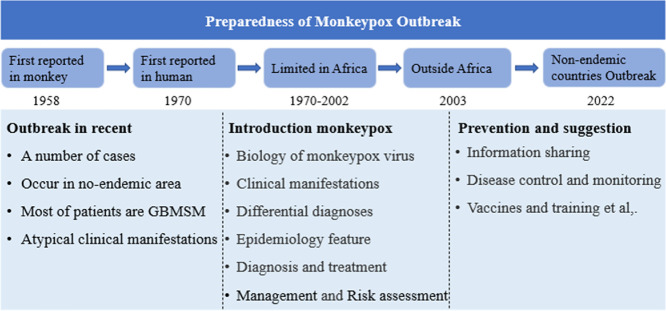
Highlights
-
•
A multi-country outbreak of the monkeypox virus which has gained global attention.
-
•
Most cases of this outbreak identified as gay, bisexual, or other men who have sex with men.
-
•
Some cases symptoms are atypical of historical monkeypox manifestations.
Keywords: Monkeypox virus, Outbreak, Infection, Treatment
Abstract
In light of the ongoing COVID-19 pandemic, the unexpected outbreak and worldwide spread of monkeypox has gained global attention. As of June 22, 2022, there were 3340 confirmed cases of monkeypox globally, which is the largest and most widespread monkeypox epidemic outside Africa. Monkeypox virus (MPXV) is transmitted from human-to-human through direct contact with infectious skin or mucosal skin lesions, respiratory droplets, or indirect contact with contaminated objects or materials, as well as mother-to-child vertical transmission. It is also possibly sexually transmitted through semen/vaginal fluid, and the possibility of community transmission cannot be ruled out. Monkeypox is a viral zoonotic disease caused by MPXV, which is an enveloped, linear, double-stranded DNA virus belonging to the Orthopoxvirus genus, of the Chordopoxvirinae subfamily, within the Poxviridae family. Monkeypox is usually a self-limiting infection, with symptoms lasting 2–4 weeks, and has a fatality rate that has historically fluctuated from 0% to 11%. Symptoms of monkeypox include intense headaches, fever, lesions, and lymphadenopathy. Although there is no specific treatment or vaccine for MPXV infection, antiviral drugs and vaccines for smallpox have been approved for use in several countries in response to the monkeypox outbreak. Before the virus can be allowed to establish efficient person-to-person transmission, rapid action must be taken to contain the local spread and, by extension, the multi-country outbreak of monkeypox.
Graphical Abstract
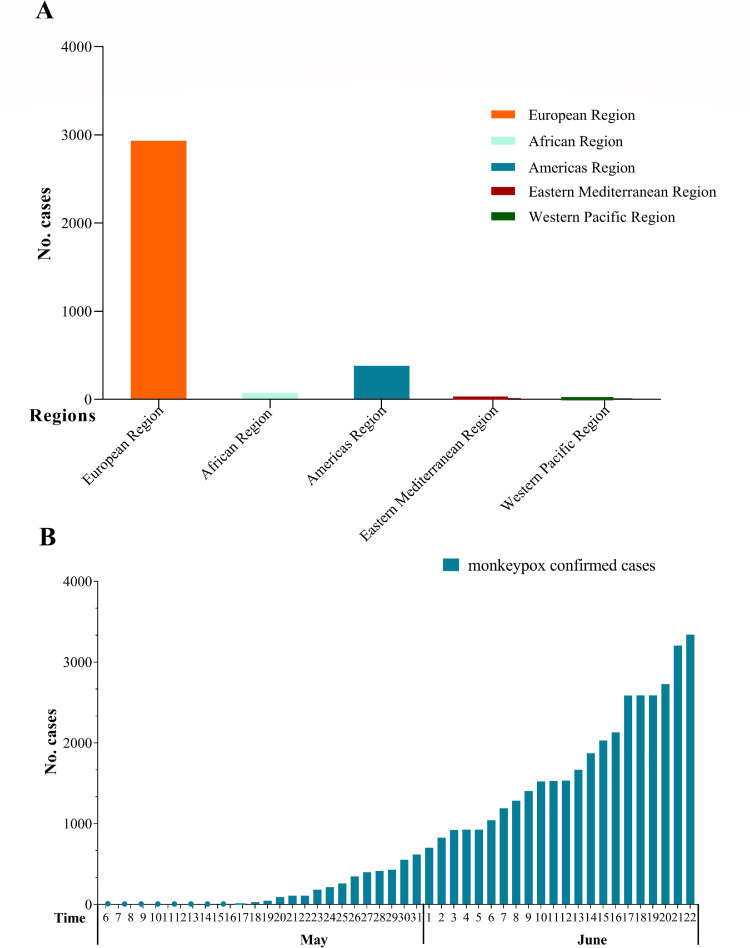
On May 7, 2022, the UK Health Security Agency (UKHSA) reported that an individual has been diagnosed with monkeypox, and that individual had a travel history to Nigeria [1]. On May 14, 2022, a family cluster of 2 cases of monkeypox was reported by the UKHSA that had no relation to the case imported from Nigeria [1]. In the following days, several non-endemic countries reported cases of monkeypox, and most confirmed cases had a travel history to countries in Europe and North America, rather than West or Central Africa where monkeypox virus (MPXV) is endemic [2]. From January 1, 2022 to June 22, 2022, there were 3413 laboratory-confirmed cases and 1 death reported to or identified by the World Health Organization (WHO) from 50 countries/territories in 5 WHO regions, and 86% (2933/3413) of cases were from the European region [2] (Fig. 1A). From May 3, 2022 to June 22, 2022, there were 3340 confirmed cases of monkeypox from 42 Member States across 4 WHO regions that were not endemic for MPXV [2,3] (Fig. 1B). On June 24, 2022, 1 case was confirmed in Taiwan, China, in a patient that had recently returned from Germany, and this was the first reported case of monkeypox in China. Epidemiological investigations are ongoing.
Fig. 1.
The number of confirmed cases of monkeypox. A. The total number of confirmed cases of monkeypox by the WHO from January 1 to June 22, 2022. B. Recent global trend in the number of confirmed cases of monkeypox from May 7 to June 22, 2022.
