Abstract
Gestational diabetes mellitus (GDM) is defined as diabetes with onset or first recognition during gestation. It is a common complication of pregnancy that has become more prevalent over the past few decades. Abnormalities in fetal growth, including increased incidence of both large and small for gestational age babies, suggest placental dysfunction. The major goal of this scoping review is to determine what is known about abnormalities in placentas delivered from GDM pregnancies, and how early in gestation these abnormalities arise. A secondary goal is to review to what extent other selected factors, particular obesity, have been found to influence or modify the reported effects of GDM on placental development, and whether these are considered in the study of GDM placentas. PubMed and Scopus databases were searched using the key terms: “gestational diabetes AND (woman OR human) AND placenta AND (ultrasound OR ultrastructure OR imaging OR histology OR pathology). Studies of gross morphology and histoarchitecture in placentas delivered from GDM pregnancies consistently report increased placental size, villous immaturity and a range of vascular lesions when compared to uncomplicated pregnancies. In contrast, a small number of ultrasound studies have examined placental development in GDM pregnancies in the second, and especially, the first trimester. Relatively few studies have analyzed interactions with maternal BMI, but these do suggest that it may play a role in placental abnormalities. Further examination of placental development early in pregnancy is needed to understand when it becomes disrupted in GDM, as a first step to identifying the underlying causes.
Introduction
Gestational diabetes mellitus (GDM) is a common complication of pregnancy, and its prevalence is increasing worldwide [1]. GDM is defined as glucose intolerance with onset or first recognition in pregnancy, although as discussed below, the procedures and criteria for diagnosing GDM have changed over time and differ amongst institutions. Pregnancies complicated by GDM are more likely to result in adverse obstetric outcomes including preterm labor, caesarean-section, macrosomia and shoulder dystocia [2]. Even for infants born at normal weight, exposure to GDM has lifelong consequences for growth patterns, obesity risk and development of diabetes [3, 4]. Additionally, although fetal overgrowth is a widely recognized consequence of GDM, there are also more babies born small for gestational age (SGA) in GDM than in normoglycemic pregnancies, and pregnancies with SGA babies are at higher risk of poor fetal outcomes [5]. Collectively, these abnormalities in fetal growth point to placental dysfunction. As will be discussed, multiple histological abnormalities have been described in GDM placentas examined after delivery. However, it is not clear when in pregnancy these abnormalities arise, or even whether they precede or follow the onset of glucose intolerance. Improvements in imaging technologies have allowed visualization of placental features progressively earlier in gestation. The goal of this scoping review is to summarize what is known about placental abnormalities in delivered GDM placentas, and how early in gestation they arise. We also ask when in pregnancy placental development has been assessed in GDM, and whether findings from pre-term imaging studies can be connected to the structural differences observed in delivered GDM placentas histologically.
A secondary goal is to review other relevant factors that influence or modify the reported effects of GDM on placental development. Do studies consider or control for maternal obesity, a risk factor for GDM that can also independently affect placental development [6–8]? Are there meaningful distinctions between GDM and pre-gestational diabetes, or amongst GDM subtypes? Was mode of delivery considered? One potentially relevant factor to consider is variable definitions of GDM. Beginning in 1964, a 100g, 3h oral glucose tolerance test, with two or more abnormal values, based on maternal risk of developing diabetes postpartum was established for diagnosing GDM[9]. These threshold values were subsequently revised downward based on work from Carpenter and Coustan in 1982[10]. In 2010, the International Association of Diabetes in Pregnancy Study Group (IADPSG) recommended that a 75g oral glucose tolerance test (OGTT) be given, with just one value greater than 92, 180 and 153 mg/dL for fasting, 1, 2 and 3 h, respectively, considered diagnostic of GDM, based on the risk of perinatal complications [11]. The IADPSG guidelines have been adopted by the WHO and the American Diabetes Association and are used in much of the world[12]. However, the screening procedure recommended by the American College of Obstetricians and Gynecologists (ACOG) and followed widely in the United States and Canada is a 50g oral glucose challenge test, which, if glucose exceeds 140 mg/dL at 1h, is followed by a 100g OGTT in which two values greater than 95, 180, 155 and 140 mg/dL for fasting, 1, 2 and 3 h, respectively, are diagnostic of GDM. Additionally, GDM may be divided into type A1, controlled by diet and exercise, and type A2, dependent on insulin, based on the White classification system[13]. Where possible, the diagnostic criteria utilized each of the reviewed studies are noted in Table 1.
Table 1:
Data Extraction table
| Author Date | Gestational age range | Maternal BMI accounted for? | Stated GDM diagnostic criteria | Delivery mode accounted for? | GDMA1 vs A2 accounted for? | Subcategory (a-f) |
|---|---|---|---|---|---|---|
| Abou-Elghait et al. 2012 [14] | 3rd, >37 weeks | no | WHO | no | no | histoarchitecture villous maturation fibrin deposition |
| Akarsu et al. 2017 [15] | 3rd, at delivery | no | 50g OGTT screen, 100g OGTT diagnostic | operative | A1 only | villous maturation |
| Arshad et al. 2015 [16] | at delivery | no | WHO | no | yes | Histoarchitecture, villous maturation, fibrin deposition |
| Ashfaq et al. 2005 [17] | 3rd, “term” | no | not specified | no | no | placental size |
| Basnet et al. 2016 [18] | 3rd | no | not specified | no | no | vascular function |
| Baumüller et al. 2015 [19] | 3rd, at delivery | yes | not specified | no | yes | vascular function |
| Berceanu et al. 2018 [20] | 2nd, 3rd, at delivery | no | not specified | no | no | placental size, histoarchitecture |
| Bhattacharjee et al. 2017 [21] | 3rd, >37 weeks | no | Carpenter and Coustan | no | no | villous maturation, villous edema, fibrin deposition |
| Brown et al. 1990 [22] | 3rd, 26–40 weeks | no | not specified | no | no | vascular function |
| Calderon et al. 2007 [23] | 3rd, >34 weeks | no | ADA | no | no | villous maturation, vascular function |
| Chan et al. 2003 [24] | 3rd, delivery | yes | WHO | no | no | placental size |
| Cosson et al. 2016 [25] | at delivery | yes | 75-g OGTT with fasting plasma glucose ≥ 5.3 mmol/L and/or a 2-h ≥ 7.8 mmol/L | no | no | placental size |
| Cvitic et al. 2018 [26] | 3rd, at delivery | no | WHO | no | no | vascular function |
| Deveci et al. 2013 [27] | 3rd, 28–35 weeks | no | not specified | no | no | cellular/subcellular |
| Dieber-Rotheneder et al. 2012 [28] | 3rd, 28–41wks | yes | 75 g OGTT: > 95 1h, > 180 1h, >155 mg/dl 2h | no | no | vascular function |
| Dollberg et al. 1997 [29] | 3rd, “term” | no | Carpenter and Coustan | no | no | vascular function |
| Dubova et al. 2011 [30] | 3rd, >37 weeks | no | not specified | no | A1 only | villous maturation |
| Edu et al. 2016 [31] | 2nd, 3rd | no | IADPSG | no | no | placental size, villous maturation, villous edema, fibrin deposition |
| Erkamp et al. 2020 [32] | 1st, 2nd, 3rd | yes (lean) | Dutch guidelines: random glucose >11.0 mmol/l, fasting ≥7.0 mmol/l, or fasting 6.1–6.9 mmol/l with abnormal GTT | no | no | vascular function |
| Fadda et al. 2001 [33] | 2nd, 3rd | no | National Diabetes Data Group (1979) | yes | yes | vascular function |
| Feng et al. 2016 [34] | 3rd, “term” | no | 75 g OGTT | no | no | cellular/subcellular |
| Figueroa et al. 1993 [35] | 3rd, “term” | no | Carpenter and Coustan | no | no | vascular function |
| Ganer Herman et al. 2018 [36] | 3rd, at delivery | yes | ACOG | yes | no | placental size |
