Abstract
Introduction
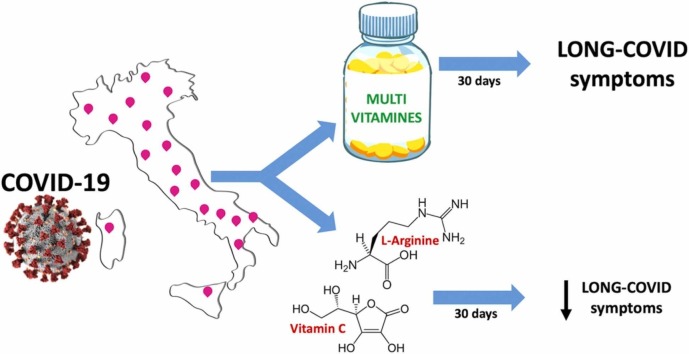
Recent evidence suggests that oxidative stress and endothelial dysfunction play critical roles in the pathophysiology of COVID-19 and Long-COVID. We hypothesized that a supplementation combining L-Arginine (to improve endothelial function) and Vitamin C (to reduce oxidation) could have favorable effects on Long-COVID symptoms.
Methods
We designed a survey (LINCOLN: L-Arginine and Vitamin C improves Long-COVID), assessing several symptoms that have been associated with Long-COVID to be administered nationwide to COVID-19 survivors; the survey also included effort perception, measured using the Borg scale. Patients receiving the survey were divided in two groups, with a 2:1 ratio: the first group included patients that received L-Arginine + Vitamin C, whereas the second group received a multivitamin combination (alternative treatment).
Results
1390 patients successfully completed the survey. Following a 30-day treatment in both groups, the survey revealed that patients in the L-Arginine + Vitamin C treatment arm had significantly lower scores compared to patients who had received the multivitamin combination. There were no other significant differences between the two groups. When examining effort perception, we observed a significantly lower value (p < 0.0001) in patients receiving L-Arginine + Vitamin C compared to the alternative-treatment arm.
Conclusions
Our survey indicates that the supplementation with L-Arginine + Vitamin C has beneficial effects in Long-COVID, in terms of attenuating its typical symptoms and improving effort perception.
Keywords: Ascorbic acid, COVID-19, Endothelial dysfunction, L-Arginine, Long-COVID, Oxidative stress, Survey
Graphical Abstract
Cartoon representing the main results of the LINCOLN survey.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has led to a global healthcare crisis [1]. The global pandemic has resulted also in job losses and economic hardships [2]. Although efficacious vaccines have been recently developed [3], [4], [5], [6], it is important to better understand SARS-CoV-2-mediated pathology, especially because many survivors are experiencing chronic symptoms of the disease, including metabolic disturbances, even months after the initial infection occurred (“Long-COVID”) [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18].
While initial studies on COVID-19 primarily focused on the pulmonary manifestations of the disease [19], [20], [21], [22], [23], [24], other organs including heart, brain, kidney, and the pancreas, were shown to be affected by COVID-19 [25], [26], [27]. In the Spring of 2020, we were among the first groups to indicate a link between COVID-19 and endothelial dysfunction [28], [29], and our view has been later confirmed by other investigators, associating the systemic manifestations of the disease to a direct or indirect involvement of the endothelium [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. Indeed, endothelial cells express all co-factors necessary for the internalization of SARS-CoV-2 in host cells, including angiotensin converting enzyme 2 (ACE2), transmembrane protease serine 2 (TMPRSS2), cathepsins B and D, neuropilin-1, transferrin receptor, and others, thereby representing a natural target of SARS-CoV-2 [28], [39], [40], [41], [42], [43], [44], [45]. Thus, COVID-19 affects not only the epithelial cells of the lung [46], [47], but also endothelial cells across the whole body, leading to a generalized endothelial damage. Endothelial cells are known to play instrumental roles in the maintenance of vascular homeostasis and in the regulation of vascular tone [48], [49], [50]. Endothelial dysfunction, caused directly by SARS-CoV-2 infection and/or by the ensuing inflammation, can shift the vascular equilibrium towards an altered vascular tone, an increased permeabilization, with subsequent tissue edema, and a pro-coagulant state, which can lead to thromboembolism; most of these findings have been substantiated by autopsies of COVID-19 patients since the outbreak of the pandemic [51], [52], [53]. Further supporting our theory of a central role of the endothelium in COVID-19, patients with endothelial dysfunction (e.g. hypertension, smoking, pre-existing diabetes, obesity, and presence of cardiovascular disease) are particularly vulnerable and have adverse outcomes in COVID-19 [54]. Equally important, clinical trials testing whether interventions that ameliorate endothelial dysfunction can have beneficial effects in COVID-19 patients are ongoing [55], [56], [57], [58], [59], [60], [61], [62], [63] and we have obtained very encouraging interim results in a randomized study testing L-Arginine oral supplementation in COVID-19 hospitalized patients [55].
