Abstract
Three-dimensional (3D) bioprinting is a rapidly developing technology that has potential to initiate a paradigm shift in the treatment of skin wounds arising from burns, ulcers, and genodermatoses. Recessive dystrophic epidermolysis bullosa (RDEB), a severe form of epidermolysis bullosa, is a rare genodermatosis that results in mechanically induced blistering of epithelial tissues that leads to chronic wounds. Currently, there is no cure for RDEB, and effective treatment is limited to protection from trauma and extensive bandaging. The care of chronic wounds and burns significantly burdens the healthcare system, further illustrating the dire need for more beneficial wound care.1 Although in its infancy, 3D bioprinting offers therapeutic potential for wound healing and could be a breakthrough technology for the treatment of rare, incurable genodermatoses like RDEB. This viewpoint essay outlines the promise of 3D bioprinting applications for treating RDEB, including skin regeneration, a delivery system for gene-edited cells and small molecules, and disease modeling. While the future of 3D bioprinting is encouraging, there are many technical challenges to overcome―including optimizing bioink and cell source―before this approach can be widely implemented in clinical practice.
Keywords: wound healing, guided tissue regeneration, regenerative medicine, tissue engineering, gene editing
Introductory Overview
The lack of accessible and advanced skin wound management has resulted in a significant yet underappreciated crisis for individuals, healthcare systems, and society. It is estimated that over 11 million people per year require burn care, with burns being one of the most common types of trauma throughout the world.2 Furthermore, the National Institutes of Health have estimated that in the United States alone around 6.5 million people are affected by slow-to-heal or chronic wounds, a statistic that is expected only to increase.3 The burden of chronic skin wounds has been referred to as a “silent epidemic,” since people at high risk for chronic wounds include older individuals and/or those with comorbidities including obesity and diabetes. Additionally, particularly rare and incurable genodermatoses, notably RDEB, that cause mechanical fragility of the skin and result in chronic wounding of epithelial tissues, require extensive, costly bandaging. Since chronic wounds are often complicated by an underlying condition, they are not identified as a disease per se. Consequently, the research funding specifically for chronic wound care is disproportionately low compared to the actual impact of chronic wounds on the healthcare system.4 The growing need for improved wound care options is reflected by the demand for wound care products. In 2014 the annual cost for wound care was estimated to be $2.8 billion with the cost expected to increase to $3.5 billion by 2021.5
Background
The significant burden skin wounds present to the individual and the healthcare system has encouraged researchers throughout the past decade to investigate a revolutionary wound-healing solution, 3D bioprinted tissue engineering. Tissue engineering aims to generate biological structures―either in an in situ or in vitro fashion―that promote healing and initiate the body’s regenerative processes. 3D bioprinting is a promising tissue engineering application that utilizes a 3D printer to achieve precision, automation, and standardization of therapeutic delivery. In traditional 3D printing, a digital template of the desired object is created using computer-aided design programs. These digital templates are converted into a script containing many thin slices or layers that, when stacked vertically, combine to create the desired object. The script is then fed to the 3D printer where the applicator dispenses the printing substance or ink layer by layer, vertically, until the object is complete. 3D printing is referred to as 3D bioprinting when the ink used includes living cells (bioink). Recent reviews thoroughly outline the bioprinting process and optimization steps.6–8 In terms of the technology, 3D bioprinting can be divided into three categories including inkjet-based, pressure-assisted, and laser-assisted bioprinting.9 Furthermore, the implementation of advanced technology is seen with freeform reversible embedding of suspended hydrogels (FRESH) bioprinting, which is designed to print bioinks within a yield-stress support bath.9 The two applications utilized for skin 3D bioprinting (outlined in Figure 1) include in situ and in vitro. In situ 3D bioprinting delivers tissue engineered therapeutics such as cells, extracellular signaling molecules, or endogenous reprogramming components directly to the target area10 In vitro 3D bioprinting refers to the printing of regenerative tissue products onto a petri dish. From there, the printed product is transplanted onto the affected area of tissue. .11 The practice of 3D bioprinting skin grafts is continually being developed, and a multitude of bioinks and cell combinations have been reported.6
Figure 1.
