Abstract
Background.
A randomized controlled trial (RCT) of 5-week stress management interventions teaching cognitive behavioral therapy (CBT) or relaxation training (RT) techniques showed decreases in stress and serum inflammatory markers over 12 months in women undergoing treatment for breast cancer (BCa). To understand the molecular mechanisms involved, we examined the effects of these interventions on the transcription factor NF-κB DNA binding activity in leukocytes in parallel with circulating inflammatory markers, stress management skill efficacy and multiple distress indicators.
Methods.
This is a secondary analysis using blood samples of 51 BCa patients (Stage 0 – III) with high cancer-specific distress selected from a completed RCT (NCT02103387). Women were randomized to one of three conditions, CBT, RT or health education control (HE). Blood samples and self-reported distress measures (Affects Balance Scale-Negative Affect [ABS-NA], Impact of Events Scale-hyperarousal [IES-H] and intrusive thoughts [IES-I]) were collected at baseline (T0) and 12-month follow-up (T2). Self-reported distress measures and perceived stress management skills (PSMS) were also measured immediately post-intervention (baseline + 2 months: T1). Repeated measures analyses compared changes in distress and NF-κB expression among conditions, controlling for age, stage of cancer, days from surgery to baseline, and receipt of chemotherapy and radiation. Regression analyses related T0 to T2 change in NF-κB expression with T0 to T1 changes in self-reported PSMS and distress measures. Exploratory regression analyses also associated change in NF-kB expression with change in serum cytokines (IL-1β, IL-6 and TNF-α); and s100A8/A9, a circulating inflammatory marker important in breast cancer progression.
Results.
There was a significant condition (CBT/RT, HE) x time (T0, T2) effect on NF-κB, F(1, 39)=5.267, p=0.036, wherein NF-κB expression significantly increased over time for HE but did not change for RT or CBT. Greater increases in PSMS from T0 to T1 were associated with less increase in NF-κB expression over 12 months (β = −0.426, t(36) = −2.637, p=0.048). We found that women assigned to active intervention (CBT/RT) had significant decreases in ABS-NA (F(1, 40)=6.537, p =0.028) and IES-I (F(1, 40)=4.391, p =0.043) from T0 to T1 compared to women assigned to HE, who showed no change over time (p’s > .10)., For women assigned to CBT or RT, lower NF-κB expression at T2 was related to less ABS-NA, IES-H, and IES-I, all p’s < .05, although T0 – T1 change in distress was not related to T0 – T2 change in NF-κB expression for those in an active intervention.
Conclusions.
Brief CBT or RT stress management interventions can mitigate increases in pro-inflammatory leukocyte NF-κB binding over 12 months of primary treatment in highly distressed BCa patients. These effects are likely brought about by improved stress management skills.
Keywords: Stress management, breast cancer, NF-κB, distress, cognitive-behavioral, relaxation training
1. INTRODUCTION
Breast cancer (BCa) is the second leading cause of cancer deaths in women in the US (Siegel et al., 2019), with the majority of deaths attributable to metastatic disease (Howlader, 2019). Host inflammatory responses are critical in facilitating BCa disease progression and metastasis (Allen and Jones, 2015) and chronic inflammation and inflammatory immune cells play an important role in tumor progression (Gonzalez et al., 2018). Particularly, the transcription factor nuclear factor-κB (NF-κB) is expressed in all immune cell types present in the tumor microenvironment and mediates a crosstalk between cancer cells and immune cells that stimulates cancer cell proliferation and survival, epithelial-to-mesenchymal transition (EMT), invasion, angiogenesis and metastasis (Taniguchi and Karin, 2018).
Biobehavioral research has tied chronic stress and distress states such as negative mood and anxiety to increased systemic inflammation, compromised cell-mediated immunity, and poor BCa outcomes. This association is believed to be mediated by stress-related neuroendocrine signaling involving the sympathetic nervous system (SNS) and hypothalamic pituitary adrenal (HPA) axis (Antoni and Dhabhar, 2019; Antoni et al., 2006a; Cole et al., 2015; Lutgendorf et al., 2010). Breast cancer diagnosis and treatment constitute major stressors including the challenges of surgery and adjuvant treatment (Spencer et al., 1999). Stress management intervention delivered early in BCa treatment can improve psychosocial adaptation and neuroimmune regulation, which in turn can affect disease progression and improve health outcomes (Andersen et al., 1994; Antoni, 2003b; Antoni and Dhabhar, 2019).
Previously we reported that breast cancer patients randomized to 10 weeks of cognitive behavioral stress management (CBSM) reported less anxiety and distress, and revealed lower late afternoon serum cortisol levels over the 12 months after surgery compared to a psycho-education control group (Antoni et al., 2006a; Antoni et al., 2006b; Phillips et al., 2008). Those in CBSM also showed downregulated leukocyte pro-inflammatory cytokine (IL1-β, IL-6, TNF-α), chemokine, and COX2 gene expression over 6-12 month follow-up (Antoni et al., 2012). Importantly, those assigned to CBSM had greater overall survival and disease-free survival at 11-year median follow-up (Stagl et al., 2015) and lower levels of pro-inflammatory gene expression during the 12 months after CBSM predicted greater 11-yr disease-free survival (Antoni et al., 2016).
Attendance data in the 10-week CBSM trial revealed that those attending 5 out of 10 weekly sessions showed improvements in adaptation equivalent to those attending 8–10 sessions. Also, the magnitude of improvements in the use of relaxation and cognitive-behavioral therapy (CBT) skills explained CBSM effects on distress and cortisol changes (Antoni et al., 2006a; Phillips et al., 2011). Therefore we conducted a dismantling trial comparing 5-week versions of CBT and relaxation training (RT) interventions with a 5-week attention-matched health education condition (HE). Patients assigned to either CBT or RT showed improved psychological adaptation (Gudenkauf et al., 2015) and reduced increases in the inflammatory biomarker and RAGE ligand s100A8/A9 (Taub et al., 2019). Greater perceived increases in stress management skills after training predicted less increase in s100A8/A9 levels over the subsequent 12 months (Taub et al., 2019). The molecular processes underlying the effects of these briefer interventions on inflammatory biomarkers remained unclear and lead to the present study where we performed secondary analyses in a subsample of the most highly distressed patients.
1.1. Present Study
The present study examined one molecular pathway that we reasoned could account for the effects of brief stress management on inflammatory signaling in breast cancer patients. Bioinformatics analyses of our prior multi-array data on changes in leukocyte gene expression after 10-week CBSM intervention inferred that downregulation of the NF-κB transcription factor in circulating leukocytes could likely explain the reduced expression of its target genes, pro-inflammatory (cytokine, chemokine, COX2) and pro-metastatic (MMP-9), over the subsequent 12 months (Antoni et al., 2012). Therefore, the current investigation focused on identifying the effects of these brief stress management interventions (CBT, RT) on changes in NF-κB activity/specific DNA binding in leukocyte cell nuclei over a 12-month period in women undergoing primary treatment for breast cancer.