In the UK, it is the first time that a sustained number of MPXV-infected cases have been reported following human-to-human transmission through close contact, thus, there is a risk of community transmission [4]. Many cases in this outbreak did not have the typical clinical manifestations of monkeypox [2]. In cases described to date, common symptoms include genital and perianal lesions, fever, swollen lymph nodes, and pain when swallowing. Although oral ulcers remain a common feature of fever and lymphadenopathy, localized anogenital rashes (with blisters, pustules, or ulcerative lesions) sometimes appear first and do not continue to spread to other parts of the body. In many cases, the initial presentation of a genital or perianal rash suggests that close physical contact during sexual contact may be the route of transmission [2,4]. No deaths have resulted from monkeypox in non-endemic countries during this outbreak; however, deaths continue to be reported in endemic countries [2].
Of the 1796 confirmed cases reported by the European Surveillance System, 99.4% (1761/1771) were male, and the majority of cases were between 31 and 40 years of age (792/1796). The UKHSA reported that 151 of the 152 men interviewed identified as gay, bisexual, or as men who have sex with men (GBMSM) [5]. As epidemiological and laboratory information is still limited, the actual number of cases is likely an underestimate. Most of the cases so far have been reported through sexual health or other health services in primary or secondary healthcare institutions, mainly involving, but not limited to GBMSM [6]. The UK has identified links to gay bars, saunas, and the use of dating apps in the UK and abroad. Investigations continue but currently, no single factor or type of exposure that links the cases has been identified [7]. At present, it is not known whether monkeypox can be transmitted through semen or vaginal fluid.
The situation is evolving rapidly and there will be more cases identified as surveillance expands in non-endemic countries, as well as in countries known to be endemic that have not recently been reporting cases. Currently, the overall public health risk at a global level is assessed as moderate considering this is the first time that monkeypox cases and clusters have been reported concurrently in widely disparate WHO geographical areas, but the symptoms are relatively mild in many cases with a localized rash and lymphadenopathy [8]. In order to identify its origin and track its dissemination, several countries have announced full-length or partial genome sequences of MPXV (https://virological.org). Sequence comparisons showed that these sequences were homologous with a strain discovered in the UK 3 years ago. The strains belong to West African strains that show weak virulence, and for which the mutation frequency has been determined. Compared to the 2018–2019 MPXV, the 2022 MPXV appeared to have a mean of 50 SNPs, which is far more than previous estimates of the substitution rate for Orthopoxviruses (1–2 substitutions per site per year) [9]. The higher-than-expected mutation rate in the genome suggests that MPXV may be becoming more adapted to humans. In light of the ongoing coronavirus disease 2019 (COVID-19) pandemic, the outbreak and global spread of MPXV infection has aroused great public concern. Therefore, to better understand MPXV and to prevent the spread of monkeypox, this paper reviews research progress on the etiology, epidemiology, clinical manifestations, laboratory diagnosis, prevention, and treatment of monkeypox.
1. The etiology of MPXV
Monkeypox is a rare disease caused by MPXV infection. MPXV is an enveloped, linear, double-stranded DNA virus, which is a member of the Orthopoxvirus genus, of the Chordopoxvirinae subfamily, within the Poxviridae family. Having the same morphological characteristics as other orthopoxviruses, virions are ovoid or brick-shaped particles [10,11]. MPXV is among the largest and most complex of animal viruses, ranging from 200 to 250 nm in length when viewed by electron microscopy [12,13]. The virus consists of 4 main components: the core, lateral bodies, the outer membrane, and the outer lipoprotein envelope. Virions are enclosed by a geometrically corrugated lipoprotein outer membrane, the core is described as biconcave and contains a large double-stranded DNA genome, with a lateral body on each side [12].
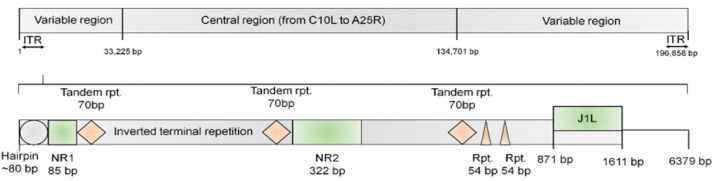
The MPXV genome is approximately 197 kb and encodes 200 proteins. The ends of the genome contain an identical but oppositely oriented sequence called an inverted terminal repeat (Fig. 2) [14]. The genome has close hairpins on both ends and contains about 190 nonoverlapping open reading frames of >180 nt in length [15]. Four open reading frames at the left side of the genome are located within the inverted terminal repeat and thus have counterparts on the right side of the genome [14]. Although MPXV is a DNA virus, its entire life cycle occurs in the cytoplasm of infected cells. All of the proteins required for viral DNA replication, transcription, virion assembly, and egress are encoded by the MPXV genome [12]. Phylogenetic trees showed that MPXV sequences were classified into 2 genetic clades, named the West African clade and the Central African clade (Congo Basin clade). The genomes of monkeypox strains from Central and West Africa were compared because of the difference in virulence and the results revealed 0.55%–0.56% nucleotide difference between the 2 types of strains [16].
Fig. 2.
The structure of the MPXV genome[10]. The ends of the genome possess a 6379-bp inverted terminal repeat (ITR), NR1, NR2, 2 short tandem repeats, and the coding region.
MPXV is resistant to ether and drying, while easily inactivated by chloroform, methanol, and formalin. In addition, heating at 56°C for 30 minutes inactivates the virus. The virus can be stored at 4°C or −20°C over a short time period or at −70°C over a long time period.
2. Clinical manifestations
MPXV and variola virus (the causative agent of smallpox) are both classified as Orthopoxviruses, and in humans, the symptoms of monkeypox are similar to, but milder than those of smallpox [17]. Monkeypox begins with a fever, headache, muscle soreness, and fatigue. The main difference between smallpox, chickenpox, and monkeypox symptoms is that monkeypox causes lymphadenopathy, while smallpox and chickenpox do not [8]. The incubation period for monkeypox (from infection to symptom onset) is usually 7–14 days, but the range is 5–21 days [18].
