Abstract
Background
Although emerging data during the SARS-CoV-2 pandemic have demonstrated robust messenger RNA vaccine–induced immunogenicity across populations, including pregnant and lactating individuals, the rapid waning of vaccine-induced immunity and the emergence of variants of concern motivated the use of messenger RNA vaccine booster doses. Whether all populations, including pregnant and lactating individuals, will mount a comparable response to a booster dose is not known.
Objective
This study aimed to profile the humoral immune response to a COVID-19 messenger RNA booster dose in a cohort of pregnant, lactating, and nonpregnant age-matched women.
Study Design
This study characterized the antibody response against ancestral Spike and Omicron in a cohort of 31 pregnant, 12 lactating, and 20 nonpregnant age-matched controls who received a BNT162b2 or messenger RNA-1273 booster dose after primary COVID-19 vaccination. In addition, this study examined the vaccine-induced antibody profiles of 15 maternal-to-cord dyads at delivery.
Results
Receiving a booster dose during pregnancy resulted in increased immunoglobulin G1 levels against Omicron Spike (postprimary vaccination vs postbooster dose; P=.03). Pregnant and lactating individuals exhibited equivalent Spike-specific total immunoglobulin G1, immunoglobulin M, and immunoglobulin A levels and neutralizing titers against Omicron compared with nonpregnant women. Subtle differences in Fc receptor binding and antibody subclass profiles were observed in the immune response to a booster dose in pregnant vs nonpregnant individuals. The analysis of maternal and cord antibody profiles at delivery demonstrated equivalent total Spike-specific immunoglobulin G1 in maternal and cord blood, yet higher Spike-specific FcγR3a-binding antibodies in the cord relative to maternal blood (P=.002), consistent with the preferential transfer of highly functional immunoglobulin. Spike-specific immunoglobulin G1 levels in the cord were positively correlated with the time elapsed since receiving the booster dose (Spearman R, .574; P=.035).
Conclusion
Study data suggested that receiving a booster dose during pregnancy induces a robust Spike-specific humoral immune response, including against Omicron. If boosting occurs in the third trimester of pregnancy, higher Spike-specific cord immunoglobulin G1 levels are achieved with greater time elapsed between receiving the booster and delivery. Receiving a booster dose has the potential to augment maternal and neonatal immunity.
Key words: antibodies, booster, COVID-19, humoral immune response, messenger RNA vaccine, immune response, immunity, SARS-CoV-2, transplacental antibody transfer, vaccination
AJOG at a Glance.
Why was this study conducted?
This study aimed to comprehensively profile SARS-CoV-2–specific immune responses to a messenger RNA (mRNA) COVID-19 booster dose in pregnant, lactating, and nonpregnant individuals and to assess SARS-CoV-2–specific antibody transfer from mother to neonate.
Key findings
Boosting in pregnant individuals induced comparable immunoglobulin (Ig)G, IgA, and IgM against ancestral and Omicron Spike compared with nonpregnant individuals, with differences in antibody profiles reflecting the unique pregnant immune state. For individuals who received primary vaccination during pregnancy, boosting increased IgG1 against Omicron. Receiving a booster in the third trimester of pregnancy resulted in the efficient transfer of highly functional antibodies to the neonate, and levels were positively correlated with the time elapsed between boosting and delivery.
What does this add to what is known?
This study has demonstrated comparable immunologic responses to receiving an mRNA booster dose in pregnant, lactating, and nonpregnant individuals and may add to the protection of the mother and infant against Omicron.
Introduction
Pregnant individuals are particularly vulnerable to COVID-19, as they are at increased risk of severe disease and adverse pregnancy outcomes, including stillbirth.1, 2, 3, 4 Despite Centers for Disease Control and Prevention and American College of Obstetricians and Gynecologists recommendations encouraging all individuals who are pregnant, recently pregnant, or considering pregnancy to receive a COVID-19 vaccine,5 , 6 vaccine coverage of pregnant individuals has lagged behind that of the general adult population, with 69% of pregnant individuals vaccinated as of February 2022, compared with 82% of the nonpregnant population.7 , 8 With the emergence of SARS-CoV-2 variants of concern and evidence of waning vaccine-induced immunity in the general population, messenger RNA (mRNA) vaccine boosters are now recommended for all adults, including pregnant individuals, at least 5 months after completion of the initial vaccine series.9 However, as of late February 2022, only 49% of fully vaccinated pregnant individuals had received a booster dose, with uptake the lowest in non-Hispanic Black and Hispanic individuals.10
Importantly, recent data from Israel indicate improved effectiveness against severe disease after the third and even a fourth booster dose in the general adult population.11 , 12 Whether pregnant and lactating individuals, who can exhibit dampened immunity to vaccines,13 mount a comparably protective response to the booster dose is not known. Studies of pregnant and lactating women receiving COVID-19 vaccination demonstrated robust immunogenicity to mRNA vaccines, comparable with nonpregnant women.14 , 15 However, comprehensive profiling of the immune response to primary vaccination in pregnant and lactating women revealed reduced Fc receptor (FcR) binding and subclass selection differences, suggesting that the development of a fully mature immune response may be delayed in these groups.16
To determine whether pregnant or lactating individuals respond effectively to a COVID-19 booster dose, we comprehensively profiled the vaccine-induced antibody response against ancestral Spike and Omicron (B.1.1.529) in a cohort of 63 individuals (31 pregnant, 12 lactating, and 20 nonpregnant age-matched control women) who received a BNT162b2 or mRNA-1273 booster dose. In addition, we characterized the transfer of vaccine-induced antibodies in 15 maternal-cord dyads at delivery.