| Georgiadis et al. 2014 [37] | 2nd, 3rd, at delivery | yes | 75g OGTT (fasting ≥ 4.8 mmol/l (until 2008) or ≥ 5.3 mmol/l (since 2009), ≥ 10.0 mmol/l 1h, and ≥ 8.6 mmol/l at 2h | no | no | vascular function |
| Han et al. 2016 [38] | 3rd, at delivery | yes | 75g OGTT -fasting glucose >92 mg/dL (5.1 mmol/L); 1h> 180 mg/dL (10.0 mmol/L); 2-h > 153 mg/dL (8.5 mmol/L) | no | no | villous maturation |
| Heidari et al. 2019 [39] | 3rd, >37 weeks | no (not different between groups) | ACOG/ Carpenter and Coustan | matched | no | placental size |
| Huynh et al. 2015 [40] | 3rd | yes | Carpenter and Coustan | no | no | histoarchitecture, villous maturation |
| Ji et al. 2017 [41] | 3rd, at delivery | no | fasting glucose >5.1 mM or 1h OGTT >10.0 mM, or 2h OGTT >8.5 mM. | no | no | cellular/subcellular |
| Jones et al. 1976 [42] | 3rd, >37 weeks | no | not specified | no | no | histoarchitecture |
| Jones et al. 1993 [43] | 3rd, >37 weeks | no | not specified | no | no | vascular function |
| Kleiner et al. 2020 [44] | 3rd, at delivery | no | ACOG | no | no | histoarchitecture |
| Kovo et al. 2016 [45] | 3rd, “term” | no | ADA | matched | yes | placental size, histoarchitecture, villous maturation |
| Kozłowska-Rup et al. 2014 | not specified | no | WHO | operative | A1 only | cellular/subcellular |
| Lao et al. 1997 [46] | 3rd, at delivery | no | WHO (1980) | no | no | placental size |
| Lao et al. 2001 [47] | 3rd, at delivery | no (groups not different) | WHO | no | no | placental size |
| Loegl et al. 2017 [48] | 3rd, at delivery | yes | 75 g OGTT, two or more >92 g/l fasting, >180 g/l 1 h, >153 g/l 2 h | no | no | vascular function |
| Magee et al. 2014 [49] | at delivery | no | ADA | operative | no | cellular/subcellular |
| Makhseed et al. 2004 [50] | 3rd, at delivery | no | 75g OGTT fasting >5.3 mmol/L or 2h >8.5 mmol/L | no | no | placental size |
| Mando et al. 2018 [51] | 3rd, “term” | yes | OGTT (not otherwise specified) | operative | no | cellular/subcellular |
| Mayhew 1998 [52] | 1st, 2nd, 3rd | no | not specified | no | yes | villous maturation, vascular function |
| Mayhew and Sisley 1998 [53] | 3rd, >37 weeks | controlled | not specified | no | yes | placental size, fibrin deposition |
| McNamara et al. 2014 [54] | 3rd, >37 weeks | yes | not specified | yes | no | placental size |
| Memon et al. 2015 [55] | 3rd, “term” | no | not specified | no | no | placental size, histoarchitecture, fibrin deposition |
| Meng et al. 2015 [56] | 3rd, “term” | no | ADA | operative | no | histoarchitecture, villous maturation, villous edema, fibrin deposition |
| Pala et al. 2016 [57] | 2nd, 3rd | no | WHO | no | no | placental size, histoarchitecture |
| Pathak et al. 2010 [58] | 3rd, >37 weeks | no | WHOhis | no | no | placental size |
| Pathak et al. 2011 | 3rd, >34 weeks | no | WHO | no | no | histoarchitecture |
| Patil et al. 2019 [59] | 2nd, 3rd | no | Diabetes in Pregnancy Study group India (DIPSI) | no | no | villous maturation |
| Pavlova et al. 2020 [60] | not specified | no | not specified | no | no | histoarchitecture |
| Perovic et al. 2012 [61] | 2nd, 3rd > 24 wks | no | ADA | not applicable | no | placental size, vascular function |
| Ramos et al. 2016 [62] | at delivery, ≥23 weeks | yes | NDDG | no | yes | placental size |
| Razak et al. 2018 [63] | 3rd, >37 weeks | no | fasting ⩾5.6mmol/L or a 2h ⩾7.8mmol/L | operative | no | vascular function |
| Saha et al. 2014 [64] | 3rd, at delivery | No | fasting glucose >126 mg/dl, random >200 mg/dl, or 100g OGTT | no | no | placental size, villous edema, fibrin deposition, vascular function |
| Sak et al. 2013 [65] | 3rd, >28 weeks | no | not specified | no | no | histoarchitecture, villous edema, fibrin deposition |
| Salafia et al. 1989 [66] | 3rd, >37 weeks | no | not specified | no | no | placental size vascular function |
| Samuel et al. 2014 [67] | 3rd, “term” | no | fasting glucose ⩾92mg/dL and 1 h ⩾ 180mg/dL and 2 h ⩾ 153mg/dL | vaginal | no | vascular function |
| Schönfelder et al. 1996 [68] | 3rd, “term” | no | NDDG criteria | no | no | vascular function |
| Scifres et al. 2017 [69] | 3rd, at delivery | yes | Carpenter-Coustan | no | no | vascular function |
| Sharma S.et al. 2014 [70] | 3rd, >36 weeks | no | fasting >90mg/dL or random >105 on first visit plus screening at 12–13 wks and 24–28 weeks | no | no | fibrin deposition, villous immaturity |
| Soygur et al. 2016 [71] | 1st (4–9w) and at delivery (not specified) | yes | fasting glucose >92 mg/dl, 75g OGTT 1 hour >180 mg/dl, and 2 hours >153 mg/dl | operative | A2 only | cellular/subcellular |
| Stanek et al. 2007 [72] | 2nd,3rd | no | not specified | no | no | histoarchitecture |
| Stanek et al. 2014 [73] | 2nd, 3rd | no | not specified | no | no | histoarchitecture |
| Stoz et al. 1988 [74] | 3rd, >37 weeks | no | 100g OGTT (J.B O’Sullivan criteria) | no | no | villous maturation, vascular function |
| Strøm-Roum et al. 2016 [75] | 3rd, at delivery | yes | 2-h 75-g OGTT with glucose ≥7.8 to <11.1 mmol/L | no | no | placental size |
| Surányi et al. 2016 [76] | 2nd, 3rd, 15–28 weeks | no | WHO | not applicable | no | placental size |
| Taricco et al. 2009 [77] | 3rd, at delivery | no | ACOG/ Carpenter and Coustan | operative only | no | placental size |
| Taricco et al. 2003 [78] | 3rd, >36 weeks | no | ACOG/ Carpenter and Coustan | no | no | placental size |
| Thunbo et al. 2018 [79] | 3rd, >35 weeks | yes | not specified | no | no | placental size |
| Tramontana et al. 2018 [80] | 1st | yes | IADPSG | no | no | vascular function |
| Vilariño-García et al. 2016 [81] | 3rd, “term” | no | ADA | operative only | no | placental size |
| Visiedo et al. 2017 [82] | 3rd, “term” | no | NDDG (1979) | operative only | no | cellular/subcellular |
| Wang et al. 2019 [83] | 3rd, “term” | no | fasting >92 mg/dl and 1h >180 mg/dl or 2h >153 mg/dl on OGTT | no | no | vascular function, cellular/subcellular |
| Wong et al. 2019 [84] | 1st, 2nd | no | National Diabetes Data Group Criteria | no | no | placental size, vascular function |
| Yavuz et al. 2015 [85] | 3rd, “term” | no | OGTT ≥140 mg/dL | no | no | histoarchitecture |
| Younes et al. 1996 [86] | 3rd, >37 weeks | no | not specified | no | no | villous maturation |
| Yuksel et al. 2016 [87] | 3rd | yes | ACOG/Carpenter and Coustan | no | yes | Histoarchitecture, vascular function |
| Zhou et al. 2016 [88] | 3rd, >37 weeks | no | WHO/IADPSG | operative only | no | vascular function |
Methodology
A systematic search strategy was utilized, supplemented by additional references and keywords suggested by the initial search results. The PubMed and Scopus databases were each searched using the key terms: “gestational diabetes AND (woman OR human) AND placenta AND (ultrasound OR ultrastructure OR imaging OR histology OR pathology).” The search was conducted for articles published through July 13th, 2020 and 956 unique publications were returned. Publications that involve pregestational diabetes but exclude GDM, that only feature GDM superimposed on other complications of pregnancy, animal studies, and studies that do not distinguish between overt, pre-gestational diabetes and gestational diabetes were excluded. Additionally, references were excluded if the full text was not available to the authors or was not available in English. Primary research articles were included if they describe placental morphology, histology or structure at the cell, tissue or organ level. An initial screen was conducted by one of two reviewers (EE or OT) to narrow the list to 196 potentially relevant results. Then, two independent reviewers (EE, OOT, LCS) conducted a detailed review of each of these papers, which identified 117 relevant, full-text publications for synthesis (Figure 1). Disagreements were resolved by a third, tie-breaking reviewer. For each publication identified, trimester of pregnancy assessed, whether maternal BMI was considered, GDM diagnostic criteria, mode of delivery, White classification and a description of the placental outcomes was recorded. Publications were then organized into categories. Some studies fit into multiple categories and were cross reviewed (Table 1).
