple juice or cider. The pH and the types of organic acids influence the growth and survival of members of the family Enterobacteriaceae (13, 17); nonetheless, fruit juices with pH >3 should be considered potential sources for pathogenic strains of Enterobacteriaceae (32). Moreover, the observations of Dingman (14, 15) and Zhao et al. (41) do not support the supposition that acidity determines the presence of E. coli in apple juice. Neither could they demonstrate the effects of °Brix (an indication of the amount of soluble sugars) (14, 15, 41). Apparently, other intrinsic factors influence the presence of E. coli in apple juice.
In this paper, the effects of two substances on survival of STEC O157:H7 are described: proline and caffeic acid. Free proline is used by E. coli for protection at high osmolarities (1). In apples, the concentration of proline is approximately 15 mg/kg (fresh weight) (28). Caffeic acid is the most important of the phenolic acids present in apples. The concentration of caffeic acid drops during ripening from approximately 1.3 g/kg (fresh weight) in July to less than 0.1 g/kg in October, when the fruit is mature (31). During cold storage, the concentration of caffeic acid decreases even further (31). Antimicrobial effects of caffeic acids and its alkyl esters have been described previously (3, 39).
The effect of these compounds was studied with a model apple juice medium (MAJ). Experiments with naturally occurring compounds are difficult to carry out with real apple juice, because of uncontrolled biological variations or labor-intensive chemical analyses. MAJ was composed to model the main intrinsic factors of apple juice, represented by pH and acid and sugar concentrations. Based on data from the literature (5, 20, 28), MAJ was created as follows. One liter of milliQ water contained fructose (66 g; Sigma), glucose (22 g; Sigma), sucrose (27 g; Sigma), sorbitol (6.0 g; Fluka), malic acid (6.0 g; Sigma), sodium citrate (0.07 g; Sigma), and K2HPO4 · 3H2O (2 g; Sigma). MAJ was adjusted to the appropriate pH with HCl and NaOH, filter sterilized (pore size, 0.2 μm; Gelman Acrodisc), and divided into 10-ml portions in sterile screw-cap vials. In all incubations, the pH was routinely checked before and after the experiment.
We used E. coli O157:H7 strain ATCC 43895, which was stored at −80°C in brain heart infusion (BHI) broth (Oxoid) with 20% (vol/vol) glycerol (Sigma). For routine cultivation, a stock was maintained in semisolid Luria-Bertani (LB) agar at room temperature, which was protected from light. Before the experiments, cultures were preincubated in 5 ml of LB agar in screw-cap bottles (37°C, 16 to 18 h, shaken at 150 rpm). Subsequently, one loopful was transferred into BHI broth(37°C, at 150 rpm) to obtain stationary-phase cultures (6 h, optical density at 620 nm [OD620] = 1.1) or log-phase cultures (3 h, OD620 = 0.25). One milliliter of the culture was centrifuged (14,000 × g). The pellet was resuspended in 1 ml of 0.85% NaCl, which was used immediately as an inoculum for all experiments. Viable counts were determined on duplicate plates of tryptone soya agar (Oxoid) supplemented with 0.5% (wt/vol) sodium pyruvate (Sigma). The plates were prepared 1 to 3 days before sampling and surface dried at 55°C for 20 min. The detection limit was 25 CFU/ml (1.4 log10 CFU/ml). Statistical analysis of the data, generally with t tests, was carried out with Microsoft Excel software. Differences were considered statistically significant at the 5% level.
Effect of proline.
Proline (Sigma) was added at a 0 (control) or 15-mg/liter final concentration to MAJ at pHs of 3.2, 3.8, 4.4, and 5.0. These media were inoculated with a 50-μl inoculum of log- or stationary-phase cultures.
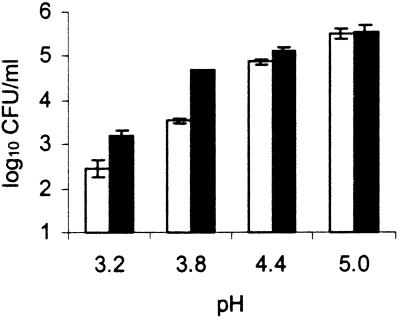
The effect of proline on the survival of log-phase-grown cells of STEC O157:H7 is shown in Fig. 1. At lower pH, the viable counts were significantly lower after 24 h of incubation. At pHs of 3.2 and 3.8, STEC O157:H7 survived significantly better with proline than without it. At pHs of 4.4 and 5.0, no effect of proline was observed, but at these pH levels, the viable counts were not reduced in the controls either, leaving no additional role for potential supportive substances, such as proline. Proline (15 mg/liter) did not influence the survival of cells in the stationary phase in MAJ at any pH (results not shown).