Materials and Methods
Participant recruitment and study design
Women at 2 tertiary care hospitals were approached for enrollment in an institutional review board–approved (protocol #2020P003538) COVID-19 pregnancy biorepository study. Eligible women were pregnant, lactating, or nonpregnant and of reproductive age (18 to 45 years) and receiving a COVID-19 mRNA vaccine booster dose (August 2021 to December 2021). Eligible participants were identified by practitioners at the participating hospitals or were self-referred. Blood was collected approximately 4 weeks after the booster dose and/or at delivery. For participants who delivered during the study period (n=15), maternal and umbilical cord blood were collected at delivery.
Antigen-specific isotype titer and Fc receptor binding
Antigen-specific isotype titer and FcR binding were measured by a multiplex Luminex, as previously described.17 Briefly, carboxylated MagPlex microspheres were covalently linked to antigen by ester-N-hydroxysulfosuccinimide (NHS) linkages using sulfo-NHS (Thermo Fisher Scientific, Waltham, MA) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC; Thermo Fisher Scientific, Waltham, MA). Immune complexes were formed by adding antigen-coupled microspheres and appropriately diluted plasma (1:100 for immunoglobulin [Ig]G2, IgG3, IgA1, and IgM; 1:500 for IgG1; and 1:1000 for FcRs). Of note, 384-well plates were incubated overnight at 4°C, shaking at 700 rpm. Plates were washed with assay buffer (1× phosphate-buffered saline with 0.1% bovine serum albumin or 0.02% Tween-20). Phycoerythrin (PE) coupled mouse antihuman detection antibodies were added to detect antigen-specific isotype titer (Southern Biotech, Birmingham, AL). To detect antigen-specific FcR-binding, Avi-tagged FcRs (Duke Human Vaccine Institute, Durham, NC) were biotinylated with a BirA500 kit (Avidity LLC, Aurora, CO). The biotinylated FcRs were fluorescently tagged using streptavidin-PE (Agilent, Santa Clara, CA) and added to immune complexes. Fluorescence was read using an iQue (IntelliCyt Corporation, Albuquerque, NM). Data have been reported as median fluorescence intensity (MFI). The assay was run in duplicate, and the average of the replicates was reported.
SARS-CoV-2 Omicron pseudovirus neutralization assay
Omicron spike pseudovirus neutralization assay was performed as previously described.18 A pseudovirus encoding Omicron Spike was produced by transfecting 293T cells with an Omicron Spike expression plasmid, a lentiviral backbone encoding CMV-Luciferase-IRES-ZsGreen and lentiviral helper plasmids. Diluted plasma was incubated with the Omicron pseudovirus for 1 hour, followed by the addition of 293T-ACE2 cells. Cells and pseudovirus were incubated at 37°C for 48 hours. Cells were lysed, and luciferase expression was assessed using a SpectraMax L luminometer (Molecular Devices, San Jose, CA). The NT50 values were analyzed in GraphPad Prism (version 8.0; GraphPad Software, Inc, San Diego, CA).
Statistical analysis
Statistical analysis was performed in R (version 4.0.0; https://developer.r-project.org/) or GraphPad Prism (version 8.0). Before multivariate analysis, Luminex data were log10-transformed, and all data were centered and scaled. For univariate analysis, significance was determined by Kruskal-Wallis or Mann-Whitney U test. For multivariate analysis, the systemseRology R package (version 1.0; https://github.com/LoosC/systems_seRology) was used. Least absolute shrinkage and selection operator (LASSO) feature selection was performed 100 times, and features selected were those chosen at least 50% of the repetitions performed.
Results
Similar vaccine-induced Spike-specific antibodies in pregnant, lactating, and nonpregnant women after booster dose
Cohort demographic characteristics and clinical information for the 31 pregnant, 12 lactating, and 20 nonpregnant age-matched individuals included in the study are reported in the Table . There was no difference in age, race, or ethnicity among groups. Of note, 3 individuals (2 pregnant and 1 nonpregnant) had a known previous SARS-CoV-2 infection. Although postbooster samples were collected at least 2 weeks from receipt of the booster dose, samples from nonpregnant individuals were collected approximately 10 days later than pregnant and lactating individuals. Of the 31 pregnant participants, 24 (77%) had completed primary vaccination before conception. Pregnant individuals delivering during the study period (n=15) had received the booster dose between 32 and 38 weeks of gestation.
Table.
Demographic characteristics and clinical information of the study cohort
| Characteristics | Lactating (n=12) | Nonpregnant (n=20) | Pregnant (n=31) | P value |
|---|---|---|---|---|
| Age (y) | 33 (4) | 37 (8) | 34 (4) | .160 |
| Race (%) | ||||
| Asian | 0 (0) | 1 (5) | 1 (3) | .650 |
| Black or African American | 1 (8) | 2 (10) | 0 (0) | |
| Other | 0 (0) | 1 (5) | 1 (3) | |
| Unknown or not reported | 0 (0) | 1 (5) | 1 (3) | |
| White | 11 (92) | 15 (75) | 28 (90) | |
| Ethnicity (%) | ||||
| Hispanic | 0 (0) | 0 (0) | 2 (6) | .247 |
| Non-Hispanic | 12 (100) | 18 (90) | 29 (94) | |
| Unknown or not reported | 0 (0) | 2 (10) | 0 (0) | |
| Primary vaccine or vaccine series (%) | ||||
| Ad26.COV2.S | 1 (8) | 0 (0) | 6 (19) | .147 |
| mRNA-1273 | 5 (42) | 7 (35) | 14 (45) | |
| BNT162b2 | 6 (50) | 13 (65) | 11 (35) | |
| Primary vaccine series was received in pregnancy (%) | 11 (92) | — | 7 (23) | |
| Booster vaccine received (%) | ||||
| mRNA-1273 | 7 (58) | 7 (35) | 18 (58) | .251 |
| BNT162b2 | 5 (42) | 13 (65) | 13 (42) | |
| Time from completion of primary vaccine series to booster dose (d) | 246 (36) | 264 (26) | 241 (37) | .076 |
| Time from booster dose to sample collection (d) | 36 (17) | 45 (13) | 35 (13) | .033 |
| Gestational age at booster dose (completed wk)a | — | — | 28 (11) | |
| Time from booster dose to delivery (d)a | — | — | 61 (83) | |
| Previous COVID-19 infection (%) | 0 (0) | 1 (5) | 2 (6) | 1.0 |
Continuous data presented as mean (standard deviation), and categorical data are presented as number (percentage).