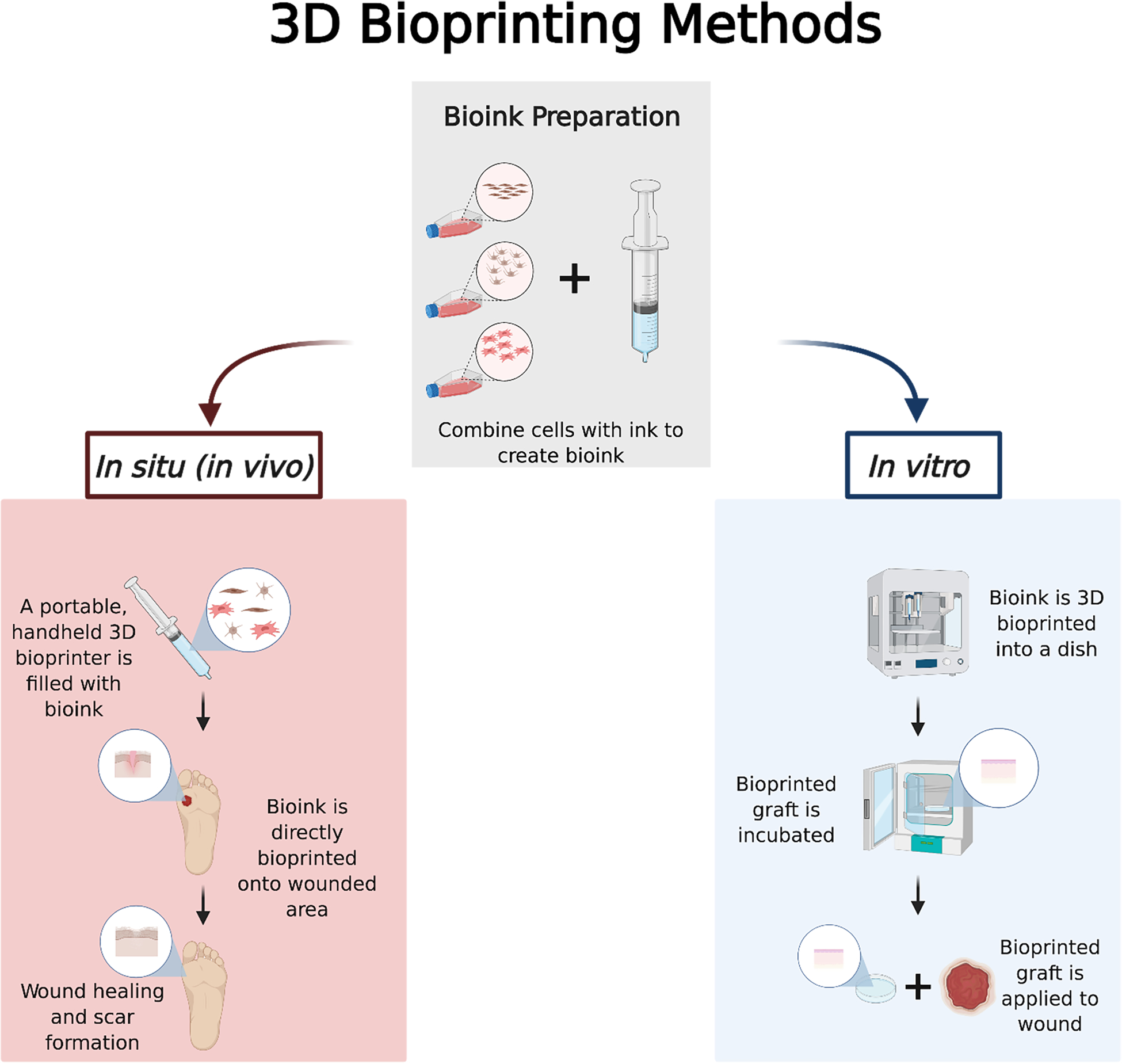
The two main methods for developing and applying 3D bioprinted therapies are in situ and in vitro. This figure displays both processes from initial cell culture to final patient application (created with BioRender.com).
Though still in its infancy, this technology holds the promise to become an integral tool in the care of chronic wounds and burns. This is also an exciting opportunity to reshape the therapeutic outlook for rare genetic skin diseases like RDEB. RDEB, a severe form of epidermolysis bullosa, is a rare genetic disease associated with mechanical fragility of the skin resulting in devastating chronic wounding of epithelial tissues. This disease is caused by mutations in the COL7A1 gene that prevent the production of functional type VII collagen (C7).12 C7 is a secreted protein vital for strengthening the dermal-epidermal junction and maintaining barrier integrity. Without functional C7, RDEB patients suffer from chronic skin wounds, pseudosyndactyly (mitten deformity), esophageal strictures, corneal abrasions, and joint contractures.13, 14 Limited treatment success was found using allogeneic hematopoietic cell transplantation (HCT) in RDEB patients.15 However, HCT has many risks such as graft vs host disease, graft failure, and the need to suppress the immune system.15, 16 Advancements in gene correction therapies―for example TALEN and CRISPR/Cas9 systems―offer a major avenue of approach as well.17, 18 Limitations of genetic therapies currently include extensive ethical concerns and lack of an effective treatment delivery system. Therefore, an innovative treatment for RDEB is necessary. As summarized in Fig. 2, this viewpoint essay outlines the applications in which 3D bioprinting could successfully be used to treat RDEB, including skin regeneration, as a delivery system for gene-edited cells and small molecules, and disease modeling.
Figure 2.
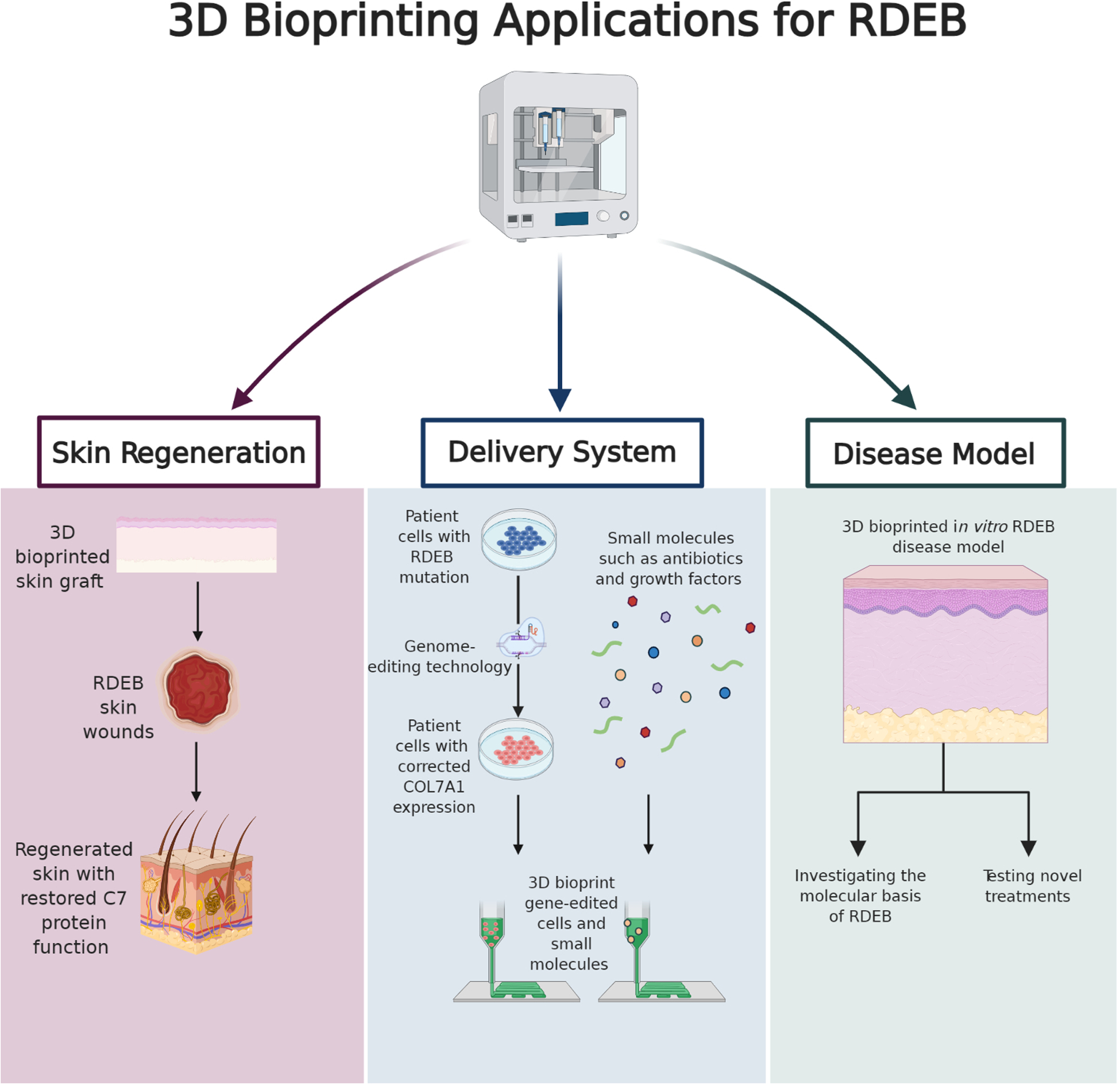
The future of 3D bioprinting shows promise for significantly impacting the study and treatment of RDEB skin wounds. This schematic displays 3D bioprinting’s possible applications for treating RDEB skin wounds including skin regeneration, a delivery system, and disease modeling (created with BioRender.com).
3D bioprinting for skin regeneration
Human skin fabrication via 3D bioprinting technology is being investigated with the goal of providing skin replacement, restoring function, and promoting healing after injury. In the case of RDEB, 3D bioprinted skin is an intriguing therapeutic option to replace damaged, blistered skin and initiate healing. With its ability to provide precise cell placement in a layer-by-layer fashion, 3D bioprinting can potentially mimic skin structure and function. The skin’s complexity comes from the spatial organization of a multitude of cell types within the extracellular matrix.19 The foundational goal of designing a tissue-engineered graft is to mimic the physical properties of skin and mirror its normal architecture to provide a structural framework hospitable for the natural healing cascade.20 Because the skin functions primarily as a protective barrier, it deploys a sophisticated and dynamic regeneration program in response to injury or infection.21 Biological insights into this healing process have largely inspired the design of 3D bioprinted grafts to include cellular diversity along with molecules crucial for skin function.19 In the last five years, there has been a push to design “pro-regenerative” skin grafts that contain cell-instructive cues to restore damaged tissues.22 Adding in these cues in the form of growth factors, antimicrobial molecules, bioactive nano-particles, cell-binding peptides, and more to the graft to foster a suitable niche for healing has been a commonly explored method.23–28