Sensitivity analyses of our 10-week CBSM trial has subsequently revealed that only women presenting with elevated cancer-specific distress (breast cancer-related intrusive thoughts) show significant effects of stress management on psychological adaptation (Wang et al., 2018). Consequently, for the present secondary analyses we restricted inclusion to women from our previous 5-week intervention trial presenting with elevated cancer-specific distress at study entry.
This study had four primary hypotheses. 1) We hypothesized that in these high-distress women, those randomly assigned to either 5 weeks of CBT or RT stress management would show a decreased rise in NF-κB DNA binding over 12 months vs those assigned to the 5-week HE control. 2) We further hypothesized that greater reports of improved perceived stress management skills after training (pre-post intervention) would relate to less NF-κB DNA binding over 12-months. 3) Additionally, we hypothesized that those randomly assigned to either 5 weeks of CBT or RT stress management would show a greater decrease in distress vs those assigned to the 5-week HE control. 4) Finally, we hypothesized that change in distress after training would relate to less NF-κB DNA binding over 12-months for those assigned to CBT or RT. Exploratory analyses examined associations between change in NF-κB nuclear expression and change in circulating inflammatory biomarkers s100A8/A9, IL-6, IL-1β and TNF-α over 12 months. Additionally, we tested the hypothesis that at 12-month follow-up, NF-kB nuclear expression would positively relate to any residual distress levels for those who received an active intervention.
2. MATERIALS AND METHODS
A single-center, single-blind, randomized controlled trial was conducted at the University of Miami. The trial was approved by the Institutional Review Board and is registered as National Institutes of Health Clinical Trial NCT02103387. The present investigation is a secondary analysis of preserved blood samples from this trial. Between 2006 to 2013, women with stage 0-III BCa age 21 or older were recruited from the Sylvester Comprehensive Cancer Center and clinics in South Florida 2-10 weeks post-surgery. Exclusion criteria included severe psychiatric illness, non-fluency in English, prior history of cancer (except non-melanoma skin cancer), stage IV BCa, other serious chronic medical conditions, and initiation of neoadjuvant or adjuvant therapy. The trial ended at the end of the funding period. Additional details of methodology including primary and secondary outcome measures and allocation to study conditions, as well as an a priori power analysis, can be found in a previously published article on the trial (Gudenkauf et al., 2015).
2.1. Participants and Procedures
During recruitment, 739 women were approached. Of these, 545 were excluded (318 for not meeting study criteria and 227 for participant refusal or non-availability). Written informed consent was provided by 194 women and they were enrolled in the study. Of the 194, 11 withdrew prior to randomization and following baseline assessment the 183 participants were randomized to either CBT, RT, or a time-attention matched Health Education control (HE). The groups sequence was pre-determined by a drawing and the generation of the random allocation sequence and assignment of participants to groups was conducted by a research coordinator not involved in intervention administration or data collection. Attrition in the trial did not differ significantly based on intervention condition, stage, number of positive lymph nodes, marital status, income, education; or hormonal treatment, chemotherapy or radiation receipt (p>0.05). Of this study sample, a distressed subsample of 51 women with the highest baseline Impact of Events Scale-Intrusive Thoughts score (median=14) at study entry and available blood samples at baseline (T0) and 12 months (T2) were identified (5-week CBT=23, 5-week RT=14, 5-week HE=14). For this subsample, we report here intensive molecular analyses using available blood samples. Data were examined for outliers. Extreme values (>3 SD from the mean) were identified for raw biological variables and removed (NF-κB T0= 1; NF-κB T2 = 1; s100A8/A9 T0 = 1; s100A8/A9 T2 = 1; IL-6 T0 = 2; IL-6 T2 = 1; IL-1β T0 = 1; IL-1β T2 = 1; TNF-α T2 = 1). Consistent with past reports with this trial sample (Gudenkauf et al., 2015), outliers for psychosocial variables were winsorized. Dependent variables for the subsample were examined for normality and skewness and kurtosis and all were within acceptable limits for study analyses (skewness < 2; kurtosis < 4) (Kim, 2013).
2.2. Study Conditions
For each of the three conditions, 90 minute in-person, group sessions (3-7 participants/group) were held once weekly for five consecutive weeks. Participants in all conditions were provided with a written participant manual corresponding to their intervention condition containing key information covered in each session. Group facilitators were pre-doctoral students in an American Psychological Association - approved Clinical Psychology Ph.D. program (total facilitators = 7) trained for approximately 20 hours in one or more of the intervention protocols (CBT, RT, HE). Facilitators attended weekly supervision where videotaped sessions were reviewed for treatment fidelity by study investigators licensed in clinical psychology.
CBT Condition: The CBT condition was derived from the CBT components of the 10-week CBSM manualized intervention (Antoni, 2003b) and was grounded in core concepts of Beck’s Cognitive Behavioral Therapy, Stress and Coping Theory, and Social Learning Theory. Session content included thought monitoring, cognitive restructuring, adaptive coping skills, communication skills, and social network building. Weekly home exercises were assigned to emphasize session material.
RT: The RT condition was derived from the relaxation components of the 10-week CBSM manualized intervention (Antoni, 2003b) and involved in-session instruction and discussion of relaxation techniques. Relaxation exercises included progressive muscle relaxation, diaphragmatic breathing, guided imagery, and meditation. Participants were also instructed to engage in at-home daily practice of relaxation exercises and at-home use was discussed at each session.
HE: The HE condition served as the time-attention matched control. It consisted of educational material related to BCa diagnosis and treatment, available resources, side effect management, and healthy lifestyle behaviors, which was discussed in session and reviewed at home.
At baseline (T0), which was prior to randomization, and again 12 months post-baseline (T2), participants completed a self-report psychosocial questionnaire assessing cancer-related distress and negative affect and provided a blood sample. Participants also completed a self-report measure of perceived stress management skills at T0 and immediately post-intervention (baseline + 2 months: T1). Cancer-related distress and negative affect was also assessed at T1. Self-report demographic information and self-report medical information was provided by the participants and verified by study staff through medical chart review.
2.3. Measures
2.3.1. NF-κB DNA Binding.
Nuclear protein extracts (5 μg) from cryopreserved unstimulated peripheral blood mononuclear cells (lymphocytes and monocytes, here referred to as leukocytes) at T0 and T2 were run in electrophoretic mobility shift assay (EMSA) using the consensus NF-κB DNA probe (sc-2505, Santa Cruz Biotechnology) labeled with [γ-32P] ATP. The protein extracts were incubated with the radiolabeled NF-κB DNA probe in the presence of poly(dI:dC) as nonspecific competitor. Samples were electrophoresed in a 6% polyacrylamide gel at 175 V for 3 h at room temperature and dried gels were exposed to Kodak x-ray films at −80 °C. Films were scanned and analyzed for integrated areas under the densitometric curves (AlphaImager gel documentation system and software, Alpha Innotech) to estimate the amount of NF-κB binding to the consensus NF-κB DNA probe. Optical density (OD) for each band was used to estimate protein binding by normalization against a control nuclear protein extract from normal leukocytes. The bands corresponded to the p65 subunit of the NF-κB protein, as confirmed by supershifting with a specific antibody.
