Abstract
Although major advancements have been made in the treatment of HIV infection, graft-versus-host reactions, and autoimmune diseases, an unexpected consequence of treatment has been the emergence of a devastating inflammatory syndrome, termed the immune reconstitution inflammatory syndrome (IRIS). The pathophysiology of the syndrome is poorly understood, and the syndrome poses unique challenges for diagnosis and treatment. We have reviewed the neurologic manifestations of IRIS in the context of HIV infection as well as in the setting of treatment of autoimmune diseases such as multiple sclerosis, in which compartmental immune suppression may occur without an obvious underlying immune suppression. The purpose of this review is to identify common themes that may assist in the diagnosis and management of these IRIS syndromes.
Keywords: HIV-1, AIDS, Encephalitis, Meningitis, Opportunistic infection, Immune deficiency, Immune reconstitution, Central nervous system
Introduction
Immune reconstitution inflammatory syndrome (IRIS) is a syndrome that emerges when the immune system recovers after an immune deficiency state. This review is focused on the central nervous system manifestations of IRIS (CNS-IRIS). IRIS emerged in the 1990s as a complication of HIV type 1 (HIV-1) infection effectively treated with combination antiretroviral therapy (cART) to lower viral load and facilitate the recovery of the CD4-expressing lymphocyte population [1]. IRIS manifests as a paradoxical deterioration in clinical status associated with the recovery of CD4+ cell counts in HIV-1–infected patients receiving cART. IRIS was initially described in 1992 in an HIV-1–infected patient who developed acute Mycobacterium avium-intracellulare (MAC) infection [2]. However, inflammatory syndromes associated with immune restoration or re-activation were described before the HIV era. Examples of this include acute hypersensitivity reactions occurring after initiation of antimicrobial therapy for syphilis (the “Jarisch-Herxheimer reaction”) [3], bacterial meningitis, leprosy, or antibiotic-induced endotoxin release in sepsis [4]. These reactions derive from a heightened immune response, and can lead to clinical deterioration with devastating consequences.
Immune Restoration and IRIS
Most immune-suppressed states are systemic, associated with decreased or defective circulating immune cells. However, monoclonal antibodies such as natalizumab that target lymphocyte trafficking have the potential to cause selective or compartmental immune suppression, such as occurs in the CNS when natalizumab-treated lymphocytes are blocked from crossing the blood–brain barrier [5]. Restoration of the immune response after systemic or compartmental immune suppression potentiates IRIS.
Restoration in HIV-1 Infection after cART Initiation
In HIV-1 infection, most patients develop IRIS within 3 to 6 months of cART initiation [6]. Restoration of immune cells after cART initiation has a biphasic course [7••]. Initial recovery includes a rapid release in memory T cells (CD4+) and a rise in circulating CD8+ T cells over approximately 1 to 2 months. These contribute to robust immune responses against pathogens known to cause opportunistic infections (OIs). Later there is increased thymic production of naïve T cells, which further increases the circulating CD4+ T-cell numbers, while CD8+ T cells return to baseline. Immune restoration takes longer in HIV-1–infected patients with very low CD4+ counts at nadir.
Clinical Categories of IRIS
Opportunistic Infection
IRIS can occur with or without an OI in the immune-suppressed patient [8]. In the HIV-infected patient, cART administration causes a rise in CD4 cell counts and an underlying OI may become evident or “unmasked” as an unusual or clinically severe presentation of the pathogen. [9]. IRIS with OI is likely the result of a reconstituted inflammatory response directed at the OI, and producing breakdown of the blood–brain barrier, leukocyte infiltration of the CNS, and inflammatory destruction of normal brain tissue. Although there may be common inflammatory mechanisms associated with IRIS, pathogen-specific, antigen-driven immune responses may drive IRIS in the setting of an OI. This may be reflected in the various types of IRIS associated with OI (Table 1) [10].
Table 1.
Clinical manifestations of IRIS in patients with HIV-1 infection on cART
| Infection | Typical clinical manifestations | Atypical clinical manifestations |
|---|---|---|
| HIV encephalitis | Asymptomatic or mild cognitive decline | Fulminant decline in mental status over days with brain swelling and inflammation |
| PML with JCV | Subacute focal neurologic deficits | Focal areas of contrast enhancement and swelling |
| Varicella zoster virus | Shingles, CNS vasculitis | Strokes without skin rash |
| Cytomegalovirus | Retinitis, cerebritis | Vasculitis |
| Epstein-Barr virus | CNS lymphoma | Optic neuropathy |
| Tuberculosis | Meningitis | Cerebral infarcts, subarachnoid hemorrhage |
| Cryptococcus | Meningitis | Enhancing mass lesions in posterior fossa |
| Candida | Meningitis | Vasculitis with strokes |
cART combination antiretroviral therapy; CNS central nervous system; IRIS immune reconstitution inflammatory syndrome; JCV JC virus; PML progressive multifocal leukoencephalopathy
In the absence of OI, cART can also be associated with paradoxical neurologic deterioration manifest as severe, progressive encephalitis. Additional manifestations of IRIS in the absence of OI may be autoimmune. Several systemic autoimmune syndromes have been described including Guillain-Barré syndrome, CNS demyelination, and axonal damage, reminiscent of multiple sclerosis (MS) [11–13].