The use of 3D bioprinted skin will also enable the inclusion of important skin components such as hair follicles, sweat glands, melanocytes, and sebaceous glands. Including these structures is key to the development of fully functional skin.29 Huang et al. bioprinted sweat glands with epidermal progenitor cells and demonstrated functional sweat glands within the extracellular matrix of the 3D graft following in vivo transplant onto murine wounds.30 Furthermore, Jorgensen et al. 3D bioprinted six different human cell phenotypes, including keratinocytes and melanocytes for the epidermis; fibroblasts, microvascular endothelial cells, and follicle dermal papilla cells for the dermis; and pre-adipocytes for the hypodermis.31 This full-thickness skin graft showed acute wound closure in a murine model after 21 days and represents a proof of concept for the inclusion of additional skin cell phenotypes. Additionally, this study demonstrated that 3D bioprinted skin once placed onto a full-thickness murine wound, can integrate, form an epidermal barrier, and recapitulate normal collagen remodeling. These findings were supported by histological analysis. A few unexpected results occurred, however, including the observation of Lamin A+C staining for human nuclei only in the dermis. An explanation could be that human keratinocytes from the bioprinted skin made up the initial epidermis and then were replaced by murine keratinocytes. Also, by day 21 there was not significant staining for adiponectin to signal the presence of preadipocytes. The study speculates these cells may have differentiated toward fibroblasts considering the dense vimentin staining. Lastly, the bioprinted skin wound center showed an increased number of capillary lumens compared with the control hydrogel wounds, which could allow for improved delivery of endogenous immune calls as well as growth factors and cytokines. A limitation of this study is only having one time point at day 21, so in a future study it would be necessary to evaluate wounds following the first few days of treatment in addition to evaluating wound healing several months post wound closure. Apart from cellular and other bioactive additives, the mechanical stiffness and porosity of the 3D bioprinted skin graft is also key in modulating cellular events. Researchers can explore different inks to fine-tune the mechanical properties of the graft in order to control cellular behavior.22 For instance, in a 3D-bioprinted graft, the ability for printed mesenchymal stem cells (MSCs) to form into tubular structures was directly dependent on the stoichiometric ratio of ink components that changed graft stiffness.32 Furthermore, sufficient porosity of 3D grafts has been shown to be important for enabling spatial distribution of cells throughout the graft.30, 33–35 Porosity increases cell interconnectivity, which aids in coordinating numerous cell fate processes, such as angiogenesis and graft take.36, 37
As explained previously, there are two different approaches to 3D bioprinting, in situ and in vitro, both of which are being studied in the context of skin replication and wound healing.38, 39 An exciting device for in situ skin bioprinting is a handheld skin bioprinter, one of which is currently being developed by Hakimi et al.40 Additionally, a group at the Wake Forest Institute for Regenerative Medicine has also developed a state-of-the-art mobile bioprinter that allows bi-layered skin to be printed directly onto a patient’s wound.39 A handheld skin bioprinter would be a remarkable addition to the current wound management options for RDEB patients, as it could provide more immediate and precise care. This portable bedside bioprinter could also be utilized by the military, as it is estimated that 10 to 30 percent of combat casualties in conventional warfare result from burn injuries.41 Although in situ bioprinting for wound reconstruction provides several advantages, such as precise graft size onto wound surface and elimination of the cost and time for in vitro differentiation, studies using in vitro bioprinting have also demonstrated positive results.35 While skin tissue engineering is an undoubtedly complex process that requires appropriate choice of cells, biomaterials, graft design, and print method, 3D bioprinting technology has the capacity to one day replicate skin’s architecture and function to revolutionize wound therapy. The use of this therapeutic advancement could significantly impact RDEB advanced wound care and provide a localized option that goes beyond bandaging for eligible cases.
Gene-edited cell and small molecule delivery system