L-Arginine is an amino acid that acts as a substrate for endothelial nitric oxide (NO) synthase (eNOS), a key enzyme in endothelial cells [64], [65], [66], [67], [68], [69], [70], [71], [72]; it has been previously shown to significantly improve endothelial function, providing a strong rationale for its use in COVID-19 patients [58]. Additionally, beneficial effects of L-Arginine on the regulation of immune responses have been reported [73], [74]. Similarly, recent clinical trials have demonstrated that Vitamin C improves the oxidative imbalance and vascular remodeling induced by different stressors and attenuates endothelial barrier permeability, an aspect that has major implications in infectious disorders (including COVID-19), which are also known to cause a systemic surge in oxidative stress [75]. So, the antioxidant roles of Vitamin C and its protective effects on endothelial permeability could come into effect especially in post-infection recovery [76], [77], [78], [79], [80], [81], [82].
Therefore, we inferred that associating Vitamin C with other nutraceuticals playing similar actions, like L-Arginine, could be useful. On these grounds, considering that both L-Arginine and Vitamin C are known to improve endothelial function and reduce vascular permeability during infectious disorders [75], [83], [84], [85], [86], and based on the emerging role of vascular permeability in Long-COVID [87], [88], we designed a survey to assess the effects of a combination of L-Arginine and Vitamin C in Long-COVID.
2. Methods
2.1. Survey design and participants
This survey (LINCOLN: L-Arginine and Vitamin C improves Long-COVID) starts from the medical need to understand Long-COVID disease. For this reason, in January 2022, we designed a questionnaire based on a seminal Nature Medicine paper published in April 2021 [89] and on the national recommendations on the management of Long-COVID. Our research group includes 16 key opinion leaders belonging to different Italian Regions; a group of physicians (please see appendix) participated in actually administering the LINCOLN survey to patients. The questionnaire was revised by the key opinion leaders and shared with the general practitioners of the nation with the aim to enroll the highest number of patients.
The LINCOLN survey included the following evaluations: patient age, sex, days from RT-qPCR negativization, and hospitalization for moderate/severe COVID-19. Furthermore, the physicians needed to assess on a scale from 1 to 3 (where 1 is absence of the symptom, 2 is the presence of a mild symptom, and 3 is the presence of a severe symptom) - the following symptoms: fatigue, shortness of breath, chest tightness, dizziness, gastrointestinal disorders, headache, anosmia, difficulties in concentrating, sleep disturbances.
Finally, effort perception was evaluated in the survey using the Borg modified 0–10 Rating of perceived exertion scale, with a score from 0 to 10 (where 10 indicate no tolerance), as we previously described [90]. The survey was administered to two groups of COVID-19 survivors who had been COVID-19 negative (confirmation to be SARS-CoV-2 negative via RT-qPCR test [91]) for at least 4 weeks. The first group included patients who had received 2 vials/day of L-Arginine 1.66 g in association with 500 mg of liposomal Vitamin C. The second group (alternative treatment) had been treated with a multivitamin combination (Vitamin B1: 388 mg; Vitamin B2: 443 mg; Nicotinamide:18 mg; Folic Acid: 200 µg; Pantothenic acid: 2493 g; Vitamin B6: 831 mg; Vitamin B12: 416 µg). Physicians were asked to maintain a ~2:1 ratio (L-Arginine + Vitamin C vs alternative treatment) in administering the survey; therefore, they were not blinded; actually, when the questionnaire was administered, patients of both groups had already completed 30 days of treatment.