FIG. 1.
Survival of log-phase-grown STEC O157:H7 ATCC 43895 in chemically defined apple juice at different pHs, with (black) or without (white) proline (15 mg/liter), after 24 h of incubation at 25°C. Error bars represent 95% confidence intervals. The initial level was 5.7 log10 CFU/ml.
Proline is known to be used by bacteria to survive and grow at high osmolarities (1). It appears that proline in the concentrations present in apples (15 mg/liter) and at the pHs characteristic of apples and juice can be used to support the survival of log-phase cells in particular as well as under conditions with high sugar and acid concentrations. Protective effects against osmotic or low-pH stress from other amino acids or derivatives, such as arginine, glutamate, and glycine betaine, are well documented (27). On the other hand, the survival of stationary-phase-grown cells was not influenced by proline. It is well known that stationary-phase cells are better adapted to acidic conditions than log-phase STEC O157:H7 cells (2, 5). While it is likely that the stationary-phase-grown cells approximate the natural condition most, these experiments suggest that for STEC O157:H7, the potential protective effect of free proline in apple juice is not relevant.
Effect of caffeic acid.
Caffeic acid (Sigma) was dissolved in alcohol to prepare a stock solution and added to MAJ at a final concentration of 0 (control), 0.2, 0.4, or 1.0 mg/ml. The concentration of alcohol was kept constant (0.5% [vol/vol]) in all incubations. First, the effect of caffeic acid on log-phase cells was studied at 25°C. MAJ was adjusted to pH 3.2, 3.8, 4.4, or 5.0 and subsequently inoculated with a 50-μl inoculum. Viable counts were determined as described above. Second, the effect of caffeic acid on the survival of log- and stationary-phase cells at 4 and 25°C was studied at a constant pH (3.7). Fifty microliters of a 100-fold dilution of the culture was used as an inoculum. Viable counts were determined immediately after inoculation, after 24, 48, and 72 h of incubation. Experiments were carried out in triplicate.
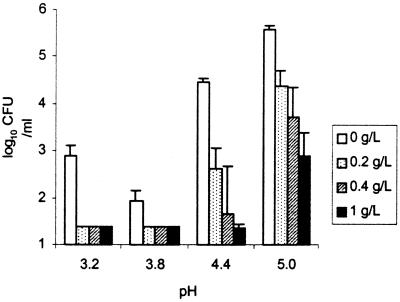
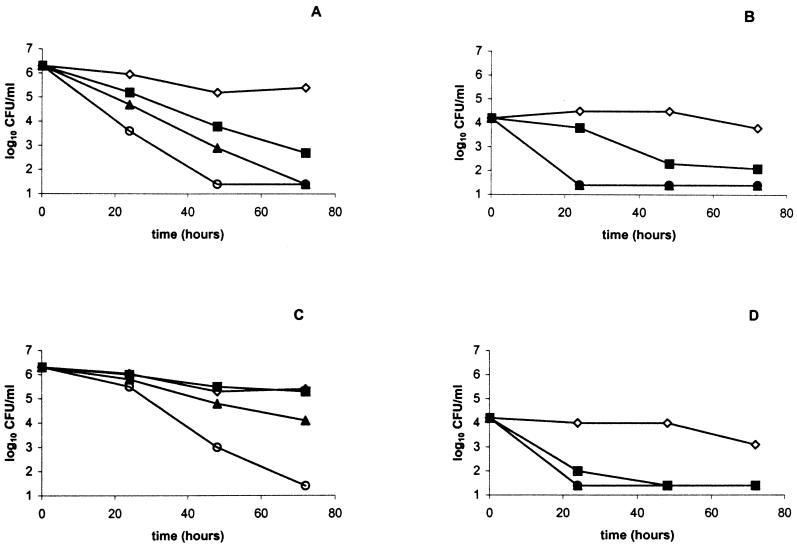
Figure 2 shows the effect of pH and caffeic acid on survival of log-phase-grown cells at 25°C. In the pH range of 3.8 to 5.0, a concentration-dependent sensitivity to caffeic acid was observed. The viable counts were reduced at least 1 log cycle in the presence of 0.2 mg of caffeic acid per milliliter, in comparison to the control samples. At the lowest pH (3.2), the viable counts decreased below the detection level (1.4 log10 CFU/liter) at any concentration of caffeic acid. Figure 3 shows the effect of caffeic acid (concentration range, 0.2 to 1.0 mg/ml) on the survival of log- or stationary-phase-grown STEC O157:H7 cells in MAJ (pH 3.7) at 4 and 25°C over a period of 72 h. At both temperatures, the stationary-phase cultures survived better than the log-phase cultures. Stationary-phase cultures declined in the presence of caffeic acid faster at 25°C than at 4°C (Fig. 3A and C). In contrast, log-phase cultures were more sensitive to caffeic acid at 4°C than at 25°C (Fig. 3B and D). The least sensitivity was observed from stationary-phase cultures at 4°C. Stationary-phase cultures were not affected by 0.2 mg of caffeic acid per ml at 4°C and could still be detected after 72 h in the presence of 0.4 mg of caffeic acid per ml (Fig. 3C). In all other cases, the viable counts of STEC O157:H7 declined below the detection limit in the presence of 0.4 mg of caffeic acid per ml or more within 72 h.
