Abstract
Background
Sepsis still threatens the lives of more than 300 million patients annually and elderly patients with sepsis usually have a more complicated condition and a worse prognosis. Existing studies have shown that both Hematocrit (HCT) and albumin (ALB) can be used as potential predictors of sepsis, and their difference HCT-ALB has a significant capacity to diagnose infectious diseases. Currently, there is no relevant research on the relationship between HCT-ALB and the prognosis of elderly sepsis patients. Therefore, this study aims to explore the association between HCT-ALB and mortality in elderly patients with sepsis.
Methods
This study was a multi-center retrospective study based on the Medical Information Mart for Intensive Care (MIMIC-IV) database and the eICU Collaborative Research Database (eICU-CRD) in elderly patients with sepsis. The optimal HCT-ALB cut-off point for ICU mortality was calculated by the Youden Index based on the eICU-CRD dataset, and multivariate logistic regressions were conducted to explore the association between HCT-ALB and ICU/hospital mortality in the two databases. Subgroup analyses were performed for different parameters and comorbidity status.
Results
The number of 16,127 and 3043 elderly sepsis patients were selected from two large intensive care databases (eICU-CRD and MIMIC-IV, respectively) in this study. Depending on the optimal cut-off point, patients in both eICU-CRD and MIMIC-IV were independently divided into low HCT-ALB (< 6.7) and high HCT-ALB (≥ 6.7) groups. The odds ratio (95%confidence interval) [OR (95CI%)] of the high HCT-ALB group were 1.50 (1.36,1.65) and 1.71 (1.58,1.87) for ICU and hospital mortality in the eICU-CRD database after multivariable adjustment. Similar trends in the ICU and hospital mortality [OR (95%CI) 1.41 (1.15,1.72) and 1.27 (1.07,1.51)] were observed in MIMIC-IV database. Subgroup analysis showed an interaction effect with SOFA score in the eICU-CRD database however not in MIMIC-IV dataset.
Conclusions
High HCT-ALB (≥ 6.7) is associated with 1.41 and 1.27 times ICU and hospital mortality risk in elderly patients with sepsis. HCT-ALB is simple and easy to obtain and is a promising clinical predictor of early risk stratification for elderly sepsis patients in ICU.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07609-7.
Keywords: Sepsis, Elderly, HCT-ALB, Mortality, Multi-center
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, which is one of the most common complications in intensive-care unit (ICU) cases [1]. In clinical practice, organ dysfunction can be indicated by an increase of 2 or more points in the Sequential Organ Failure Assessment (SOFA) score [2, 3] Although the awareness of sepsis and medical technology have improved significantly recently, the number of sepsis patients each year still exceeds 30 million worldwide, and the total mortality rate is 17% [4], creating a high global disease burden. Sepsis is very common in elderly patients, with more than 50% of sepsis patients in the United States being over 65 years old [5]. Due to advanced age, immune system aging [6, 7], prolonged host inflammatory response time [8], and other factors, elderly patients have a more complicated disease occurrence and development and relatively difficult clinical treatment, making their mortality rate higher than that of nonelderly sepsis patients [9].
Synthesized in the liver, albumin (ALB) is the most abundant plasma protein, accounting for 40–60% of total plasma protein. ALB has various physiological functions, including expanding blood volume, maintaining plasma colloidal osmotic pressure, and providing nutrition and transportation. Serum ALB measurements are standard predictors in the prognosis of various diseases [10]. Hypoalbuminemia is common in critically ill patients and often causes a poor prognosis, with previous studies suggesting a correlation between serum ALB level and sepsis patient prognosis [11, 12]. Hematocrit (HCT) is the volume percentage of red blood cells. HCT can be increased by massive fluid loss leading to hemoconcentration and by the presence of chronic hypoxia in the patient. This relationship can also be observed in hemorrhagic fever, which is caused by certain viruses [13]. Several published studies have shown that HCT is a variable that can predict the risk of death in patients with sepsis and septic shock [14, 15]. The HCT and plasma ALB levels of healthy people are stable under normal conditions, and their values are in the ranges of 40–45% and 35–45 g/l [16], respectively. Sepsis can be caused by any type of infection, and it is common in conditions such as pneumonia, peritonitis, cholangitis, urinary system infection, cellulitis, meningitis, and abscesses. Its pathogenic microorganisms include bacteria, fungi, viruses, and parasites [17]. Infectious factors can activate the monocyte-macrophage system and other inflammatory response cells to produce and release many inflammatory mediators, including vasoactive substances, cytokines, chemokines, oxygen free radicals, and acute-phase reaction substances. Extensive damage to certain body systems and organs and increased capillary permeability leads to leakage of ALB and HCT fluctuations, altering their proportions [18]. In addition to the above effects of infection, the patient’s hypermetabolic state causes increased albumin consumption, intestinal dysfunction causes increased albumin loss, and patients with chronic hypoxic diseases can cause an increase in HCT, as well as blood loss and anemia, which are all factors that cause changes in HCT-ALB. The numerical difference value between HCT (%) and ALB (g/L) levels (HCT-ALB) is highly sensitive and specific among patients with infectious diseases and is, therefore, a potential indicator for the differential diagnosis of infections [19]. The relationship between HCT-ALB and the prognosis of elderly sepsis patients remains unclear. Therefore, the purpose of this study is to investigate the relationship between HCT-ALB and the prognosis of elderly patients with sepsis, and to provide a basis for clinical decision-making.
Method
Data source
All study data were extracted from the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database and the eICU Collaborative Research Database (eICU-CRD). The MIMIC database is derived from the electronic medical records of the Beth Israel Deaconess Medical Center and includes data of basic demographics, ICU monitoring records, laboratory test results, and treatment prescriptions [20, 21]. MIMIC-IV version 0.4 was released in 2020 and includes over 250,000 case admissions from 2008 to 2019. eICU-CRD contains the electronic medical records of more than 200,000 patients admitted to the ICU of 208 hospitals in the United States in 2014 and 2015 [22]. Both databases were IRB pre-approved and de-identified databases under the requirements of the United States Health Insurance Portability and Accountability Act (HIPAA) to ensure the anonymity of patients’ personal information so informed consent is not necessary. All data were extracted by structured query language (SQL) Server.