2.2. Inclusion and exclusion criteria
Eligible patients were men and non-pregnant women who were at least 18-year-old, screened by applying the following eligibility criteria:
Inclusion criteria
Presence of the following conditions:
Previous diagnosis of COVID-19 confirmed by RT-qPCR;
COVID-19 negativization confirmed by RT-qPCR from at least 4 weeks;
Presence of COVID-19 sequelae that extend beyond four weeks after initial infection.
Exclusion criteria
-
•
Refusal to participate;
-
•
History of intolerance to L-Arginine, Vitamin C, or components of the multivitamin combination;
-
•
Pregnancy or breastfeeding;
-
•
Cancer;
-
•
Diagnosis of chronic pulmonary disease - currently under treatment;
-
•
Use of immunosuppressive drugs, or cytotoxic chemotherapy within the previous three weeks;
-
•
Enrollment into an investigational treatment study for COVID-19 in the 30 days before screening.
2.3. Ethical aspects
The survey was distributed to patients who had already received the treatments. A clear and informative description of the survey and an explanation of how collected data would have been used were given to respondents; participation was voluntary. According to the Italian regulatory framework, all of the data were anonymized and aggregated, and no personally identifiable information was collected.
2.4. Statistical analysis
The main characteristics of the participants are reported as mean ± SD or percentage. Statistical significance was determined by a p value <0.05. In the statistical analysis, differences for continuous variables were evaluated using two-sample t-test for approximately normally distributed variables and Mann-Whitney U test for severely skewed variables. Chi-square or Fisher tests were used to measure associations between dichotomous and categorical variables. All analyses were performed using SPSS 26.0.
3. Results
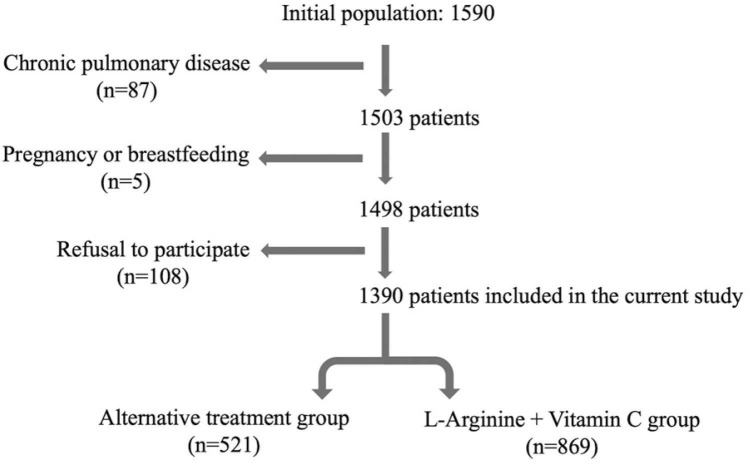
1590 patients were initially enrolled, of which 1390 fulfilled the eligibility criteria and successfully completed the survey, as shown in the flow chart in Fig. 1.
Fig. 1.
Flow chart.
There were no significant differences in terms of age, male sex, or hospitalization due to COVID-19 between the group treated with L-Arginine + Vitamin C and the arm treated with the alternative treatment ( Table 1). Notably, no difference in the time from SARS-Cov-2 negativization was noted between the two groups of patients (33.8 ± 24.9 vs 31.5 ± 24.0 days; ns).
Table 1.
Main characteristics of the two populations.
| Alternative Treatment | L-Arginine + Vitamin C | p | |
|---|---|---|---|
| N | 521 | 869 | - |
| Age (y) | 55.5±15.6 | 55.4±15.8 | 0.854 |
| Male sex (%) | 51.8 | 49.8 | 0.471 |
| Hospitalization for COVID-19 (%) | 9.2 | 7.9 | 0.408 |
Following a 30-day treatment with L-Arginine + Vitamin C, the survey revealed that patients in this treatment group had significantly lower scores (which means less severe long-COVID symptoms) compared to the other group (L-Arginine + Vitamin C: 8.15 ± 1.3 vs Alternative treatment: 13.9 ± 2.3, p < 0.001); remarkably, the treatment with L-Arginine + Vitamin C had favorable effects on all the symptoms explored by the survey ( Table 2).
Table 2.