Figure 1:
Scoping review strategy
Placental Size
GDM placentas are most consistently found to be larger than controls at term, in weight [17, 25, 31, 39, 47, 54, 64, 66, 78, 81, 89], volume [39, 64, 90], thickness, and diameter [17, 55, 64], although there are some exceptions [50, 53] placental weights below the 10th percentile are also more common in GDM [91]. The increase in volume is a result of increased parenchymal villous tissue and intervillous spaces, as well as extravillous trophoblasts, but not placental membranes or maternal decidual tissue, suggesting an increase in the functional trophoblast exchange area [39, 90, 92]. However, as discussed below, this is accompanied by changes in villous architecture that would likely reduce maternal-fetal exchange efficiency. Larger placental weight in GDM is a predictor of larger infant birth weight [35], which may reflect increased placental delivery of nutrients or may simply result from GDM independently increasing both fetal and placental growth. Results from 2D and 3D ultrasonography suggest that placental overgrowth generally arises in the second trimester in GDM, and worsens or becomes more prevalent as gestation progresses [20, 31, 57, 61, 76, 84]. No differences in placental volume have been detected in GDM pregnancies at 11–14 weeks, but they are significantly larger by 21–24 weeks [84]. Elevated placental thickness is likewise apparent by 24–28 weeks via ultrasound assessment, and continues to increase through term [20]. Abnormal placental thickness was more prevalent in GDM than in type I diabetic pregnancies [20].
These data suggest that placental size increases in tandem with increasing glycemia, as the majority of women diagnosed with GDM don’t meet the WHO definition of glucose intolerance before 24 weeks, although many have evidence of some level of hyperglycemia as early as the first trimester detected by OGTT, fasting glucose or HbA1C [93, 94]. At delivery, placental weights have been found to be directly proportional to the degree of glucose intolerance [46]; placental weights in women with GDM or pre-gestational diabetes are closer to those of uncomplicated pregnancies with good glycemic control [79, 95]. Placental weight also is inversely correlated with total caloric intake from protein, as estimated from food diaries over five days at 28–30 weeks in pregnancies diagnosed with GDM [24]. Maternal body mass index appears to contribute to placental overgrowth in GDM, but does not fully explain it. While placental weight increases with BMI amongst women with GDM [36, 62, 75], and one study found elevated placental weights in GDM only in women with pre-gestational BMI > 30 and or gestational weight gain > 20 kg [36], others have shown that GDM results in greater placental weights even when comparing to BMI-matched controls [25].
At the cellular level, increased placental size is associated with changes in both trophoblast cell death and proliferation rates in delivered placentas, but there is essentially no information on these processes in GDM prior to delivery, while the placenta is still growing. Immunohistochemistry of term GDM placentas reveal elevated protein expression of markers of cytotrophoblast cell proliferation KI-67 and PCNA [82, 96], consistent with greater placental volumes. However, increased TUNEL staining, a more apoptotic gene profile, and greater caspase activity and expression have all been detected in GDM placentas at term [15, 49, 82, 97], although at least one study has reported a more anti-apoptotic protein profile in GDM pregnancies carrying large for gestational age (LGA) fetuses [96]. Placental autophagy, the process by which cell components are recycled for energy or growth, also has a controversial relationship to GDM. While autophagic vacuoles and autophagolysomes have been observed in GDM placentas by electron microscopy, increased placental expression of autophagic markers is reported in some studies but no difference or lower expression is reported in others [41, 96, 98].
Villous Structure
Placental villi first form at approximately four weeks of gestation (2 weeks post-conception), as columns of cytotrophoblast grow towards the outward edge of the primitive syncytial mass [99]. These columns fill with a mesenchymal core, and within a week, hemangioblastic cells, vascular precursors, begin to form cords within the villous cores [100]. Cords are transformed into blood-filled capillaries by the sixth week of gestation, with vasculogenesis continuing throughout the first trimester [101]. By the second trimester, stem villi, attached to the chorionic plate, branch into mostly intermediate villi, which are characterized by a nearly continuous cytotrophoblast layer, a relatively thick syncytiotrophoblast, and somewhat central capillaries. As the placenta matures well into the third trimester, these villi further branch to form the smaller terminal villi, characterized by a discontinuous cytotrophoblast layer, thinned syncytiotrophoblast, more peripheral capillaries, existing capillaries that continue to elongate and branch and a greater surface area-to-volume ratio [102] (Figure 2).
Figure 2:
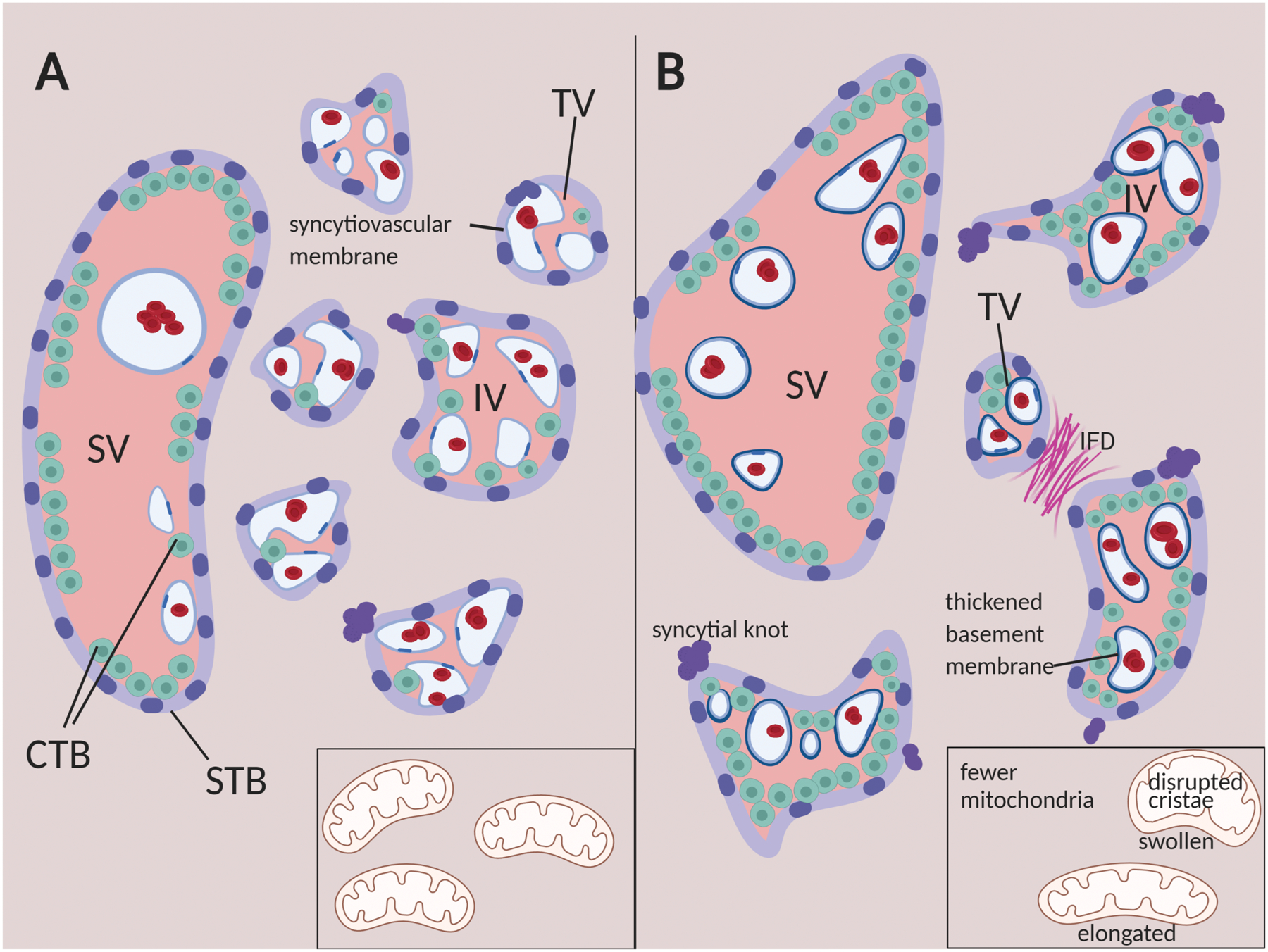
Summary of histopathological anomolies in GDM placentas (A) Illustrated cross-section of placental villous tissue at term. (B) Abnormalities frequently observed in GDM placentas include villous immaturity (reduced terminal villous surface area, reduced syctyiovascular membranes, increased cytotrophoblast), vascular lesions like intervillous fibrin deposition (IFD), and excess syncytial knots. SV = stem villus, IV= intermediate villus, TV=terminal villus, CTB=cytotrophoblast, STB=syncytiotrophoblast. Inset: mitochondrial damage is also a feature of GDM placental trophoblast cells. Illustration created with BioRender.