Study population
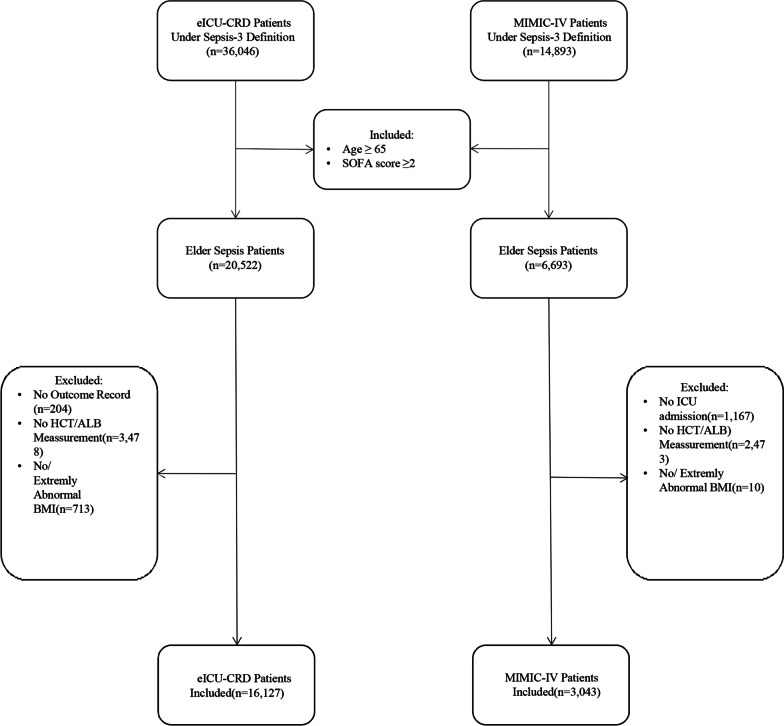
The code to derive the SOFA score of MIMIC-IV and eICU-CRD was sourced from MIT Laboratory for Computational Physiology (https://github.com/MIT-LCP), which referenced the SOFA definition of previous researches [21, 22]. The number of elderly patients (age > 65 years) with sepsis meeting the Sepsis-3 definition (infected and with SOFA ≥ 2) [3] in the MIMIC-IV and eICU-CRD databases were 20,522 and 6693, respectively. Patients not admitted to ICU, without discharge status, without HCT-ALB records, and without BMI values or with an extremely abnormal BMI [BMI is associated with mortality in sepsis patients and is suggested to be adjusted for baseline characteristics [23]. However, as retrospective databases across multi-center, MIMIC-IV and eICU-CRD may have data entry errors and ununiformed units which may induce extremely abnormal BMI. To avoid potential bias, we removed patients with a BMI greater than 100.] were excluded, leaving 16,127 and 3043 patients for the study from the eICU-CRD database and MIMIC-IV for further analysis, respectively (Fig. 1).
Fig. 1.
Inclusion and Exclusion Flowchart of the Study
Outcomes and covariates
The primary outcome of this study was ICU mortality, and the secondary outcome was hospital mortality. The included variables were age, sex, HCT-ALB, BMI, SOFA score, ventilation use, dialysis use, vasopressor use, comorbidities: congestive heart failure (CHF), chronic pulmonary disease, diabetes, renal disease, malignant cancer, liver disease, and metastatic solid tumor. The HCT and ALB were extracted at the first record after ICU admission. BMI were grouped into underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30.0 kg/m2) [24].
Statistical analysis
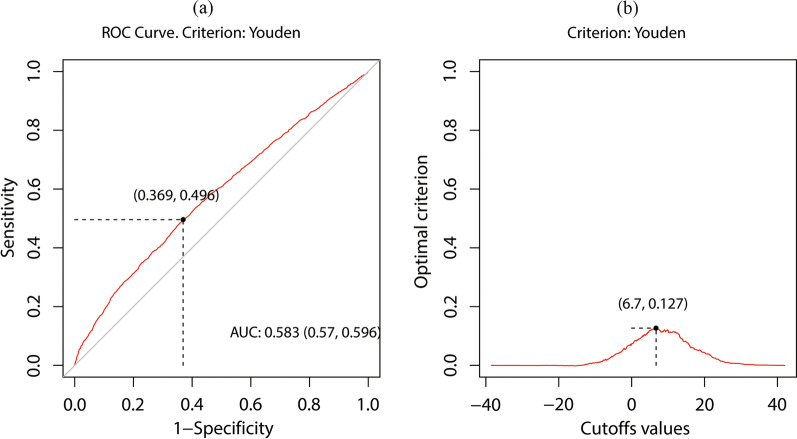
The optimal HCT-ALB cut-off point for ICU mortality was calculated by the Youden Index of ROC curve based on the eICU-CRD dataset (Fig. 2) [25]. Depending on the optimal cut-off point (6.7), patients in both eICU-CRD and MIMIC-IV were independently divided into low HCT-ALB and high HCT-ALB groups. The median and interquartile range (IQR), counts, and percentages, described continuous and categorical variables, respectively. Kruskal–Wallis tests were conducted to analyze the differences between HCT-ALB groups in continuous variables, and chi-square tests were conducted to compare the categorical variables. Univariate and multivariate (adjusted by age, sex, HCT-ALB, BMI, SOFA score, ventilation use, dialysis use, vasopressor use, and comorbidities as potential confounders) logistic regressions were conducted to investigate the raw and adjusted between HCT-ALB groups and ICU/hospital mortality. Datasets of eICU-CRD and MIMIC-IV were analyzed individually to test the consistency of results in different populations.
Fig. 2.
The result of optimal cut-off value calculated by Youden Index. a Receiver operating characteristic (ROC) curve for HCT-ALB. The black point represented the position of optimal point in ROC curve where sensitivity was 0.496 and specificity was 0.631. b The relationship between HCT-ALB and the position of max Youden Index. The black point represented the position of optimal cut-off value and the related Youden Index. The optimal cut-off point of HCT-ALB was 6.7; The max Youden Index was 0.127
To further exam the stability of results, subgroup analyses were performed in subgroups including Age (< 65 and ≥ 65 years), gender (male and female), SOFA score (< 6 and ≥ 6), BMI (underweight, normal weight, overweight, and obese) and comorbidities (no, yes) and tested the interaction effects.
A two-sided P value of < 0.05 was considered statistically significant. All data were analyzed using the R statistical software.