2.3.2. Circulating pro-inflammatory cytokines and s100A8/A9.
Serum concentrations of the pro-inflammatory cytokines, interleukin 1-beta (IL-1β), Tumor Necrosis Factor-alpha (TNF-α), and IL-6 were measured using Quantikine High Sensitivity Enzyme-linked Immunosorbent Assay (ELISA) kits from R&D Systems (USA), according to manufacturer’s instructions. The assays have good reliability (IL-1β: inter-assay coefficient of reliability (CV) = 8.1% and intra-assay CV = 3.6%; IL-6: inter-assay CV = 6.5% and intra-assay CV = 4.1%; TNF-α: inter-assay CV = 6.5% and intra-assay CV =2.0%). We also quantified levels of s100A8/A9 at T0 and T2, which we have previously analyzed (Taub et al., 2019) with ultra-sensitive ELISA (HycultBiotech Calprotectin Human ELISA). The assay was conducted in accordance with the manualized protocol (inter-assay CV = 4.3%; intra-assay CV = 5.3%).
2.3.3. Phenotype Analysis.
To examine the proportion of major cell types in our study samples, blood mononuclear cells were stained and analyzed by flow cytometry. Specific cell markers were used to analyze total T cells (CD3+ CD56−), CD4+ T cells (CD3+ CD4+), CD8+ T cells (CD3+ CD8+), natural killer (NK) cells (CD56+CD3−) and B cells (CD19+). The following fluorochrome conjugated antibodies were used for labeling the cells, all from BD Biosciences: anti-human CD3 (clone UCHT1), anti-human CD4 (clone SK3), anti-human CD8 (clone SK1), anti-human CD56 (clone B159) and anti-human CD19 (clone HIB19). Events (5 x 105/sample) were acquired with a BD LSR-II instrument and analyzed with BD FACSDiva software (BD Biosciences).
2.3.4. Cancer-specific distress.
The Intrusion (IES-I) and Hyperarousal (IES-H) subscales of the Impact of Event Scale—Revised (IES-R) (Weiss, 2007) were used to assess cancer-related distress with regard to breast cancer in the past week. Sample items of the Intrusion subscale include, “I thought about it when I didn’t mean to” and for the Hyperarousal subscale, “Reminders of it caused me to have physical reactions such as sweating, trouble breathing, nausea, or a pounding heart.” Response options range from 0 (not at all) to 4 (extremely) and the total subscale score is the average of the responses for the subscale items. The IES-R has been used in previous reports examining stress and stress management in breast cancer patients (Antoni et al., 2006b; Gudenkauf et al., 2015). In the current sample, Cronbach’s Alpha (α) = .78 for IES-I and α=.76 for IES-H averaged across time points.
2.3.5. Negative Affect.
Negative affect was assessed using the Negative Affect subscale of the 40-item Affects Balance Scale (ABS-NA).(Derogatis, 1975) Participants were shown a list of emotions and asked to indicate on a scale of 1 (Never) to 5 (Always) the degree to which they experienced each during the past week. The Negative Affect subscale is calculated as the average of scores on the four negative dimensions (anxiety, depression, guilt and hostility). The ABS has been used in previously published reports of stress management trials in BCa (Antoni et al., 2006a; Gudenkauf et al., 2015). In the current sample, α=.93 for ABS-NA, averaged across time points.
2.3.6. Perceived Stress Management Skills (PSMS).
To measure participants’ self-assessed confidence in using stress management skills targeted by the interventions, a subset of relevant items from the Measure of Current Status-Part A (MOCS-A) (Carver, 2006) was used. Participants rated items on a scale of 1 (“I cannot do this at all”) to 5 (“I can do this extremely well”). The items were summed for a composite score with a maximum of 20. Previous reports on both this trial (Taub et al., 2019) and a previous CBSM trial in breast cancer (Antoni et al., 2006a; Phillips et al., 2011) have shown changes in MOCS-A items to be associated with changes in psychological and physiological adaptation during primary BCa treatment.
2.4. Statistical Analyses
The Statistical Package for the Social Sciences (SPSS)-version 25 was utilized for analyses. Analyses included all participants from the distressed subsample from the parent trial for whom T0 and T2 blood samples and psychosocial data were available, including participants who did not attend all sessions, consistent with an Intent-to-Treat approach. Available data for variables used varied between 47-51 out of total of 51 participants. Available data for variables used in primary analyses was as follows: NF-κB OD T0 =48, T2=48; PSMS T0=51, T1=47; ABS-NA T0=51, T1=47; age=51; stage of cancer=51; time from surgery to baseline=51; receipt of chemotherapy=50; receipt of radiation=50). Four primary analyses were conducted using the Benjamini–Hochberg procedure to adjust for multiple comparisons (Benjamini and Hochberg, 1995).
For the first hypothesis, to examine intervention effects on NF-κB OD levels, 2 (Active Condition [CBT, RT] vs Control [HE]) by 2 (Time: T0, T2) repeated-measures analysis of co-variance (RANCOVA) was used controlling for age (years), stage of cancer (0 vs I-III), time from surgery to baseline (days), and receipt of chemotherapy and radiation (yes/no) based on their prior association with stress adaptation (Montazeri, 2008) and inflammatory markers (Blomberg et al., 2009; Bouchard et al., 2016; O’Connor et al., 2009). Secondary RANCOVAs for CBT vs HE, RT vs HE, and CBT vs RT were conducted to further probe intervention effects on NF-κB OD levels. After conducting the RANCOVA analyses, mean NF-κB OD values were compared over time (T0 – T2) in each condition (CBT, RT and HE) with paired t-tests.
For the second hypothesis, to associate changes in perceived stress management skills (PSMS) with change in NF-kB expression, regression was used to correlate T0 to T1 change in PSMS with T0 to T2 changes in NF-κB across all participants in the subsample. To further investigate the relationship between stress management skill confidence and NF-κB OD levels over time, active intervention participants (CBT/RT) were divided into two groups based on whether they showed the expected change in NF-κB OD from T0 to T2. For active stress management intervention groups (CBT/RT), expected change was NF-κB OD: T2<=T0 (64% of the participants) and unexpected change was NF-κB OD: T2>T0 (36% of the participants) following the hypothesis that the stress management interventions would attenuate increases in NF-κB OD during the primary treatment period.
For the third hypothesis, intervention effects on negative affect (ABS-NA) were examined using 2 (Active Condition [CBT, RT] vs Control [HE]) by 2 (Time: T0, T1) repeated-measures analysis of co-variance. For the fourth hypothesis, regression was used to test whether change in negative affect from T0 to T1 related to T0 to T2 change in NF-κB OD.
Supplementary exploratory analyses used the same methods to investigate T0 to T1 effects on cancer-specific distress (IES-I; IES-H). Supplemental analyses looked at the relationship between T2 NF-κB OD and distress levels for participants who received active intervention (CBT/RT). Finally, we examined relationships between T0 to T2 change in NF-κB OD and other inflammatory markers, including s100A8/A9 and serum cytokines (TNF-α, IL-1β, IL-6) using regression.