Monkeypox is usually a self-limiting illness, with symptoms recovering within 2–4 weeks [19]. However, the disease can be severe in some individuals, such as children, pregnant women, or those who are immunocompromised [20,21]. The infection can be divided into 2 periods: the invasion period and the skin eruption period. The invasion period (lasting between 0 and 5 days) is characterized by a fever, intense headache, chills, exhaustion, asthenia, lymphadenopathy (swelling of the lymph nodes), back pain, and myalgia. The skin eruption period usually begins within 1–3 days of the appearance of a fever. Previous studies and studies during this outbreak reported that a rash may also precede a fever [6,22]. The rash often begins on the face and quickly spreads centrifugally over the body, tending to be more concentrated on the face and extremities rather than on the trunk, and including the face (in 95% of cases), palms of the hands and soles of the feet (in 75% of cases), oral mucous membranes (in 70% of cases), genitalia (30%), and conjunctivae (20%), as well as the cornea [1]. Monkeypox lesions then progress through several stages from blister-like lesions to scabs, each lasting 1–2 days. The rash evolves sequentially from macules (lesions with a flat base) to papules (slightly raised firm lesions), vesicles (lesions filled with clear fluid), pustules (lesions filled with yellowish fluid), and crusts that dry up and fall off, with the number of lesions varying from a few to several thousand [23,24]. In severe cases, lesions can coalesce until large sections of skin slough off [1]. In addition, these different lesions may appear at the same time. After their lesion scabs fall off to reveal healthy tissue underneath, which usually takes 2–4 weeks after symptom onset, the individual is no longer infectious. Because monkeypox is a disease that involves a rash, it needs to be clearly distinguished from other illnesses with a rash (Table 1). Complications of monkeypox can include secondary infections, respiratory distress, bronchopneumonia, sepsis, encephalitis, loss of vision due to corneal infections, gastrointestinal involvement, vomiting, and diarrhea with dehydration [18].
Table 1.
Characteristics of diseases with a rash.
| Illness | Pathogeny | Incubation period | Clinical manifestation | Rash | Susceptible ppopulation | Course of disease | Vaccine |
|---|---|---|---|---|---|---|---|
| Monkeypox |
Monkeypox virus | 5–21 days | Fever (often>38.5°C), intense headache, chills, exhaustion, asthenia, lymphadenopathy, back pain, myalgia, lesions | Centrifugal Type:Macules, papules, vesicles Progression: Begin face, extremities, and over the body. Lesions are often in one stage (slow progression with each stage) |
Unvaccinated smallpox vaccine | 2–4 weeks | No smallpox vaccine |
| Smallpox | Smallpox virus | 7–17 days | Fever (often >40°C), headache, backpain, chills, vomiting, or severe abdominal pain c |
Centrifugal Type: Maculopapular (face and neck), vesicular and pustular (after 1–2 days). Progression: First appear on face, oral mucosa, or forearms, Lesions in the same stage of development (slow progression with each stage) |
Generally | 15–20 days | Yes |
| Chickenpox | Varicella-zoster virus | 10–21 days | Fever (up to 38.8°C), chills, sore throat, vomiting, exhaustion | Centripetal Type: rash, round tense blister. Spots, mounds, blisters, scabs appear simultaneously Progression: Face-trunk-extremities, multiple stages of development on the body (fast progression) |
6–9 years old | 5–7 days | Yes |
| Scarlet Fever | Group A B hemolytic streptococcus |
1–7 days | Fever, malaise, and sore throat, Abdominal pain and vomiting, tender anterior cervical lymph nodes, circumoral pallor, strawberry tongue, partridge line | Type: tiny red papules Progression: first appear on forehead, armpit, and then groin-whole body (slow progression) |
2–8 years old | 2–5 days | No |
| Herpes Simplex | Herpes simplex virus (HSV) | 2–12 days | HSV-1: Oral herpes infection is mostly asymptomatic, tingling, itching or burning sensation around their mouth HSV-2: fever, body aches and swollen lymph nodes |
Type: blisters or ulcers that can recur over time. Progression: HSV-1: oral herpes/genital herpes. HSV-2: genital herpes (slow progression) |
Infant Immuno- compromised |
7 to14 days | No |
| Measles | Measles virus | 6–21 days | Fever (>39°C), Catarrhal symptoms due to upper respiratory tract and ocular conjunctivitis, and Koplik spots | Type: maculopapular skin rash Progression: appear behind the ear hairline, forehead, face, neck, later on trunk and then extremities (slow progression) |
0.5–5 years old | 7–14 days | Yes |
| Syphilis | Treponema Pallidum | 9–90 days | Primary: lesions on the genitals or other body sites Secondary: fever, headache, muscular soreness, Lymph nodes |
Type: maculopapular rash Progression: on the flank, shoulders, arm, chest or back and that often involves the palms of the hands and soles of the feet (very slow progression) |
A history of unclean sexual intercourse or multiple sexual partners | / | No |
| Scabies | Mites | 2–6 weeks | a markedly pruritic papular or papulovesicular rash (nocturnal crescendo) |
Type: papular or papulo-vesicular rash Progression: occurred body regions with a thin stratum corneum and a low density of sebaceous gland follicles (slow progression) |
Immuno-suppression, Children and elderly | Weeks or months | No |
| Medication-associated Allergies | / | 4–20 days | Fever, rhinorrhea, erubescence, face swelled up, scratchy or ache, dizziness, palpitations, dyspnea | Type: skin rash, hives or itching Progression: The rash is extensive and varied (quickly or slow) |
Specific allergic constitution and exposed to the drug before | 2–4 weeks | No |
| Rickettsia Vesicles | Mites of Rickettsia | 7–14 days | Headache, fatigue, chills, rash | Type: Papules or pimple herpes Progression: localized and extremities not, |
Generally | Within 2 weeks | No |
| Rubella | Rubella virus | 5–25 days | Fever, cough, rhinorrhea, occipital lymph node enlargement | Type: tiny papule (roseola) Progression: first appeared on head, face, then on trunk, lase on extremities All over the body for 24 hours (fast progression) |
1–5 years old | 3–7 days | Yes |
| Roseola Infantum | human herpesvirus-6 or 7 | 8–14 days | Fever (39–41°C for 3–5 days) | Type: pink papular rash (nonpruritic) Progression: begins on the trunk with a rapid defervescence of the fever (fast progression) |
Under 1 years old | 8–10 days | No |
| Hand-Foot-and-Mouth Disease | Enterovirus 71 | 2–7 days | Fever and flu-like symptoms, sore throat, eating or drinking less, feeling unwell, herpangina (painful), Only wanting to drink cold fluids | Type: red spots, skin rash, blisters Progression: start as mouth, then skin rash on the palms of the hands and soles of the feet (slow progression) |
Under 5 years old | 7–10 days | Yes |
MPXV appears to present differently in the current outbreak. The prodromal symptoms of these patients may be mild or even remain unnoticed. Compared with the clinical characteristics in previously-reported African monkeypox patients, most cases in this outbreak have atypical clinical characteristics. The initial presentation is of a genital or perianal rash among men who have sex with men. Some cases have also been described as pustules preceded by constitutional symptoms (eg, a fever) and lesions at different stages of development, both of which are atypical of historical monkeypox manifestations. Most cases develop localized lesions, such as on oral mucous membranes, the genitals, and/or the anus and surrounding areas. Some patients develop proctitis [25].