Result
Baseline clinical characteristics
In eICU-CRD dataset, the optimal cut-off point of HCT-ALB was 6.7 with a max Youden Index equal to 0.127. The confusion matrix for HCT, ALB, and HCT-ALB groups with optimal cut-off points was shown in (Additional file 1: Table S1). Compared to the confusion matrix of HCT and ALB, HCT-ALB was more balanced in sensitivity and specificity and had the highest balanced accuracy. The baseline characteristics of the study population were listed in (Table. 1). A total of 6238 (38.7%) eICU-CRD patients and 1073 (35.3%) MIMIC-IV patients were divided into high HCT-ALB groups. The median (IQR) of age in eICU-CRD and MIMIC-IV were 76 (70, 83) and 75 (69,82). The HCT level, ALB level, SOFA score, ventilation use, vasopressor use, CHF status, diabetes status, renal disease status, ICU mortality, and hospital mortality were significantly different between the HCT-ALB groups in the two datasets. Patients in high HCT-ALB groups had significantly higher HCT and lower ALB levels in all datasets. In addition, the difference in gender, BMI groups, dialysis use, liver disease status, and metastatic solid tumor status between HCT-ALB groups were significant for the eICU-CRD dataset while age and malignant cancer status were significant for the MIMIC-IV dataset.
Table 1.
The Baseline clinical characteristics of eICU-CRD and MIMIC-IV patients
| eICU-CRD | MIMIC-IV | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 16127) | HCT-ALB < 6.7 (n = 9899) | HCT-ALB ≥ 6.7 (n = 6238) | P value | Total (n = 3043) | HCT-ALB < 6.7 (n = 1970) | HCT-ALB ≥ 6.7 (n = 1073) | P value | |
| Age | 76 (70,83) | 76 (70,83) | 76 (70,83) | 0.532 | 75 (69,82) | 75 (69, 81) | 76 (70, 82) | 0.048 |
| HCT (%) | 30.8 (27.0,35.4) | 28. (25.7,32.6) | 34.3 (30.4,38.8) | < 0.001 | 32.0 (27.6,36.3) | 29.7 (25.9, 33.5) | 36.3 (32.9, 40.1) | < 0.001 |
| ALB (g/L) | 27.0 (22.0,32.0) | 29.0 (25.0,34.0) | 22.0 (19.0,26.0) | < 0.001 | 28.0 (24.0,32.0) | 30.0 (26.0, 34.0) | 24.0 (21.0, 28.0) | < 0.001 |
| SOFA | 7 (5,9) | 7 (5,9) | 7 (5,9) | < 0.001 | 7 (5,10) | 7 (4, 10) | 8 (5, 11) | < 0.001 |
| Gender (%) | 0.010 | 0.445 | ||||||
| Male | 8607 (53.4) | 5198 (52.6) | 3409 (54.6) | 1686 (55.4) | 1102 (55.9) | 584 (54.4) | ||
| Female | 7520 (46.6) | 4691 (47.4) | 2829 (45.4) | 1357 (44.6) | 868 (44.1) | 489 (45.6) | ||
| BMI (%) | < 0.001 | 0.322 | ||||||
| Underweight | 828 (5.1) | 449 (4.5) | 379 (6.1) | 154 (5.1) | 89 (4.5) | 65 (6.1) | ||
| Normalweight | 5110 (31.7) | 3022 (30.6) | 2088 (33.5) | 965 (31.7) | 628 (31.9) | 337 (31.4) | ||
| Overweight | 4520 (28.0) | 2843 (28.7) | 1677 (26.9) | 876 (28.8) | 573 (29.1) | 303 (28.2) | ||
| Obese | 5669 (35.2) | 3575 (36.2) | 2094 (33.6) | 1048 (34.4) | 680 (34.5) | 368 (34.3) | ||
| Ventilation (%) | < 0.001 | 0.001 | ||||||
| No | 11,202 (69.5) | 7061 (71.4) | 4141 (66.4) | 356 (11.7) | 259 (13.1) | 97 (9.0) | ||
| Yes | 4925 (30.5) | 2828 (28.6) | 2097 (33.6) | 2687 (88.3) | 1711 (86.9) | 976 (91.0) | ||
| Dialysis (%) | < 0.001 | 0.712 | ||||||
| No | 14,389 (89.7) | 8656 (87.4) | 5743 (92.1) | 2724 (89.5) | 1760 (89.3) | 964 (89.8) | ||
| Yes | 1738 (10.3) | 1243 (12.6) | 495 (7.9) | 319 (10.5) | 210 (10.7) | 109 (10.2) | ||
| Vasopressor (%) | < 0.001 | < 0.001 | ||||||
| No | 11,271 (69.9) | 7205 (72.8) | 4066 (65.2) | 1218 (40.0) | 859 (43.6) | 359 (33.5) | ||
| Yes | 4856 (30.1) | 2684 (27.2) | 2172 (34.8) | 1825 (60.0) | 1111 (56.4) | 714 (66.5) | ||
| Congestive heart Failure | < 0.001 | 0.040 | ||||||
| No | 12,162 (75.4) | 7310 (73.9) | 4852 (77.8) | 1712 (56.3) | 1081 (54.9) | 631 (58.8) | ||
| Yes | 3965 (24.6) | 2579 (26.1) | 1386 (22.2) | 1331 (43.7) | 889 (45.1) | 442 (41.2) | ||
| Chronic pulmonary disease (%) | 0.941 | 0.450 | ||||||
| No | 12,804 (79.4) | 7849 (79.4) | 4955 (79.4) | 2108 (69.3) | 1355 (68.8) | 753 (70.2) | ||
| Yes | 3323 (20.6) | 2040 (20.6) | 1283 (20.6) | 935 (30.7) | 615 (31.2) | 320 (29.8) | ||
| Diabetes (%) | < 0.001 | 0.001 | ||||||
| No | 13,409 (83.1) | 8097 (81.9) | 5312 (85.2) | 1923 (63.2) | 1204 (61.1) | 719 (67.0) | ||
| Yes | 2718 (16.9) | 1792 (18.1) | 926 (14.8) | 1120 (36.8) | 766 (38.9) | 354 (33.0) | ||
| Renal disease (%) | < 0.001 | < 0.001 | ||||||
| No | 14,669 (91.0) | 8867 (89.7) | 5802 (93.0) | 2025 (66.5) | 1240 (62.9) | 785 (73.2) | ||
| Yes | 1458 (9.0) | 1022 (10.3) | 436 (7.0) | 1018 (33.5) | 730 (37.1) | 288 (26.8) | ||
| Malignant cancer (%) | 0.314 | 0.006 | ||||||
| No | 12,699 (78.7) | 7761 (78.5) | 4938 (79.2) | 2428 (79.8) | 1542 (78.3) | 886 (82.6) | ||