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Pregnant cohort only.
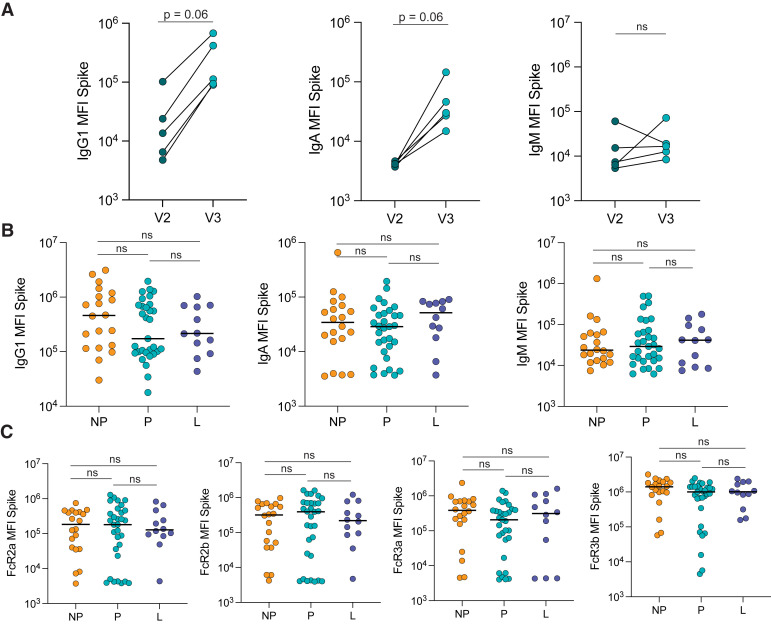
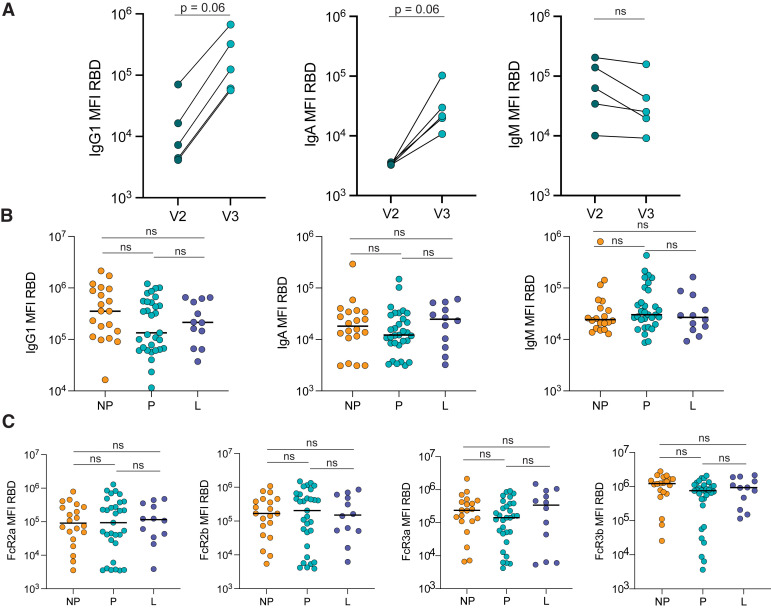
To determine how the antibody response to a booster dose compared with the response produced after the primary vaccine series in pregnant individuals, we plotted the antibody response in a subset of 5 pregnant individuals 2 to 6 weeks after completion of primary mRNA vaccination (V2) and the response in the same individuals 4 weeks after the booster dose (V3). The booster dose induced higher IgG1 and IgA levels than the primary vaccination, against both the ancestral SARS-CoV-2 Spike (IgG1 V2 vs V3: P=.06; IgA V2 vs V3: P=.06) and the receptor-binding domain (RBD) (IgG1 V2 vs V3: P=.06; IgA V2 vs V3: P=.06), and a stable IgM response against Spike and RBD (Figure 1 , A; Figure 2 , A).
Figure 1.
Similar Spike-specific antibody responses after boosting in pregnant, lactating, and non-pregnant
A, The dot plots show the peak IgG1, IgA, and IgM response against Spike in 5 pregnant individuals after receiving the second dose of a primary mRNA vaccine series (V2) and after the booster dose (V3). The lines connect samples from the same individual. Significance was determined by a Wilcoxon signed-rank test. The differences did not reach statistical significance (P=.06 or ns for P>.1 is indicated). B, The dot plots show IgG1, IgA, and IgM levels against Spike in NP, P, and L individuals. Horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparison was significant (ns). C, The dot plots show the FcR-binding of antibodies against Spike in NP, P, and L individuals. Horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparison was significant (ns).
FcR, Fc receptor; IgA, immunoglobulin A; IgG1, immunoglobulin G1; IgM, immunoglobulin M; L, lactating; mRNA, messenger RNA; NP, nonpregnant; ns, not significant; P, pregnant.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Figure 2.