FIG. 2.
Survival of log-phase-grown STEC O157:H7 ATCC 43895 in chemically defined apple juice at different pHs and different concentrations of caffeic acid after 24 h of incubation at 25°C. Numbers below 1.4 log10 CFU/ml indicate that the viable counts were below the level of detection. Error bars represent 95% confidence intervals. The initial level was 5.7 log10 CFU/ml.
FIG. 3.
The survival of stationary-phase (A and C) or log-phase (B and D) STEC O157:H7 ATCC 43895 in chemically defined apple juice (pH 3.7) at 25°C (A and B) or 4°C (C and D) in the presence of caffeic acid at concentrations of 0 (open diamonds), 0.2 (solid squares), 0.4 (solid triangles), or 1.0 (open circles) g/liter. Counts below 1.4 log10 CFU/ml indicate that STEC O157:H7 was not detected.
Caffeic acid reduced the numbers of STEC O157:H7 cells, grown to either the log or stationary phase and at either 4 or 25°C. From this study, it can be concluded that phenolic acids such as caffeic acid may play an important role in the survival and growth of E. coli in apple juice or apple cider. Caffeic acid is known to occur in apples in the highest concentrations of all phenolic acids (31). In addition, it is the most effective of the phenolic acids against STEC O157:H7 cells and other foodborne pathogens (39). In apples, the concentration of caffeic acid decreases during ripening, ranging from an average of 1.3 g/kg (fresh weight) in July to less than 0.1 g/kg in October. During storage, the concentration of caffeic acid can decrease even further (31). Apples or apple juice can become contaminated throughout the year at several stages of production (7, 33, 35). Our study indicates that the survival of E. coli would be inhibited to a large extent in apples and apple products when harvested and processed between July and September, in contrast to apples harvested in October. This might explain why outbreaks linked to apple juice or cider have predominantly occurred in October or November and why contamination of apple juice with E. coli is associated with apples harvested between mid-October and mid-November, but not with apples harvested before mid-October (14). From the data presented in this study, it can be hypothesized that phenolic acids such as caffeic acid in apples may play an important role in the propagation of STEC O157:H7 in apple juice or cider. The association between the occurrence of E. coli and the concentration of phenolic acids in (commercial) unpasteurized apple juice and apple cider should be studied to test this hypothesis.
Many methods to reduce the numbers of STEC O157:H7 in apple juice or cider have been described. These include the use of preservatives (6, 18, 26, 41), irradiation (8), heating (23, 36), fermentation (34), and pulsed electric fields (16). Cultures of Pseudomonas syringae help to control outgrowth of STEC O157:H7 in apple wounds before processing (25). Nevertheless, treatment of apple juice and cider is not desired by some consumers and producers because of the loss of the unique characteristics of the untreated product (33). Perhaps caffeic acid or other naturally occuring phenolic acids (19) would be acceptable alternatives to the currently used methods for preservation.