In addition to providing a skin replacement that promotes wound healing, 3D bioprinting technology can also be an efficient delivery system for gene-edited cells to help specifically correct the phenotype of RDEB along with other rare and incurable genodermatoses. The genetic etiology of RDEB is well defined, which makes it an obvious candidate for genome-editing applications such as CRISPR/Cas9 and TALEN.42 RDEB primary patient-derived cells have been successfully gene-edited in several in vitro studies.17, 43–45 Optimizing the mechanism of delivery for these gene-edited cells is under investigation, with many researchers focusing on the use of bioengineered skin grafts. Compared to direct injection of cells in tissue, 3D bioengineered in situ scaffolds have been shown to enhance the delivery and retention of cells within the tissue.46 In 2016, a phase I clinical trial involved the delivery of autologous RDEB keratinocytes that had been transduced with a retroviral vector to correctly express the COL7A1 gene.47 These cells were assembled into epidermal sheet grafts and applied to patient wounds. Results showed that grafts were well tolerated and some, but not all, demonstrated improved wound healing in addition to C7 expression at the dermal-epidermal basement membrane. More recently, Jackow et al. reported a CRISPR/Cas9-mediated correction of the COL7A1 gene in patient-derived induced pluripotent stem cells that were differentiated into fibroblasts and keratinocytes.48 These cells were then used to generate 3D human skin equivalents that were grafted onto murine wounds. Two months post grafting, normal C7 expression at the basement membrane zone and anchoring fibrils were reported. The use of skin grafts to deliver gene-edited cells is a promising systematic pathway to restore C7 expression to RDEB wounds. While laboratory-grown skin grafts have shown to be a viable cell delivery option, the automation and standardization of 3D bioprinting offers an advantage when it comes to cell placement. By 3D bioprinting the cells layer by layer, the arrangement of the gene-edited cells within the graft can be controlled and offer a more precise delivery to affected areas of the epidermis and dermis. The combination of genome editing and 3D bioprinting technologies has the potential to correct chronic skin wounds of genetic origin and must be further explored.
Additionally, 3D bioprinting has recently gained momentum as a small molecule delivery system, another hopeful application for treating RDEB. Specific to mitigating the RDEB phenotype, the therapeutic targets of these small molecules can include skin barrier restoration, infection control, immune response, and interference with epigenetic drivers of the disease. Small molecule-based therapies for RDEB that have been investigated in clinical studies and shown positive results include the use of gentamicin and botulin.49, 50 There are several ongoing clinical trials examining small molecules such as pregabalin and rigosertib as potential RDEB therapies.51, 52 The best delivery method for small molecule-based therapies has yet to be determined; however, 3D bioprinting is a realistic and innovative option that has the advantage of precise organization and placement of components. Importantly, 3D bioprinted matrices have been proven to be suitable for the delivery of bioactive molecules like growth factors, antibiotics, and other types of small molecules.53, 54 Small molecules that have been shown to be therapeutic against RDEB, mentioned above, could be integrated into a 3D bioprinted matrix for delivery to wound sites. By using a 3D printer, the small molecules can be incorporated into the matrix in precise patterns based on the scaffold’s morphology as well as the small molecule’s release profile in order to promote a targeted, efficacious delivery. In a study looking at bone tissue regeneration, released small molecules from a loaded 3D printed scaffold successfully enhanced the angiogenesis and targeted MSCs for osteogenic differentiation.55 In a similar study, growth factors were 3D bioprinted into spatiotemporally defined patterns, tightly controlling the release of the growth factors in order to enhance the bone tissue regeneration.56 The control over the placement and release of small molecules within a 3D printed scaffold cannot be obtained with other traditional delivery methods such as injected 3D in situ scaffolds. With encouraging and ongoing clinical studies examining small molecules to treat RDEB, 3D bioprinting should be considered as a delivery system.