| Yes | 3428 (21.3) | 2128 (21.5) | 1300 (20.8) | 615 (20.2) | 428 (21.7) | 187 (17.4) | ||
| Liver disease (%) | < 0.001 | 0.271 | ||||||
| No | 15,352 (95.2) | 9,461 (95.7) | 5891 (94.4) | 2586 (85.0) | 1685 (85.5) | 901 (84.0) | ||
| Yes | 775 (4.8) | 428 (4.3) | 347 (5.6) | 457 (15.0) | 285 (14.5) | 172 (16.0) | ||
| Metastatic solid tumor (%) | 0.069 | 0.247 | ||||||
| No | 15,591 (96.7) | 9,581 (96.9) | 6010 (96.3) | 2779 (91.3) | 1790 (90.9) | 989 (92.2) | ||
| Yes | 536 (3.3) | 308 (3.1) | 228 (3.7) | 264 (8.7) | 180 (9.1) | 84 (7.8) | ||
| ICU mortality (%) | < 0.001 | < 0.001 | ||||||
| No | 13,899 (86.2) | 8766 (88.6) | 5133 (82.3) | 2377 (78.1) | 1601 (81.3) | 776 (72.3) | ||
| Yes | 2228 (13.8) | 1123 (11.4) | 1105 (17.7) | 666 (21.9) | 369 (18.7) | 297 (27.7) | ||
| Hospital mortality (%) | < 0.001 | < 0.001 | ||||||
| No | 12,585 (78.0) | 8128 (82.2) | 4457 (71.4) | 2065 (67.9) | 1391 (70.6) | 674 (62.8) | ||
| Yes | 3542 (22.0) | 1761 (17.8) | 1781 (28.6) | 978 (32.1) | 579 (29.4) | 399 (37.2) | ||
Continuous variables were expressed by median (interquartile), categorical variables were expressed by count (percentage)
P values were calculated to compare difference between HCT-ALB groups for each dataset separately
Logistic regression
(Table. 2) showed that compared to the reference group, the high HCT-ALB group had increased univariate ICU and hospital mortality risk [OR (95%CI) = 1.68 (1.54,1.84) and 1.84 (1.71,1.99)] in the eICU- CRD datasets. After adjusted by co-variables, the OR (95%CI) for ICU and hospital mortality for the eICU-CRD population were 1.50 (1.36,1.65) and 1.71 (1.58,1.87), demonstrating that high HCT-ALB was independently associated and had 42% and 66% elevated risk of ICU and hospital mortality, respectively. The results of the MIMIC-IV data set also showed high consistency with eICU-CRD. The univariate regressions result revealed that high HCT-ALB was also positively correlated to ICU and hospital mortality [OR (95%CI) = 1.66 (1.39,1.98) and 1.42 (1.22,1.66)] in MIMIC-IV dataset. After multivariate adjustment, the OR (95%CI) for MIMIC-IV population were 1.41 (1.15,1.72) and 1.27 (1.07,1.51), respectively. MIMIC-IV result showed that higher HCT-ALB group, divided by the cut-off value from eICU-CRD dataset, was also independently had a 41% and 26% higher risk of ICU and hospital mortality in external dataset. The results of all co-variables were showed in (Additional file 1: Tables S2,3).
Table 2.
Analysis for association between HCT-ALB group and outcomes
| ICU mortality | Hospital mortality | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| eICU-CRD | ||||
| Univariable model | 1.68 (1.54, 1.84) | < 0.001 | 1.84 (1.71, 1.99) | < 0.001 |
| Multivariable model | 1.50 (1.36, 1.65) | < 0.001 | 1.71 (1.58, 1.87) | < 0.001 |
| MIMIC-IV | ||||
| Univariable model | 1.66 (1.39, 1.98) | < 0.001 | 1.42 (1.22, 1.66) | < 0.001 |
| Multivariable model | 1.41 (1.15, 1.72) | 0.001 | 1.27 (1.07, 1.51) | 0.007 |
Multivariate models were adjusted by Age, Gender, BMI group, SOFA score, Ventilation, Dialysis, Vasopressor use, Comorbidities (CHF, Chronic Pulmonary Disease, Diabetes, Renal disease, Malignant cancer, Liver disease, and Metastatic solid tumor), excluding models belonging subgroup variate
OR odds ratio, CI confidence interval
The OR (95CI%) were calculated when low HCT-ALB (< 6.7) groups set as reference
Subgroup analysis
The subgroup analyses revealed the association between HCT-ALB and outcomes under different statuses in eICU-CRD and MIMIC-IV (Table. 3) (Table. 4). In eICU-CRD dataset, ORs for ICU mortality were not statistically significant for patients with normalweight, with liver disease, and with metastatic solid tumor. In the MIMIC-IV dataset, the association between the high HCT-ALB group and ICU/hospital mortality was not significant in subgroups including patients over 80, male, underweight/obese, without CHF, without diabetes, with chronic pulmonary disease, with malignant cancer, and with metastatic solid tumor. No meaning ORs for hospital mortality were also observed in overweight, without renal disease, and with liver disease. However, the only significant interaction effects were SOFA scores for hospital mortality (P = 0.040) in eICU-CRD while no interaction effect for any subgroup was observed in the MIMIC-IV dataset. In summary, the association between HCT-ALB and outcomes was consistently positive in different subgroups.
Table 3.