Villous immaturity is a common histopathological finding in GDM, with decreased number and total surface area of terminal villi, thickened basement membranes, and more centrally-located capillaries in term placentas [20, 21, 23, 30, 31, 40, 74, 86, 89, 91, 92]. These immature villi in GDM are also associated with abnormally formed and less numerous microvilli on the syncytiotrophoblast surface [15, 38, 56]. All of these changes increase the barrier to maternal-fetal exchange of oxygen and nutrients. The timeline of villous maturation in GDM vs. non-GDM placentas is not clear. Placental maturity in the second and third trimesters may be judged by ultrasound using the Grannum scale, which is based largely on the degree of folding of the chorionic plate, and the frequency of calcifications and hyperechoic areas, particularly in the basal plate [103]. While ultrasound maturity has been found to relate to oxygen exchange efficiency in normal term placentas [104], detailed comparison found essentially no relationship between the classic histological features of placental maturation assessed after delivery and the ultrasound definition of maturity [105]. Nonetheless, GDM placentas appear immature by ultrasound criteria in the second and third trimesters [20]and ultrasound findings of placental immaturity after 26 weeks have a sensitivity of 84.8–88.6% and specificity of 87.5–88.3% for detection of GDM [59, 61]. When immaturity is assessed in combination with placental thickness and fetal characteristics, GDM prediction on the basis of ultrasound screening is both sensitive (90.9 – 93.2%) and specific (89.6–92%) [20, 59, 61].
Another villous structural abnormality observed in GDM is villous edema, which has been correlated with reduced placental function [31, 56, 64]. While the cause is unknown, increased expression of water channel aquaporin 9 has also been observed, suggesting a mechanism for the accumulation of fluid, though vascular abnormalities, or inflammation could also be responsible [81]. The degree of placental histological change is not directly correlated to the degree of hyperglycemia, suggesting there are other factors contributing to edema [31].
Alterations in villous structure are also observed in pre-gestational diabetes, although there is no consensus on whether it is worse [30], similar [65], or less severe [15, 86] than in GDM, with at least one study reporting accelerated villous maturation in pre-gestational diabetes [106]. These findings, along with the relatively early age at which villous maturation defects are detected in GDM, suggest that hyperglycemia may not be the major cause of delayed maturation, and many of the defects persist even when there is good glycemic control [42]. There are almost no data with which to assess the contribution of obesity to villous maturation defects in GDM, although placental microvillous abnormalities are less frequent following a management program that decreases gestational weight gain in non-obese women with GDM [38].
Vascular function and vascular lesions
GDM pregnancies exhibit increased incidence of placental vascular lesions (histological changes related to blood flow) in the third trimester [69, 89, 91]. Evidence includes fetal thrombotic vasculopathy (obstruction of arteries and veins in the fetal-side placental vasculature) [107], elevated fibrinoid deposition in intervillous spaces [21, 86, 108], and fibrinoid necrosis [14, 31, 55, 61, 109]. It has been proposed that higher incidence of intervillous thrombi is associated with localized hemorrhage, due to the presence of fetal hemoglobin within these blood collections, but the causes are unknown [18]. Some of the pathological findings in GDM placental villous vasculature, including pericyte detachment and pericyte ghost cells, are also observed in the retinal vasculature in patients with type I or type II diabetes [27, 67]. In a prospective study of over a thousand patients, GDM placentas had significantly higher rates of chorangiosis (elevated capillary density in terminal villi), and fetal thrombotic vasculopathy than uncomplicated pregnancies, but it should be noted that 80% of GDM placentas had normal histologic findings (absence of any type of pathological lesions) compared to just 72% of placentas from uncomplicated pregnancies at or near term [108]. Thus, the vast majority of GDM term placentas lack such lesions, and placentas with pathologic findings do not always result in abnormal outcomes.
Although abnormalities in villous architecture are detectable in GDM only from the second trimester onward, and the precise branching of villous capillaries can only be assessed after delivery, 3D Power Doppler ultrasound has been used to detect reduced vascular indices and vascular flow indices in GDM placentas as early as 12 weeks gestation, suggesting that villous vascular abnormalities begin even prior to hyperglycemia [84]. In contrast, umbilical, pulsatility is not significantly different in GDM pregnancies and nitric oxide (NO) synthase activity is normal in cord artery and vein and chorionic plate artery and vein in delivered GDM placentas [22, 29].
Alterations in placental vascular function are also apparent at the cellular and molecular level. Loss of adherens junctions and reduced vascular-endothelial cadherin expression are consistent with reduced barrier function [19, 110]. Ex vivo contractility of chorionic vessels in response to adenosine is impaired, even in women with well controlled GDM, and expression of endothelin1 and endothelin-A, potent vasoconstrictors, is significantly decreased in whole term GDM placental lysates [28, 63, 111]. In contrast, in vitro smooth muscle relaxation and contractions in response to hypoxia-reoxygenation were exaggerated in arteries and veins isolated from GDM placentas after delivery [35]. Vasodilatory endothelial nitric oxide synthase (eNOS) activity is reduced in stem villous vessels from GDM placentas [29], while iNOS, which is not normally expressed in the placenta, is present in both endothelial cells and trophoblasts in GDM [68]. In culture, the migratory capacity and proliferative responses of vascular endothelial cells from GDM pregnancies are also impaired [83, 88]. Both arterial and venous villous endothelium exhibit global alterations in DNA methylation and gene expression, particularly in actin organization and pathways regulating cell morphology and barrier function [26] in the GDM placenta.
Other Pathologic Features
Other pathologic features have been observed in GDM placentas in one or more studies. GDM placentas exhibit higher central and peripheral elasticity by shear wave elastography, a measure of placental stiffness that correlates with histopathological changes like fibrosis [87, 112]. GDM placentas have more syncytial knots than controls [14, 56, 64, 85]. Although syncytial knots occur with increasing frequency as normal placentas mature, excessive numbers are associated with placental malperfusion [113]. Abnormalities are also observed at the ultrastructural level in term placentas from GDM pregnancies, particularly in mitochondria, although altered endoplasmic reticulum has also been reported [38, 42, 56, 65]. Mitochondrial abnormalities include swelling or dilation, fracturing, reduced matrix density and disrupted cristae [38, 51, 56, 65] (Figure 2). In cytotrophoblast cells, significantly reduced mitochondria numbers and size, as well as mitochondrial elongation were measured in placentas from both diet-controlled and insulin-controlled GDM pregnancies relative to controls, whereas mitochondrial density was reduced in both syncytiotrophoblast and fetal endothelium [114]. These structural abnormalities are consistent with the 50% reduction in mitochondrial respiration measured in A2 GDM placentas compared to BMI -matched controls, and accompanying decreases in protein levels of mitochondrial electron transport chain complexes I,II,III and IV [115].
Studies of mitochondrial structure and function in GDM often do not account for BMI. However, mitochondrial abnormalities have been observed in fetal endothelial cells in GDM placentas even compared to placentas from obese controls [67]. In contrast, mitochondrial DNA copy numbers in maternal plasma, which provide at least an indirect indication of placental mitochondrial number and health, are significantly higher at term in GDM pregnancies when compared to normal weight controls, but not when compared to obese controls [116]. While there are understandably no studies of placental mitochondria in GDM pregnancies prior to term, mtDNA in maternal plasma can be measured at any gestational age. At 20 weeks, just as at term, there are higher circulating mtDNA levels in women with GDM compared to lean (BMI = 24.64 +/− 3.94) but not BMI-matched (BMI = 28.07 +/− 5.32) controls [117]. Thus, the increase in mtDNA probably reflects BMI more closely than GDM. Further, because mitochondrial numbers and function are reduced in GDM placentas at term, it is likely that these higher circulating mtDNA levels reflect cell death or loss of mitochondria in the second and third trimesters. Although placental mtDNA can be detected in maternal plasma in the first trimester [118], it has not yet been measured in pregnancies that go on to develop GDM. Thus, it is not known when in pregnancy mitochondrial abnormalities begin. However, strict management of GDM and improved glycemic control after diagnosis at 24–28 weeks reduced ultrastructural abnormalities by half [38], suggesting that some of the mitochondrial damage arises after the onset of hyperglycemia.