Similar RBD-specific antibody responses after boosting in pregnant, lactating, and non-pregnant
A, The dot plots show the peak IgG1, IgA, and IgM response against RBD in 5 pregnant individuals after receiving the second dose of a primary mRNA vaccine series (V2) and after the booster dose (V3). The lines connect samples from the same individual. The significance was determined by a Wilcoxon signed-rank test. The differences did not reach statistical significance (P=.06 or ns for P>.1 is indicated). B, The dot plots show the IgG1, IgA, and IgM-titer against RBD in NP, P, and L individuals. The horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparison was significant (ns). C, The dot plots show the FcR-binding of antibodies against RBD in NP, P, and L individuals. The horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparison was significant (ns).
FcR, Fc receptor; IgA, immunoglobulin A; IgG1, immunoglobulin G1; IgM, immunoglobulin M; L, lactating; mRNA, messenger RNA; NP, nonpregnant; ns, not significant; P, pregnant; RBD, receptor-binding domain.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Previous data from our group revealed that after the primary mRNA vaccine series, pregnant and lactating women induced similar IgG, IgA, and IgM levels after the second dose but slower evolution of Fcγ receptor (FcγR)-binding antibodies to Spike than nonpregnant women.16 Therefore, we aimed to determine whether a booster dose could compensate for this observed deficit in immunity observed in pregnant individuals. We observed a slightly, but not significantly, lower IgG1 against Spike (pregnant: 1.7×105 MFI; nonpregnant: 4.6×105 MFI; lactating: 2.2×105 MFI) and RBD (pregnant: 1.3×105 MFI; nonpregnant: 3.5×105 MFI; lactating: 2.1×105 MFI) in pregnant women than in nonpregnant and lactating women, although similar IgA and IgM levels were observed in all 3 groups (Figure 1, B; Figure 2, B). Moreover, FcγR binding against Spike and RBD was nearly equivalent across the groups (Figure 1, C; Figure 2, C).
A booster dose induces an increase in Omicron-specific immunoglobulin G1 and an equivalent Omicron-specific antibody response between pregnant and nonpregnant individuals
Emerging data point to a crucial role for boosting not only in augmenting the absolute amount of antibodies to ancestral Spike but also in improving the breadth of the response to variants of concern (VOCs).18, 19, 20 In particular, the ability of the mRNA booster dose to protect against Omicron, which has become the predominant strain in the United States, is crucial. Boosting resulted in a robust increase in Omicron Spike-specific IgG1 (median IgG1 V2 vs V3: 1.4×104 MFI vs 1.1×105 MFI; P=.03) but not in IgA or IgM (Figure 3 , A). The comparison of Omicron-specific binding profiles across nonpregnant, pregnant, and lactating women pointed to slightly lower Omicron Spike-specific IgG1 in pregnant individuals (pregnant: 1.2×105 MFI; nonpregnant: 2.0×105 MFI; lactating: 1.5×105 MFI) but equivalent IgA and IgM titers and neutralizing activity across the 3 groups (Figure 3, B and C). Omicron Spike-specific Fcγ receptor binding was equivalent among groups (Figure 3, D).
Figure 3.
Similar Omicron-Spike specific antibody responses after boosting in pregnant, lactating, and non-pregnant
A–B, The dot plots show the peak IgG1, IgA, and IgM response against Omicron Spike in 5 pregnant individuals after receiving the second dose of a primary mRNA vaccine series (V2) and after the booster dose (V3). The lines connect samples from the same individual. Significance was determined by a Wilcoxon signed-rank test, asterisk represents P<.05 (ns). B, The dot plots show IgG1, IgA, and IgM levels against Omicron Spike in NP, P, and L individuals. The horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparison was significant (ns). C, The dot plots show the NT50 against an Omicron Spike pseudovirus in NP, P, and L individuals. The horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparison was significant (ns). D, The dot plots show the FcR-binding of antibodies against Omicron Spike in NP, P, and L individuals. The horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. No comparison was significant.
FcR, Fc receptor; IgA, immunoglobulin A; IgG1, immunoglobulin G1; IgM, immunoglobulin M; L, lactating; mRNA, messenger RNA; NP, nonpregnanct; ns, not significant; P, pregnant.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Differences in antibody class switching are observed in pregnant individuals
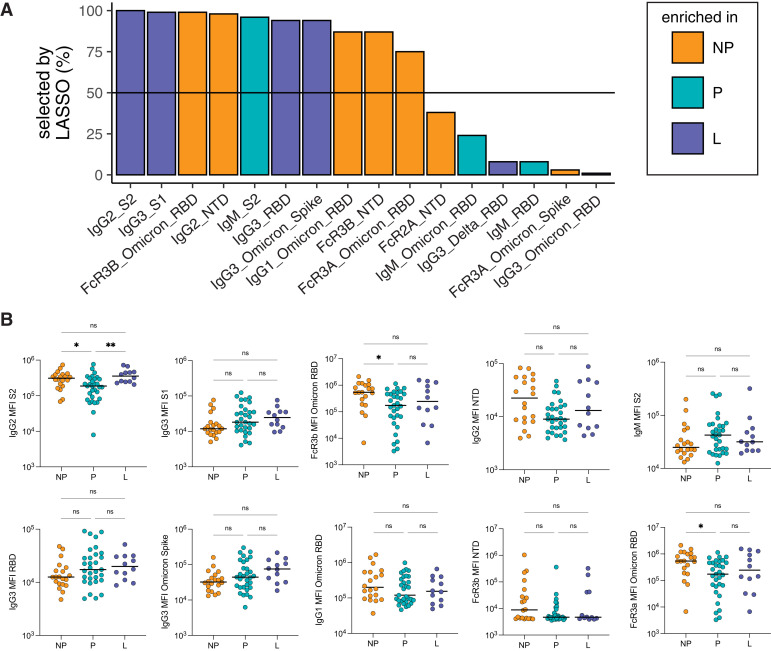
The observation of subtle univariate differences in vaccine-induced IgG1 responses after boosting (Figure 1) prompted the further dissection of differences by multivariate modeling. First, to understand if certain antibody characteristics were different among the 3 groups, we used LASSO to define the minimal set of antibody features that were the most different among groups. LASSO is a regression technique that is used to select the minimal set of features that provide the best separation between 2 groups.21 From this analysis, LASSO selected 10 of 75 antibody features per sample that separated the antibody profiles in each of the 3 groups (Figure 4 , A). Next, we performed a univariate analysis for each of the LASSO-selected features (Figure 4, B). From this analysis, we identified that there was an elevation of FcγR binding or IgG1 against Omicron RBD and FcγR binding or IgG2 ancestral strain N-terminal domain (NTD) in nonpregnant vs pregnant individuals. Moreover, we observed a shift toward an elevation of IgG3 in lactating and pregnant individuals and a slight elevation of IgM in pregnant individuals. Overall, these data may suggest a more robust vaccine-induced selection of preexisting memory B cells and antibody class switching in nonpregnant individuals vs enhanced selection of naïve B-cell responses in pregnant individuals (see Comment). The significant increase in IgG2 S2 in lactating individuals vs pregnant individuals suggests a return toward the nonpregnant immune state during lactation.