Subgroup analysis for association between HCT-ALB group and outcomes for eICU-CRD
| ICU mortality | Hospital mortality | ||||||
|---|---|---|---|---|---|---|---|
| Subgroups | N | OR (95%CI) | P value | P—interaction | OR (95%CI) | P value | P—interaction |
| Total | 16,127 | 1.50 (1.36, 1.65) | < 0.001 | / | 1.71 (1.58, 1.87) | < 0.001 | / |
| Age | 0.390 | 0.861 | |||||
| 65–80 | 10,624 | 1.60 (1.41, 1.80) | < 0.001 | 1.76 (1.59, 1.96) | < 0.001 | ||
| > 80 | 5503 | 1.43 (1.22, 1.69) | < 0.001 | 1.73 (1.52, 1.98) | < 0.001 | ||
| Gender | 0.353 | 0.208 | |||||
| Male | 8607 | 1.43 (1.25, 1.64) | < 0.001 | 1.63 (1.46, 1.83) | < 0.001 | ||
| Female | 7052 | 1.57 (1.36, 1.82) | < 0.001 | 1.82 (1.62, 2.06) | < 0.001 | ||
| SOFA | 0.064 | 0.040 | |||||
| < 6 | 5592 | 1.65 (1.29, 2.11) | < 0.001 | 1.89 (1.59, 2.26) | < 0.001 | ||
| ≥ 6 | 10,535 | 1.50 (1.30, 1.67) | < 0.001 | 1.70 (1.55, 1.68) | < 0.001 | ||
| BMI | 0.324 | 0.658 | |||||
| Underweight | 828 | 1.93 (1.29, 2.91) | 0.001 | 1.86 (1.35, 2.61) | < 0.001 | ||
| Normalweight | 5110 | 1.55 (0.68, 3.60) | 0.299 | 2.00 (1.13, 3.56) | 0.017 | ||
| Overweight | 4520 | 1.78 (0.95, 3.35) | 0.007 | 2.32 (1.51, 3.95) | < 0.001 | ||
| Obese | 5669 | 1.91 (1.20, 3.02) | 0.006 | 2.01 (1.45, 2.78) | < 0.001 | ||
| Congestive heart failure | 0.809 | 0.499 | |||||
| No | 12,162 | 1.48 (1.32, 1.66) | < 0.001 | 1.69 (1.54, 1.86) | < 0.001 | ||
| Yes | 3965 | 1.54 (1.27, 1.88) | < 0.001 | 1.81 (1.54, 2.14) | < 0.001 | ||
| Chronic pulmonary disease | 0.753 | 0.262 | |||||
| No | 12,804 | 1.49 (1.33, 1.69) | < 0.001 | 1.68 (1.53, 1.85) | < 0.001 | ||
| Yes | 3323 | 1.56 (1.26, 1.94) | < 0.001 | 1.87 (1.56, 2.23) | < 0.001 | ||
| Diabetes | 0.577 | 0.621 | |||||
| No | 13,409 | 1.54 (1.39, 1.72) | < 0.001 | 1.73 (1.53, 1.89) | < 0.001 | ||
| Yes | 2718 | 1.66 (1.33, 2.05) | < 0.001 | 1.72 (1.38, 2.13) | < 0.001 | ||
| Renal disease | 0.387 | 0.206 | |||||
| No | 14,669 | 1.49 (1.34, 1.65) | < 0.001 | 1.70 (1.56, 1.85) | < 0.001 | ||
| Yes | 1458 | 1.65 (1.17, 2.32) | < 0.001 | 1.99 (1.50, 2.66) | < 0.001 | ||
| Malignant cancer | 0.984 | 0.721 | |||||
| No | 12,699 | 1.49 (1.34, 1.67) | < 0.001 | 1.71 (1.55, 1.88) | < 0.001 | ||
| Yes | 3428 | 1.47 (1.21, 1.81) | < 0.001 | 1.76 (1.49, 2.09) | < 0.001 | ||
| Liver disease | 0.735 | 0.435 | |||||
| No | 15,352 | 1.50 (1.36, 1.67) | < 0.001 | 1.71 (1.57, 1.86) | < 0.001 | ||
| Yes | 775 | 1.39 (0.97, 2.01) | 0.073 | 1.99 (1.44, 1.78) | < 0.001 | ||
| Metastatic solid tumor | 0.953 | 0.324 | |||||
| No | 15,591 | 1.50 (1.35, 1.66) | < 0.001 | 1.70 (1.57,1.86) | < 0.001 | ||
| Yes | 536 | 1.51 (0.92, 2.48) | 0.104 | 2.21 (1.47, 3.34) | < 0.001 | ||
Multivariate models were adjusted by Age, Gender, BMI group, SOFA score, Ventilation, Dialysis, Vasopressor use, Comorbidities (CHF, Chronic Pulmonary Disease, Diabetes, Renal disease, Malignant cancer, Liver disease, and Metastatic solid tumor), excluding models belonging subgroup variate
BMI subgroup were defined as underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30.0 kg/m2)
OR odds ratio, CI confidence interval
The OR (95CI%) were calculated when low HCT-ALB (< 6.7) groups set as reference
Table 4.
Subgroup analysis for association between HCT-ALB group and outcomes for MIMIC-IV
| ICU mortality | Hospital mortality | ||||||
|---|---|---|---|---|---|---|---|
| Subgroups | N | OR (95%CI) | P value | P—interaction | OR (95%CI) | P value | P—interaction |
| Total | 3043 | 1.41 (1.15, 1.72) | 0.001 | / | 1.26 (1.07, 1.51) | 0.007 | / |
| Age | 0.707 | 0.438 | |||||
| 65–80 | 2156 | 1.61 (1.27, 2.05) | < 0.001 | 1.43 (1.15, 1.746) | 0.001 | ||
| > 80 | 887 | 1.14 (0.79, 1.64) | 0.473 | 1.01 (0.73, 1.38) | 0.964 | ||
| Gender | 0.189 | 0.051 | |||||
| Male | 1686 | 1.28 (0.97, 1.68) | 0.075 | 1.09 (0.86, 1.38) | 0.488 | ||
| Female | 1357 | 1.64 (1.21, 2.22) | 0.001 | 1.54 (1.20, 1.99) | 0.001 | ||
| SOFA | 0.433 | 0.446 | |||||
| < 6 | 994 | 2.37 (1.37, 4.10) | 0.002 | 1.56 (1.07, 2.24) | 0.018 | ||
| ≥ 6 | 2049 | 1.39 (1.12, 1.71) | 0.002 | 1.25 (1.03, 1.52) | 0.022 | ||
| BMI | 0.683 | 0.405 | |||||
| Underweight | 154 | 1.03 (0.35, 2.94) | 0.954 | 1.01 (0.42, 2.39) | 0.986 | ||
| Normalweight | 965 | 8.94 (1.55, 73.6) | 0.021 | 2.72 (1.01, 7.25) | 0.044 | ||
| Overweight | 876 | 8.20 (1.51, 58.6) | 0.018 | 2.32 (0.94, 5.69) | 0.065 | ||
| Obese | 1048 | 1.23 (0.43, 3.36) | 0.689 | 0.84 (0.42, 1.61) | 0.609 | ||
| Congestive heart failure | 0.302 | 0.214 | |||||
| No | 1712 | 1.25 (0.94, 1.65) | 0.119 | 1.13 (0.89, 1.42) | 0.324 | ||
| Yes | 1331 | 1.62 (1.21, 2.18) | 0.001 | 1.46 (1.12, 1.89) | 0.005 | ||
| Chronic pulmonary disease | 0.395 | 0.623 | |||||
| No | 2108 | 1.49 (1.16, 1.91) | 0.002 | 1.31 (1.06, 1.61) | 0.013 | ||
| Yes | 935 | 1.28 (0.90, 1.82) | 0.162 | 1.20 (0.88, 1.64) | 0.254 | ||
| Diabetes | 0.226 | 0.136 | |||||
| No | 1923 | 1.28 (0.99, 1.64) | 0.056 | 1.14 (0.92, 1.42) | 0.214 | ||
| Yes | 1120 | 1.75 (1.24, 2.46) | 0.001 | 1.54 (1.15, 2.07) | 0.004 | ||
| Renal disease | 0.401 | 0.269 | |||||
| No | 2025 | 1.32 (1.03, 1.70) | 0.027 | 1.16 (0.94, 1.45) | 0.179 | ||
| Yes | 1018 | 1.60 (1.13, 2.26) | 0.008 | 1.47 (1.09, 1.98) | 0.012 | ||
| Malignant cancer | 0.758 | 0.072 | |||||
| No | 2428 | 1.47 (1.17, 1.84) | < 0.001 | 1.39 (1.15, 1.69) | < 0.001 | ||
| Yes | 615 | 1.41 (0.88, 2.24) | 0.148 | 0.925 (0.61, 1.39) | 0.713 | ||
| Liver disease | 0.362 | 0.554 | |||||
| No | 2586 | 1.39 (1.11, 1.73) | 0.004 | 1.25 (1.03, 1.51) | 0.027 | ||
| Yes | 457 | 1.73 (1.05, 2.87) | 0.031 | 1.46 (0.94, 2.26) | 0.091 | ||
| Metastatic solid tumor | 0.639 | 0.875 | |||||
| No | 2779 | 1.44 (1.17, 1.79) | < 0.001 | 1.28 (1.06, 1.53) | 0.009 | ||