3. Distribution of the disease
3.1. Sources of infection
Monkeypox is a zoonotic infectious disease, which usually occurs sporadically in forested areas of Central and West Africa [26]. It is caused by MPXV of the Orthopoxvirus family. Monkeypox can be spread by contact and by large droplets of exhaled fluid. Most cases of infection occur from a reservoir host or infected animals. Humans are the sole reservoir host of variola virus, with no known animal reservoirs, whereas MPXV has a wide range of permissible animal reservoir(s), including rodents, mammals, and primates [27]. Specific animal reservoirs include Kenyan vervet monkeys, chimpanzees, African elephants, wild boar, antelope, Gambian poached rats, pet prairie dogs, West African squirrels, and anteaters. The natural reservoir of monkeypox has not yet been identified, and the mode of transmission in nature remains unknown. However, African rodents are suspected to play a role in the transmission of monkeypox to humans [28,29].
3.2. The mode of transmission
MPXV spreads when a person is exposed to the virus from an infected animal, infected person, or virus-contaminated material. Transmission of MPXV occurs via the animal-to-human and human-to-human routes [12]. The virus can also spread from a mother's placenta to a fetus. MPXV may be transmitted from animal-to-human through direct contact with the blood, bodily fluids, or cutaneous or mucosal lesions from bites or scratches of infected animals, treatment of wild animals, or the use of products made from infected animals [30,31]. Monkeypox is mainly transmitted from person-to-person through direct contact with infectious ulcers, scabs or bodily fluids, or materials that have been exposed to bodily fluids or ulcers, such as clothing or linen [21]. In long-term face-to-face contact, it can also be transmitted through respiratory secretions. MPXV also can spread during close contact between people, including during sexual intercourse, kissing, hugging, or touching body parts with monkeypox.
Table 2 summarizes the MPXV transmission mode from 1970 to 2019. Throughout history, monkeypox mainly occurred in Africa, and the mode of transmission was animal-to-human, while human-to-human transmission was limited, with cases of secondary human-to-human transmission accounting for about 28% of cases [32]. However, during this outbreak, the mode of transmission is human-to-human transmission, and most cases have been diagnosed in GBMSM. Although the disease is not generally considered a sexually-transmitted infection, inter-human transmission has been proven through close contact [33]. Therefore, there is a risk of community transmission.
Table 2.
Transmission mode of monkeypox in recent years.
| Decades | Central countries | West countries | Other countries |
|---|---|---|---|
| 1970–1979 | Cameroon: unknown DRC: Both |
Liberia: unknown Nigeria: unknown Nigeria: human-to-human Sierra Leone: unknown |
|
| 1980–1989 | CAR: animal-to-human DRC: Both Gabon: unknown |
Ivory Coast: unknown |
|
| 1990–1999 | DRC: Both Gabon: unknown |
||
| 2000–2009 | DRC: animal-to-human RC: animal-to-human |
||
| 2010–2019 | CAR: Both DRC: animal-to-human RC: unknown |
Nigeria: Both Sierra Leone: unknown Sierra Leone: animal |
Israel: animal-to-human Singapore: animal South Sudan: human-to-Human UK: unknow, human- to-Human US: animal-to-human |
CAR, Central African Republic; DRC,Democratic Republic of the Congo; RC, Republic of the Congo.
3.3. Susceptible population
The contemporary susceptible population is composed mainly of individuals who are unvaccinated for vaccinia virus (approximately 80%–96% of the population). Although the vaccinated population has some immunity to MPXV, protection may have waned over time, and there is still a risk of infection [34]. Historically, vaccination against smallpox (vaccinia) was shown to be 85% effective in preventing MPXV and the case fatality rate of people who are unvaccinated against vaccinia is 9.8% [35].
4. Epidemiology
4.1. Discovery of MPXV
MPXV belongs to the genus Orthopoxvirus, which also includes the smallpox virus (causing smallpox), vaccinia virus (used for the smallpox vaccine), and cowpox virus [36]. Monkeypox was first discovered in a Danish laboratory in 1958 [37]. At that time, there were 2 outbreaks of disease with pox in the monkeys used for research, so it was named “monkeypox.” The first human case was confirmed in 1970 in a 9-month-old boy who was suspected of having smallpox during the eradication of smallpox in the Democratic Republic of the Congo (DRC) [38]. Since then, monkeypox has been reported in several other Central and West African countries, where it is endemic and responsible for thousands of cases each year.
4.2. Number of cases throughout history
By the end of 2019, a total of 1347 cases and an additional 28,815 suspected cases of monkeypox had been reported [39]. In the 1980s, the number of confirmed and probable monkeypox cases in the DRC had increased 9-fold compared with that in the 1970s. Monkeypox cases continued to increase in the 1990s. From 2000 to 2009, 3 African countries reported 92 confirmed cases, while during 2009–2019, 7 African countries reported 277 confirmed cases. Compared with the last 3 decades of the 20th century, outbreaks as of the year 2000 were greater in terms of the total number of cases, but there were fewer single case reports [39]. The number of monkeypox cases and the associated countries are shown in Table 3.
Table 3.