Temporal Distinctions
IRIS can be distinguished on a temporal basis with respect to immune reconstitution and the manifestation of OIs in the previously immune-suppressed patient. Simultaneous IRIS is seen in patients who develop OI after starting cART or after initiation of immune reconstitution by other mechanisms (eg, removal of immune suppression). The mechanism of simultaneous IRIS may involve elimination or suppression of pathogen-specific T cells by a hyperstimulated immune response following reconstitution [7••]. Delayed IRIS appears in patients who have already manifest OI and are subsequently started on cART, or in non-HIV-1 patients in whom immune suppression is withdrawn. In these patients the clinical deterioration is attributed to the enhanced inflammation at the site of the OI.
Clinical Severity
IRIS can be classified according to the severity of the clinical state [7••]. Asymptomatic IRIS is not detected by clinical examination. Radiologic studies show increased contrast enhancement. Symptomatic IRIS is accompanied by objective deterioration in neurologic function; this is typically accompanied by new changes on brain imaging studies. Catastrophic IRIS includes severe neurologic decline, brain edema, coma, and/or imminent signs of brain herniation.
Risk Factors for IRIS
IRIS tends to occur in patients with OI, either subclinical or detectable, and particularly if these are present prior to immune reconstitution. Particularly at-risk patients have very low immune cell counts at nadir, and a rapid increase in immune cell counts after immune reconstitution. In the HIV-1–infected population, IRIS patients typically have a low CD4+ nadir, then a rapid response to cART, with associated decline in viral load and increase in the CD4+ T-cell count [9, 14]. Risk for IRIS may also be influenced by the expression of proinflammatory cytokines, which has a genetic basis for variation among subpopulations of HIV-1–infected patients [15]. Levels of plasma cytokine interleukin (IL)-6 and soluble IL-6 receptor may be indicators of the risk for and severity of IRIS in HIV-1–infected patients [16, 17].
Diagnosis of IRIS in the CNS
IRIS should be considered in any patient with unexpected neurologic deterioration after immune reconstitution. Like the many diseases of the immune suppressed, IRIS can have varying presentations and severities; this makes diagnosis difficult. Johnson and Nath [7••] suggested diagnostic criteria for HIV-1–infected patients that combine clinical assessment with radiologic and immunologic findings. With slight modification, these criteria can be applied to the diagnosis of CNS-IRIS in the broader immune-suppressed population:
History of immune suppression, either systemic or compartmental;
Neurologic deterioration by clinical examination, unexplained by previous illness or side effects of therapy;
New neuroradiologic findings (with or without enhancement), or increased enhancement. The optimal screening neuroradiologic imaging modality in these cases would be MRI with gadolinium;
Increased immune cells counts, particularly CD4+ lymphocytes, either systemically or in the cerebrospinal fluid (CSF), after initiation of immune reconstitution;
If available, biopsy demonstrating T-cell infiltration into the CNS, to confirm the diagnosis of CNS-IRIS.
IRIS in the Setting of HIV-1 Infection
Epidemiology
Meta-analysis of 54 cohort studies including 1699 cases of IRIS concluded that 16% of unselected AIDS patients starting cART develop any type of IRIS, and 4.5% of IRIS patients died [18]. There was substantial variability among results from different studies, possibly due to nonstandardized diagnostic criteria for IRIS. Previous studies estimated that 15% to 45% of HIV-1–infected patients receiving cART develop any type of IRIS within a few months of therapy, with almost three-fourths of IRIS patients presenting within 90 days [9, 19]. About 1% of patients develop CNS-IRIS [20], suggesting that CNS-IRIS is uncommon. However, estimates of the incidence of and death rates attributable to CNS-IRIS vary according to the various OI [18]. Meta-analysis confirms that the risk of all types of IRIS is associated with CD4 cell count prior to cART, with the highest risk in patients with less than 50 cells/μL.
Differential Diagnosis of CNS-IRIS
IRIS is one of several conditions that can present during HIV-1 infection as worsening or new symptoms after cART initiation. These can include drug toxicities, non-compliance with medication, or advancing HIV-1 infection due to drug-resistant viral strains.
HIV-1 Encephalitis as CNS-IRIS
A severe, progressive encephalitis after cART associated with cerebral edema and inflammatory cell infiltration has been described, and may represent IRIS due to cytotoxic T cells [6, 21, 22]. This is often fatal, although it may improve with corticosteroid treatment; however, the long-term prognosis is poor [21]. This clinical picture can also occur with failure of cART to control viral replication in the CNS, and thus can present a diagnostic dilemma. It is being increasingly recognized that activated T cells can be found in the CSF of patients well controlled on cART, but who have mild or asymptomatic neurocognitive dysfunction, suggesting that more indolent forms of IRIS may be commonly present.