| Yes | 264 | 1.28 (0.65, 2.50) | 0.464 | 1.36 (0.73, 2.52) | 0.328 | ||
Multivariate models were adjusted by Age, Gender, BMI group, SOFA score, Ventilation, Dialysis, Vasopressor use, Comorbidities (CHF, Chronic Pulmonary Disease, Diabetes, Renal disease, Malignant cancer, Liver disease, and Metastatic solid tumor), excluding models belonging subgroup variate
BMI subgroup were defined as underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30.0 kg/m2)
OR odds ratio, CI confidence interval
The OR (95CI%) were calculated when low HCT-ALB (< 6.7) groups set as reference
Discussion
In this multi-center retrospective study based on two large public databases MIMIC-IV and eICU-CRD, HCT-ALB was related to the prognosis of elderly sepsis patient. We used the eICU-CRD database to calculate the optimal cut-off point and grouped patients from two datasets independently. Consistent results were obtained in both datasets, and the external dataset result demonstrated that when HCT-ALB was greater than 6.7, the risk of death in the ICU and in-hospital of elderly patients with sepsis will increase by about 1.41 and 1.27 times. Although previous studies had studied the relationship between HCT-ALB and infectious diseases/hypertension in pregnancy [26, 27], our research firstly explored the relationship between this indicator and the prognosis of elderly sepsis patients in the ICU. Elderly patients with sepsis have high mortality rates and are often delayed in diagnosis due to complications characteristics [28], weakened immunity, and atypical clinical manifestations [29]. Studies have shown that the mortality rate of sepsis patients increases with age, and the mortality rate of elderly patients (≥ 80 years old) is higher than that of patients 65–79 years old [30].
In critically ill patients, hypoproteinemia is a common phenomenon [31, 32]. It is firstly associated with patients being bedridden for long periods, in a state of fasting and drinking, with inadequate nutritional intake [33, 34]. When patients suffered from sepsis, the pathogenic microorganisms in the foci of infection and the various toxins released by them can stimulate the immune system of the body to release a large number of inflammatory mediators such as tumor necrosis factor and interleukins [35], which can impair the function of the vascular endothelial barrier, increase capillary permeability and cause a large amount of plasma extravasation, resulting in a decrease in blood volume and a decrease in the concentration of ALB in plasma [36]. Under normal conditions, the gap between capillary endothelial cells is 6–7 nm [37, 38], and the diameter of red blood cells is 7–8 μm (1000 times larger than the gap), meaning that red blood cells cannot pass through, and so maintaining HCT levels. However, patients with sepsis can experience a relative increase in HCT due to increased vascular permeability, which leads to massive extravasation of body fluids. In addition to these, the depletion produced by the hypermetabolic state of sepsis patients and the gastrointestinal dysfunction can lower the level of ALB [39]. When the patient's plasma albumin decreases due to the above-mentioned various factors, the plasma colloid osmotic pressure in turn will decrease, a large amount of liquid will be retained in the interstitial space [40], and the effective circulating blood volume will be further reduced, red blood cells will aggregate, blood viscosity will increase, and microcirculation disorders will be aggravated [41, 42]. It can lead to organ ischemia and focal necrosis, which can lead to organ failure and aggravate the condition of sepsis patients if it is severe or lasts for a long time [43]. Therefore, in elderly patients with sepsis, the HCT-ALB difference can reflect the degree of the patient's condition, and the corresponding therapeutic measures, such as albumin infusion and replenishment of effective circulating blood volume, should be taken according to the level of the difference to improve the patient's prognosis.
The results of subgroup analysis indicated that in the eICU-CRD dataset, in the group with HCT-ALB greater than 6.7, compared with people with SOFA ≥ 6, hospital death in patients with SOFA score < 6 is significantly higher, and there was an interaction, which was not observed in the MIMIC-IV database. Among patients with SOFA greater than 6 in the eICU-CRD dataset, the decrease of HCT may be due to the aggravation of the patient's condition, the increase of erythrocyte destruction and the change of plasma volume caused by the increase of fluid supplement, thus reducing the HCT-ALB difference [44, 45]. However, as the optimal value was calculated from the eICU-CRD dataset, this interaction effect may be biased which can also explain why similar result was not observed in external datasets. In addition, the heterogeneity of databases leads to different results, which is understandable. The ORs for mortality were not statistically significant for subgroups including BMI, liver disease, and metastatic solid tumor in eICU-CRD dataset while in the MIMIC-IV dataset, the association between the high HCT-ALB group and mortality was not significant in subgroups including age, gender, BMI, CHF, diabetes, chronic pulmonary disease, renal disease, malignant cancer, and metastatic solid tumor. The heterogeneity between databases is one of the reasons for this phenomenon, and secondly this may be due to the aging and physiological decline of the immune system in patients over 80 years of age [46, 47], hence the relatively small difference in HCT-ALB reflecting the level of inflammation.