The changing number of cases of human monkeypox over time.
| Decade | Confirmed | Suspected | Death | Countries |
|---|---|---|---|---|
| 1970–1979 | 48 | / | 8 | DRC, Cameroon, Côte d'Ivoire, Liberia, Nigeria, and Sierra Leone |
| 1980–1989 | 357 | / | 36 | DRC, Central African Republic, Gabon, Cameroon, Côte d'Ivoire |
| 1990–1999 | 520 | / | 3 | DRC, Gabon |
| 2000–2009 | 139 | 10027 | 1 | DRC, Congo, United States, South Sudan |
| 2010–2019 | 283 | 18788 | 30 | DRC, Central African Republic, Nigeria, Congo, Liberia, United Kingdom, Cameroon, Sierra Leone, Israel, Singapore |
Note: From the year 2000, the DRC started primarily reporting the number of suspected cases.
In Africa, outbreaks of monkeypox have been frequent. From 1996 to 1997, an unprecedented outbreak occurred in the DRC (511 cases), with a peak incidence rate in August 1996. The majority of cases were secondary cases resulting from person-to-person transmission, with clinically milder disease [40]. In September 2017, the largest monkeypox outbreak was recorded in Nigeria, which was caused by a strain belonging to the West Africa clade [34]. This resurgence occurred after 40 years of no cases being reported. Between 2017 and 2019, 183 cases were diagnosed, with most cases being among those aged 21–40 years, whereas historically, the disease was more prevalent among <15-year-olds [40,41]. A total of 502 confirmed cases and 8 deaths were reported from September 2017 to October 2021 [42].
Recently, monkeypox outbreaks have occurred in non-endemic countries, with most cases having no direct travel links to an endemic area. Whereas, prior to this outbreak, confirmed cases in non-endemic countries usually had a travel history to endemic countries. It was not until 2003 that the first monkeypox outbreak occurred outside of Africa, with 47 confirmed or possible cases being reported in the USA. These cases occurred after contact with an infected pet marmot, which was infected with MPXV from an infected exotic animal imported from Ghana [28]. Seven international monkeypox cases were confirmed from 2018 to 2021, and all cases had a history of travel and exposure in Nigeria, of these: 3 were in the UK (May 2021) [23], 2 were in the USA [43,44] (July 2021 and November 2021), one was in Israel [45] (October 2018), and one was in Singapore [46] (May 2019).
Before the 2017 Nigeria outbreak, most human monkeypox cases occurred in rural, forested areas in Africa. However, monkeypox cases occurred in urban areas in Nigeria [34]. This change indicated that human-to-human transmission increases the risk of MPXV spread.
4.3. Case fatality rate and phylogeographical analyses
In endemic countries, the case fatality rate (CFR) of monkeypox ranges from 0% to 11%, and a previous study calculated a pooled estimate of CFR of 8.7% [20,39]. From 1970 to 1999, 100% (47/47) of the deaths occurred in children less than 10 years old, whereas in 2000–2009, only 37.5% (6/16) of deaths occurred in this age group. In recent years, deaths have occurred in adults, with an average age of 27 years [22,39]. Grant et al. [47] established a mathematical model of human-to-human transmission, and found that monkeypox has epidemic potential, with R0>1.
The two clades of monkeypox strains are geographically separated by Cameroon and have defined epidemiological and clinical differences [48]. Geographically, West African strain is restricted to Nigeria, Ghana, Liberia, Cote d'Ivoire, and Sierra Leone, while the Central African strain is restricted to DRC, Republic of the Congo, Cameroon, and Gabon [48]. The CFR of the Central African strain (10.6%, 95% confidence interval: 8.4%–13.3%) is significantly higher than that of the West African strain (3.6%, 95% confidence interval: 1.7%–6.8%) [39]. For the Central African strain, the longest documented chain of transmission in a community has risen from 6 to 9 successive person-to-person infections [21]. Experimental animal studies also support that the West African strain is less virulent, and no human-to-human transmission was documented for the West African clade previously [49]. However, the current endemics are caused by West African strains, and transmission is mainly human-to-human. This suggests that the virus may have evolved to infect and be spread among humans.
5. Diagnosis and detection
5.1. Diagnosis
To diagnose cases of monkeypox, epidemiological and clinical characteristics are required. Usually, patients have traveled to an endemic country or have been in contact with infected animals and patients in the previous 21 days. Outbreaks of MPXV infection have been ongoing in non-endemic countries since the beginning of May 2022. Moreover, almost all of these cases had no travel history to endemic countries. Therefore, the WHO has developed surveillance case definitions for the current monkeypox outbreak in non-endemic countries [6] (Table 4).
Table 4.
The definitions of cases of MPXV infection.
| Type of case | Definitions |
|---|---|
| Suspected | A person of any age presenting in a monkeypox non-endemic country with an unexplained acute rash. AND 1. Acute onset of fever (>38.5°C), Headache, Lymphadenopathy, Myalgia, Back pain, Asthenia. 2. The common causes of acute rash do not explain the clinical picture, such as: varicella zoster, herpes zoster, measles, Zika, dengue, chikungunya, herpes simplex, bacterial skin infections, and so on. |
| Probable | A person meeting the case definition for a suspected case AND 1. Has an epidemiological link; direct physical contact with skin or skin lesions in the 21 days before symptom onset. 2. Reported travel history to a monkeypox endemic country1 in the 21 days before symptom onset. 3. Had multiple or anonymous sexual partners in the 21 days before symptom onset. 4. Has a positive result of an orthopoxvirus serological assay, in the absence of smallpox vaccination or other known exposure to orthopoxviruses. |
| Confirmed | A case meeting the definition of either a suspected or probable case and is laboratory confirmed for monkeypox virus by detection of unique sequences of viral DNA either by real-time polymerase chain reaction (PCR) and/or sequencing. |
| Discarded | A suspected or probable case for which laboratory testing by PCR and/or sequencing is negative for monkeypox virus. |
5.2. Detection
The early and accurate laboratory testing of samples from cases is an essential part of the diagnosis and surveillance of this emerging infection. Confirmation of monkeypox depends on the type and quality of the specimen and the type of laboratory test. The recommended clinical specimen type for laboratory confirmation of MPXV includes specimens from skin lesion material, such as swabs of the lesion surface, exudate, or roof from more than one lesion, or lesion crust. Nasopharyngeal swabs and saliva are also important specimens for detection, while blood specimens are not usually used for diagnostic purposes [6,18].