Opportunistic Infections and CNS-IRIS
Well-known viral, bacterial, fungal, and parasitic OIs complicate HIV-1 infection. The incidence of these declined in the cART era, as patients sustained lower viral loads and higher CD4+ lymphocyte counts. In the post-cART era, these OIs cause high morbidity when associated with IRIS.
Progressive multifocal leukoencephalopathy (PML)
Progressive multifocal leukoencephalopathy (PML) PML is a well-known leukoencephalopathy due to glial infection by the ubiquitous polyoma virus, JC virus (JCV). Although HIV-1 infection accounts for about 80% of cases of PML, the widening use of therapeutic immunosuppression is expanding the epidemiology of JCV infection of the CNS [23]. Even in the cART era it remains a devastating OI in HIV-1/AIDS, with mortality in excess of 40% and no effective antiviral treatment available [24]. The incidence of IRIS in PML associated with AIDS is 17% based on multiple studies [18]. The onset is usually between 4 and 8 weeks after cART initiation, but may occur up to 2 years later [25]. A study of eight patients presenting with PML within 6 months of initiating cART included three cases of IRIS involving posterior fossa lesions exclusively [26]. IRIS in PML can be distinguished by contrast enhancement of lesions on MRI scan (Fig. 1), which likely reflects white matter infiltration by predominantly CD8+ T cells, although macrophages and CD4+ T cells may also be present [27]. However, contrast enhancement may not be seen in all IRIS patients. Patients with IRIS typically show a clinical response to corticosteroids [25].
Fig. 1.
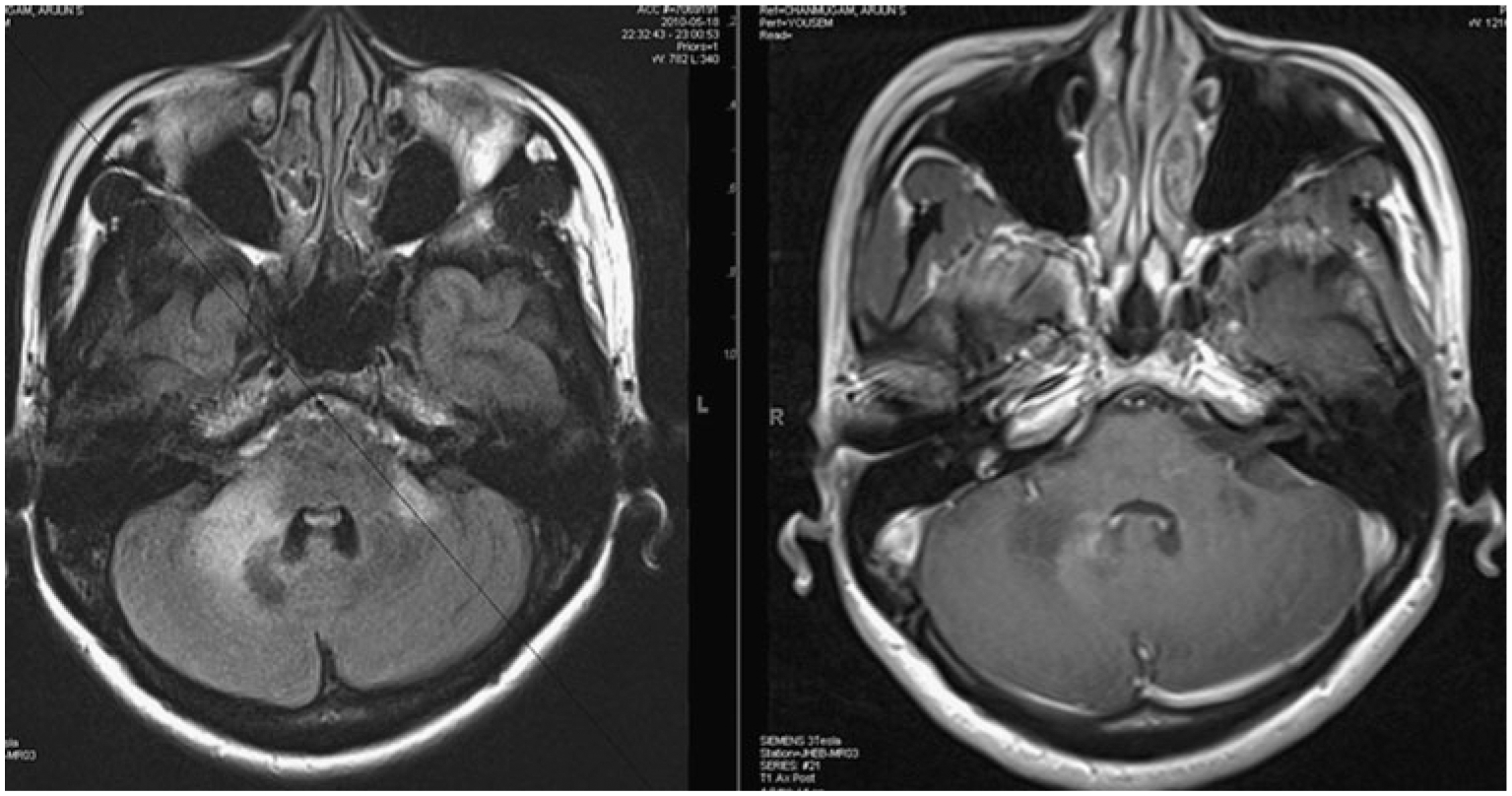
Progressive multifocal leukoencephalopathy–immune reconstitution inflammatory syndrome (PML-IRIS) with HIV infection: MRI scan with fluid-attenuated inversion recovery (FLAIR) sequence on the left shows high signal intensity lesions at the cerebellopontine junction on both sides. The corresponding contrast-enhanced T1-weighted image on the right shows an area of gadolinium enhancement in the peripheral margins of the lesion, most prominent along the medial border. This image was obtained at the time of diagnosis of PML without antiretroviral treatment and thus would be called simultaneous IRIS
Herpesvirus infections
IRIS may occur in association with multiple herpesviruses. OIs are associated with HIV-1 infection, the most prominent being cytomegalovirus (CMV) and varicella zoster virus (VZV). CMV is commonly associated with retinitis, but CMV-IRIS can include retinitis and uveitis [28]. Approximately 38% of CMV retinitis cases develop IRIS when median CD4 lymphocyte counts are less than 50/μL [18]. CMV lumbosacral radiculitis has not been reported with IRIS. A case report suggests that CMV-IRIS could occur as a vasculitis causing multiple small infarcts [29]. Approximately 12% of HIV-1–associated VZV infections develop IRIS, which is more frequent than other OI-IRIS after the first 3 months of successful cART therapy, suggesting that VZV-IRIS immune responses are mediated by naïve CD4+ lymphocytes that emerge later after starting cART [30]. VZV can cause a CNS vasculitis resulting in stroke, which may or may not be accompanied by vesicular skin rash in the immune-suppressed patient. Stroke after initiation of cART may result from VZV-IRS [31]. Although herpes simplex virus and Epstein-Barr virus (EBV) are well-known causative