Strengths and limitations of the study
To the best of our knowledge, this is the first study on the relationship between HCT-ALB and mortality in elderly patients with sepsis in the ICU. HCT-ALB is a relatively easy-to-measure index, which can facilitate risk stratification and serve as a reference for early clinical intervention decisions. As a large-sample multi-center study, our results are representative, reliable, and extrapolative. Just as we segmented the HCT-ALB with the best threshold in the eICU-CRD, we got consistent results in the MIMIC-IV database. There are, of course, some limitations to this study. First, it is a retrospective study, and some confounding bias is inevitable. Second, the disease severity scoring system used in this study was the SOFA score, and the Acute Physiology and Chronic Health Evaluation (APACHE) II score may make the study more robust in the future when circumstances permit. Third, in this study, liver disease was adjusted as a covariate, and it might be better to exclude patients with liver dysfunction in future studies. Fourth, we did not assess the nutritional and anemia status of the patients. In addition, as an external validation dataset, the ICU/hospital mortality risks of the high HCT-ALB group in the MIMIC-IV dataset were lower than that of eICU-CRD. In the subgroup analysis, although only the SOFA subgroup in the eICU-CRD dataset showed a significant interaction, there was heterogeneity in the ORs observed in the eICU-CRD between MIMIC-IV subgroups, which also weakened the association of the HCT-ALB and mortality risks. Therefore, more studies are needed to further validate the interaction between subgroup analysis populations.
Conclusions
High HCT-ALB (≥ 6.7) is associated with 1.41 and 1.27 times of ICU and hospital mortality risk in elderly patients with sepsis. HCT-ALB is simple and easy to obtain and is a promising clinical predictor of early risk stratification for elderly sepsis patients in ICU.
Supplementary Information
Additional file 1: Table S1. The evaluation metrics of confusion matrix of three univariables. Table S2. The result of multivariate regressions of eICU-CRD dataset. Table S3. The result of multivariate regressions of MIMIC-IV dataset.
Acknowledgements
Not applicable.
Abbreviations
- ICU
Intensive care units
- HCT
Hematocrit
- ALB
Albumin
- HCT-ALB
Hematocrit albumin difference
- MIMIC-IV
Medical Information Mart for Intensive Care-IV
- eICU-CRD
EICU Collaborative Research Database
- SOFA
Sequential Organ Failure Assessment
- OR
Odds ratio
- CI
Confidence interval
- APACHE
Acute Physiology and Chronic Health Evaluation
Author contributions
ZW created the study protocol, performed the statistical analyses and wrote the first manuscript draft. LZ conceived the study and critically revised the manuscript. SL and FX assisted with the study design and performed data collection. DH assisted with data collection and manuscript editing. HW confirmed the data and assisted with the statistical analyses TH maintained the localized database and worked on SQL coding. JL and HY contributed to data interpretation and manuscript revision. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82072232; 81871585), the Natural Science Foundation of Guangdong Province (No. 2018A030313058), Technology and Innovation Commission of Guangzhou Science, China (No.201804010308), Guangdong Provincial Key Laboratory of Traditional Chinese Medicine Informatization (2021B1212040007).
Data availability
The data were available on the MIMIC-IV website at https://mimic.physionet.org/,10.13026/C2HM2Qand eICU-CRD at. The data in this article can be reasonably applied to the corresponding author.
Declarations
Ethics approval and consent to participate
The Medical Information Mart for Intensive Care-IV database was supported by grants from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH) under award numbers R01-EB001659 (2003–2013) and R01-EB017205 (2014–2018) and approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The eICU Collaborative Research Database was released under the Health Insurance Portability and Accountability Act (HIPAA) safe harbor provision. The re-identification risk was certified as meeting safe harbor standards by Privacert (Cambridge, MA) (HIPAA Certification no. 1031219–2). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Informed consent
Data extracted from the MIMIC-IV and eICU-CRD database do not require individual informed consent because the research data is publicly available, and all patient data are de-identified.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zichen Wang and Luming Zhang contributed equally to this work
Contributor Information
Haiyan Yin, Email: yinhaiyan1867@126.com.
Jun Lyu, Email: lyujun2020@jnu.edu.cn.
References
- 1.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A, Evans LE, Alhazzani W, Levy MM, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 5.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21(4):418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris JR, Korolchuk VI, editors. Biochemistry and Cell Biology of Ageing: Part II Clinical Science. Singapore: Springer; 2019. [Google Scholar]
- 8.Thomas R, Wang W, Su DM. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun Ageing. 2020 doi: 10.1186/s12979-020-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang SY. Sepsis and other infectious disease emergencies in the elderly. Emerg Med Clin North Am. 2016;34(3):501–522. doi: 10.1016/j.emc.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Infusino I, Panteghini M. Serum albumin: accuracy and clinical use. Clin Chim Acta. 2013;419:15–18. doi: 10.1016/j.cca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Kendall H, Abreu E, Cheng AL. Serum albumin trend is a predictor of mortality in icu patients with sepsis. Biol Res Nurs. 2019;21(3):237–244. doi: 10.1177/1099800419827600. [DOI] [PubMed] [Google Scholar]
- 12.Arnau-Barrés I, Güerri-Fernández R, Luque S, Sorli L, Vázquez O, Miralles R. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur Clin Microbiol Infect. 2019;38(4):743–746. doi: 10.1007/s10096-019-03478-2. [DOI] [PubMed] [Google Scholar]
- 13.Balasubramanian S, Anandnathan K, Shivbalan S, Datta M, Amalraj E. Cut-off hematocrit value for hemoconcentration in dengue hemorrhagic fever. J Trop Pediatr. 2004;50:123–124. doi: 10.1093/tropej/50.2.123. [DOI] [PubMed] [Google Scholar]
- 14.Block S, Büchler M, Bittner R, Beger HG. Sepsis indicators in acute pancreatitis. Pancreas. 1987;2(5):499–505. doi: 10.1097/00006676-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Juncal VR, de BrittoNeto LA, Camelier AA, Messeder OHC, de Farias AMC. Clinical impact of sepsis at admission to the ICU of a private hospital in Salvador Brazil. JBP. 2011;37(1):85–92. doi: 10.1590/s1806-37132011000100013. [DOI] [PubMed] [Google Scholar]