3. RESULTS
Participants were on average middle aged (M=53.6 years), middle-class, and well-educated. The majority of women were Non-Hispanic White (45.1%) or Hispanic (39.2%) and married or partnered (70.6%). Of the sample, 15.7% were stage 0, 52.9% stage I, 27.5% stage II, and 3.9% stage III. In terms of surgery, 45.1% received a lumpectomy and 54.9% received a mastectomy. For adjuvant treatments, 35.3% received chemotherapy, 41.2% radiation, and 70.6% anti-hormonal therapy. There were no differences in demographic or medical variables by study arm (see Table 1). Chi-squared tests showed no difference in frequency between groups (CBT/RT vs HE) for receipt of chemotherapy or radiation treatment in the six months leading up to the 12 month time point (T2). Within the 6 months prior to T2, only 2 women were still on chemotherapy (CBT/RT = 1, HE = 1) and 6 received radiation (CBT/RT = 5, HE = 1). Ten women had reconstructive surgery (CBT/RT = 9, HE = 1) within 6 months of T2 and receipt of reconstructive surgery was not significantly associated with NF-κB expression at T2. Across all study conditions, attendance at the 5 weekly sessions was high (Mean=4.07, SD=1.30) and the number of sessions attended did not significantly differ across the study conditions, F(2, 40)=0.681, p=0.512). Session attendance was not significantly associated with the magnitude of T0 to T2 changes in NF-κB OD, F(1, 39)=0.366, p=0.549. There was no baseline difference in NF-κB OD among women assigned to CBT, RT or HE conditions, F(2, 45)=0.142, p=0.868.
Table 1: Baseline characteristics of the participants in the study.
(CBT: Cognitive Behavioral Training; RT: Relaxation Training; HE: Health Education)
| Demographic/Medical Variable |
Total (N=51) | CBT (N=23) | RT (N=14) | HE (N=14) | Statistic | P |
|---|---|---|---|---|---|---|
| Age (years), 35-73 | 53.65(8.17) | 54.52(7.30) | 52.93(9.19) | 52.93(8.91) | F(2,48)=0.23 | 0.793 |
| Surgery to baseline (days) | 35.20 (21.35) | 36.52 (25.50) | 37.57 (21.13) | 30.64 (13.25) | F(2,48)=0.44 | 0.647 |
| Race/Ethnicity | X2(6)=5.15 | 0.524 | ||||
| Non-Hispanic White | 23(45.1%) | 8(34.8%) | 7(50.0%) | 8(57.1%) | ||
| Hispanic | 20(39.2%) | 9(39.1%) | 6(42.9%) | 5(35.7%) | ||
| African American | 3(5.9%) | 2(8.7%) | 0(0.0%) | 1(7.1%) | ||
| Other | 5(9.8%) | 4(17.4%) | 1(7.1%) | 0(0.0%) | ||
| Income (thousands) | 109.92 (68.50) | 110.27 (65.08) | 84.46 (30.08) | 134.80 (92.79) | F(2, 48)=1.96 | 0.151 |
| Education (years) | 15.73 (3.06) | 16.30 (2.27) | 15.29 (4.55) | 15.21 (2.36) | F(2, 48)=0.74 | 0.481 |
| Married/partnered | 36(70.6%) | 17(73.9%) | 9(64.3%) | 10(71.4%) | X2(2)=0.40 | 0.821 |
| Stage | X2(6)=7.27 | 0.297 | ||||
| 0 | 8(15.7%) | 5(21.7%) | 1(7.1%) | 2(14.3%) | ||
| I | 27(52.9%) | 12(52.2%) | 8(57.1%) | 7(50.0%) | ||
| II | 14(27.5%) | 5(21.7%) | 5(35.7%) | 4(28.6%) | ||
| III | 2(3.9%) | 1(4.3%) | 0(0.0%) | 1(7.1%) | ||
| Positive Nodes | 7(13.8%) | 2(8.7%) | 2(14.3%) | 3(21.3%) | X2(2)=0.80 | 0.672 |
| Hormonal Status | ||||||
| ER Positive | 37(72.5%) | 16(69.6%) | 12(85.7%) | 9(64.3%) | X2(2)=4.42 | 0.110 |
| PR Positive | 28(54.9%) | 13(56.5%) | 8(57.1%) | 9(64.3%) | X2(2)=0.76 | 0.684 |
| Surgical Procedure | X2(2)=0.19 | 0.910 | ||||
| Lumpectomy | 23(45.1%) | 10(43.5%) | 6(42.9%) | 7(50.0%) | ||
| Mastectomy | 28(54.9%) | 13(56.5%) | 8(57.1%) | 7(50.0%) | ||
| Adjuvant treatment | ||||||
| Chemotherapy | 18(35.3%) | 7(30.4%) | 4(28.6%) | 7(50.0%) | X2(2)=1.49 | 0.475 |
| Radiation | 21(41.2%) | 10(43.5%) | 4(28.6%) | 7(50.0%) | X2(2)=1.13 | 0.569 |
| Antihormonal Therapy | 36(70.6%) | 19(82.6%) | 9(64.3%) | 8(57.1%) | X2(2)=2.83 | 0.243 |
| Psychological Measures | ||||||
| Mean (SD) | ||||||
| Total (N=51) | CBT (N=23) | RT (N=14) | HE (N=14) | Statistic | P | |
| IES-I | 2.04(.57) | 2.07(.57) | 1.90(.46) | 2.12(.66) | F(2, 48)=0.60 | 0.551 |
| Total (N=51) | CBT (N=23) | RT (N=14) | HE (N=14) | Statistic | P | |
| IES-H | 1.28(.72) | 1.27(.65) | 1.16(.81) | 1.41(.77) | F(2, 48)=0.41 | 0.669 |
| Total (N=51) | CBT (N=23) | RT (N=14) | HE (N=14) | Statistic | P | |
| ABS-NA | 2.25(.56) | 2.18(.56) | 2.38(.53) | 2.25(.61) | F(2, 48)=0.51 | 0.605 |
| Biological Measures | ||||||
| Mean (SD) | ||||||
| Total (N=50) | CBT (N=22) | RT (N=14) | HE (N=14) | Statistic | P | |
| IL-1β (pg/ml) | 2.21(4.11) | 3.62(5.76) | 1.40(1.77) | .80(.83) | F(2, 47)=2.534 | 0.090 |
| Total (N=49) | CBT (N=21) | RT (N=14) | HE (N=14) | Statistic | P | |
| IL-6 (pg/ml) | 2.67(3.13) | 2.95(2.80) | 2.79(4.47) | 2.12(1.92) | F(2, 46)=.269 | 0.745 |
| Total (N=51) | CBT (N=23) | RT (N=14) | HE (N=14) | Statistic | P | |
| TNF-α (pg/ml) | 1.82(1.44) | 1.78(1.34) | 1.99(1.29) | 1.72(1.80) | F(2, 48)=.136 | 0.873 |
| Total (N=48) | CBT (N=22) | RT (N=13) | HE (N=13) | Statistic | P | |
| NF-κB OD | 137.74(107.16) | 145.76(126.04) | 125.47(63.70) | 136.44(113.30) | F(2, 45)=.142 | 0.868 |
| Total (N=49) | CBT (N=23) | RT (N=12) | HE (N=14) | Statistic | P | |