5.2.1. Nucleic acid amplification testing
Identification of MPXV infection is based on nucleic acid amplification testing, using real-time or conventional polymerase chain reaction as the primary detection method for the detection of unique sequences of MPXV viral DNA [50]. The WHO suggested that if a specific MPXV test is unavailable, a positive polymerase chain reaction result for Orthopoxvirus is considered confirmation in non-endemic countries [51].
5.2.2. Antibody detection
Antibodies of plasma or serum should not be used alone for diagnosis. Acute and convalescent sera can be used for MPXV-specific immunoglobulin M detection and immunoglobulin G detection. However, there is antigenic cross-reactivity between MPXV and other orthopoxviruses [8].
5.2.3. Electron microscopy
Electron microscopy can be used to visualize potential poxvirus in a sample, but cannot distinguish MPXV from poxvirus. Furthermore, this method is highly technical and complex, requires expensive equipment and facilities, and only offers low detection sensitivity.
5.2.4. Virus isolation
To date, the standard diagnostic method of infection is the isolation of MPXV from clinical samples. However, MPXV should only be performed in laboratories with appropriate experience and containment facilities. Hence, this method is not optimal as a routine diagnostic procedure [6,18]. Virus isolation should be carried out in P2-level biosafety laboratories but technicians should take personal protective measures according to the standards of P3-level laboratories.
6. Treatment
As described above, many individuals infected with MPXV have a mild self-limiting disease course without specific treatment. However, the prognosis of monkeypox depends on many factors, such as initial health status, concurrent diseases, complications, and previous vaccination status.
People who should be considered for treatment may include[52]: (1) those suffering from serious diseases (such as hemorrhagic diseases, confluent lesions, sepsis, encephalitis, or other diseases requiring hospitalization); (2) the immunocompromised population (for example, those with human immunodeficiency virus/acquired immunodeficiency syndrome infection, tumors, transplantation, and those receiving radiotherapy or high-dose corticosteroids); (3) the pediatric population, especially those under 8 years old; (4) pregnant or breastfeeding women; (5) those with a history of allergic dermatitis or allergic dermatitis, and those with other active exfoliative skin diseases (such as burns, pustulosis, varicella-zoster virus infection, herpes simplex virus infection, severe acne); (6) those with one or more complications (eg, secondary bacterial skin infection; bronchopneumonia; concurrent diseases, or other comorbidities); (7) those with abnormal MPXV infection, including accidental implantation into eyes, mouth, or other anatomical parts (such as the genitals or anus), where MPXV infection may pose special hazards.
At present, there is no specific treatment for MPXV infection. However, antiviral drugs developed for smallpox patients may be beneficial. Clinical treatment is primarily symptomatic and supportive, including alleviating symptoms, managing complications, and minimizing long-term sequelae [8]. It also needs to prevent secondary bacterial infections and monitor respiratory status [44]. For severe cases, the antivirals tecovirimat, brincidofovir, and cidofovir are potential options. Tecovirimat (known as tpoxx) is an antiviral drug approved by the US Food and Drug Administration (FDA) for the treatment of smallpox in adults and children. The US Centers for Disease Control and Prevention allows the use of stored tecorvir to treat monkeypox during monkeypox outbreaks as “compassionate use” [10,20]. Brincidofivir (known as tembexa) is another antiviral drug approved by the US FDA to treat smallpox in adults and children, including newborns, though it is not included in the US Strategic National Stockpile. Cidofovir (known as vistide) is also allowed by the US CDC to treat orthopoxviruses (including monkeypox) during outbreaks [52]. Only tecovirimat has been licensed by the European Medicines Agency to treat monkeypox and has shown safety in clinical trials, while limited data exist on its efficacy [10,20].
7. Management of cases and contacts
Patients with confirmed monkeypox should be isolated immediately until the rash scabs fall off. Cases should use designated household items (clothes, bed linen, towels, eating utensils, plates, glasses), which should not be shared with other members of the household [20]. A monkeypox case should be monitored daily, during which medical face masks should be worn and the rash should be covered. Patients should also be instructed to avoid close or intimate contact with other people until their rash heals completely [21].
A person identified as being in close contact with a monkeypox case should start daily self-monitoring for monkeypox symptoms and fever for 21 days after their last exposure. If any symptoms appear during this period, they should self-isolate, except for attending medical assessments or testing. In general, contacts that have symptoms should be isolated until monkeypox infection is excluded [21].
8. Risk assessment
The infection source of the current epidemic is unknown at present, so the risk of further spread of the epidemic in Nigeria, Europe, and North America cannot be ruled out. Once monkeypox is detected, the authorities should immediately take appropriate public health measures, including isolating cases and tracing contacts. If appropriate measures are implemented, the potential forward transmission risk associated with this case will be reduced. As the source of infection in Nigeria is still unknown, monkeypox remains prevalent, and Nigeria still presents a risk of further transmission. Concerns should be raised about the importation of cases with a travel history from Delta state in the Nigerian Delta.
Understanding monkeypox will help to ensure that as few people as possible are affected, and that future outbreaks are prevented. Some cases have been identified through sexual health clinics in GBMSM. It is worth noting that the risk of monkeypox is not limited to men who have sex with men. Anyone who has close contact with an infectious person is at risk. The risk is high for laboratory personnel and healthcare workers that are not using personal protective equipment [20].
9. Prevention of monkeypox
At present, the multi-country monkeypox outbreak that is occurring in non-endemic countries, alongside the COVID-19 pandemic, has attracted global attention. With the rapid growth of global trade and tourism, communicable diseases have no borders. In Asia, cases of monkeypox have been reported in Singapore and South Korea, and imported cases of monkeypox were reported in China on June 24, 2022. Asian countries therefore need to pay close attention to any changes in the epidemic. Meanwhile, stringent import control and quarantine systems are in place to prevent more cases of importation of the disease into China.
9.1. Information sharing
In response to this outbreak, the WHO initiated a clinical and public health incident response to coordinate comprehensive case discovery, contact tracking, laboratory investigation, clinical management, isolation, and the implementation of infection prevention and control measures [8]. Endemic countries fully share information and jointly respond to outbreaks under WHO guidance. Some European countries (Belgium, Finland, France, Germany, Israel, Italy, the Netherlands, Portugal, Slovenia, Spain, Switzerland, Great Britain and Northern Ireland) and the USA have announced full-length or partial genome sequences of MPXV strains isolated during this outbreak [2].