Discussion
Altogether, it is clear that GDM disrupts placental development, likely through hyperglycemia, and other, as yet unknown metabolic or endocrine mechanisms, such as insulin resistance. While placental overgrowth is common, so are structural abnormalities like villous immaturity and vascular dysfunction that are likely to offset the fetal growth-promoting effects of a larger placenta. The major goal of this review was to summarize the structural placental abnormalities that have been reported in GDM, and determine what is known about when in pregnancy these placental pathologies develop. The most consistently reported pathologies are summarized in Figure 2. We found that placental overgrowth is both the most frequently reported abnormality, and the one that could most convincingly be linked to hyperglycemia, through timing, occurrence in pre-gestational diabetes, and responsiveness to glycemic control. While placental overgrowth has been observed in GDM as early as the second trimester, there is as yet no evidence for this earlier in gestation.
In contrast, a variety of vascular abnormalities, including chorangiosis, fetal thrombotic vasculopathy, and intervillous fibrin deposition, and even altered contractile and vasodilatory responses have been reported in GDM placentas, but with more variation amongst studies. Relatively few studies in this area examine common endpoints, or utilize with common approaches. Limited evidence from imaging studies of pre-term placental morphology suggests that vascular abnormalities and maturation defects in the GDM placentamay begin even before 24–28 weeks, raising the possibility that sensitive measures of placental function may in the future be used to predict the disease. However, we were able to find only one, relatively small (n<50) study examining GDM placentas by ultrasound in the first trimester[84]. Thus, there is a clear need for additional study of the placenta early in GDM pregnancies, to better understand when placental development is altered.
The secondary goal of this review was to discover to what extent other relevant factors that may modify the influence of GDM on placental development have been considered. While a number of studies did consider maternal BMI in their analyses, this remained the exception rather than the rule (Table 1). Results from studies that did consider BMI, however, suggest that it is a relevant factor that ought to be considered in future. Almost none of the studies in our search compared diet-controlled and insulin-treated diabetics when reporting placental outcomes (Table 1). While no meta-analysis or truly systematic comparison was made here, broadly similar placental findings were reported across studies regardless of which GDM diagnostic criteria were used. In conclusion, there is a consistent, broad evidence base supporting the idea that placental structure is disrupted in GDM, but there is still a need for studies that address when in development these disruptions occur and what additional factors influence them.
Acknowledgements
This work has been supported by the National Institutes of Health R03HD105831
References
- [1].Ferrara A, Increasing prevalence of gestational diabetes mellitus: a public health perspective, Diabetes Care 30 Suppl 2 (2007) S141–6. [DOI] [PubMed] [Google Scholar]
- [2].Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, Jacqueminet S, Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012, Diabetologia 60(4) (2017) 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P, High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia, Diabetes Care 31(2) (2008) 340–6. [DOI] [PubMed] [Google Scholar]
- [4].Baptiste-Roberts K, Nicholson WK, Wang NY, Brancati FL, Gestational diabetes and subsequent growth patterns of offspring: the National Collaborative Perinatal Project, Matern Child Health J 16(1) (2012) 125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, Dunne F, Lawlor DA, Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis, BMJ 354 (2016) i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wallace JM, Horgan GW, Bhattacharya S, Placental weight and efficiency in relation to maternal body mass index and the risk of pregnancy complications in women delivering singleton babies, Placenta 33(8) (2012) 611–8. [DOI] [PubMed] [Google Scholar]
- [7].Leon-Garcia SM, Roeder HA, Nelson KK, Liao X, Pizzo DP, Laurent LC, Parast MM, LaCoursiere DY, Maternal obesity and sex-specific differences in placental pathology, Placenta 38 (2016) 33–40. [DOI] [PubMed] [Google Scholar]
- [8].Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, Dietz PM, Maternal obesity and risk of gestational diabetes mellitus, Diabetes Care 30(8) (2007) 2070–6. [DOI] [PubMed] [Google Scholar]
- [9].O’Sullivan JB, Mahan CM, Criteria for the Oral Glucose Tolerance Test in Pregnancy, Diabetes 13 (1964) 278–85. [PubMed] [Google Scholar]
- [10].Carpenter MW, Coustan DR, Criteria for screening tests for gestational diabetes, Am J Obstet Gynecol 144(7) (1982) 768–73. [DOI] [PubMed] [Google Scholar]
- [11].D. International Association of, P. Pregnancy Study Groups Consensus, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI, International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy, Diabetes Care 33(3) (2010) 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li-Zhen L, Yun X, Xiao-Dong Z, Shu-Bin H, Zi-Lian W, Adrian Sandra D, Bin L, Evaluation of guidelines on the screening and diagnosis of gestational diabetes mellitus: systematic review, BMJ Open 9(5) (2019) e023014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].White P, Pregnancy complicating diabetes, Am J Med 7(5) (1949) 609–16. [DOI] [PubMed] [Google Scholar]
- [14].Abou-Elghait AT, Histological and immunohistochemical changes in placental chorionic villi of patients with poorly controlled gestational diabetes, The Egpytian J of Histology 35(2) (2012). [Google Scholar]
- [15].Akarsu S, Bagirzade M, Omeroglu S, Buke B, Placental vascularization and apoptosis in Type-1 and gestational DM, J Matern Fetal Neonatal Med 30(9) (2017) 1045–1050. [DOI] [PubMed] [Google Scholar]
- [16].S.F. Arshad R., Shanim MO, Karim N, Azam F, Placental Histology in Diet and Insulin Treated Gestational Diabetics, Med Forum 26(4) (2015) 21–25. [Google Scholar]
- [17].Ashfaq M, Janjua MZ, Channa MA, Effect of gestational diabetes and maternal hypertension on gross morphology of placenta, J Ayub Med Coll Abbottabad 17(1) (2005) 44–7. [PubMed] [Google Scholar]
- [18].Basnet KM, Bentley-Lewis R, Wexler DJ, Kilic F, Roberts DJ, Prevalence of Intervillous Thrombi Is Increased in Placentas from Pregnancies Complicated by Diabetes, Pediatr Dev Pathol 19(6) (2016) 502–505. [DOI] [PubMed] [Google Scholar]
- [19].Baumuller S, Lehnen H, Schmitz J, Fimmers R, Muller AM, The impact of insulin treatment on the expression of vascular endothelial cadherin and Beta-catenin in human fetoplacental vessels, Pediatr Dev Pathol 18(1) (2015) 17–23. [DOI] [PubMed] [Google Scholar]
- [20].Berceanu C, Tetileanu AV, Ofiteru AM, Bratila E, Mehedintu C, Voicu NL, Szasz FA, Berceanu S, Vladareanu S, Navolan DB, Morphological and ultrasound findings in the placenta of diabetic pregnancy, Rom J Morphol Embryol 59(1) (2018) 175–186. [PubMed] [Google Scholar]
- [21].Bhattacharjee D, Mondal SK, Garain P, Mandal P, Ray RN, Dey G, Histopathological study with immunohistochemical expression of vascular endothelial growth factor in placentas of hyperglycemic and diabetic women, J Lab Physicians 9(4) (2017) 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brown MA, North L, Hargood J, Uteroplacental Doppler ultrasound in routine antenatal care, Aust N Z J Obstet Gynaecol 30(4) (1990) 303–7. [DOI] [PubMed] [Google Scholar]