agents of human infection, there are few reports of CNS-IRIS associated with these herpes viruses, and these may manifest with atypical manifestations such as optic neuropathy associated with EBV infection. [7••, 10]. Herpes viral infections are more commonly associated with IRIS outside the CNS. Genital herpes lesions are increased four- to fivefold in the first 4 months after cART [32]. Polymorphisms in genes related to the inflammatory cytokine tumor necrosis factor-α may predispose to the development of herpes-associated IRIS [15].
Fungal infections Cryptococcus neoformans
Fungal infections Cryptococcus neoformans remains one of the most important pathogens associated with CNS-IRIS, typically emerging between 3 and 20 months after the cART initiation [7••, 33]. Cryptococcal CNS-IRIS presents as an aseptic recurrence of meningitis or rarely as intracranial cryptococcoma. Meta-analysis indicated that 19.5% of AIDS patients with cryptococcal meningitis develop IRIS, and about 21% of those with IRIS die [18]. A recent prospective multicenter study of 101 AIDS patients with cryptococcal meningitis found no significant association between the timing of cART initiation and the diagnosis of IRIS [34]. Serum cryptococcal antigen titer was the only risk factor for cryptococcal IRIS. The odds ratio for serum titer (as log to base 2) was 1.37. CSF findings associated with the diagnosis of cryptococcal IRIS included negative cultures, increased opening pressure, and higher neutrophil count. Another prospective study of 65 AIDS patients with cryptococcal meningitis starting cART after antifungal treatment found CNS-IRIS occurred in 17% of patients at a median 29 days after cART [35]. These cryptococcal IRIS patients had a greater increase in CD4 counts after 6 months of cART. Fulminant Candida meningitis with brainstem strokes and diffuse vascular and parenchymal CD8+ lymphocyte infiltration has been reported in an AIDS patient started on cART prior to clinical deterioration [36].
Mycobacterial infections
Mycobacterial agents associated with HIV-1 infection and IRIS include Mycobacterium tuberculosis, Mycobacterium leprae, and M. avium complex (MAC). Mycobacterial-associated CNS-IRIS may be simultaneous or delayed, but usually occurs about 5 to 10 months after the initiation of cART, about 2 months later than non-CNS-IRIS [14]. A meta-analysis suggests that approximately 16% of AIDS patients coinfected with tuberculosis develop IRIS, and 3% of those die [18]. Other estimates suggest delayed tuberculosis-associated IRIS occurs in greater than 45% of coinfected patients [37••]. Tuberculosis-associated systemic IRIS manifests primarily with pulmonary symptoms and lymphadenitis, whereas CNS-IRIS includes tuberculoma and/or tuberculous meningitis. These CNS complications have been estimated to occur in 12% of coinfected patients, and approximately 13% of these die [38]. Tuberculous meningitis in HIV-infected patients typically has reduced inflammatory responses, extensive vasculopathy, and absent or minimal meningeal enhancement. Therefore, CNS-IRIS should be considered if the coinfected tuberculosis patient develops meningeal signs and meningeal enhancement on MRI scan after starting cART [7••]. The low probability of physical and mental recovery from CNS-IRIS with tuberculosis, approximately 16% [38], is particularly troubling in resource-limited settings.