Survey results in the two groups of patients.
| Alternative Treatment (N=521) | L-Arginine + Vitamin C (N=869) | p | ||
|---|---|---|---|---|
| Asthenia | Absent (%) | 0.4 | 94.9 | <0.0001 |
| Mild (%) | 5.2 | 4.0 | ||
| Severe (%) | 94.4 | 1.1 | ||
| Dyspnea | Absent (%) | 5.4 | 74.2 | <0.0001 |
| Mild (%) | 55.1 | 25.4 | ||
| Severe (%) | 39.5 | 0.4 | ||
| Chest tightness | Absent (%) | 26.3 | 86.1 | <0.0001 |
| Mild (%) | 50.9 | 13.4 | ||
| Severe (%) | 22.8 | 0.5 | ||
| Dizziness | Absent (%) | 66.6 | 87.3 | <0.0001 |
| Mild (%) | 25.9 | 11.6 | ||
| Severe (%) | 7.5 | 1.1 | ||
| Gastrointestinal disorders | Absent (%) | 63.3 | 87.7 | <0.0001 |
| Mild (%) | 26.7 | 11.7 | ||
| Severe (%) | 10.0 | 0.6 | ||
| Headache | Absent (%) | 39.2 | 81.8 | <0.0001 |
| Mild (%) | 44.1 | 16.8 | ||
| Severe (%) | 16.7 | 1.4 | ||
| Anosmia | Absent (%) | 52.0 | 87.2 | <0.0001 |
| Mild (%) | 34.0 | 11.0 | ||
| Severe (%) | 14.0 | 1.8 | ||
| Concentration difficulty | Absent (%) | 32.8 | 79.1 | <0.0001 |
| Mild (%) | 46.8 | 19.4 | ||
| Severe (%) | 20.4 | 1.5 | ||
| Sleeplessness | Absent (%) | 39.5 | 80.7 | <0.0001 |
| Mild (%) | 42.6 | 17.5 | ||
| Severe (%) | 17.9 | 1.8 |
When examining the effort perception (modified Borg scale), we observed a significantly lower value (p < 0.0001; Fig. 2) in patients receiving L-Arginine + Vitamin C, compared to the alternative-treatment arm, indicating that L-Arginine + Vitamin C led to a better effort perception.
Fig. 2.
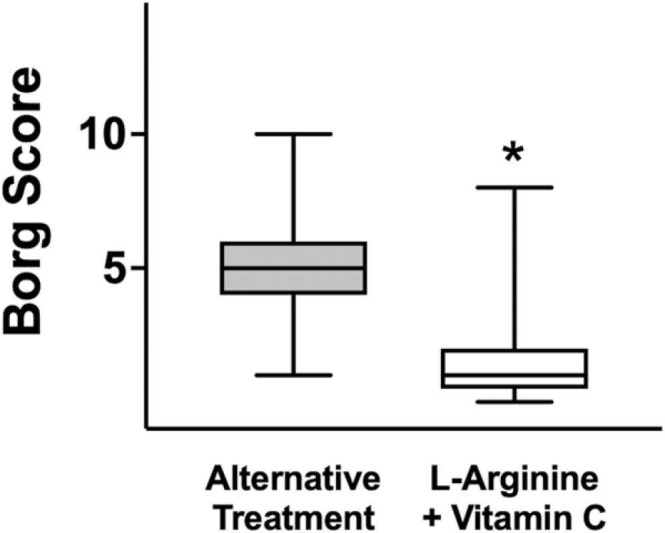
Borg score assessed after 1 month in the groups of patients receiving L-Arginine + Vitamin C or alternative treatment. Box plots indicate upper/lower quartiles, the line in the middle of each box is the mean, and the whiskers represent the min–max of values; *:P < 0.0001.
4. Discussion
This survey is the first to show the beneficial effects of the combination of L-Arginine and Vitamin C in Long-COVID. Our investigation was based on a robust rationale, i.e. targeting endothelial dysfunction in Long-COVID. Indeed, endothelial cell infection with consecutive inflammatory cell recruitment and endothelial dysfunction could explain the impaired microcirculation observed across vascular beds in COVID-19, triggering vasoconstriction, ischemia, and a pro-coagulant state [92], [93], [94], [95]. Consistent with our view, several investigators had proposed that endotheliitis could be a critical mechanism underlying systemic impaired microcirculatory function observed in different vascular beds in patients experiencing Long-COVID symptoms [92], [96].