Disease model
RDEB is a challenging disease to research due to the small patient cohort and limited access to patient specimens. An accurate and consistent method for generating an RDEB skin model is needed for disease modeling and drug testing, which could be accomplished with 3D bioprinting technology. Although RDEB mice have been generated to be used as an animal model, few wound healing studies have been successfully performed on these in vivo models because of short life expectancy due to the RDEB phenotype.57, 58 Additionally, the differences in skin physiology between humans and mice restrains the direct clinical translation of such experiments.59 Optimizing a 3D in vitro diseased skin model is necessary and should be prioritized. The lack of essential skin basement membrane zone structures results in increased tissue fragility and difficulty handling during wound closure studies.59 Recently, an in vitro dermal-epidermal junction model was successfully developed using ECM proteins and seeded RDEB keratinocytes and fibroblasts.60 This model could be used to test mechanical adhesion between skin layers. A more physiologically relevant tissue could also be achieved by the addition of vasculature, which would be important for testing systemic delivery of drugs.61 While constructing a useful and intact in vitro model is not simple due to the nature of this disease, 3D bioprinting technology could allow for consistent generation of these tissues due to its standardization and automation. Beyond the devastating skin phenotype of RDEB, bioprinting allows for the modeling of the associated cutaneous squamous cell carcinoma (SCC) that develops at lesion sites. RDEB-associated SCC is the major cause of death in RDEB individuals, due to its high metastatic potential and therapy-resistant nature.62 3D in vitro RDEB models for drug testing and researching RDEB-associated SCC show promise but are not commonly used.63, 64 In studying SCC, 3D spheroids have significant potential for modeling the pervasive cancer phenotype demonstrated in RDEB.57, 65, 66 However, the use of these organotypic technologies, despite their pervasiveness in wound-healing studies, remain notably underrepresented in RDEB research.67 This is most likely a result of the difficulty of producing and maintaining an in vitro 3D model using RDEB cells. The continued advancement of 3D bioprinting technology has the potential to transform current in vitro RDEB models and thus lead to new, advantageous therapies.
Important to add, other complicated and severe genodermatoses that bioengineered skin could be useful for include Kindler syndrome, Gorlin syndrome, Netherton syndrome, and mechanobullous diseases.68 Furthermore, skin models are beneficial for the modeling of many more common physiological and pathological conditions like ultraviolet irradiation response, inflammation, psoriasis, and skin cancers, in addition to pharmaceutical screenings for potential therapies.69–71 In fact, a groundbreaking study used 3D bioprinting technology to produce skin tissue with pathophysiological signs of type 2 diabetes. The study found that the diseased skin tissue model displayed the characteristic insulin resistance, adipocyte hypertrophy, inflammatory reaction, and vascular dysfunction in a hyperglycemic environment. The model was also verified for future disease drug screening and represents a major step in the direction of modeling human diseases using 3D bioprinting technologies.72
Major Open Questions
While 3D bioprinting could radically change the study and treatment of skin wounds, there are many technical challenges to overcome before clinical practice of 3D bioprinting is a reality. One challenge is optimization of bioink to meet requirements for printability, reproducibility, and spatial organization of the graft. Further research investigating the molecular signaling molecules required for activation of innate healing mechanisms is needed to narrow the disparity between the in vitro and in vivo microenvironments. In order to have successful transplantation to the patient, immunological barriers such as the human leukocyte antigen (HLA) or ABO blood group must be matched or circumvented. Ideally, autologous cells such as iPSCs would be used. In the case of genetic disorders, this is challenging. The banking of allogenic cells is an idea that has been proposed to provide cellular therapy support. However, broad HLA diversity makes the idea of establishing haplobanks seem unrealistic. This can be circumvented by using immunomodulatory therapy as an adjunct to allogenic transplant.73 For clinical translation, active monitoring of cell yields and maintenance of quality parameters including purity, potency, and viability of the cell types during printing would be critical protocol measures.19 These intricate challenges must be addressed before 3D bioprinting technology can meet its full potential. Additionally, the field of 3D bioprinting for wound healing faces a unique challenge in regards to conducting clinical trials. In order to conduct a clinical trial, there must be genuine equipoise as to the efficacy of different treatments being considered.74, 75 Generally, it is thought that autografts, grafts harvested from the intended recipient, are the ideal for replacing damaged skin tissue.76 In the case of rare genetic diseases like RDEB, however, the generalized superiority of autografts is diminished, thus prompting researchers and practitioners to pursue alternative therapeutic technologies like 3D bioprinting.