Parasitic infections
Parasitic infections infrequently cause CNS-IRIS with HIV-1 infection, with Toxoplasma gondii, the causative organism of toxoplasmosis, being the major cause [39]. Cerebral toxoplasmosis can present with seizures, headaches, confusion, or focal motor findings. Cerebral edema associated with this infection can cause mass effects or even herniation in catastrophic cases. Leishmaniasis, caused by a protozoan transmitted by species of sandfly, has been reported in cutaneous and visceral presentations after cART initiation [39]. Because parasitic infections are more prevalent in resource-poor countries, the increasing global accessibility of cART should lead to an increased risk of parasite-associated CNS-IRIS.
Management of CNS-IRIS Associated with HIV-1 Infection
Management of the various CNS-associated IRIS is complicated by the clinical heterogeneity of the various IRIS syndromes, lack of standardized definitions for IRIS, and the lack of evidence-based therapeutic protocols.
cART: The Timing of Treatment
Because immune reconstitution after cART is the proximate cause of IRIS associated with HIV-1 infection, there is a rationale for delaying or discontinuing cART when symptoms of IRIS emerge, particularly when it occurs in the context of an OI for which effective antimicrobial therapy is available [40]. However, some patients may manifest OI on initiation of cART (simultaneous IRIS), so delaying cART might not prevent the development of OI-associated IRIS.
The Use of Corticosteroids
Currently, the use of corticosteroids is controversial. In the setting of an OI, the inflammatory immune response helps control the infection, but a fulminant inflammatory response can result in injury to normal tissue. In non-HIV-infected patients with IRIS, there is evidence for the efficacy of adjunctive corticosteroid therapy. However, corticosteroids may cause worsening of immunocompromised patients with undetected OI. If corticosteroids are used for a short duration (3–5 days), there may be recurrence of IRIS upon stopping therapy. Corticosteroids are used effectively for short time intervals to reduce or prevent vasogenic brain edema until an OI is adequately treated with a specific antimicrobial. This is seen in patients with “catastrophic” CNS-IRIS, in which massive inflammation associated with OI risks brain herniation [7••]. These patients need high-dose corticosteroids (eg, 1–2 mg/kg/d of prednisone or 1 g/d of methylprednisolone for 5 days), or dose equivalents of other corticosteroids such as dexamethasone followed by a taper over 4 to 6 weeks. Even in patients with PML-IRIS in which no specific antimicrobial therapy is available, corticosteroid may be necessary until the immune response against JCV becomes effective, usually within 4 to 6 weeks [25]. The use of steroids in HIV encephalitis needs to be evaluated in controlled clinical trials. Immunosuppressed patients on corticosteroids should be maintained on prophylaxis for pneumocystis pneumonia and for fungal infections (eg, candidiasis) or on isoniazid in regions where tuberculosis is endemic.
Other Immunomodulatory Drugs
Other potentially useful anti-inflammatory agents include nonsteroidal anti-inflammatory drugs (eg, chloroquine, pentoxifylline, and thalidomide). These drugs suppress inflammatory cytokine production, but their broad role in IRIS has yet to be established [7••].
IRIS Not Associated with HIV-1 Infection
Although the term IRIS was only recently introduced in the context of HIV infection, the phenomenon of a heightened immune response that may be damaging to the host following the initiation of antimicrobial therapy for various infections has been well recognized since antimicrobials were first introduced. The recent use of immune-modulatory drugs for treatment of autoimmune diseases has resulted in the development of OIs and thus the subsequent development of IRIS following the withdrawal of the drugs. Management of these patients poses special challenges, because the very immune cells needed to control the infection are also mediators of the autoimmune disease.
PML-IRIS in Patients with MS Treated with Natalizumab
Neurologic IRIS is most recognized and reported as a complication of HIV-1 infection. However, IRIS can occur in other settings. CNS-IRIS has recently been recognized as a complication of PML in MS patients who received monoclonal antibody therapy with natalizumab [41••]. Natalizumab is a monoclonal antibody targeting the α−4 subunit of the integrin adhesion molecule on T lymphocytes. Natalizumab blocks binding of lymphocytes to endothelial cells, and thereby inhibits trafficking across the blood–brain barrier. MS patients on natalizumab therapy may be subject to compartmental immune suppression [5]. Because JCV infection is usually acquired in childhood, and PML results from the reactivation of this virus in the brain, seropositivity for JCV is a potential risk factor.
PML occurs in approximately 1:1000 MS patients treated with natalizumab. No cases have occurred in the first 12 months of treatment. The treatment of PML in these patients involves the removal of natalizumab by plasmapheresis so as to restore the trafficking of lymphocytes into the brain. This results in a massive inflammation at the site of PML, leading to IRIS. Nearly 100% of patients to date treated in this manner have developed IRIS. The consequences of IRIS in this population may be devastating, leading to death or severe morbidity.