A common guideline should be developed to support each country in awareness raising, surveillance, laboratory diagnosis and testing, case investigation and contact tracing, clinical management and infection prevention and control, vaccines and immunization, risk communication, and community participation.
9.2. Disease control and monitoring
Understanding monkeypox will help to reduce the risk of future outbreaks. It is important to maintain respiratory etiquette and hand hygiene in epidemic areas and public places. In particular, entry-exit personnel and people in epidemic-related territories should pay close attention to the epidemic dynamics of monkeypox, and avoid contact with infected people, rodents, and non-human primates in the wild, as well as their blood and excretions. Care should also be taken to eat only adequately cooked meat and other animal products from potentially infected animals. If necessary, protective coverings, such as gloves, should be worn. Other measures may include strengthening communication and coordination between airlines and ports, enhancing health education, and correctly informing the public.
The implementation of surveillance measures and rapid identification of new cases are critical to contain disease outbreaks. The key objectives of surveillance for monkeypox are to rapidly identify cases and prevent further transmission. To achieve this, the following measures may need to be taken. Strengthening entry checks and quarantine procedures, including strictly implementing health declarations, temperature monitoring, medical inspection, sampling and testing, and other health and quarantine measures for entry personnel. Restricting the import of rodents and primates from Africa to reduce the risk of virus transmission. On entry, monkeypox cases should be detected and monitored, with a standard 21-day return visit for symptoms and signs. Strengthening the quarantine of goods carried into a country, especially consignments of rodents, to establish safe detection and management procedures for MPXV.
9.3. Vaccines
Since the eradication of smallpox in 1980, smallpox vaccination has ceased. However, the protection provided by the vaccine may wane over time, and the number of unvaccinated cohorts is growing. The susceptible population is therefore growing globally, creating conditions that may permit MPXV to infect humans. The USA approved the use of ACAM2000 and JYNNEOS (also known as imvamune or imvanex) vaccines to prevent MPXV. JYNNEOS is a live attenuated virus vaccine, which has been approved by the US FDA for some individuals at risk of being exposed to poxvirus. However, ACAM2000 has serious side effects, and is no longer licensed in the European Union [20]. These 2 vaccines have mainly been used to vaccinate the close contacts of patients diagnosed with monkeypox.
9.4. Training
The training of laboratory workers and healthcare providers is essential for identifying and preventing further secondary cases. The requirement for “early detection, early reporting, early diagnosis, early investigating, and early treatment” should be fulfilled. If cases occur, immediate action is required to control further spread among at-risk groups. Disinfection measures should also be taken, as MPXV is sensitive to all commonly used disinfectants.
10. Summary
The sudden and unexpected appearance of monkeypox simultaneously in several non-endemic countries suggests that there might have been undetected transmission for some unknown duration of time, followed by recent amplifier events. Fortunately, some measures introduced to control COVID-19 may also effectively control monkeypox transmission, such as social distancing, mask wearing, surface disinfection, and hand washing [53,54].
Monkeypox is a viral zoonotic disease that was first discovered in humans in 1970. Historically, this disease mostly affects tropical rainforests in Africa, afflicting the poorest and most marginalized communities. It did not become a major public health concern until 2003. There are 3 main reasons for the recent outbreak. First, the symptoms were mild and therefore transmission was not controlled in time. Second, MPXV sequences underwent mutation faster than expected during this outbreak. Third, since universal smallpox vaccination programs were discontinued in the 1970s, herd immunity has declined over time.
As a result of the abrupt and portentous surge in the number of cases across the West, healthcare workers should be on high alert and the smallpox vaccine may need to be made available in case of an emergency. Specific antiviral drugs for MPXV need to be developed immediately. Finally, we should establish control methods and measures for monkeypox and strengthen intersectoral coordination, including health, forestry, agricultural, environmental protection, customs and other departments. Medical and technical support may also need to be strengthened in certain at-risk areas with weak public health to improve their epidemic handling capacity, so as to avoid the spread and spillover risk to corresponding regions.
Author contribution
Qin Luo did collect and analysis of relevant literature, and wrote the manuscript. Jun Han participated in literature collection, supervision, validation, and reviewed the manuscript.
Acknowledgments
We apologize to any scientist whose excellent work could not be discussed because of space limitations.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding sources
This work was supported by a grant from the National Pathogen Resource Collection Center (NPRC-32) and the SKLID Development Grant (2011SKLID104).
Ethic statement
An ethical statement is not required as there were no human subjects involved in this study.
Informed consent
Not applicable.
Data available statement
No data, models, or code were generated or used during the study.