Figure 4.
Boosting induces differences in antibody profiles between pregnant and non-pregnant
A, LASSO was used to select the antibody features that separated the 3 groups: NP, P, and L individuals. LASSO was performed 100 times, and the barplot shows the percentage that each feature was selected (for features selected at least once). The horizontal line represents the 50% cutoff used to define the top features. The color of the bar represents the group in which the feature is the most elevated. B, The dot plots show the univariate analysis of the LASSO-selected features (A). The horizontal line represents the median for each group. Significance was determined by a Kruskal-Wallis test. P values were corrected for multiple testing by the Benjamini-Hochberg method, single asterisk represents P<.05 and double asterisks represent P<.01.
L, lactating; LASSO, least absolute shrinkage and selection operator; NP, nonpregnant; P, pregnant.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Boosting results in transfer of antibodies to the cord in a time-dependent manner
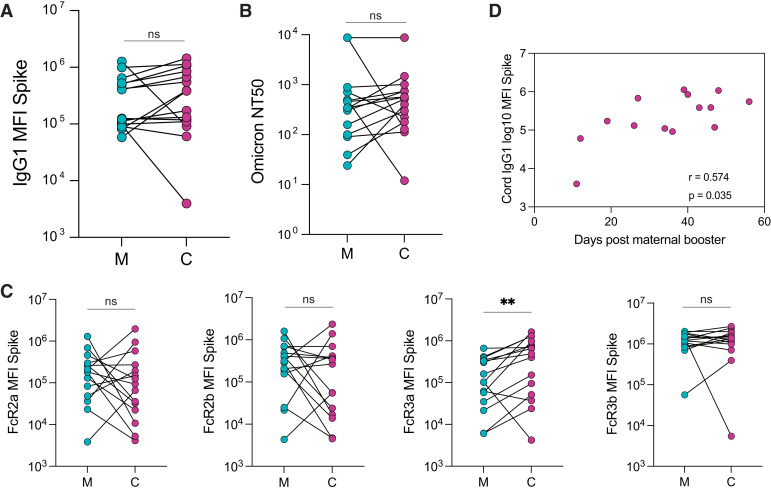
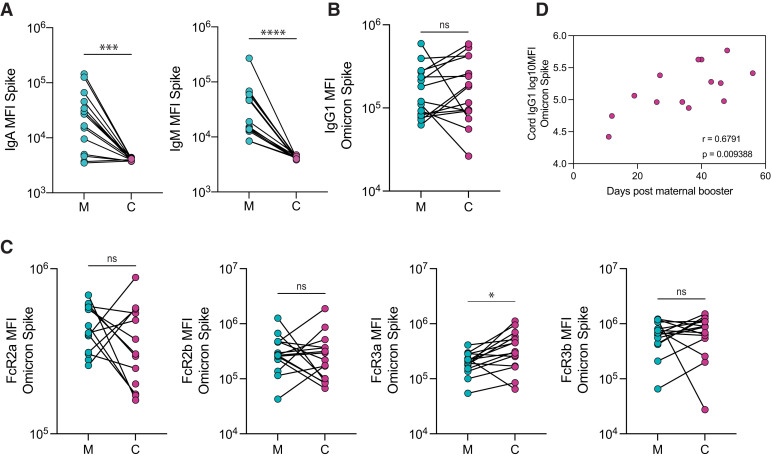
IgG1 levels against ancestral Spike in matched maternal and cord plasma obtained at delivery from 15 pregnant individuals who delivered during the study period were equivalent (Figure 5 , A). Similarly, we observed equivalent levels of neutralizing antibodies against Omicron in both maternal and cord blood (Figure 5, B). IgA and IgM against ancestral Spike were not transferred to the cord, as expected (Figure 6 , A). The analysis of FcγR-binding antibodies revealed that although levels of FcγR2a-, FcγR2b-, and FcγR3b-binding antibodies against Spike were equivalent in maternal and cord blood, a higher concentration of FcγR3a-binding antibodies was observed in cord blood relative to maternal blood (cord: 4.9×105 MFI: maternal: 1.0×105 MFI; P=.002), consistent with efficient transplacental transfer of these antibodies (Figure 5, C). We observed similar transfer patterns of antibodies against Omicron Spike, with equivalent levels of Omicron Spike-specific IgG1 in maternal and cord plasma (Figure 6, B), and a higher concentration of FcγR3a-binding antibodies in the cord plasma than in maternal plasma (cord: 3.1×105; maternal: 2.1×105; P=.007) (Figure 6, C).