Of note, a recent clinical study has demonstrated that COVID-19 patients develop endothelial dysfunction, which remains significantly impaired compared to healthy controls subjects at a 6-month follow-up [97], implicating that endothelial dysfunction is a main player in both acute COVID-19 and Long-COVID. Interestingly, increased numbers of circulating endothelial cells, a biomarker of vascular injury [98], most likely detached from the vessel wall due to pathological insults, were found to significantly correlate with the severity of COVID-19 outcome [99]. Strikingly, elevated levels of circulating endothelial cells persisted in recovered COVID-19 convalescent patients [100], denoting long-term detrimental effects of SARS-CoV-2 infection on endothelial function. Actual signs of endothelial dysfunction (denoted by glycocalyx damage) have been reported in convalescent COVID-19 patients after mild disease progression without hospitalization [101]. Other studies evidenced that sustained endotheliopathy is common in convalescent COVID-19 subjects [102], and that Long-COVID symptoms, specifically non-respiratory symptoms, are mainly due to persistent endothelial dysfunction [103].
Our data are fully in agreement with previous reports implying that after the acute phase of COVID-19, the disease is dominated by immunopathological pro-inflammatory elements [104], [105], [106], [107], [108], [109], [110]. In fact, previous investigations have actually demonstrated that reduced levels of L-Arginine increase the generation of reactive oxygen species (ROS), aggravating inflammation [111]. Besides, in vitro assays revealed that the T cell proliferative capacity is significantly reduced among COVID-19 patients and can be restored through L-Arginine supplementation [112]. COVID-19 patients with severe symptoms present an increased level of myeloid-derived suppressor cells, directly correlated to an enhanced activity of arginase – the enzyme responsible for metabolizing L-Arginine to ornithine and urea [112], [113]. Another recent investigation has demonstrated an inverse correlation between L-Arginine level and platelet activation [114], a major contributor to thromboembolic complications of COVID-19. While eNOS produces physiological levels of NO, the inducible NO synthase (iNOS) is mainly expressed under inflammatory stimuli in parenchymal cells and leucocytes, producing much larger amounts of NO, and its exact role in COVID-19 remains to be fully clarified [115], [116].
As a critical driver of inflammation and oxidative stress [117], endothelial dysfunction has also been involved in the pathophysiology of the neurological symptoms of COVID-19 and Long-COVID [118], [119], [120]. Fatigue is a prevailing symptom in Long-COVID patients [121], and previous trials have evidenced a significant decrease in fatigue in subjects treated with Vitamin C [122], [123], fully consistent with our present results. Supporting our strategy to combine L-Arginine to Vitamin C, previous investigations have shown that ascorbic acid can synergistically improve the effects of other agents: for example, if added to glucagon-like peptide 1 (GLP-1) agonists, it reduces ROS generation in diabetic patients [124], in combination with metformin, it reduces macro- and microvascular diabetic complications [125].
Strengths of this survey include the large population enrolled and the fact that the questionnaire was administered in multiple centers throughouth Italy. Nevertheless, we do reckon that our research is not exempt from limitations. For instance, we did not assess blood levels of nitrates and nitrite, Vitamin C, or citrulline in our patients; moreover, we did not administer the survey at baseline but only after 30 days. Hence, one may argue that the patients who received L-Arginine + Vitamin C could have been somehow healthier than the other group before starting the treatment. However, albeit we do not have full clinical data for all patients, this hypothesis seems unlikely since patients were enrolled by their family physicians who had prescribed different treatments to patients with similar clinical characteristics only on account of little guidance on managing Long-COVID. Further dedicated interventional studies are warranted to endorse our findings.