References
- 1.UK Health Security Agency; 2022. Monkeypox Cases Confirmed in England – Latest Updates.https://www.gov.uk/government/news/monkeypox-cases-confirmed-in-england-latest-updates Available at: Accessed June 29, 2022. [Google Scholar]
- 2.World Health Organization; 2022. Multi-country Monkeypox Outbreak: Situation Update.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON392 Available at: Accessed June 10, 2022. [Google Scholar]
- 3.Mathieu E, Dattani S, Ritchie H, et al. 2022. Available at: https://ourworldindata.org/monkeypox. Accessed June 28, 2022
- 4.UK Health Security Agency; 2022. Public Health Agencies Issue Monkeypox Guidance to Control Transmission.https://www.gov.uk/government/news/public-health-agencies-issue-monkeypox-guidance-to-control-transmission Available at: Accessed June 13, 2022. [Google Scholar]
- 5.UK Health Security Agency; 2022. Monkeypox Outbreak: Technical Briefings.https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings Available at: Accessed June 12, 2022. [Google Scholar]
- 6.World Health Organization; 2022. Multi-country Monkeypox Outbreak in Non-endemic Countries.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 Available at: Accessed June 27, 2022. [Google Scholar]
- 7.UK Health Security Agency; 2022. UKHSA Latest Findings into Monkeypox Outbreak.https://www.gov.uk/government/news/ukhsa-latest-findings-into-monkeypox-outbreak Available at: Accessed June 28, 2022. [Google Scholar]
- 8.World Health Origanization; 2022. Multi-country Monkeypox Outbreak in Non-endemic Countries: Update.https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON388 Available at: Accessed June 29, 2022. [Google Scholar]
- 9.Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022 doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena SK, Ansari S, Maurya VK, et al. Re-emerging human monkeypox: a major public-health debacle. J Med Virol. 2022 doi: 10.1002/jmv.27902. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Subramaniam G, Karuppanan K. Human monkeypox outbreak in 2022. J Med Virol. 2022 doi: 10.1002/jmv.27894. [DOI] [PubMed] [Google Scholar]
- 12.Alakunle E, Moens U, Nchinda G, et al. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11) doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973;37(1):1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shchelkunov SN, Totmenin AV, Safronov PF, et al. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–194. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kugelman JR, Johnston SC, Mulembakani PM, et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20(2):232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 18.Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33(4):1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabanillas B, Valdelvira R, Akdis CA. Monkeypox outbreak in Europe, UK, North America, and Australia: a changing trend of a zoonotic disease. Allergy. 2022 doi: 10.1111/all.15393. [DOI] [PubMed] [Google Scholar]
- 20.European Centre for Disease Prevention and Control; 2022. Risk Assessment: Monkeypox Multi-country Outbreak.https://www.ecdc.europa.eu/sites/default/files/documents/Monkeypox-multi-country-outbreak.pdf Available at: Accessed June 12, 2022. [Google Scholar]
- 21.World Health Organization; 2022. Monkeypox.https://www.who.int/news-room/fact-sheets/detail/monkeypox Available at: Accessed June 10, 2022. [Google Scholar]
- 22.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobson G, Adamson J, Adler H, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26(32) doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK Health Security Agency; 2022. Factsheet for Health Professionals on Monkeypox.https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals Available at: Accessed June 27, 2022. [Google Scholar]
- 25.Harris E. What to know about monkeypox. JAMA. 2022 doi: 10.1001/jama.2022.9499. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds MG, Doty JB, McCollum AM, et al. Monkeypox re-emergence in Africa: a call to expand the concept and practice of one health. Expert Rev Anti Infect Ther. 2019;17(2):129–139. doi: 10.1080/14787210.2019.1567330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of monkeypox - West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67(10):306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease C, Prevention Multistate outbreak of monkeypox–Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(23):537–540. [PubMed] [Google Scholar]
- 29.Nolen LD, Osadebe L, Katomba J, et al. Introduction of monkeypox into a Community and household: risk factors and zoonotic reservoirs in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93(2):410–415. doi: 10.4269/ajtmh.15-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Saene HK, Stoutenbeek CC, Stoller JK. Selective decontamination of the digestive tract in the intensive care unit: current status and future prospects. Crit Care Med. 1992;20(5):691–703. doi: 10.1097/00003246-199205000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Ihekweazu C, Yinka-Ogunleye A, Lule S, et al. Importance of epidemiological research of monkeypox: is incidence increasing? Expert Rev Anti Infect Ther. 2020;18(5):389–392. doi: 10.1080/14787210.2020.1735361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shchelkunov SN, Totmenin AV, Babkin IV, et al. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509(1):66–70. doi: 10.1016/s0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivancos R, Anderson C, Blomquist P, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen PY, Ajisegiri WS, Costantino V, et al. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017-2020. Emerg Infect Dis. 2021;27(4) doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.L'Vov DK, Zverev VV, Gintsburg AL, et al. [Smallpox is a dormant volcano] Vopr Virusol. 2008;53(4):4–8. [PubMed] [Google Scholar]
- 36.Babkin IV, Babkina IN, Tikunova NV. An update of orthopoxvirus molecular evolution. Viruses. 2022;14(2) doi: 10.3390/v14020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arita I, Gispen R, Kalter SS, et al. Outbreaks of monkeypox and serological surveys in nonhuman primates. Bull World Health Organ. 1972;46(5):625–631. [PMC free article] [PubMed] [Google Scholar]
- 38.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 39.Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 41.Monkeypox Monthly Situational Report, December 2019: Nigeria Centre for Disease Control and Prevention. Available at: https://ncdc.gov.ng/themes/common/files/sitreps/5a1a9820f21136842ba43f186b8d09e7.pdf. Accessed June 15, 2022
- 42.Update on Monkeypox (Mpx) in Nigeria: Nigeria Centre for Disease Control and Prevention. Available at: https://ncdc.gov.ng/themes/common/files/sitreps/75e3b8532d48167c0e58fcd0eca03062.pdf. AccessedJune 15, 2022
- 43.Rao AK, Schulte J, Chen TH, et al. Monkeypox in a traveler returning from Nigeria - Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep. 2022;71(14):509–516. doi: 10.15585/mmwr.mm7114a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costello V, Sowash M, Gaur A, et al. Imported monkeypox from international traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022;28(5):1002–1005. doi: 10.3201/eid2805.220292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng OT, Lee V, Marimuthu K, et al. A case of imported monkeypox in Singapore. Lancet Infect Dis. 2019;19(11):1166. doi: 10.1016/S1473-3099(19)30537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manes NP, Estep RD, Mottaz HM, et al. Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J Proteome Res. 2008;7(3):960–968. doi: 10.1021/pr070432+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sklenovska N, Van Ranst M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front Public Health. 2018;6:241. doi: 10.3389/fpubh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iizuka I, Saijo M, Shiota T, et al. Loop-mediated isothermal amplification-based diagnostic assay for monkeypox virus infections. J Med Virol. 2009;81(6):1102–1108. doi: 10.1002/jmv.21494. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Zhao H, Wilkins K, et al. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169(1):223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization; 2022. Laboratory Testing for the Monkeypox Virus: Interim Guidance.https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1 Available at: Accessed June 11, 2022. [Google Scholar]
- 52.Centers for Disease Control and Prevention; 2022. Interim Clinical Guidance for the Treatment of Monkeypox.https://www.cdc.gov/poxvirus/monkeypox/treatment.html Available at: Accessed June 15, 2022. [Google Scholar]
- 53.Vitiello L, Ilari S, Sansone L, et al. Preventive measures against pandemics from the beginning of civilization to nowadays-how everything has remained the same over the millennia. J Clin Med. 2022;11(7) doi: 10.3390/jcm11071960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Memariani M, Memariani H. Multinational monkeypox outbreak: what do we know and what should we do? Ir J Med Sci. 2022 doi: 10.1007/s11845-022-03052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data, models, or code were generated or used during the study.