The diagnosis of PML in patients with MS can be challenging, because both diseases cause demyelination and thus produce high signal intensity lesions on T2-weighted or fluid-attenuated inversion recovery (FLAIR) sequences of MRI scans. PML lesions typically do not show contrast enhancement or edema, unless there is accompanying IRIS. PML characteristically involves the uncinate fibers, leading to cortical signs such as aphasia and apraxia, which are seldom seen in MS. Conversely, PML does not involve the optic nerve and rarely involves the spinal cord, whereas these two structures are often involved in MS. However, conclusive diagnosis can only be made by demonstration of the presence of JCV in the CSF or brain tissue. JC viral load in CSF in the natalizumab-treated patients is often lower than that seen in HIV-infected patients; thus, a sensitive polymerase chain reaction assay capable of detection of up to 10 copies of JCV DNA/mL of CSF should be used. This will also allow for early diagnosis of PML. Such highly sensitive assays are currently available in few laboratories but efforts are underway to set up centralized diagnostic facilities in North America and Europe to provide this service.
In comparison to PML-IRIS in HIV-infected patients, the natalizumab-treated patients develop IRIS more frequently, and the IRIS is much more severe. This is consistent with an intact systemic immune system in the natalizumab-treated patients, which provides a rapid influx of lymphocytes from blood to brain, once the natalizumab is removed. In the natalizumab-associated PML-IRIS patients, high-dose corticosteroids (1 g/d of methylprednisolone for 5 days) followed by a prolonged oral taper over 1 to 2 months may dampen the CNS inflammation. There is some concern that the use of corticosteroids may obliterate the immune responses necessary to keep the JCV in check. However, the IRIS can be fulminant and result in significant mortality and morbidity. Thus, the use of corticosteroids is warranted. Further, some patients who underwent repeated spinal fluid evaluations following the development of PML-IRIS did not show any evidence of increase in JC viral load following the administration of corticosteroids (Tan and Nath, Unpublished observations). IRIS may develop within days or up to 2 months following the withdrawal of natalizumab. To prevent the development of IRIS, some have advocated for the use of corticosteroids at the time of initiation of plasmapheresis to remove natalizumab. The theoretic disadvantage of this approach is that if all inflammation was completely prevented, the PML lesion would continue to advance, since the immune response is necessary to control the infection. Such prophylactic approaches would be useful if effective antiviral compounds against JCV were available.
Leprosy
It is well known that about 30% of patients with borderline leprosy will develop a type 1 lepra reaction following initiation of antimicrobial therapy for leprosy [42•]. These type 1 reactions are immunologically mediated episodes localized in skin and nerves, and are a major cause of nerve injury. Nerve damage may result in disability and deformity. Oral corticosteroids are used for the treatment of this reaction; however, there is no consensus on dosage or duration of use. In HIV-infected patients, type 1 lepra reaction presents as IRIS following the use of cART.
Tuberculous Meningitis
Meningitis due to M. tuberculosis often worsens clinically with increased cells in the CSF following initiation of antimycobacterial therapy. This immune-mediated phenomenon has been termed a hypersensitivity reaction. The use of corticosteroids to treat this reaction has remained controversial. A recent meta-analysis independently assessed search results and methodologic quality [43]. Seven trials involving 1140 participants (with 411 deaths) met the inclusion criteria. All used dexamethasone or prednisolone. Overall, corticosteroids reduced the risk of death. Data on disabling residual neurologic deficit from three trials showed that corticosteroids reduced the risk of death or disabling residual neurologic deficit. Patients with mycobacterial meningitis may also develop cerebral infarcts during the first few weeks following antimicrobial therapy [44]. The blood vessels involved range from small penetrating blood vessels resulting in lacunar infarcts to involvement of the large vessels around the circle of Willis. Inflammation within the walls of the blood vessels resulting in a vasculitis/arteritis is the major cause of the vessel occlusion. Occasionally, necrosis of the vessel wall may result in a subarachnoid hemorrhage. The efficacy of corticosteroids for treatment of this complication has yet to be proven. In one study of serial MRI scans of 43 adults, the proportion of patients with infarcts was halved in the dexamethasone group where it was used prophylactically at the time of initiation of antituberculous therapy [45]. Together, these studies favor the use of corticosteroids in conjunction with antimycobacterial therapy early in the course of treatment to prevent an IRIS-like phenomenon.
Bacterial Meningitis
The incidence of bacterial meningitis is 2.6 to 6 adults per 100,000 per year in developed countries, and is up to 10 times more prevalent in some developing countries. Severe disability or death can occur despite appropriate antibiotic treatment. The profound inflammatory response associated with the infection has been implicated in causing neuronal injury. For these reasons, corticosteroids have been advocated as an adjunctive treatment [46]. However, in these patients the immune response is not due to immune restoration. Rather, animal models suggest that it is a response to products released by bacterial lysis due to antibiotics. This is consistent with the observation that corticosteroid therapy is most effective if instituted within the first few hours of antibacterial treatment. The use of corticosteroids is supported by a prospective, randomized, double-blind, multicenter, placebo-controlled trial in which patients received dexamethasone (10 mg) or placebo 15 to 20 min before or in conjunction with the first dose of antibiotic, and were subsequently given every 6 h for 4 days. The dexamethasone group showed a significant decrease in morbidity and mortality [46].