Figure 5.
The transfer of Spike-specific antibodies to the cord after third trimester vaccination
A, The dot plots show the IgG1 against Spike in M and C blood. The lines connect matched maternal-to-cord dyads (n=15). Significance was determined by a Wilcoxon signed-rank test (ns). B, The dot plots show the NT50 against an Omicron Spike pseudovirus in M and C blood. The lines connect matched maternal-to-cord dyads (n=15). Significance was determined by a Wilcoxon signed-rank test (ns). C, The dot plots show the FcR-binding titer against Spike in M and C blood. The lines connect matched maternal-to-cord dyads (n=15). Significance was determined by a Wilcoxon signed-rank test followed by a Benjamini-Hochberg correction for multiple testing, double asterisks represent P<0.01 (ns). D, The scatter plot shows the correlation of cord IgG1 titer against Spike vs days from maternal booster to delivery. The r value reflects a Spearman correlation. Of note, 1 dyad was excluded because of missing information about time from booster to delivery.
C, cord blood; FcR, Fc receptor; IgG1, immunoglobulin G1; M, maternal blood; ns, not significant.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Figure 6.
Omicron Spike-specific antibody transfer to the umbilical cord after third trimester boosting
A, The dot plots show IgA and IgM against Spike in M and C blood. The lines connect matched maternal-to-cord dyads (n=15), triple asterisks denote P<.001 and four asterisks denote P<.0001 (ns). B–C, The dot plots show the IgG1 (B) and FcR-binding (C) titer against Omicron Spike in M and C blood. The lines connect matched maternal-to-cord dyads (n=15). Significance was determined by a Wilcoxon signed-rank test followed by a Benjamini-Hochberg correction for multiple testing, single asterisk denotes P<.05 (ns). D, The scatter plot shows the correlation of log-transformed cord IgG1 levels against Omicron Spike vs days from maternal booster to delivery. The r value reflects a Spearman correlation.
C, cord blood; FcR, Fc receptor; IgA, immunoglobulin A; IgG1, immunoglobulin G1; IgM, immunoglobulin M; M, maternal blood; ns, not significant.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Ancestral Spike-specific IgG1 levels in the cord were positively correlated with the time elapsed since receiving the booster dose (r=0.57; P=.035) (Figure 5, D). Furthermore, Omicron Spike-specific IgG1 cord levels were significantly positively correlated with time elapsed since boosting (r=0.68; P=.009) (Figure 6, D).
Comment
Principal findings
Here, we showed that receiving an mRNA booster dose induces equivalent IgG1, IgA, and IgM responses to ancestral and Omicron Spike in pregnant, lactating, and nonpregnant individuals. For women who received primary vaccination during pregnancy, a booster dose given in the third trimester of pregnancy significantly increased IgG1 levels against Omicron. Multivariate analysis revealed differences in Spike-specific epitope coverage and antibody class switching unique to pregnancy. Third-trimester boosting resulted in equivalent maternal and cord IgG1 levels, with the most efficient transfer of FcγR3a-binding IgG1 to the cord. In individuals boosted in the third trimester of pregnancy, cord IgG1 levels against ancestral Spike and Omicron were positively correlated with increasing time from boost to delivery. Overall, the results suggested that boosting in pregnancy has the potential to augment SARS-CoV-2 immune protection in pregnant individuals and their neonates, particularly against Omicron.
Results in the context of what is known
Although previously regarded as a generalized immune tolerant state, pregnancy is marked by immunomodulatory changes aimed at balancing wound healing and pathogen surveillance, with bidirectional immune crosstalk between the mother and the fetus resulting in both immune tolerance, and protection of the pregnancy from potential invading pathogens.22, 23, 24, 25, 26 Previous work from our group has demonstrated subtle alterations in the humoral response to COVID-19 vaccines in pregnant individuals compared with nonpregnant individuals and differences by trimester of vaccination.16 , 27 Similarly, pregnant individuals display alterations in their cellular response to SARS-CoV-2 compared with nonpregnant individuals,28 and pregnancy-specific alterations in both humoral and cellular immunity are important to consider when evaluating response to vaccines. Here, the primary differences observed in the humoral immune response between pregnant and nonpregnant individuals after boosting were related to differences in the breadth of epitopes recognized and isotype and subclass levels. Specifically, nonpregnant women exhibited a broader targeting of the NTD and S2 domains by functional antibodies, both of which may play a crucial role in the general immune response to the Spike antigen. These antibody profiles may confer enhanced immunity in the event of significant mutation in the RBD by VOCs,29 and nonneutralizing protection against the disease should transmission occur.30 Whether additional boosters, or alternative vaccine platforms, might drive enhanced epitope coverage in pregnancy and breach the immunodominance of RBD will be important to assess. However, the observed enhancement in immunity to RBD is likely key to the augmented protection against VOCs induced by boosting during pregnancy.
In addition, the significant differences in antibody subclass or isotype selection across nonpregnant and pregnant women in response to boosting may point to a difference in booster-induced B-cell selection between pregnant and nonpregnant individuals. Specifically, B-cell class switching progresses from IgM to IgG3 > IgG1 > IgA > IgG2 > IgG4.31 The selective induction of more functional IgG1 and IgG2 in nonpregnant women points to enhanced functionalization or class switching in memory IgG B cells. Conversely, the selection of largely IgM and IgG3 responses in pregnant women points to either (1) the selective induction of naïve (new) B-cell responses in pregnancy or (2) a blockade of further IgG class switching and the interruption of germinal center memory B-cell activation and expansion. Given that IgM and IgG3 have potent antimicrobial and antiviral activity because of their inherent higher affinity for complement and FcRs, respectively,32 , 33 these data may point to a unique hallmark of the immune state that emerges during pregnancy. Interestingly, our data suggested that lactating women exhibit an intermediate profile between pregnant and nonpregnant women.