| s100A8/A9 (ng/mL) | 4167.66(3077.40) | 4636.94(3600.95) | 4437.75(2970.63) | 3165.21(2004.32) | F(2, 46)=1.059 | 0.355 |
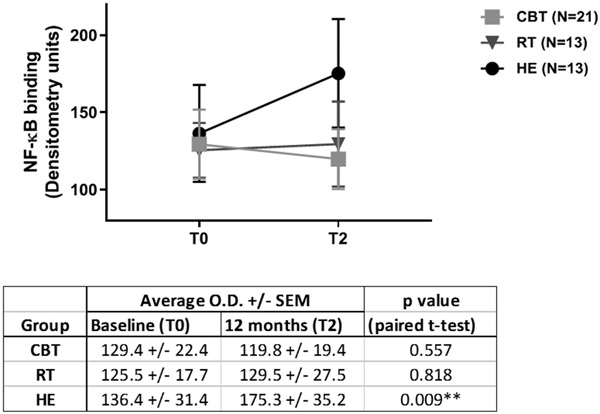
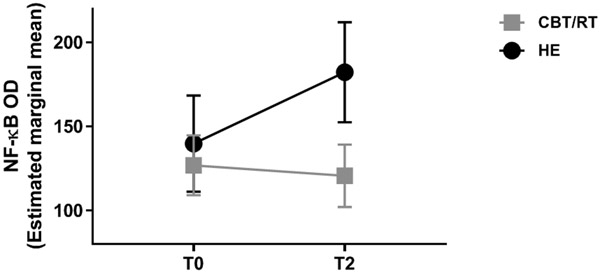
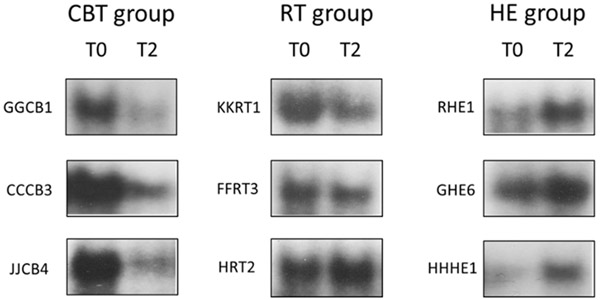
Our first hypothesis tested whether assignment to either of the active treatment conditions (CBT/RT) vs control (HE) was associated with differences in NF-κB OD over 12 months. Figure 1 shows the mean NF-κB OD values at each time point for each study condition. As hypothesized, we found a significant condition x time interaction effect, F(1, 39)=5.267, p=0.036, partial eta2 =.119, a medium effect size (see Figure 2). As can be seen, while the HE condition showed rises in NF-κB binding over time, there were slight declines in those assigned to CBT/RT. The contrast in NF-κB OD changes between CBT and HE was significant, F(1, 27)=5.304, p=0.029. The contrast between RT and HE was also significant, F(1, 18)=5.453, p=0.031. The contrast between CBT vs RT was not significant, F(1, 26)=0.147, p=0.704. Within condition tests showed that mean NF-κB OD significantly increased over time (T0 to T2) for HE, (T0 M=136.4, T2 M=175.3, t[12]=−3.096, p=0.009) and did not change for those assigned to RT (T0 M=125.5, T2 M=129.5, t[12]=−0.235, p=0.818) or CBT (T0 M=129.4, T2 M=119.8, t[20]=0.597, p=0.557). Representative EMSA gel scans show decreased or unchanged NF-κB binding at 12-month follow-up in CBT and RT groups, and increased binding in the HE group (see Figure 3).
Figure 1. Mean NF-κB transcription factor expression in O.D. units (+/− SEM) in breast cancer patients assigned to the 3 study conditions.
There is a statistically significant increase at 12-month follow-up (T2) versus baseline (T0) in the HE control group while no change in the active intervention groups (CBT, RT). CBT indicates cognitive behavioral therapy; RT indicates relaxation training; HE indicates health education.
Figure 2. Effects of active intervention (CBT/RT) on NF-κB activity over 12 months.
Group assignment was associated with changes in NF-κB over 12 months as indicated by the significant group (CBT/RT vs HE control) x time (0 [T0] vs 12 months [T2]) interaction effect, F(1, 39)=5.267, p=0.027, partial eta2 =.119. CBT/RT indicates the combined data from those assigned to either cognitive behavioral therapy (CBT) or relaxation training (RT) and HE indicates those assigned to the Health Education control.
Figure 3: Representative EMSA gel scans from breast cancer patients assigned to the 3 study conditions.
EMSA gel scans show some decrease in NF-κB DNA binding at 12-month follow-up (T2) (not statistically significant) in women assigned to the cognitive behavioral therapy (CBT) group, unchanged binding in the relaxation training (RT) group, and increased binding in the health education (HE) group compared to baseline (T0). Individual participant ID keys are shown to the left of each scan.
Because the NF-κB assay involved multiple cell types within the peripheral blood mononuclear cell sample, we used flow cytometry to examine specific cell phenotypes at baseline and over time. There were no baseline differences in numbers or percent of total T cells, B cells, CD4+ T cells, CD8+ T cells or NK cells among women assigned to CBT, RT and HE after controlling for age, disease stage and time since surgery, all p’s > .05. We also saw no significant changes in any of these cell numbers or percentages by study condition over the 12-month period, with or without controlling for age, disease stage, time since surgery, receipt of chemotherapy or radiation, all p’s > .05.
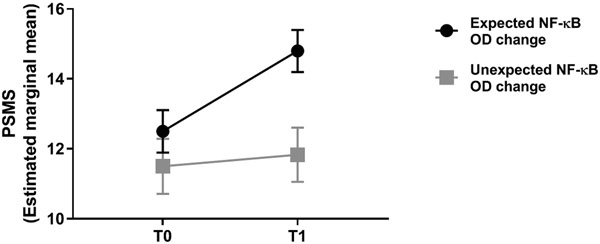
For our second hypothesis, greater increases in PSMS from T0 to T1 were associated with less increase in NF-κB OD over the 12-month follow-up period (F(6, 36) = 1.816, p = .123, R2=.232, β = −0.426; t(36) = −2.637; p=.048). For participants assigned to an active condition (CBT or RT), there was a significant interaction between sub-group (expected non-increase/decrease [64% of cases] vs. unexpected rise [36% of cases] in NF-κB OD) and PSMS, such that increases in PSMS were greater for those who showed the expected change (decrease or non-increase) in NF-κB OD compared to those who showed the unexpected change (increase) F(1, 30)=4.512, p=0.042, suggesting improvements in PSMS attenuated increases in NF-κB OD during primary treatment (see Figure 4).
Figure 4. Relationship between perceived stress management skills and NF-κB response.
For active intervention participants (CBT/RT), there was a significant interaction between group (expected vs. unexpected change in NF-κB OD over 12 months) and change in perceived stress-management skills (PSMS) pre (T0) to post intervention (T1), such that increases in PSMS were greater for those who showed the expected change (no increase/ decrease) in NF-κB OD compared to those who showed the unexpected change (increase), F(1, 30)=4.512, p=0.042.