Conclusions
IRIS in the CNS can have devastating consequences because it is associated with inflammation and edema in the cranial cavity. Because it usually occurs in immune-compromised patients with underlying OIs, careful evaluation is needed to establish the diagnosis. Diagnostic clues include atypical presentation of these infections and increased contrast enhancement on MRI scanning. Treatment involves the use of corticosteroids because of their anti-inflammatory effects and their ability to stabilize the blood–brain barrier. However, the treatment is not ideal because it may compromise the immune system. In the appropriate patient antimicrobial therapy for the underlying OI and coverage for common OI while on chronic corticosteroid therapy may be necessary.
Acknowledgment
Dr. Avindra Nath has received a grant from the US National Institutes of Health.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Micheline McCarthy, Department of Neurology, Miller School of Medicine, University of Miami, 1201 NW 16th Street, Miami, FL 33125, USA; Bruce Carter Veterans Affairs Medical Center, 1201 NW 16th Street, Miami, FL 33125, USA.
Avindra Nath, Department of Neurology, Johns Hopkins School of Medicine, 509 Pathology, 600 North Wolfe Street, Baltimore, MD 21287, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Shelburne SA 3rd, Hamill RJ, Rodriguez-Barradas MC, et al. : Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine 2002, 81:213–227. [DOI] [PubMed] [Google Scholar]
- 2.French MA, Mallal SA, Dawkins RL: Zidovudine-induced restoration of cell-mediated immunity to mycobacteria in immunodeficient HIV-infected patients. AIDS 1992, 6:1293–1297. [DOI] [PubMed] [Google Scholar]
- 3.Pound MW, May DB: Proposed mechanisms and preventative options of Jarisch-Herxheimer reactions. J Clin Pharm Ther 2005, 30:291–295. [DOI] [PubMed] [Google Scholar]
- 4.Holzheimer RG: Antibiotic induced endotoxin release and clinical sepsis: a review. J Chemother 2001, 13 Spec No 1:159–172. [DOI] [PubMed] [Google Scholar]
- 5.Stuve O, Marra CM, Jerome KR, et al. : Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006, 59:743–747. [DOI] [PubMed] [Google Scholar]
- 6.Miller RF, Isaacson PG, Hall-Craggs M, et al. : Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol 2004, 108:17–23. [DOI] [PubMed] [Google Scholar]
- 7. ••.Johnson T, Nath A: Neurological complications of immune reconstitution in HIV-infected populations. Ann N Y Acad Sci 2010, 1184:106–120. [DOI] [PubMed] [Google Scholar]; This is a comprehensive review of the neurologic manifestations of CNS-IRIS in HIV-infected patients.
- 8.Riedel DJ, Pardo CA, McArthur J, Nath A: Therapy insight: CNS manifestations of HIV-associated immune reconstitution inflammatory syndrome. Nat Clin Pract Neurol 2006, 2:557–565. [DOI] [PubMed] [Google Scholar]
- 9.Shelburne SA, Visnegarwala F, Darcourt J, et al. : Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS 2005, 19:399–406. [DOI] [PubMed] [Google Scholar]
- 10.French MA, Lenzo N, John M, et al. : Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med 2000, 1:107–115. [DOI] [PubMed] [Google Scholar]
- 11.French MA: Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep 2007, 4:16–21. [DOI] [PubMed] [Google Scholar]
- 12.Langford TD, Letendre SL, Marcotte TD, et al. : Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 2002, 16:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corral I, Quereda C, Garcia-Villanueva M, et al. : Focal monophasic demyelinating leukoencephalopathy in advanced HIV infection. Eur Neurol 2004, 52:36–41. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch DM, Venter WD, Feldman C, Van Rie A: Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS 2008, 22:601–610. [DOI] [PubMed] [Google Scholar]
- 15.Price P, Morahan G, Huang D, et al. : Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS 2002, 16:2043–2047. [DOI] [PubMed] [Google Scholar]
- 16.Stone SF, Price P, Keane NM, et al. : Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med 2002, 3:21–27. [DOI] [PubMed] [Google Scholar]
- 17.Kestens L, Sedikki N, Bohjanen PR: Immunopathogenesis of the immune reconstitution disease in HIV patients responding to antiretroviral therapy. Curr Opin HIV AIDS 2008, 3:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller M, Wandel S, Colebunders R, et al. : Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010, 10:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shelburne SA, Montes M, Hamill RJ: Immune reconstitution inflammatory syndrome: more answers, more questions. J Anti-microb Chemother 2006, 57:167–170. [DOI] [PubMed] [Google Scholar]
- 20.McCombe JA, Auer RN, Maingat FG, et al. : Neurologic immune reconstitution inflammatory syndrome in HIV/AIDS: outcome and epidemiology. Neurology 2009, 72:835–841. [DOI] [PubMed] [Google Scholar]
- 21.Venkataramana A, Pardo CA, McArthur JC, et al. : Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 2006, 67:383–388. [DOI] [PubMed] [Google Scholar]