Conclusions and Perspectives
The overwhelming amount of research focused on developing 3D bioprinting applications shows the transformative potential of this technology. Although in its early stages, 3D bioprinting is projected to transcend the accomplishments of current tissue engineering and lead to a shift in the treatment of skin injuries. The clinical burden, particularly of chronic wounds and burns, is significant and in need of more efficient and effective wound care options. In the case of RDEB, because of its rarity and complexity, both the treatment and study of this disease are limited. 3D bioprinting could offer several applications―namely skin regeneration, cell and molecule delivery systems, and disease modeling―to circumvent these limitations and improve patient outcomes. With continued research, these applications could revolutionize the outlook of skin wound healing.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Nussbaum SR, Carter MJ, Fife CE, et al. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health. Jan 2018;21(1):27–32. doi: 10.1016/j.jval.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 2.Peck MD. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns. Nov 2011;37(7):1087–100. doi: 10.1016/j.burns.2011.06.005 [DOI] [PubMed] [Google Scholar]
- 3.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. Jan-Feb 2009;17(1):1–18. doi: 10.1111/j.1524-475X.2008.00436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen CK. Wound Healing. 4th ed. vol 1. Plastic Surgery. Elsevier; 2017. [Google Scholar]
- 5.Settipalli S A Robust market rich with opportunities: advanced wound dressings. PM360. June ed. Online2015. [Google Scholar]
- 6.Daly AC, Prendergast ME, Hughes AJ, Burdick JA. Bioprinting for the Biologist. Cell. Jan 7 2021;184(1):18–32. doi: 10.1016/j.cell.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barros N, Kim H, Goudie MJ, et al. Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication. Jul 10 2020;doi: 10.1088/1758-5090/aba503 [DOI] [PubMed] [Google Scholar]
- 8.Kim BS, Das S, Jang J, Cho DW. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem Rev. Oct 14 2020;120(19):10608–10661. doi: 10.1021/acs.chemrev.9b00808 [DOI] [PubMed] [Google Scholar]
- 9.Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. Sep 20 2016;14:271. doi: 10.1186/s12967-016-1028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaharwar AK, Singh I, Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Review. Nat Rev Mater. September 2020. 2020;5:686–705. doi: 10.1038/s41578-020-0209-x [DOI] [Google Scholar]
- 11.Singh S, Choudhury D, Yu F, Mironov V, Naing MW. In situ bioprinting - Bioprinting from benchside to bedside? Acta Biomater. Jan 1 2020;101:14–25. doi: 10.1016/j.actbio.2019.08.045 [DOI] [PubMed] [Google Scholar]
- 12.Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. Jan 2010;28(1):93–105. doi: 10.1016/j.det.2009.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuttner V, Mack C, Rigbolt KT, et al. Global remodelling of cellular microenvironment due to loss of collagen VII. Mol Syst Biol. Apr 16 2013;9:657. doi: 10.1038/msb.2013.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack MR, Wendelschafer-Crabb G, McAdams BD, Hordinsky MK, Kennedy WR, Tolar J. Peripheral neuro-immune pathology in recessive dystrophic epidermolysis bullosa. J Invest Dermatol. Apr 2015;135(4):1193–1197. doi: 10.1038/jid.2014.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner JE, Ishida-Yamamoto A, McGrath JA, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. Aug 12 2010;363(7):629–39. doi: 10.1056/NEJMoa0910501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geyer MB, Radhakrishnan K, Giller R, et al. Reduced Toxicity Conditioning and Allogeneic Hematopoietic Progenitor Cell Transplantation for Recessive Dystrophic Epidermolysis Bullosa. J Pediatr. Sep 2015;167(3):765–9 e1. doi: 10.1016/j.jpeds.2015.05.051 [DOI] [PubMed] [Google Scholar]
- 17.Osborn MJ, Starker CG, McElroy AN, et al. TALEN-based gene correction for epidermolysis bullosa. Mol Ther. Jun 2013;21(6):1151–9. doi: 10.1038/mt.2013.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webber BR, Osborn MJ, McElroy AN, et al. CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. NPJ Regen Med. 2016;1doi: 10.1038/npjregenmed.2016.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Valle A, Del Amo C, Andia I. Overview of Current Advances in Extrusion Bioprinting for Skin Applications. Int J Mol Sci. Sep 12 2020;21(18)doi: 10.3390/ijms21186679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pourmoussa A, Gardner DJ, Johnson MB, Wong AK. An update and review of cell-based wound dressings and their integration into clinical practice. Ann Transl Med. Dec 2016;4(23):457. doi: 10.21037/atm.2016.12.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med. Jan 5 2015;5(1):a023267. doi: 10.1101/cshperspect.a023267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouhan D, Dey N, Bhardwaj N, Mandal BB. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials. Sep 2019;216:119267. doi: 10.1016/j.biomaterials.2019.119267 [DOI] [PubMed] [Google Scholar]
- 23.Chouhan D, Thatikonda N, Nileback L, Widhe M, Hedhammar M, Mandal BB. Recombinant Spider Silk Functionalized Silkworm Silk Matrices as Potential Bioactive Wound Dressings and Skin Grafts. ACS Appl Mater Interfaces. Jul 18 2018;10(28):23560–23572. doi: 10.1021/acsami.8b05853 [DOI] [PubMed] [Google Scholar]
- 24.Blazquez R, Sanchez-Margallo FM, Alvarez V, Uson A, Casado JG. Surgical meshes coated with mesenchymal stem cells provide an anti-inflammatory environment by a M2 macrophage polarization. Acta Biomater. Feb 2016;31:221–230. doi: 10.1016/j.actbio.2015.11.057 [DOI] [PubMed] [Google Scholar]