- [23].Calderon IM, Damasceno DC, Amorin RL, Costa RA, Brasil MA, Rudge MV, Morphometric study of placental villi and vessels in women with mild hyperglycemia or gestational or overt diabetes, Diabetes Res Clin Pract 78(1) (2007) 65–71. [DOI] [PubMed] [Google Scholar]
- [24].Chan KK, Ho LF, Lao TT, Nutritional intake and placental size in gestational diabetic pregnancies--a preliminary observation, Placenta 24(10) (2003) 985–8. [DOI] [PubMed] [Google Scholar]
- [25].Cosson E, Diallo A, Docan M, Sandre-Banon D, Banu I, Cussac-Pillegand C, Chiheb S, Pharisien I, Valensi P, Carbillon L, Fetal gender is not associated with either gestational diabetes mellitus or placental weight: A cohort study, Diabetes Metab 42(4) (2016) 276–9. [DOI] [PubMed] [Google Scholar]
- [26].Cvitic S, Novakovic B, Gordon L, Ulz CM, Muhlberger M, Diaz-Perez FI, Joo JE, Svendova V, Schimek MG, Trajanoski S, Saffery R, Desoye G, Hiden U, Human fetoplacental arterial and venous endothelial cells are differentially programmed by gestational diabetes mellitus, resulting in cell-specific barrier function changes, Diabetologia 61(11) (2018) 2398–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deveci E, Turgut A, Aktas A, Ayaz E, Sak S, Seker U, The Immunohistochemical and Ultrastructural Evaluation of Pericytes in Human Full Term Placentas of Gestasyonal Diabetes Mellitus, Acta Medica Mediterranea 29 (2013) 697–700. [Google Scholar]
- [28].Dieber-Rotheneder M, Beganovic S, Desoye G, Lang U, Cervar-Zivkovic M, Complex expression changes of the placental endothelin system in early and late onset preeclampsia, fetal growth restriction and gestational diabetes, Life Sci 91(13–14) (2012) 710–5. [DOI] [PubMed] [Google Scholar]
- [29].Dollberg S, Brockman DE, Myatt L, Nitric oxide synthase activity in umbilical and placental vascular tissue of gestational diabetic pregnancies, Gynecol Obstet Invest 44(3) (1997) 177–81. [DOI] [PubMed] [Google Scholar]
- [30].Dubova EA, Pavlov KA, Yesayan RM, Nagovitsyna MN, Tkacheva ON, Shestakova MV, Shchegolev AI, Morphometric characteristics of placental villi in pregnant women with diabetes, Bull Exp Biol Med 151(5) (2011) 650–4. [DOI] [PubMed] [Google Scholar]
- [31].Edu A, Teodorescu C, Dobjanschi CG, Socol ZZ, Teodorescu V, Matei A, Albu DF, Radulian G, Placenta changes in pregnancy with gestational diabetes, Rom J Morphol Embryol 57(2) (2016) 507–12. [PubMed] [Google Scholar]
- [32].Erkamp JS, Geurtsen ML, Duijts L, Reiss IKM, Mulders A, Steegers EAP, Gaillard R, Jaddoe VWV, Associations of Maternal Early-Pregnancy Glucose Concentrations With Placental Hemodynamics, Blood Pressure, and Gestational Hypertensive Disorders, Am J Hypertens 33(7) (2020) 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fadda GM, D’Antona D, Ambrosini G, Cherchi PL, Nardelli GB, Capobianco G, Dessole S, Placental and fetal pulsatility indices in gestational diabetes mellitus, J Reprod Med 46(4) (2001) 365–70. [PubMed] [Google Scholar]
- [34].Feng H, Su R, Song Y, Wang C, Lin L, Ma J, Yang H, Positive Correlation between Enhanced Expression of TLR4/MyD88/NF-kappaB with Insulin Resistance in Placentae of Gestational Diabetes Mellitus, PLoS One 11(6) (2016) e0157185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Figueroa R, Omar HA, Tejani N, Wolin MS, Gestational diabetes alters human placental vascular responses to changes in oxygen tension, Am J Obstet Gynecol 168(5) (1993) 1616–22. [DOI] [PubMed] [Google Scholar]
- [36].Ganer Herman H, Dekalo A, Jubran L, Schreiber L, Bar J, Kovo M, Obstetric outcomes and placental findings in gestational diabetes patients according to maternal prepregnancy weight and weight gain, J Matern Fetal Neonatal Med 32(10) (2019) 1682–1687. [DOI] [PubMed] [Google Scholar]
- [37].Georgiadis L, Keski-Nisula L, Harju M, Raisanen S, Georgiadis S, Hannila ML, Heinonen S, Umbilical cord length in singleton gestations: a Finnish population-based retrospective register study, Placenta 35(4) (2014) 275–80. [DOI] [PubMed] [Google Scholar]
- [38].Han Y, Zheng YL, Wu AM, Liu HB, Su JB, Lu XY, Han YW, Ji JL, Ji JH, Shi Y, Effects of management in gestational diabetes mellitus with normal prepregnancy body mass index on pregnancy outcomes and placental ultrastructures: a prospective cohort study, Endocrine 54(3) (2016) 691–699. [DOI] [PubMed] [Google Scholar]
- [39].Heidari Z, Mahmoudzadeh-Sagheb H, Narouei M, Sheibak N, Effects of gestational diabetes mellitus on stereological parameters and extravillous trophoblast cells of placenta compared to the control group, J Obstet Gynaecol 39(7) (2019) 928–933. [DOI] [PubMed] [Google Scholar]
- [40].Huynh J, Yamada J, Beauharnais C, Wenger JB, Thadhani RI, Wexler D, Roberts DJ, Bentley-Lewis R, Type 1, type 2 and gestational diabetes mellitus differentially impact placental pathologic characteristics of uteroplacental malperfusion, Placenta 36(10) (2015) 1161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ji L, Chen Z, Xu Y, Xiong G, Liu R, Wu C, Hu H, Wang L, Systematic Characterization of Autophagy in Gestational Diabetes Mellitus, Endocrinology 158(8) (2017) 2522–2532. [DOI] [PubMed] [Google Scholar]
- [42].Jones CJ, Fox H, An ultrastructural and ultrahistochemical study of the placenta of the diabetic woman, J Pathol 119(2) (1976) 91–9. [DOI] [PubMed] [Google Scholar]
- [43].Jones CJ, Desoye G, Glycogen distribution in the capillaries of the placental villus in normal, overt and gestational diabetic pregnancy, Placenta 14(5) (1993) 505–17. [DOI] [PubMed] [Google Scholar]
- [44].Kleiner I, Ram S, Kovo M, Schreiber L, Barber E, Levy M, Fainstein N, Bar J, Weiner E, Pregnancy outcomes in association with placental histopathology in pregnancies complicated by macrosomia in diabetic vs. non-diabetic women, Eur J Obstet Gynecol Reprod Biol 248 (2020) 24–29. [DOI] [PubMed] [Google Scholar]
- [45].Kovo M, Schreiber L, Ben-Haroush A, Gold E, Golan A, Bar J, The placental component in early-onset and late-onset preeclampsia in relation to fetal growth restriction, Prenat Diagn 32(7) (2012) 632–7. [DOI] [PubMed] [Google Scholar]
- [46].Lao TT, Lee CP, Wong WM, Placental weight to birthweight ratio is increased in mild gestational glucose intolerance, Placenta 18(2–3) (1997) 227–30. [DOI] [PubMed] [Google Scholar]
- [47].Lao TT, Ho LF, Perinatal morbidity and placental size in gestational impaired glucose tolerance, J Soc Gynecol Investig 8(6) (2001) 347–50. [PubMed] [Google Scholar]
- [48].Loegl J, Nussbaumer E, Cvitic S, Huppertz B, Desoye G, Hiden U, GDM alters paracrine regulation of feto-placental angiogenesis via the trophoblast, Lab Invest 97(4) (2017) 409–418. [DOI] [PubMed] [Google Scholar]
- [49].Magee TR, Ross MG, Wedekind L, Desai M, Kjos S, Belkacemi L, Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta, J Diabetes Complications 28(4) (2014) 448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Makhseed MA, Ahmed MA, Musini VM, Impaired gestational glucose tolerance. Its effect on placental pathology, Saudi Med J 25(9) (2004) 1241–4. [PubMed] [Google Scholar]
- [51].Mando C, Anelli GM, Novielli C, Panina-Bordignon P, Massari M, Mazzocco MI, Cetin I, Impact of Obesity and Hyperglycemia on Placental Mitochondria, Oxid Med Cell Longev 2018 (2018) 2378189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mayhew TM, Thinning of the intervascular tissue layers of the human placenta is an adaptive response to passive diffusion in vivo and may help to predict the origins of fetal hypoxia, Eur J Obstet Gynecol Reprod Biol 81(1) (1998) 101–9. [DOI] [PubMed] [Google Scholar]
- [53].Mayhew TM, Sisley I, Quantitative studies on the villi, trophoblast and intervillous pores of placentae from women with well-controlled diabetes mellitus, Placenta 19(5–6) (1998) 371–7. [DOI] [PubMed] [Google Scholar]