- 22.Petito CK, Torres-Munoz JE, Zielger F, McCarthy M: Brain CD8 + and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J Neurovirol 2006, 12:272–283. [DOI] [PubMed] [Google Scholar]
- 23.Bag AK, Cure JK, Chapman PR, et al. : JC virus infection of the brain. AJNR Am J Neuroradiol 2010. Mar 18 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyen C, Hoffmann C, Schmeisser N, et al. : Progressive multifocal leukencephalopathy in patients on highly active antiretroviral therapy: survival and risk factors of death. J Acquir Immune Defic Syndr 2004, 37:1263–1268. [DOI] [PubMed] [Google Scholar]
- 25.Tan K, Roda R, Ostrow L, et al. : PML-IRIS in patients with HIV infection. Clinical manifestations and treatment with steroids. Neurology 2009, 72:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidhu N, McCutchan JA: Unmasking of PML by HAART: unusual clinical features and the role of IRIS. J Neuroimmunol 2010, 219:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vendrely A, Bienvenu B, Gasnault J, et al. : Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol 2005, 109:449–455. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen QD, Kempen JH, Bolton SG, et al. : Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am J Ophthalmol 2000, 129:634–639. [DOI] [PubMed] [Google Scholar]
- 29.Anderson AM, Fountain JA, Green SB, et al. : Human immunodeficiency virus-associated cytomegalovirus infection with multiple small vessel cerebral infarcts in the setting of early immune reconstitution. J Neurovirol 2010, 16:179–184. [DOI] [PubMed] [Google Scholar]
- 30.Espinosa E, Pena-Jimenez A, Ormsby CE, et al. : Later onset of herpes zoster-associated immune reconstitution inflammatory syndrome. HIV Med 2009, 10:454–457. [DOI] [PubMed] [Google Scholar]
- 31.Newsome SD, Nath A: Varicella-zoster virus vasculopathy and central nervous system immune reconstitution inflammatory syndrome with human immunodeficiency virus infection treated with steroids. J Neurovirol 2009, 15:288–291. [DOI] [PubMed] [Google Scholar]
- 32.Couppie P, Sarazin F, Clyti E, et al. : Increased incidence of genital herpes after HAART initiation: a frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care STDS 2006, 20:143–145. [DOI] [PubMed] [Google Scholar]
- 33.Skiest DJ, Hester LJ, Hardy RD: Cryptococcal immune reconstitution inflammatory syndrome: report of four cases in three patients and review of the literature. J Infect 2005, 51: e289–e297. [DOI] [PubMed] [Google Scholar]
- 34.Sungkanuparph S, Filler SG, Chetchotisakd P, et al. : Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis 2009, 49:931–934. [DOI] [PubMed] [Google Scholar]
- 35.Bicanic T, Meintjes G, Rebe K, et al. : Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J Acquir Immune Defic Syndr 2009, 51:130–134. [DOI] [PubMed] [Google Scholar]
- 36.Berkeley JL, Nath A, Pardo CA: Fatal immune reconstitution inflammatory syndrome with human immunodeficiency virus infection and Candida meningitis: case report and review of the literature. J Neurovirol 2008, 14:267–276. [DOI] [PubMed] [Google Scholar]
- 37. ••.Murdoch DM, Venter WD, Van Rie A, Feldman C: Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther 2007, 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a comprehensive review of the systemic manifestations of IRIS.
- 38.Pepper DJ, Marais S, Maartens G, et al. : Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis 2009, 48: e96–e107. [DOI] [PubMed] [Google Scholar]
- 39.Lawn SD: Immune reconstitution disease associated with parasitic infections following initiation of antiretroviral therapy. Cur Opin Infect Dis 2007, 20:482–488. [DOI] [PubMed] [Google Scholar]
- 40.Leone S, Nicastri E, Giglio S, et al. : Immune reconstitution inflammatory syndrome associated with Mycobacterium tuberculosis infection: a systematic review. Int J Infect Dis 2010, 14: e283–e291. [DOI] [PubMed] [Google Scholar]
- 41. ••.Clifford DB, De Luca A, Simpson DM, et al. : Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 2010, 9:438–446. [DOI] [PubMed] [Google Scholar]; This is a review of the clinical manifestations and outcome of the different forms of PML-IRIS in patients with MS treated with natalizumab.
- 42. •.Walker SL, Lockwood DN: Leprosy type 1 (reversal) reactions and their management. Lepr Rev 2008, 79:372–386. [PubMed] [Google Scholar]; This paper describes the forms of lepra reactions and IRIS-like syndrome associated with the treatment of leprosy.
- 43.Prasad K, Singh MB: Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev 2008, (1):CD002244. [DOI] [PubMed] [Google Scholar]
- 44.Lammie GA, Hewlett RH, Schoeman JF, Donald PR: Tuberculous cerebrovascular disease: a review. J Infect 2009, 59:156–166. [DOI] [PubMed] [Google Scholar]
- 45.Thwaites GE, Macmullen-Price J, Chau TT, et al. : Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol 2007, 6:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitch MT, van de Beek D: Drug insight: steroids in CNS infectious diseases—new indications for an old therapy. Nat Clin Pract Neurol 2008, 4:97–104. [DOI] [PubMed] [Google Scholar]