REFERENCES
- 1.Amezaga R M, Booth I R. Osmoprotection of Escherichia coli by peptone is mediated by the uptake and accumulation of free proline but not of proline-containing peptides. Appl Environ Microbiol. 1999;65:5272–5278. doi: 10.1128/aem.65.12.5272-5278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold K W, Kaspar C W. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:2037–2039. doi: 10.1128/aem.61.5.2037-2039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baranowski J D, Nagel C W. Properties of alkyl hydroxycinnamates and effects on Pseudomonas fluorescens. Appl Environ Microbiol. 1982;45:218–222. doi: 10.1128/aem.45.1.218-222.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belitz H D, Grosch W. Food chemistry. 2nd ed. Berlin, Germany: Springer Verlag; 1987. [Google Scholar]
- 5.Benjamin M M, Datta A R. Acid tolerance of enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1995;61:1669–1672. doi: 10.1128/aem.61.4.1669-1672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besser R E, Lett S M, Weber J T, Doyle M P, Barrett T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 7.Beuchat L R, Ryu J-H. Produce handling and processing practices. Emerg Infect Dis. 1997;3:459–465. doi: 10.3201/eid0304.970407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan R L, Edelson S G, Snipes K, Boyd G. Inactivation of Escherichia coli O157:H7 in apple juice by irradiation. Appl Environ Microbiol. 1998;64:4533–4535. doi: 10.1128/aem.64.11.4533-4535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. Salmonella typhimurium outbreak traced to a commercial apple cider—New Jersey. Morb Mortal Wkly Rep. 1975;24:87–88. [Google Scholar]
- 10.Centers for Disease Control and Prevention. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami—Washington and California, 1994. Morb Mortal Wkly Rep. 1995;44:157–159. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. Morb Mortal Wkly Rep. 1997;46:4–8. [PubMed] [Google Scholar]
- 12.Cody S, Glynn M K, Farrar J A, Cairns K L, Griffin P M, Kobayashi J, Fyfe M, Hoffmann R, King A S, Lewis J H, Swaminathan B, Bryant R G, Vugia D J. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Ann Intern Med. 1999;130:202–203. doi: 10.7326/0003-4819-130-3-199902020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Conner D E, Kotrola J S. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl Environ Microbiol. 1994;61:382–385. doi: 10.1128/aem.61.1.382-385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dingman D. Prevalence of Escherichia coli in apple cider manufactured in Connecticut. J Food Prot. 1999;62:567–573. doi: 10.4315/0362-028x-62.6.567. [DOI] [PubMed] [Google Scholar]
- 15.Dingman D. Growth of Escherichia coli O157:H7 in bruised apple (Malus domestica) tissue as influenced by cultivar, date of harvest, and source. Appl Environ Microbiol. 2000;66:1077–1083. doi: 10.1128/aem.66.3.1077-1083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evrendilek G A, Zhang Q H, Richter E R. Inactivation of Escherichia coli O157:H7 and Escherichia coli 8739 in apple juice by pulsed electric fields. J Food Prot. 1999;62:793–796. doi: 10.4315/0362-028x-62.7.793. [DOI] [PubMed] [Google Scholar]
- 17.Fisher T L, Golden D A. Fate of Escherichia coli O157:H7 in ground apples used in cider production. J Food Prot. 1998;62:1372–1374. doi: 10.4315/0362-028x-61.10.1372. [DOI] [PubMed] [Google Scholar]
- 18.Fisher T L, Golden D A. Survival of Escherichia coli O157:H7 in apple cider as affected by dimethyl dicarbonate, sodium bisulfite, and sodium benzoate. J Food Prot. 1998;62:904–906. [Google Scholar]
- 19.Friedman M, Jurgens H S. Effect of pH on the stability of plant phenolic compounds. J Agric Food Chem. 2000;48:2101–2110. doi: 10.1021/jf990489j. [DOI] [PubMed] [Google Scholar]
- 20.Fuleki T, Pelabo E, Pelabay R B. Carboxylic acid composition of varietal juices produced from fresh and stored apples. J Agric Food Chem. 1995;43:598–607. [Google Scholar]