Boosting in the third trimester of pregnancy resulted in 1:1 transplacental transfer of total Spike-specific IgG1 to the cord, as evidenced by equivalent levels in the maternal and cord blood at delivery. Although maximal transfer efficiency was not observed for Spike-specific IgG1, that is, higher antibody concentrations in cord blood relative to maternal blood,34, 35, 36 this is likely due, in part, to the relatively short interval between boost and delivery (2–8 weeks). However, we did observe the selective, efficient transfer of FcγR3a-binding antibodies, which are able to activate natural killer (NK) cells, the most mature and functional innate immune cell subset present in the neonate at birth.36 Recent data on boosting in pregnancy suggests that compared with natural infection or primary mRNA vaccination in the third trimester of pregnancy, receiving a booster dose can result in greater maternal and cord IgG titers against the ancestral SARS-CoV-2 Spike at delivery.37 Expanding on these data, our results demonstrate that for pregnant individuals vaccinated before pregnancy or in early pregnancy, boosting augments IgG1 against ancestral Spike and Omicron, and for those boosted in the third trimester of pregnancy, boosting drives the preferential transfer of highly functional FcγR3a-binding IgG1 to the cord, with greater cord antibody levels observed with increasing time from booster dose to delivery.
Clinical implications
Here, the data reported demonstrating comparable overall immunogenicity of the booster dose in pregnant and lactating individuals compared with nonpregnant individuals may help inform uptake of boosters among these high-risk groups, which remain vulnerable to infection with emerging VOCs that can escape neutralization.
Beyond the impact of vaccination on driving pathogen-specific immunity to protect pregnant individuals, vaccine-induced antibody transfer via the placenta is a crucial means to provide immunity to the infant.38 , 39 Receiving a primary COVID-19 mRNA vaccine series during pregnancy was associated with a reduction in newborn hospitalization from COVID-19 in the first 6 months of life,40 related to persistent maternal IgG in the newborn circulation.41 Here, compared with pregnant individuals who received a primary vaccination, pregnant individuals who received a booster dose demonstrated an increase in IgG1 levels against Omicron and preferential transfer of NK cell-activating FcγR3a-binding antibodies to the cord. In the subset of maternal-neonatal dyads analyzed, in which a booster dose was received in the third trimester of pregnancy, the highest cord IgG1 level was observed with increased time from boost to delivery. Because COVID-19 vaccines are only recommended for individuals 5 years and older, and infants under 6 months of age will likely not receive a COVID-19 vaccination in the near future, strategies that augment maternal vaccine-induced antibody transfer and optimize infant protection against emerging VOCs are extremely important.
Research implications
These data pointing to subtle alterations in epitope targeting and subclass or isotype selection in pregnancy suggest that future studies investigating both humoral and cellular immune responses to additional boosters or novel vaccines will be important to gain a comprehensive understanding of how the immune response to vaccination is altered in pregnancy. Future investigation into the impact of boosting across all trimesters on the maternal immune response and antibody transfer efficiency at delivery will be important, as will assessment of heterologous boost (receiving a booster that does not match the primary vaccine received) vs homologous boost in pregnant vs nonpregnant individuals.
Strengths and limitations
Leveraging the systems serology approach, we were able to comprehensively profile the immune response to COVID-19 mRNA boosting across pregnant, lactating, and nonpregnant age-matched controls, enabling both broad and deep assessment of the humoral response to boosting against both ancestral Spike and Omicron. Data on boosting in pregnancy, particularly on the specificity of the antibody response to Omicron, remain extremely limited.
Our study had several limitations. The study population was predominantly White and non-Hispanic, consistent with well-documented reduced initial vaccine uptake in marginalized racial and ethnic communities, and may limit the generalizability of our findings. An approximately 10-day difference in time from booster dose administration to sample collection in nonpregnant women was observed but is unlikely to be meaningful, as all samples were collected at least 2 weeks the booster dose was received, when peak responses are expected to occur. All pregnant individuals who delivered during the study period received the booster dose in the third trimester of pregnancy, precluding comparisons of transplacental transfer efficiency by trimester of boosting. Most pregnant individuals in this study were vaccinated before conception, limiting our ability to compare the booster response between individuals vaccinated during or before pregnancy. However, the data in our study were most applicable to booster-eligible individuals who are currently pregnant or considering pregnancy in the United States, as nearly 70% of reproductive-aged women have completed primary vaccination.42
Conclusions
Study data have suggested that COVID-19 boosting in pregnant and lactating individuals induces a robust humoral immune response against ancestral and Omicron Spike, comparable with that observed in nonpregnant individuals. Moreover, antibodies were transferred to the cord in a time-dependent manner, suggesting that boosting earlier in pregnancy may be beneficial both for augmenting immunity in the pregnant individual and for optimal transfer of immunity to the infant.
Footnotes
C.A. and L.L.S. contributed equally to this work.
G.A. and A.G.E. jointly supervised this work.