- 16.Vercueil A, Grocott MPW, Mythen MG. Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically ill patients. Transfus Med Rev. 2005;19(2):93–109. doi: 10.1016/j.tmrv.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 18.Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1(8432):781–784. doi: 10.1016/S0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 19.Dai DM, Wang D, Hu D, Wan WL, Su Y, Yang JL, et al. Difference in hematocrit and plasma albumin levels as an additional biomarker in the diagnosis of infectious disease. Arch Med Sci. 2019;16(3):522–530. doi: 10.5114/aoms.2019.86898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson AEW, Pollard TJ, Shen L, Lehman LWH, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Li Y, Liu Q, Li L, Lyu J. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13(9578):57–69. doi: 10.1111/jebm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018;5(1):180178. doi: 10.1038/sdata.2018.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalani C, Venigalla T, Bailey J, Udeani G, Surani S. Sepsis patients in critical care units with obesity: is obesity protective? Cureus. 2020 doi: 10.7759/cureus.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 36 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–953. doi: 10.1016/S2213-8587(18)30288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perkins NJ, Schisterman EF. The youden index and the optimal cut-point corrected for measurement error. Biom J. 2005;47(4):428–441. doi: 10.1002/bimj.200410133. [DOI] [PubMed] [Google Scholar]
- 26.Dai D, Cao J, Yang HM, Sun H, Su Y, Chen Y, et al. Hematocrit and plasma albumin levels difference may be a potential biomarker to discriminate preeclampsia and eclampsia in patients with hypertensive disorders of pregnancy. Clin Chim Acta. 2017;464:218–222. doi: 10.1016/j.cca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Dai D, Hu R, Xu W, Su Y, Li M, Duan H, et al. Feasibility study of the difference between hematocrit and albumin for identifying hemorrhagic shock and septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30(12):1137–1140. doi: 10.3760/cma.j.issn.2095-4352.2018.012.007. [DOI] [PubMed] [Google Scholar]
- 28.Boonmee P, Ruangsomboon O, Limsuwat C, Chakorn T. Predictors of mortality in elderly and very elderly emergency patients with sepsis: a retrospective study. Western J Emerg Med. 2020;21(6):210–218. doi: 10.5811/westjem.2020.7.47405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He W, Xiao K, Fang M, Xie L. Immune cell number, phenotype, and function in the elderly with sepsis. Aging Dis. 2021;12(1):277–296. doi: 10.14336/AD.2020.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonmee P, Ruangsomboon O, Limsuwat C, Chakorn T. Predictors of mortality in elderly and very elderly emergency patients with sepsis: a retrospective study. West J Emerg Med. 2020;21(6):210–218. doi: 10.5811/westjem.2020.7.47405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erstad BL. Serum albumin levels: who needs them? Ann Pharmacother. 2020;55(6):798–804. doi: 10.1177/1060028020959348. [DOI] [PubMed] [Google Scholar]
- 32.Wei Li NLSL. Relationship between postoperative immediate serum albumin level and postoperative acute kidney injury after major abdominal surgery in critically ill patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(8):955–961. doi: 10.3760/cma.j.cn121430-20200730-00554. [DOI] [PubMed] [Google Scholar]
- 33.Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, et al. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2623 hospitalized cases. Sci China Life Sci. 2020;63(11):1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baig MA, Raza MM, Baig M, Baig MU. Serum albumin levels monitoring in ICU in early days and mortality risk association in patients with moderate to severe COVID-19 pneumonia. Pakistan J Med Sci. 2022;38(3):612–616. doi: 10.12669/pjms.38.3.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minasyan H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J Crit Care. 2017;40:229–242. doi: 10.1016/j.jcrc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Berg D, Gerlach H. Recent advances in understanding and managing sepsis. F1000Res. 2018;7:F1000 Faculty Rev-1570.
- 37.Piety NZ, Reinhart WH, Stutz J, Shevkoplyas SS. Optimal hematocrit in an artificial microvascular network. Transfusion (Paris). 2017;57(9):2257–2266. doi: 10.1111/trf.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westerman M, Porter JB. Red blood cell-derived microparticles: an overview. Blood Cells Mol Dis. 2016;59:134–139. doi: 10.1016/j.bcmd.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Xu JY, Chen QH, Xie JF, Pan C, Liu SQ, Huang LW, et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: A meta-analysis of randomized clinical trials. Crit Care. 2014;18(6):702. doi: 10.1186/s13054-014-0702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddiqui SS, Chakraborty N, Muzaffar SN, Gurjar M. Albumin Kinetics in Sepsis and COVID-19. Crit Care Explorations. 2022;4(3):e0651. doi: 10.1097/CCE.0000000000000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J Parenter Entera Nutr. 2019;43:181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Sarmiento J, Schlapbach LJ, Acevedo L, Santana CR, Acosta Y, Diana A, et al. Endothelial damage in sepsis: the importance of systems biology. Front Pediatr. 2022;9:10. doi: 10.3389/fped.2021.796504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panda A, Revadi G, Sharma JP, Pakhare A, Singhai A, Joshi R, et al. On admission, microcirculation abnormality is an independent predictor of sepsis and sepsis-related mortality: a hospital-based study. Indian J Crit Care Med. 2022;26(3):294–301. doi: 10.5005/jp-journals-10071-24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ping ZL, Sheng HY, He T, Gang HX. Clinical study on hematocrit used as a predictor for evaluation of resuscitation effect in the early shock stage after burn. Zhonghua Shao Shang Za Zhi. 2013;29(3):235–238. [PubMed] [Google Scholar]
- 45.Li L, Jin T, Wen S, Shi N, Zhang R, Zhu P, et al. Early rapid fluid therapy is associated with increased rate of noninvasive positive-pressure ventilation in hemoconcentrated patients with severe acute pancreatitis. Dig Dis Sci. 2020;65(9):2700–2711. doi: 10.1007/s10620-019-05985-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RydyznskiModerbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fulop T, Larbi A, Pawelec G, Cohen AA, Provost G, Khalil A, et al. Immunosenescence and altered vaccine efficiency in older subjects: a myth difficult to change. Vaccines (Basel). 2022;10(4):607. doi: 10.3390/vaccines10040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The evaluation metrics of confusion matrix of three univariables. Table S2. The result of multivariate regressions of eICU-CRD dataset. Table S3. The result of multivariate regressions of MIMIC-IV dataset.
Data Availability Statement
The data were available on the MIMIC-IV website at https://mimic.physionet.org/,10.13026/C2HM2Qand eICU-CRD at. The data in this article can be reasonably applied to the corresponding author.