For our third hypothesis, we found that women assigned to active intervention (CBT/RT) had greater decreases in ABS-NA (M(SD) T0 = 2.26(0.55), T1 = 1.93(0.53) compared to women assigned to HE (M(SD) T0 = 2.25(0.61), T1 = 2.17(0.57), controlling for age, stage, time since surgery, chemotherapy, and radiation, F(1, 40)=6.537, p =0.028). Women assigned to active intervention (CBT/RT) were also found to have a significant decrease in IES-I versus HE (CBT/RT: M(SD) T0 = 2.00(0.54), T1 = 1.38(0.81); HE: M(SD) T0 = 2.12(0.66), T1 = 1.93(0.66); F(1, 40)=4.391, p =0.043) and a non-significant decrease in IES-H (CBT/RT: M(SD) T0 = 1.23(0.71), T1 = 0.82(0.66); HE: M(SD) T0 = 1.41(0.77), T1 = 1.26(0.55); F(1, 40)=1.723, p =0.197) compared to HE. Within condition analyses revealed highly significant T0 to T1 decreases in IES-H t(32)=4.07, p<.001, IES-I t(32) = 4.68, p<.001, and ABS-NA t(32)=4.841, p<.001 in those assigned to CBT/RT; and non-significant changes in IES-H t(13)=0.92, p = 0.375, IES-I t(13) = 1.272, p=0.226, and ABS-NA t(13)=1.502, p=0.157 in the HE group.
For our fourth hypothesis, for active intervention participants, change in ABS-NA from T0 to T1 did not predict NF-κB OD at T2 controlling for covariates and baseline levels of NF-κB OD (β=−.079, t(22)=−.502, p =.621). Changes in IES-I from T0 to T1 also did not predict NF-κB OD at T2 (β=.236, t(22)=1.463, p =.158), nor did changes in IES-H (β=.027, t(22)=.172, p=.865).
In supplemental analyses, among those assigned to CBT/RT, IES-H, IES-I and ABS-NA scores at T2 were significantly concurrently associated with NF-κB expression at T2 (IES-H: F(1, 32)=4.232, β=.342, t(32)=2.057, p=.048, R2 = .117; IES-I: F(1, 32)=4.258, β=.343, t(32)=2.063, p=.047, R2 = .117; ABS-NA: F(1, 32)=6.276, β=.405, t(32)=2.505, p=.018, R2 = .164). Using natural log transformed values for cytokines, change in NF-κB OD over the T0 – T2 period in the entire sample was positively associated with T0 – T2 change in ln IL-6 F(1, 39)=4.520, β=.322, t(39)=2.126, p=.040, R2 = .104. Change in NF-κB OD over the T0 – T2 period was marginally associated with change in ln IL-1β, F(1, 42)=3.723, β=.285, t(42)=1.930, p=.060, and not related to change in ln TNF-α, F(1, 42)=.142, β=−.058, t(42)=−.377, p=.708. Finally, we found a significant positive relationship between change in s100A8/A9 and NF-κB OD, β=0.314, t(42)=2.141, p=.038 over this period.
In sum, women in either of the stress management conditions showed greater T0 to T1 distress reduction and showed less increase in NF-κB DNA binding over 12 months of primary treatment vs HE controls. The extent to which women reported greater PSMS improvements after stress management training was associated with less increase in NF-κB binding over time. Also, within the CBT/RT conditions women who showed a pattern of no increase or decreased NF-κB DNA binding over 12 months (the non-inflammatory pattern) had greater reported T0 to T1 improvements in PSMS than women who showed increased NF-κB binding over this period (the inflammatory pattern). Although T0 to T1 changes in distress were not associated with the magnitude of NF-kB changes over 12 months, we did find that lower T2 cancer-specific distress and negative affect were concurrently associated with lower NF-κB expression at T2. Finally, changes in NF-κB DNA binding over 12 months were associated with changes in IL-6 and with s100A8/A9, the latter of which has previously been shown to be reduced by these stress management interventions in a larger sample of women with BCa in the parent study (Taub et al., 2019).
4. DISCUSSION
Previously we showed a 5-week group-based stress management intervention using CBT or RT techniques decreased distress (Gudenkauf et al., 2015) and the circulating inflammatory marker, s100A8/A9 (Taub et al., 2019) in breast cancer (BCa) patients during primary treatment. Here we had four major findings. First, we showed for the first time that BCa patients assigned to a brief stress management condition focused on either CBT or RT showed mitigation of the rise in NF-κB expression over the 12 months of primary treatment as compared to a time-matched health education (HE) control. Second, greater increases in perceived ability to use stress management skills was associated with less increase in NF-κB expression over the 12 months of primary treatment. This suggested that acquisition of specific skills targeted by RT and CBT was accounting in part for the changes in NF-κB expression. Third we found that women assigned to CBT or RT vs HE showed greater pre-post intervention decreases in distress, including a composite of negative affect and a well established cancer-specific distress scale (IES-intrusive thoughts). Finally, among women assigned to CBT or RT less negative affect and cancer-specific distress at 12 month follow-up were concurrently associated with less NF-κB DNA binding. This suggested that women who were able to maintain lower distress levels up to a year after the intervention had less concurrent inflammatory signaling. Supplementary analyses supported that NF-κB changes over the study period covaried with serum inflammatory markers. Importantly, changes in NF-κB binding over this 12-month period mirrored changes in circulating s100A8/A9 levels, consistent with the mitigating effects of CBT/RT on rises in this marker over the same 12-month period seen in a larger sample (Taub et al., 2019), thus providing evidence for a molecular mechanism for stress management effects.
The present study was designed such that the HE condition provided attention and useful health information in a supportive group, while not providing training in stress management skills that were the target of the two active treatment conditions (RT and CBT) of this trial. The fact that we demonstrated significant effects of our active stress management conditions (CBT, RT) against this form of control suggests that effects were attributable to some aspect of stress management training and not simply being in a supportive group. We should note that although it was not associated with improvements in inflammatory signaling here, health education can facilitate healthy behaviors and provide access to information that is useful to cancer patients during treatment.
We showed for the first time the effects of brief stress management interventions during primary breast cancer treatment on reducing the increase in the transcription factor NF-κB, which regulates downstream inflammatory responses (Ahmed et al., 2015; Mitchell and Carmody, 2018). The more that these women sensed that they had mastered these stress management skills and could apply them, the less the increases in NF-κB over time over the first year of treatment. Women with the lowest levels of distress at 12 months also revealed the lowest NF-κB expression suggesting that intervention effects were likely brought about by stress management skill efficacy and distress reduction. It was expected that inflammation would increase over time in this group of BCa patients, in part as a consequence of chemotherapy and radiation therapy. Chemotherapy and radiation therapy have been associated with increased inflammation in BCa patients, leading to symptoms such as depression, fatigue and neurocognitive deficits (Bower et al., 2011b; Cheung et al., 2015; Pomykala et al., 2013). Inflammation may last for several months after the end of the treatment, contributing to the persistence of treatment side effects (Shibayama et al., 2014; Smith et al., 2014; Tromp et al., 2020). Several studies have identified the transcription factor NF-κB as a mediator of BCa treatment-related inflammation and its subsequent effects (Bower et al., 2011a; Smith et al., 2014; Torres et al., 2013).