- 21.Goverd K A, Beech F W, Hobbs R P, Shannon R. The occurrence and survival of coliforms and salmonellas in apple juice and cider. J Appl Bacteriol. 1979;46:521–530. doi: 10.1111/j.1365-2672.1979.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 22.Hilborn E D, Mshar P A, Fiorentino T R, Dembek Z F, Barrett T J, Howard R T, Carter M L. An outbreak of Escherichia coli O157:H7 infections and haemolytic uraemic syndrome associated with consumption of unpasteurized apple cider. Epidemiol Infect. 2000;124:31–36. doi: 10.1017/s0950268899003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingham S C, Uljas H E. Prior storage conditions influence the destruction of Escherichia coli O157:H7 during heating of apple cider and juice. J Food Prot. 1998;61:390–394. doi: 10.4315/0362-028x-61.4.390. [DOI] [PubMed] [Google Scholar]
- 24.Janisiewicz W J, Conway W S, Brown M W, Sapers G M, Fratamico P, Buchanan R L. Fate of Escherichia coli O157:H7 on fresh-cut apple tissue and its potential for transmission by fruit flies. Appl Environ Microbiol. 1999;65:1–5. doi: 10.1128/aem.65.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janisiewicz W J, Conway W S, Leverentz B. Biological control of postharvest decay of apple can prevent growth of Escherichia coli O157:H7 in apple wounds. J Food Prot. 1999;62:1372–1375. doi: 10.4315/0362-028x-62.12.1372. [DOI] [PubMed] [Google Scholar]
- 26.Lin C M, Kim J, Du W X, Wei C I. Bactericidal activity of isothiocyanate against pathogens on fresh produce. J Food Prot. 2000;63:25–30. doi: 10.4315/0362-028x-63.1.25. [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Smith M P, Chapin K C, Baik H S, Bennett G N, Foster J W. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1996;62:3094–3100. doi: 10.1128/aem.62.9.3094-3100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattick L R, Moyer J C. Composition of apple juice. J AOAC. 1983;66:1251–1255. [PubMed] [Google Scholar]
- 29.Mead P S, Griffin P M. Escherichia coli O157:H7. Lancet. 1998;352:1207–1211. doi: 10.1016/S0140-6736(98)01267-7. [DOI] [PubMed] [Google Scholar]
- 30.Morgan D, Newman C P, Hutchinson D N, Walkes A M, Rowe B, Majid F. Verotoxin-producing Escherichia coli O157:H7 infections associated with the consumption of yoghurt. Epidemiol Infect. 1993;111:181–187. doi: 10.1017/s0950268800056880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosel H-D, Herrmann K. Changes in catechins and hydroxycinnamic acid derivatives during development of apples and pears. J Sci Food Agric. 1974;25:251–256. doi: 10.1002/jsfa.2740250304. [DOI] [PubMed] [Google Scholar]
- 32.Mossel D A A, De Bruin A S. The survival of Enterobacteriaceae in acid liquid foods stored at different temperatures. Ann Inst Pasteur. 1960;11:65–72. [Google Scholar]
- 33.Parish E M. Crit. Rev. Microbiol. 23:109–119. 1997. Public health and nonpasteurized fruit juices. [DOI] [PubMed] [Google Scholar]
- 34.Semanchek J J, Golden D A. Survival of Escherichia coli O157:H7 during fermentation of apple cider. J Food Prot. 1996;59:1256–1259. doi: 10.4315/0362-028X-59.12.1256. [DOI] [PubMed] [Google Scholar]
- 35.Senkel I A, Jr, Henderson R A, Jolbitado B, Meng J. Use of hazard analysis of critical control point and alternative treatments in the production of apple cider. J Food Prot. 1999;62:778–785. doi: 10.4315/0362-028x-62.7.778. [DOI] [PubMed] [Google Scholar]
- 36.Splittstoesser D F, McLellan M R, Churey J J. Heat resistance of Escherichia coli O157:H7 in apple juice. J Food Prot. 1996;59:226–229. doi: 10.4315/0362-028x-59.3.226. [DOI] [PubMed] [Google Scholar]
- 37.Steele B T, Murphy N, Arbus G S, Rance C P. An outbreak of hemolytic uremic syndrome associated with ingestion of fresh apple juice. J Pediatr. 1982;101:963–965. doi: 10.1016/s0022-3476(82)80021-8. [DOI] [PubMed] [Google Scholar]
- 38.Tamblyn S, DeGrosbois J, Taylor D, Stratton J. An outbreak of Escherichia coli O157:H7 infection asociated with unpasteurized non-commercial, custom-pressed apple cider—Ontario, 1998. Can Commun Dis Rep. 1999;25:113–120. [PubMed] [Google Scholar]
- 39.Tunçel G, Nergiz C. Antimicrobial effect of some olive phenols in a laboratory medium. Lett Appl Microbiol. 1993;17:300–302. [Google Scholar]
- 40.Wallace J S. The ecological cycle of Escherichia coli O157:H7. In: Stewart C S, Flint H J, editors. Escherichia coli O157 in farm animals. Wallingford, United Kingdom: Cabi Publishing; 1999. [Google Scholar]
- 41.Zhao T, Doyle M P, Besser R E. Fate of enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl Environ Microbiol. 1993;59:2526–2530. doi: 10.1128/aem.59.8.2526-2530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]