- [54].McNamara H, Hutcheon JA, Platt RW, Benjamin A, Kramer MS, Risk factors for high and low placental weight, Paediatr Perinat Epidemiol 28(2) (2014) 97–105. [DOI] [PubMed] [Google Scholar]
- [55].G.P. Memon S, Lata H, Gross and Histological Alteration in the Placenta of Mothers Suffering from Gestational Diabetes, J Liaquat Uni Med Health Sci 14(1) (2015) 16–20. [Google Scholar]
- [56].Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao C, Gao L, Liu J, Cui Y, Ultrastructure of Placenta of Gravidas with Gestational Diabetes Mellitus, Obstet Gynecol Int 2015 (2015) 283124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pala HG, Artunc-Ulkumen B, Koyuncu FM, Bulbul-Baytur Y, Three-dimensional ultrasonographic placental volume in gestational diabetes mellitus, J Matern Fetal Neonatal Med 29(4) (2016) 610–4. [DOI] [PubMed] [Google Scholar]
- [58].Pathak S, Hook E, Hackett G, Murdoch E, Sebire NJ, Jessop F, Lees C, Cord coiling, umbilical cord insertion and placental shape in an unselected cohort delivering at term: relationship with common obstetric outcomes, Placenta 31(11) (2010) 963–8. [DOI] [PubMed] [Google Scholar]
- [59].Patil V, Srinivas G, Ms S, Kiran Das S, Hiremath R, Shabadi N, Diagnostic Significance of Ultrasonographic Markers and Score in Detection of Gestational Diabetes Mellitus in the Indian Subcontinent, Ultrasound Q (2019). [DOI] [PubMed] [Google Scholar]
- [60].Pavlova T.V. PVA, Kaplin AN, Malyutina ES, Zemoliankala LO, New Approaches in Assessing the Clinical and Pathomorphological Aspects of Obstetric Pathology in the Structures of the Mother-Placenta-Fetus using Atomic Force Research, Sys Rev Pharm 11(6) (2020) 21–25. [Google Scholar]
- [61].Perovic M, Garalejic E, Gojnic M, Arsic B, Pantic I, Bojovic DJ, Fazlagic A, Gardiner H, Sensitivity and specificity of ultrasonography as a screening tool for gestational diabetes mellitus, J Matern Fetal Neonatal Med 25(8) (2012) 1348–53. [DOI] [PubMed] [Google Scholar]
- [62].Ramos A, Caimari F, Pujol IM, Garcia-Patterson A, Ginovart G, Adelantado JM, Corcoy R, In women with gestational diabetes mellitus factors influencing growth have a larger effect on placental weight than on birth weight, Eur J Obstet Gynecol Reprod Biol 202 (2016) 60–5. [DOI] [PubMed] [Google Scholar]
- [63].Razak AA, Leach L, Ralevic V, Impaired vasocontractile responses to adenosine in chorionic vessels of human term placenta from pregnant women with pre-existing and gestational diabetes, Diab Vasc Dis Res 15(6) (2018) 528–540. [DOI] [PubMed] [Google Scholar]
- [64].Saha S, Biswas S, Mitra D, Adhikari A, Saha C, Histologic and morphometric study of human placenta in gestational diabetes mellitus, Ital J Anat Embryol 119(1) (2014) 1–9. [PubMed] [Google Scholar]
- [65].Sak ME, Deveci E, Evsen MS, Kalkanhi S, Baran O, Ozekinci S, Seker U, Expression of beta human chorionic gonadotropin in the placenta of gestational diabetic mothers: an immunohistochemistry and ultrastructural study, Anal Quant Cytopathol Histpathol 35(1) (2013) 52–6. [PubMed] [Google Scholar]
- [66].Salafia CM, Silberman L, Placental pathology and abnormal fetal heart rate patterns in gestational diabetes, Pediatr Pathol 9(5) (1989) 513–20. [DOI] [PubMed] [Google Scholar]
- [67].Samuel R, Ramanathan K, Mathews JE, Seshadri MS, Back to the future: examining type 2 diabetic vasculature using the gestational diabetic placenta, Diab Vasc Dis Res 11(5) (2014) 363–5. [DOI] [PubMed] [Google Scholar]
- [68].Schonfelder G, John M, Hopp H, Fuhr N, van Der Giet M, Paul M, Expression of inducible nitric oxide synthase in placenta of women with gestational diabetes, FASEB J 10(7) (1996) 777–84. [DOI] [PubMed] [Google Scholar]
- [69].Scifres CM, Parks WT, Feghali M, Caritis SN, Catov JM, Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus, Placenta 49 (2017) 10–15. [DOI] [PubMed] [Google Scholar]
- [70].G.S. Sharma S, Biswas S, Chakravarty S, Effect of Gestational Diabetes on Microanatomy of Placenta, J Forensic Medicine & Toxicology 31(2) (2014) 65–69. [Google Scholar]
- [71].Soygur B, Moore H, Expression of Syncytin 1 (HERV-W), in the preimplantation human blastocyst, embryonic stem cells and trophoblast cells derived in vitro, Hum Reprod 31(7) (2016) 1455–61. [DOI] [PubMed] [Google Scholar]
- [72].Stanek J, Weng E, Microscopic chorionic pseudocysts in placental membranes: a histologic lesion of in utero hypoxia, Pediatr Dev Pathol 10(3) (2007) 192–8. [DOI] [PubMed] [Google Scholar]
- [73].Stanek J, Biesiada J, Trzeszcz M, Clinicoplacental phenotypes vary with gestational age: an analysis by classical and clustering methods, Acta Obstet Gynecol Scand 93(4) (2014) 392–8. [DOI] [PubMed] [Google Scholar]
- [74].Stoz F, Schuhmann RA, Haas B, Morphohistometric investigations in placentas of gestational diabetes, J Perinat Med 16(3) (1988) 205–9. [DOI] [PubMed] [Google Scholar]
- [75].Strom-Roum EM, Tanbo TG, Eskild A, The associations of maternal body mass index with birthweight and placental weight. Does maternal diabetes matter? A population study of 106 191 pregnancies, Acta Obstet Gynecol Scand 95(10) (2016) 1162–70. [DOI] [PubMed] [Google Scholar]
- [76].Suranyi A, Kozinszky Z, Molnar A, Nemeth G, Placental volume relative to fetal weight estimated by sonography in diabetic pregnancies, J Matern Fetal Neonatal Med 29(8) (2016) 1229–32. [DOI] [PubMed] [Google Scholar]
- [77].Taricco E, Radaelli T, Rossi G, Nobile de Santis MS, Bulfamante GP, Avagliano L, Cetin I, Effects of gestational diabetes on fetal oxygen and glucose levels in vivo, BJOG 116(13) (2009) 1729–35. [DOI] [PubMed] [Google Scholar]
- [78].Taricco E, Radaelli T, Nobile de Santis MS, Cetin I, Foetal and placental weights in relation to maternal characteristics in gestational diabetes, Placenta 24(4) (2003) 343–7. [DOI] [PubMed] [Google Scholar]
- [79].Thunbo MO, Sinding M, Bogaard P, Korsager AS, Frokjaer JB, Ostergaard LR, Petersen A, Sorensen A, Postpartum placental CT angiography in normal pregnancies and in those complicated by diabetes mellitus, Placenta 69 (2018) 20–25. [DOI] [PubMed] [Google Scholar]
- [80].Tramontana A, Pablik E, Stangl G, Hartmann B, Dieplinger H, Hafner E, Combination of first trimester serum afamin levels and three-dimensional placental bed vascularization as a possible screening method to detect women at-risk for adverse pregnancy complications like pre-eclampsia and gestational diabetes mellitus in low-risk pregnancies, Placenta 62 (2018) 9–15. [DOI] [PubMed] [Google Scholar]
- [81].Vilarino-Garcia T, Perez-Perez A, Dietrich V, Fernandez-Sanchez M, Guadix P, Duenas JL, Varone CL, Damiano AE, Sanchez-Margalet V, Increased Expression of Aquaporin 9 in Trophoblast From Gestational Diabetic Patients, Horm Metab Res 48(8) (2016) 535–9. [DOI] [PubMed] [Google Scholar]
- [82].Visiedo F, Santos-Rosendo C, Mateos-Bernal RM, Gil-Sanchez MD, Bugatto F, Aguilar-Diosdado M, Segundo C, Lopez-Tinoco C, Characterization of NO-Induced Nitrosative Status in Human Placenta from Pregnant Women with Gestational Diabetes Mellitus, Oxid Med Cell Longev 2017 (2017) 5629341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang P, Wang H, Li C, Zhang X, Xiu X, Teng P, Wang Z, Dysregulation of microRNA-657 influences inflammatory response via targeting interleukin-37 in gestational diabetes mellitus, J Cell Physiol 234(5) (2019) 7141–7148. [DOI] [PubMed] [Google Scholar]
- [84].Wong CH, Chen CP, Sun FJ, Chen CY, Comparison of placental three-dimensional power Doppler indices and volume in the first and the second trimesters of pregnancy complicated by gestational diabetes mellitus, J Matern Fetal Neonatal Med 32(22) (2019) 3784–3791. [DOI] [PubMed] [Google Scholar]