One possible neuroendocrine mechanism underlying the effects of stress management intervention on inflammation may involve the HPA axis (Antoni and Dhabhar, 2019). Conditions of chronic stress are associated with persisting elevations in the glucocorticoid cortisol (McEwen, 1998, 2017). In situations where chronic stress and activation disrupt the endocrine-immune balance synergistic effects between leukocyte glucocorticoid receptor (GR) and NF-κB have been described (Rao et al., 2011). Several studies have shown a dysregulation of GR and NF-κB in blood cells from patients with chronic stress or mood disorders (Miller et al., 2002; Miller et al., 2014; Pace et al., 2012). The experience of chronic stress due to dealing with the impact of diagnosis and treatment of breast cancer may translate into leukocyte GR resistance and subsequent upregulation of pro-inflammatory NF-κB activity, due to the inability of glucocorticoids to effectively suppress NF-κB (McEwen, 1998, 2017; Miller et al., 2002; Miller et al., 2014; Pace et al., 2012; Rao et al., 2011).
We have found previously that greater negative affect, possibly reflecting greater chronic stress, is associated with greater leukocyte pro-inflammatory gene expression in non-metastatic BCa patients in the period after surgery (Antoni et al., 2012). Patients who underwent a 10-wk CBSM intervention showed reversed negative affect-related upregulation of pro-inflammatory transcripts in parallel with reductions in negative affect over a 12-month period (Antoni et al., 2012). More specifically, CBSM upregulated transcripts showed an underrepresentation of the NF-κB response element in their promoters, together with an overexpression of GR response elements, suggesting a decreased transcriptional activity of genes modulated by NF-κB, coupled with an increased expression of GR responsive genes. In the present trial, we have shown that shorter forms of stress management (5-week CBT or RT) are associated with decreases in distress and less NF-κB expression increases over a 12-month period in tandem with improved perceived stress management skills. This may be due to a modulation of GR activity that translates into a mitigation of NF-κB activation and therefore decreased inflammation.
Another plausible neuroendocrine mechanism for the observed effects of stress reduction on inflammation is the reduction in the activation of the sympathetic nervous system (SNS), particularly decreased catecholamine hormone signaling effects. Chronic stress may lead to low grade activation of the SNS that in turn may promote immune dysfunction and tumor progression (Antoni and Dhabhar, 2019; Cole et al., 2015; Lutgendorf et al., 2010). It has been previously shown that a 10-week CBSM intervention in patients with HIV infection reduces urinary noradrenaline output mediated by decreases in negative mood. This reduction was associated with positive changes in immunologic status that may have positive health consequences for HIV infection (Antoni, 2003a). Recent clinical studies have demonstrated that pharmacological blockade of adrenergic signaling around the time of surgery in BCa patients improves markers of inflammation (including downregulation of NF-κB and IL-6), immune function, poor prognosis and metastasis (Haldar et al., 2018; Hiller et al., 2020). Although our intervention is provided after surgery, it is possible that the observed reduction in distress and negative affect may have reduced the low grade SNS chronic activation that these highly distressed patients may be experiencing, which may in turn relate to less inflammation.
We previously also found that BCa patients assigned to a 10-week CBSM showed longer disease free survival (DFS) at 8 – 15 years (Stagl et al., 2015), and longer DFS was associated with a smaller rise in inflammatory gene expression (including NF-κB transcripts: NFKB1, NFKB2, REL, RELA, RELB) over the 12 months after this intervention (Antoni et al., 2016). Thus, the mitigated increase in NF-κB nuclear binding observed in the present 5-wk stress management interventions may relate to future long-term outcomes. We are currently following these patients in the long term to evaluate possible effects of brief stress management on health outcomes and cancer progression.
Study strengths were the recruitment of a diverse sample of BCa patients reflecting the catchment area, the use of an attention-time matched control, the inclusion of theoretically-determined disease and treatment covariates, and analyses to identify psychological mechanisms that might underlie these intervention effects. Study weaknesses were the relatively small sample size, and the lack of information on anti-inflammatory medications, and other factors that could contribute to stress-related changes in NF-κB expression such as social support, sleep, and physical activity, among others.
In summary, we showed that two brief forms of stress management, cognitive behavior therapy and relaxation training, administered post-surgically to women with non-metastatic breast cancer, are associated with a reduction in distress after the intervention and less increases in leukocyte NF-κB expression over the 12-month period of primary treatment. Future work should examine behavioral and neuroendocrine mechanisms that may underlie these effects in larger-scale trials in women with breast cancer and other persons dealing with cancer.
Funding and acknowledgements
This study is supported by a grant from the National Cancer Institute/ National Institutes of Health (R01 CA64710), Florida Breast Cancer Foundation (FBCF) C140027 and the Sylvester Comprehensive Cancer Center, to whom we are thankful. The funding sources did not have any involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declaration of Competing Interest
Michael H. Antoni reports royalties from a book on stress management in patients with breast cancer, is an inventor of the intellectual property used in this study, and serves as a compensated consultant for Blue Note Therapeutics. The other authors declare no potential conflicts of interest.
CRediT authorship contribution statement
Alain Diaz: Data curation, formal analysis, investigation, methodology, validation, visualization, writing - original draft. Chloe J. Taub: Data curation, formal analysis, investigation, methodology, validation, visualization, writing - original draft, writing - review & editing. Michael H. Antoni: Conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, writing - original draft, writing - review & editing. Marc E. Lippman: Conceptualization, methodology, writing - review & editing. Bonnie B. Blomberg: Conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, writing - review & editing.
REFERENCES
- Ahmed AU, Williams BR, Hannigan GE, 2015. Transcriptional Activation of Inflammatory Genes: Mechanistic Insight into Selectivity and Diversity. Biomolecules 5, 3087–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MD, Jones LJ, 2015. The role of inflammation in progression of breast cancer: Friend or foe? (Review). Int J Oncol 47, 797–805. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Kiecolt-Glaser JK, Glaser R, 1994. A biobehavioral model of cancer stress and disease course. Am Psychol 49, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, 2003a. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress 6, 173–188. [DOI] [PubMed] [Google Scholar]
- Antoni MH, 2003b. Stress Management Intervention for Women with Breast Cancer, American psychological association. [Google Scholar]
- Antoni MH, Bouchard LC, Jacobs JM, Lechner SC, Jutagir DR, Gudenkauf LM, Carver CS, Lutgendorf S, Cole SW, Lippman M, Blomberg BB, 2016. Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: An exploratory analysis. Psychoneuroendocrinology 74, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Dhabhar FS, 2019. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 125, 1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly SR, Sifre T, Urcuyo KR, Phillips K, Gluck S, Carver CS, 2006a. How stress management improves quality of life after treatment for breast cancer. Journal of Consulting and Clinical Psychology 74, 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JM, Cole SW, 2012. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry 71, 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, Phillips K, Smith RG, Petronis VM, Guellati S, Wells KA, Blomberg B, Carver CS, 2006b. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. American Journal of Psychiatry 163, 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B 57, 289–300. [Google Scholar]
- Blomberg BB, Alvarez JP, Diaz A, Romero MG, Lechner SC, Carver CS, Holley H, Antoni MH, 2009. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: An exploratory study. Journal of psychosomatic research 67, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard LC, Antoni MH, Blomberg BB, Stagl JM, Gudenkauf LM, Jutagir DR, Diaz A, Lechner S, Gluck S, Derhagopian RP, Carver CS, 2016. Postsurgical Depressive Symptoms and Proinflammatory Cytokine Elevations in Women Undergoing Primary Treatment for Breast Cancer. Psychosom Med 78, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW, 2011a. Fatigue and gene expression in human leukocytes: increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun 25, 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW, 2011b. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 29, 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, 2006. Measure of Current Status. http://www.psy.miami.edu/faculty/ccarver/sclMOCS.html.
- Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, Lee JA, Fan G, Tan YP, Yong WS, Madhukumar P, Loo SK, Ang SF, Wong M, Chay WY, Ooi WS, Dent RA, Yap YS, Ng R, Chan A, 2015. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol 26, 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK, 2015. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 15, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L, 1975. Affects balance scale. Baltimore: Clinical Psychometric Research. [Google Scholar]
- Gonzalez H, Hagerling C, Werb Z, 2018. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32, 1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudenkauf LM, Antoni MH, Stagl JM, Lechner SC, Jutagir DR, Bouchard LC, Blomberg BB, Glück S, Derhagopian RP, Giron GL, 2015. Brief cognitive–behavioral and relaxation training interventions for breast cancer: A randomized controlled trial. Journal of consulting and clinical psychology 83, 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar R, Shaashua L, Lavon H, Lyons YA, Zmora O, Sharon E, Birnbaum Y, Allweis T, Sood AK, Barshack I, Cole S, Ben-Eliyahu S, 2018. Perioperative inhibition of beta-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav Immun 73, 294–309. [DOI] [PubMed] [Google Scholar]
- Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang JB, Henderson MA, Nightingale SS, Ho KM, Myles PS, Fox S, Riedel B, Sloan EK, 2020. Preoperative beta-Blockade with Propranolol Reduces Biomarkers of Metastasis in Breast Cancer: A Phase II Randomized Trial. Clin Cancer Res 26, 1803–1811. [DOI] [PubMed] [Google Scholar]
- Howlader NN, A.M.; Krapcho M; Miller D; Brest A; Yu M; Ruhl J; Tatalovich Z; Mariotto A; Lewis DR; Chen HS; Feuer EJ; Cronin KA (eds). 2019. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. Accessed on December 18, 2019. [Google Scholar]
- Kim HY, 2013. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod 38, 52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK, Antoni MH, 2010. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol 28, 4094–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2017. Allostasis and the Epigenetics of Brain and Body Health Over the Life Course: The Brain on Stress. JAMA Psychiatry 74, 551–552. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK, 2002. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol 21, 531–541. [DOI] [PubMed] [Google Scholar]
- Miller GE, Murphy ML, Cashman R, Ma R, Ma J, Arevalo JM, Kobor MS, Cole SW, 2014. Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain Behav Immun 41, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Carmody RJ, 2018. NF-kappaB and the Transcriptional Control of Inflammation. Int Rev Cell Mol Biol 335, 41–84. [DOI] [PubMed] [Google Scholar]
- Montazeri A, 2008. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. Journal of experimental & clinical cancer research 27, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, 2009. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain, behavior, and immunity 23, 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM, 2012. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 26, 13–17. [DOI] [PubMed] [Google Scholar]
- Phillips KM, Antoni MH, Carver CS, Lechner SC, Penedo FJ, McCullough ME, Gluck S, Derhagopian RP, Blomberg BB, 2011. Stress management skills and reductions in serum cortisol across the year after surgery for non-metastatic breast cancer. Cognitive Therapy and Research 35, 595–600. [Google Scholar]
- Phillips KM, Antoni MH, Lechner SC, Blomberg BB, Llabre MM, Avisar E, Gluck S, DerHagopian R, Carver CS, 2008. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosom Med 70, 1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, Mallam S, Cheng I, Ahn R, Breen EC, Irwin MR, Silverman DH, 2013. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav 7, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, McCalman MT, Moulos P, Francoijs KJ, Chatziioannou A, Kolisis FN, Alexis MN, Mitsiou DJ, Stunnenberg HG, 2011. Coactivation of GR and NFKB alters the repertoire of their binding sites and target genes. Genome Res 21, 1404–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama O, Yoshiuchi K, Inagaki M, Matsuoka Y, Yoshikawa E, Sugawara Y, Akechi T, Wada N, Imoto S, Murakami K, Ogawa A, Akabayashi A, Uchitomi Y, 2014. Association between adjuvant regional radiotherapy and cognitive function in breast cancer patients treated with conservation therapy. Cancer Med 3, 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2019. Cancer statistics, 2019. CA Cancer J Clin 69, 7–34. [DOI] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Pace TW, Mister D, Felger JC, Kilaru V, Akel MJ, Vertino PM, Miller AH, Torres MA, 2014. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun 38, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, Derhagopian RP, Ironson G, Love N, 1999. Concerns about breast cancer and relations to psychosocial well-being in a multiethnic sample of early-stage patients. Health Psychol 18, 159–168. [DOI] [PubMed] [Google Scholar]
- Stagl JM, Lechner SC, Carver CS, Bouchard LC, Gudenkauf LM, Jutagir DR, Diaz A, Yu Q, Blomberg BB, Ironson G, Gluck S, Antoni MH, 2015. A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast Cancer Res Treat 154, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, Karin M, 2018. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 18, 309–324. [DOI] [PubMed] [Google Scholar]
- Taub CJ, Lippman ME, Hudson BI, Blomberg BB, Diaz A, Fisher HM, Nahin ER, Lechner SC, Kwak T, Hwang GH, Antoni MH, 2019. The effects of a randomized trial of brief forms of stress management on RAGE-associated S100A8/A9 in patients with breast cancer undergoing primary treatment. Cancer 125, 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Pace TW, Liu T, Felger JC, Mister D, Doho GH, Kohn JN, Barsevick AM, Long Q, Miller AH, 2013. Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer 119, 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp J, Boerman LM, Sama IE, Maass S, Maduro JH, Hummel YM, Berger MY, de Bock GH, Gietema JA, Berendsen AJ, van der Meer P, 2020. Long-term survivors of early breast cancer treated with chemotherapy are characterized by a pro-inflammatory biomarker profile compared to matched controls. Eur J Heart Fail 22, 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AW, Bouchard LC, Gudenkauf LM, Jutagir DR, Fisher HM, Jacobs JM, Blomberg BB, Lechner SC, Carver CS, Antoni MH, 2018. Differential psychological effects of cognitive-behavioral stress management among breast cancer patients with high and low initial cancer-specific distress. J Psychosom Res 113, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, 2007. The impact of event scale: revised, Cross-cultural assessment of psychological trauma and PTSD. Springer, pp. 219–238. [Google Scholar]