- 25.Devalliere J, Chang WG, Andrejecsk JW, et al. Sustained delivery of proangiogenic microRNA-132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J. Feb 2014;28(2):908–22. doi: 10.1096/fj.13-238527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo J, Sun W, Kim JP, et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. May 2018;72:35–44. doi: 10.1016/j.actbio.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nileback L, Chouhan D, Jansson R, Widhe M, Mandal BB, Hedhammar M. Silk-Silk Interactions between Silkworm Fibroin and Recombinant Spider Silk Fusion Proteins Enable the Construction of Bioactive Materials. ACS Appl Mater Interfaces. Sep 20 2017;9(37):31634–31644. doi: 10.1021/acsami.7b10874 [DOI] [PubMed] [Google Scholar]
- 28.Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl Med. Mar 2017;6(3):736–747. doi: 10.5966/sctm.2016-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varkey M, Visscher DO, van Zuijlen PPM, Atala A, Yoo JJ. Skin bioprinting: the future of burn wound reconstruction? Burns Trauma. 2019;7:4. doi: 10.1186/s41038-019-0142-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Yao B, Xie J, Fu X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. Mar 1 2016;32:170–177. doi: 10.1016/j.actbio.2015.12.039 [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen AM, Varkey M, Gorkun A, et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng Part A. May 2020;26(9–10):512–526. doi: 10.1089/ten.TEA.2019.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendez JJ, Ghaedi M, Sivarapatna A, et al. Mesenchymal stromal cells form vascular tubes when placed in fibrin sealant and accelerate wound healing in vivo. Biomaterials. Feb 2015;40:61–71. doi: 10.1016/j.biomaterials.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng WL, Qi JTZ, Yeong WY, Naing MW. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication. Jan 23 2018;10(2):025005. doi: 10.1088/1758-5090/aa9e1e [DOI] [PubMed] [Google Scholar]
- 34.Mori N, Morimoto Y, Takeuchi S. Skin integrated with perfusable vascular channels on a chip. Biomaterials. Feb 2017;116:48–56. doi: 10.1016/j.biomaterials.2016.11.031 [DOI] [PubMed] [Google Scholar]
- 35.Cubo N, Garcia M, Del Canizo JF, Velasco D, Jorcano JL. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication. Dec 5 2016;9(1):015006. doi: 10.1088/1758-5090/9/1/015006 [DOI] [PubMed] [Google Scholar]
- 36.Eke G, Mangir N, Hasirci N, MacNeil S, Hasirci V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials. Jun 2017;129:188–198. doi: 10.1016/j.biomaterials.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 37.Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. Dec 2013;19(6):485–502. doi: 10.1089/ten.TEB.2012.0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pourchet LJ, Thepot A, Albouy M, et al. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv Healthc Mater. Feb 2017;6(4)doi: 10.1002/adhm.201601101 [DOI] [PubMed] [Google Scholar]
- 39.Albanna M, Binder KW, Murphy SV, et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci Rep. Feb 12 2019;9(1):1856. doi: 10.1038/s41598-018-38366-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hakimi N, Cheng R, Leng L, et al. Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab Chip. May 15 2018;18(10):1440–1451. doi: 10.1039/c7lc01236e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas SJ, Kramer GC, Herndon DN. Burns: military options and tactical solutions. J Trauma. May 2003;54(5 Suppl):S207–18. doi: 10.1097/01.TA.0000065013.27877.F3 [DOI] [PubMed] [Google Scholar]
- 42.March OP, Kocher T, Koller U. Context-Dependent Strategies for Enhanced Genome Editing of Genodermatoses. Cells. Jan 2 2020;9(1):112. doi: 10.3390/cells9010112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chamorro C, Mencia A, Almarza D, et al. Gene Editing for the Efficient Correction of a Recurrent COL7A1 Mutation in Recessive Dystrophic Epidermolysis Bullosa Keratinocytes. Mol Ther Nucleic Acids. Apr 5 2016;5(4):e307. doi: 10.1038/mtna.2016.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgiadis C, Syed F, Petrova A, et al. Lentiviral Engineered Fibroblasts Expressing Codon-Optimized COL7A1 Restore Anchoring Fibrils in RDEB. J Invest Dermatol. Jan 2016;136(1):284–92. doi: 10.1038/JID.2015.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osborn MJ, Lees CJ, McElroy AN, et al. CRISPR/Cas9-Based Cellular Engineering for Targeted Gene Overexpression. Int J Mol Sci. Mar 22 2018;19(4):946. doi: 10.3390/ijms19040946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh J, Griffin DR, Archang MM, et al. Enhanced In Vivo Delivery of Stem Cells using Microporous Annealed Particle Scaffolds. Small. Sep 2019;15(39):e1903147. doi: 10.1002/smll.201903147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siprashvili Z, Nguyen NT, Gorell ES, et al. Safety and Wound Outcomes Following Genetically Corrected Autologous Epidermal Grafts in Patients With Recessive Dystrophic Epidermolysis Bullosa. JAMA. Nov 1 2016;316(17):1808–1817. doi: 10.1001/jama.2016.15588 [DOI] [PubMed] [Google Scholar]
- 48.Jackow J, Guo Z, Hansen C, et al. CRISPR/Cas9-based targeted genome editing for correction of recessive dystrophic epidermolysis bullosa using iPS cells. Proc Natl Acad Sci U S A. Dec 9 2019;116:201907081. doi: 10.1073/pnas.1907081116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodley DT, Cogan J, Hou Y, et al. Gentamicin induces functional type VII collagen in recessive dystrophic epidermolysis bullosa patients. J Clin Invest. Aug 1 2017;127(8):3028–3038. doi: 10.1172/JCI92707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwieger-Briel A, Kiritsi D, Schempp C, Has C, Schumann H. Betulin-Based Oleogel to Improve Wound Healing in Dystrophic Epidermolysis Bullosa: A Prospective Controlled Proof-of-Concept Study. Dermatol Res Pract. 2017;2017:5068969. doi: 10.1155/2017/5068969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahuja RB, Gupta GK. A four arm, double blind, randomized and placebo controlled study of pregabalin in the management of post-burn pruritus. Burns. Feb 2013;39(1):24–9. doi: 10.1016/j.burns.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 52.Atanasova VS, Pourreyron C, Farshchian M, et al. Identification of Rigosertib for the Treatment of Recessive Dystrophic Epidermolysis Bullosa-Associated Squamous Cell Carcinoma. Clin Cancer Res. Jun 1 2019;25(11):3384–3391. doi: 10.1158/1078-0432.CCR-18-2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M, Duan XP, Li YM, Yang DP, Long YZ. Electrospun nanofibers for wound healing. Mater Sci Eng C Mater Biol Appl. Jul 1 2017;76:1413–1423. doi: 10.1016/j.msec.2017.03.034 [DOI] [PubMed] [Google Scholar]