This study received funding from the Eunice Kennedy ShriverNational Institute of Child Health and Human Development (grant numbers 1R01HD100022-01 and 3R01HD100022-02S2 [A.G.E.] and grant number 1K12HD103096 [L.L.S.]); the National Institute of Allergy and Infectious Diseases (grant numbers U19AI167899-01 [G.A. and A.G.E.] and grant numbers 3R37AI080289-11S1 [G.A.], R01AI146785, U19AI42790-01, and U19AI135995-02 [A.G.E.]); the March of Dimes Grant (grant number 6-FY20-223 [A.G.E.]); the National Institutes of Health/National Heart, Lung, and Blood Institute (grant number K08HL1469630-03 and 3K08HL146963-02S1 [K.J.G.]); the Ragon Institute of MGH, MIT and Harvard and the MGH ECOR Scholars award (G.A.); the Nancy Zimmerman, SAMANA Kay MGH Research Scholars award (G.A.); an anonymous donor, the Massachusetts Consortium on Pathogen Readiness (grant numbers 1U01CA260476-01 and CIVIC5N93019C00052 [G.A.]); the Gates Foundation Global Health Vaccine Accelerator Platform funding (grant number OPP1146996 and INV-001650 [G.A.]); and the Musk Foundation.
K.J.G. has consulted for Illumina, BillionToOne, Aetion, and Roche outside the scope of the submitted work. G.A. is the founder of SeromYx. A.G.E. reported serving as a medical advisor for Mirvie. A.F. reported serving as a cofounder of and owning stock in Alba Therapeutics and serving on scientific advisory boards for NextCure and Viome outside the submitted work. All other authors report no conflict of interest.
Cite this article as: Atyeo C, Shook LL, Nziza N, et al. COVID-19 booster dose induces robust antibody response in pregnant, lactating, and nonpregnant women. Am J Obstet Gynecol 2023;228:68.e1-12.
Supplementary Data
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
References
- 1.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodworth K.R., Olsen E.O., Neelam V., et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSisto C.L., Wallace B., Simeone R.M., et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1640–1645. doi: 10.15585/mmwr.mm7047e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . 2022. COVID-19 vaccines while pregnant or breastfeeding.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Available at: [Google Scholar]
- 6.The American College of Obstetricians and Gynecologists . 2021. COVID-19 vaccination considerations for obstetric–gynecologic care.https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care Available at: [Google Scholar]
- 7.Blakeway H., Prasad S., Kalafat E., et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226:236.e1–236.e14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention COVID-19 vaccination among pregnant people aged 18-49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink,∗ United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women Available at:
- 9.Centers for Disease Control and Prevention . 2021. COVID-19 vaccine booster.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html Available at: [PubMed] [Google Scholar]
- 10.Razzaghi H., Meghani M., Crane B., et al. Receipt of COVID-19 booster dose among fully vaccinated pregnant individuals aged 18 to 49 years by key demographics. JAMA. 2022;327:2351–2354. doi: 10.1001/jama.2022.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. 2022;386:1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421–2430. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kay A.W., Blish C.A. Immunogenicity and clinical efficacy of influenza vaccination in pregnancy. Front Immunol. 2015;6:289. doi: 10.3389/fimmu.2015.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray K.J., Bordt E.A., Atyeo C., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225:303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collier A.Y., McMahan K., Yu J., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atyeo C., DeRiso E.A., Davis C., et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abi8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown E.P., Dowell K.G., Boesch A.W., et al. Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles. J Immunol Methods. 2017;443:33–44. doi: 10.1016/j.jim.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Then E., Lucas C., Monteiro V.S., et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Zhao X., Song J., et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microbes Infect. 2022;11:477–481. doi: 10.1080/22221751.2022.2030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc B. 2011;73:273–282. [Google Scholar]
- 22.Wegmann T.G., Lin H., Guilbert L., Mosmann T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 23.Trowsdale J., Betz A.G. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Raya B., Michalski C., Sadarangani M., Lavoie P.M. Maternal immunological adaptation during normal pregnancy. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mor G., Aldo P., Alvero A.B. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 26.Mold J.E., Michaëlsson J., Burt T.D., et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atyeo C.G., Shook L.L., Brigida S., et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat Commun. 2022;13:3571. doi: 10.1038/s41467-022-31169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Lopez N., Romero R., Tao L., et al. Distinct cellular immune responses to SARS-CoV-2 in pregnant women. J Immunol. 2022;208:1857–1872. doi: 10.4049/jimmunol.2101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss W.N., Hou Y.J., Johnson N.V., et al. Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitopes. Science. 2021;372:1108–1112. doi: 10.1126/science.abg5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amanat F., Thapa M., Lei T., et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell. 2021;184:3936–3948.e10. doi: 10.1016/j.cell.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stavnezer J., Guikema J.E., Schrader C.E. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennewein M.F., Alter G. The immunoregulatory roles of antibody glycosylation. Trends Immunol. 2017;38:358–372. doi: 10.1016/j.it.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Lu L.L., Suscovich T.J., Fortune S.M., Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonçalves G., Cutts F.T., Hills M., Rebelo-Andrade H., Trigo F.A., Barros H. Transplacental transfer of measles and total IgG. Epidemiol Infect. 1999;122:273–279. doi: 10.1017/s0950268899002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munoz F.M., Bond N.H., Maccato M., et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311:1760–1769. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y.C., Lin S.J. Neonatal natural killer cell function: relevance to antiviral immune defense. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/427696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y.J., Murphy E.A., Singh S., et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet Gynecol. 2022;139:373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 38.Chu H.Y., Englund J.A. Maternal immunization. Clin Infect Dis. 2014;59:560–568. doi: 10.1093/cid/ciu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saso A., Kampmann B. Maternal immunization: nature meets nurture. Front Microbiol. 2020;11:1499. doi: 10.3389/fmicb.2020.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halasa N.B., Olson S.M., Staat M.A., et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months - 17 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shook L.L., Atyeo C.G., Yonker L.M., et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention . 2022. Trends in demographic characteristics of people receiving COVID-19 vaccinations in the United States.https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends Available at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.
Atyeo. Antibody response to COVID-19 booster dose in pregnancy. Am J Obstet Gynecol 2023.