- [85].B.D. Yavuz D, Ekinci C, Tahaoglu AE, Togrul C, Goruk N, Aktas A, Karaman E, Expression of VEGF and CD68 in the Placenta of Gestational Diabetic Mothers (Immunohistochemistry and Ultrastructural Study), Int J Morphol 33(2) (2015) 522–526. [Google Scholar]
- [86].Younes B, Baez-Giangreco A, al-Nuaim L, al-Hakeem A, Abu Talib Z, Basement membrane thickening in the placentae from diabetic women, Pathol Int 46(2) (1996) 100–4. [DOI] [PubMed] [Google Scholar]
- [87].Yuksel MA, Kilic F, Kayadibi Y, Alici Davutoglu E, Imamoglu M, Bakan S, Mihmanli I, Kantarci F, Madazli R, Shear wave elastography of the placenta in patients with gestational diabetes mellitus, J Obstet Gynaecol 36(5) (2016) 585–8. [DOI] [PubMed] [Google Scholar]
- [88].Zhou J, Ni X, Huang X, Yao J, He Q, Wang K, Duan T, Potential Role of Hyperglycemia in Fetoplacental Endothelial Dysfunction in Gestational Diabetes Mellitus, Cell Physiol Biochem 39(4) (2016) 1317–28. [DOI] [PubMed] [Google Scholar]
- [89].Kovo M, Granot Y, Schreiber L, Divon M, Ben-Haroush A, Bar J, Pregnancy outcome and placental pathology differences in term gestational diabetes with and without hypertensive disorders, J Matern Fetal Neonatal Med 29(9) (2016) 1462–7. [DOI] [PubMed] [Google Scholar]
- [90].Akhter F, Ferdausi R, Quantitative macroscopic study on preterm placenta in gestational diabetes mellitus and pregnancy induced hypertension, Mymensingh Med J 20(2) (2011) 280–6. [PubMed] [Google Scholar]
- [91].Weiner E, Barber E, Feldstein O, Schreiber L, Dekalo A, Mizrachi Y, Bar J, Kovo M, The placental component and neonatal outcome in singleton vs. twin pregnancies complicated by gestational diabetes mellitus, Placenta 63 (2018) 39–44. [DOI] [PubMed] [Google Scholar]
- [92].Carrasco-Wong I, Moller A, Giachini FR, Lima VV, Toledo F, Stojanova J, Sobrevia L, San Martin S, Placental structure in gestational diabetes mellitus, Biochim Biophys Acta Mol Basis Dis 1866(2) (2020) 165535. [DOI] [PubMed] [Google Scholar]
- [93].Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, Datta M, Gestational diabetes mellitus manifests in all trimesters of pregnancy, Diabetes Res Clin Pract 77(3) (2007) 482–4. [DOI] [PubMed] [Google Scholar]
- [94].Immanuel J, Simmons D, Screening and Treatment for Early-Onset Gestational Diabetes Mellitus: a Systematic Review and Meta-analysis, Curr Diab Rep 17(11) (2017) 115. [DOI] [PubMed] [Google Scholar]
- [95].Clarson C, Tevaarwerk GJ, Harding PG, Chance GW, Haust MD, Placental weight in diabetic pregnancies, Placenta 10(3) (1989) 275–81. [DOI] [PubMed] [Google Scholar]
- [96].Hung TH, Huang SY, Chen SF, Wu CP, Hsieh TT, Decreased placental apoptosis and autophagy in pregnancies complicated by gestational diabetes with large-for-gestational age fetuses, Placenta 90 (2020) 27–36. [DOI] [PubMed] [Google Scholar]
- [97].Mejia JF, Hirschi KM, Tsai KYF, Long MG, Tullis BC, Bitter EEK, Bikman BT, Reynolds PR, Arroyo JA, Differential placental ceramide levels during gestational diabetes mellitus (GDM), Reprod Biol Endocrinol 17(1) (2019) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Avagliano L, Massa V, Terraneo L, Samaja M, Doi P, Bulfamante GP, Marconi AM, Gestational diabetes affects fetal autophagy, Placenta 55 (2017) 90–93. [DOI] [PubMed] [Google Scholar]
- [99].James JL, Carter AM, Chamley LW, Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation?, Placenta 33(5) (2012) 327–34. [DOI] [PubMed] [Google Scholar]
- [100].Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A, Fetal vasculogenesis and angiogenesis in human placental villi, Acta Anat (Basel) 136(3) (1989) 190–203. [DOI] [PubMed] [Google Scholar]
- [101].Zygmunt M, Herr F, Munstedt K, Lang U, Liang OD, Angiogenesis and vasculogenesis in pregnancy, Eur J Obstet Gynecol Reprod Biol 110 Suppl 1 (2003) S10–8. [DOI] [PubMed] [Google Scholar]
- [102].Turowski G, Vogel M, Re-view and view on maturation disorders in the placenta, APMIS 126(7) (2018) 602–612. [DOI] [PubMed] [Google Scholar]
- [103].Grannum PA, Berkowitz RL, Hobbins JC, The ultrasonic changes in the maturing placenta and their relation to fetal pulmonic maturity, Am J Obstet Gynecol 133(8) (1979) 915–22. [DOI] [PubMed] [Google Scholar]
- [104].Burton GJ, Jauniaux E, Sonographic, stereological and Doppler flow velocimetric assessments of placental maturity, Br J Obstet Gynaecol 102(10) (1995) 818–25. [DOI] [PubMed] [Google Scholar]
- [105].Yin TT, Loughna P, Ong SS, Padfield J, Mayhew TM, No correlation between ultrasound placental grading at 31–34 weeks of gestation and a surrogate estimate of organ function at term obtained by stereological analysis, Placenta 30(8) (2009) 726–30. [DOI] [PubMed] [Google Scholar]
- [106].Whittington JR, Cummings KF, Ounpraseuth ST, Aughenbaugh AL, Quick CM, Dajani NK, Placental changes in diabetic pregnancies and the contribution of hypertension, J Matern Fetal Neonatal Med (2020) 1–9. [DOI] [PubMed] [Google Scholar]
- [107].Kraus FT, Fetal Thrombotic Vasculopathy: Perinatal Stroke, Growth Restriction, and Other Sequelae, Surg Pathol Clin 6(1) (2013) 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pathak S, Lees CC, Hackett G, Jessop F, Sebire NJ, Frequency and clinical significance of placental histological lesions in an unselected population at or near term, Virchows Arch 459(6) (2011) 565–72. [DOI] [PubMed] [Google Scholar]
- [109].Shams F, Rafique M, Samoo NA, Irfan R, Fibrinoid necrosis and hyalinization observed in normal, diabetic and hypertensive placentae, J Coll Physicians Surg Pak 22(12) (2012) 769–72. [PubMed] [Google Scholar]
- [110].Babawale MO, Lovat S, Mayhew TM, Lammiman MJ, James DK, Leach L, Effects of gestational diabetes on junctional adhesion molecules in human term placental vasculature, Diabetologia 43(9) (2000) 1185–96. [DOI] [PubMed] [Google Scholar]
- [111].Swiderski S, Celewicz Z, Miazgowski T, Ogonowski J, Maternal endothelin-1 and cyclic guanosine monophosphate concentrations in pregnancies complicated by pregravid and gestational diabetes mellitus, Gynecol Obstet Invest 69(1) (2010) 46–50. [DOI] [PubMed] [Google Scholar]
- [112].Wu S, Nan R, Li Y, Cui X, Liang X, Zhao Y, Measurement of elasticity of normal placenta using the Virtual Touch quantification technique, Ultrasonography 35(3) (2016) 253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Loukeris K, Sela R, Baergen RN, Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks’ gestational age, Pediatr Dev Pathol 13(4) (2010) 305–9. [DOI] [PubMed] [Google Scholar]
- [114].Abbade J, Klemetti MM, Farrell A, Ermini L, Gillmore T, Sallais J, Tagliaferro A, Post M, Caniggia I, Increased placental mitochondrial fusion in gestational diabetes mellitus: an adaptive mechanism to optimize feto-placental metabolic homeostasis?, BMJ Open Diabetes Res Care 8(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Muralimanoharan S, Maloyan A, Myatt L, Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: role of miR-143, Clin Sci (Lond) 130(11) (2016) 931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Anelli GM, Cardellicchio M, Novielli C, Antonazzo P, Mazzocco MI, Cetin I, Mando C, Mitochondrial content and hepcidin are increased in obese pregnant mothers, J Matern Fetal Neonatal Med 31(18) (2018) 2388–2395. [DOI] [PubMed] [Google Scholar]
- [117].McElwain C, McCarthy CM, Investigating mitochondrial dysfunction in gestational diabetes mellitus and elucidating if BMI is a causative mediator, Eur J Obstet Gynecol Reprod Biol 251 (2020) 60–65. [DOI] [PubMed] [Google Scholar]
- [118].Cushen SC, Sprouse ML, Blessing A, Sun J, Jarvis SS, Okada Y, Fu Q, Romero SA, Phillips NR, Goulopoulou S, Cell-free mitochondrial DNA increases in maternal circulation during healthy pregnancy: a prospective, longitudinal study, Am J Physiol Regul Integr Comp Physiol 318(2) (2020) R445–R452. [DOI] [PMC free article] [PubMed] [Google Scholar]