- 54.Gil ES, Panilaitis B, Bellas E, Kaplan DL. Functionalized silk biomaterials for wound healing. Adv Healthc Mater. Jan 2013;2(1):206–17. doi: 10.1002/adhm.201200192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Shi W, Wu S, et al. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication. Jun 12 2020;12(3):035020. doi: 10.1088/1758-5090/ab906e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freeman FE, Pitacco P, van Dommelen LHA, et al. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv. Aug 2020;6(33):eabb5093. doi: 10.1126/sciadv.abb5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mittapalli VR, Kuhl T, Kuzet SE, et al. STAT3 targeting in dystrophic epidermolysis bullosa. Br J Dermatol. May 2020;182(5):1279–1281. doi: 10.1111/bjd.18639 [DOI] [PubMed] [Google Scholar]
- 58.Nystrom A, Velati D, Mittapalli VR, Fritsch A, Kern JS, Bruckner-Tuderman L. Collagen VII plays a dual role in wound healing. J Clin Invest. Aug 2013;123(8):3498–509. doi: 10.1172/JCI68127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zomer HD, Trentin AG. Skin wound healing in humans and mice: Challenges in translational research. J Dermatol Sci. Apr 2018;90(1):3–12. doi: 10.1016/j.jdermsci.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 60.Jung JP, Lin WH, Riddle MJ, Tolar J, Ogle BM. A 3D in vitro model of the dermoepidermal junction amenable to mechanical testing. J Biomed Mater Res A. Dec 2018;106(12):3231–3238. doi: 10.1002/jbm.a.36519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abaci HE, Guo Z, Coffman A, et al. Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells. Adv Healthc Mater. Jul 2016;5(14):1800–7. doi: 10.1002/adhm.201500936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Condorelli AG, Dellambra E, Logli E, Zambruno G, Castiglia D. Epidermolysis Bullosa-Associated Squamous Cell Carcinoma: From Pathogenesis to Therapeutic Perspectives. Int J Mol Sci. Nov 14 2019;20(22):5707. doi: 10.3390/ijms20225707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lincoln V, Cogan J, Hou Y, et al. Gentamicin induces LAMB3 nonsense mutation readthrough and restores functional laminin 332 in junctional epidermolysis bullosa. Proc Natl Acad Sci U S A. Jul 10 2018;115(28):E6536–E6545. doi: 10.1073/pnas.1803154115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sporrer M, Prochnicki A, Tolle RC, et al. Treatment of keratinocytes with 4-phenylbutyrate in epidermolysis bullosa: Lessons for therapies in keratin disorders. EBioMedicine. Jun 2019;44:502–515. doi: 10.1016/j.ebiom.2019.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng YZ, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. Jul 15 2012;72(14):3522–34. doi: 10.1158/0008-5472.CAN-11-2996 [DOI] [PubMed] [Google Scholar]
- 66.Wimmer M, Zauner R, Ablinger M, et al. A cancer stem cell-like phenotype is associated with miR-10b expression in aggressive squamous cell carcinomas. Cell Commun Signal. Apr 10 2020;18(1):61. doi: 10.1186/s12964-020-00550-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guttmann-Gruber C, Bauer JW, Piñón Hofbauer J. Hereditary bullous diseases: current and innovative models to study the skin blistering disease epidermolysis bullosa. Drug Discovery Today: Disease Models. 2020/December/01/ 2020;32:17–25. doi: 10.1016/j.ddmod.2020.10.001 [DOI] [Google Scholar]
- 68.Sarkiri M, Fox SC, Fratila-Apachitei LE, Zadpoor AA. Bioengineered Skin Intended for Skin Disease Modeling. Int J Mol Sci. Mar 20 2019;20(6)doi: 10.3390/ijms20061407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guerrero-Aspizua S, Conti CJ, Zapatero-Solana E, Larcher F, Rio MD. Current applications for bioengineered skin. In: Laurence J, Baptista P, Atala A, eds. Translating Regenerative Medicine to the Clinic. 1st ed. Academic Press; 2016:chap 107–120. [Google Scholar]
- 70.Lu G, Huang S. Bioengineered skin substitutes: key elements and novel design for biomedical applications. Int Wound J. Aug 2013;10(4):365–71. doi: 10.1111/j.1742-481X.2012.01105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MacNeil S Progress and opportunities for tissue-engineered skin. Nature. Feb 22 2007;445(7130):874–80. doi: 10.1038/nature05664 [DOI] [PubMed] [Google Scholar]
- 72.Kim BS, Ahn M, Cho WW, Gao G, Jang J, Cho DW. Engineering of diseased human skin equivalent using 3D cell printing for representing pathophysiological hallmarks of type 2 diabetes in vitro. Biomaterials. May 2021;272:120776. doi: 10.1016/j.biomaterials.2021.120776 [DOI] [PubMed] [Google Scholar]
- 73.de Rham C, Villard J. Potential and limitation of HLA-based banking of human pluripotent stem cells for cell therapy. J Immunol Res. 2014;2014:518135. doi: 10.1155/2014/518135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Freedman B Equipoise and the ethics of clinical research. N Engl J Med. Jul 16 1987;317(3):141–5. doi: 10.1056/NEJM198707163170304 [DOI] [PubMed] [Google Scholar]
- 75.Cook C, Sheets C. Clinical equipoise and personal equipoise: two necessary ingredients for reducing bias in manual therapy trials. J Man Manip Ther. Feb 2011;19(1):55–7. doi: 10.1179/106698111X12899036752014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janis JE, Kwon RK, Attinger CE. The new reconstructive ladder: modifications to the traditional model. Plast Reconstr Surg. Jan 2011;127 Suppl 1:205S–212S. doi: 10.1097/PRS.0b013e318201271c [DOI] [PubMed] [Google Scholar]