CRediT authorship contribution statement
Raffaele Izzo: Writing - original draft, Writing - review & editing. Valentina Trimarco: Conceptualization, Data curation, Formal analysis, Software. Pasquale Mone: Visualization, Writing – review & editing. Teresita Aloè: Investigation, Visualization. Massimo Capra Marzani: Investigation, Visualization. Antonio Diana: Investigation, Visualization. Giovanni Fazio: Investigation, Visualization. Mario Mallardo: Investigation, Visualization. Mauro Maniscalco: Investigation, Visualization. Giuseppe Marazzi: Investigation, Visualization. Nunzia Messina: Investigation, Visualization. Simone Mininni: Investigation, Visualization. Chiara Mussi: Investigation, Visualization. Girolamo Pelaia: Investigation, Visualization. Alfio Pennisi: Investigation, Visualization. Pierachille Santus: Investigation, Visualization. Francesco Scarpelli: Investigation, Visualization. Francesco Tursi: Investigation, Visualization. Alessandro Zanforlin: Investigation, Visualization. Gaetano Santulli: Writing - original draft, Writing - review & editing. Bruno Trimarco: Writing - original draft.
Declaration of Competing Interest
None.
Acknowledgements
We thank Dr. J. Gambardella for helpful discussion. The study did not receive any funding.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2022.106360.
Appendix A. Supplementary material
Supplementary material.
.
List of physicians who participated in the LINCOLN survey and their affiliations.
Data availability
Data will be made available on request.
References
- 1.Page J.H., et al. Trends in characteristics and outcomes among US adults hospitalised with COVID-19 throughout 2020: an observational cohort study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolahchi Z., De Domenico M., Uddin LQ., Cauda V., Grossmann I., Lacasa L., Grancini G., Mahmoudi M., Rezaei R. COVID-19 and Its Global Economic Impact. Adv. Exp. Med. Biol. 2021;1318:825–837. doi: 10.1007/978-3-030-63761-3_46. [DOI] [PubMed] [Google Scholar]
- 3.Boggiano C., et al. Update on and future directions for use of anti-SARS-CoV-2 antibodies: National Institutes of Health Summit on Treatment and Prevention of COVID-19. Ann. Intern. Med. 2022;175:119–126. doi: 10.7326/M21-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morens D.M., Taubenberger J.K., Fauci A.S. Universal coronavirus vaccines - an urgent need. N. Engl. J. Med. 2022;386:297–299. doi: 10.1056/NEJMp2118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sievers B.L., et al. Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses. Sci. Transl. Med. eabn7842. 2022 doi: 10.1126/scitranslmed.abn7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauci A.S. The story behind COVID-19 vaccines. Science. 2021;372:109. doi: 10.1126/science.abi8397. [DOI] [PubMed] [Google Scholar]
- 7.Desai A.D., Lavelle M., Boursiquot B.C., Wan E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022;322:C1–C11. doi: 10.1152/ajpcell.00375.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaffney A.W. The long COVID conundrum. Am. J. Med. 2022;135:5–6. doi: 10.1016/j.amjmed.2021.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raveendran A.V., Misra A. Post COVID-19 syndrome (“Long COVID”) and diabetes: challenges in diagnosis and management. Diabetes Metab. Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann P., Pittet L.F., Curtis N. Long COVID in children and adolescents. BMJ. 2022;376:o143. doi: 10.1136/bmj.o143. [DOI] [PubMed] [Google Scholar]
- 12.Nalbandian A., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegelman J.N. Reflections of a COVID-19 Long Hauler. JAMA. 2020;324:2031–2032. doi: 10.1001/jama.2020.22130. [DOI] [PubMed] [Google Scholar]
- 14.Feldman E.L., et al. COVID-19 and diabetes: a collision and collusion of two diseases. Diabetes. 2020;69:2549–2565. doi: 10.2337/dbi20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raveendran A.V., Jayadevan R., Sashidharan S. Long COVID: an overview. Diabetes Metab. Syndr. 2021;15:869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokes A.C., et al. COVID-19 and excess mortality in the United States: a county-level analysis. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sathish T., Anton M.C., Sivakumar T. New-onset diabetes in “long COVID”. J. Diabetes. 2021;13:693–694. doi: 10.1111/1753-0407.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montefusco L., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021 doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N. Engl. J. Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paules C.I., Fauci A.S. COVID-19: the therapeutic landscape. Med. (N. Y.) 2021;2:493–497. doi: 10.1016/j.medj.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoehl S., et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N. Engl. J. Med. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou L., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheahan T.P., Frieman M.B. The continued epidemic threat of SARS-CoV-2 and implications for the future of global public health. Curr. Opin. Virol. 2020;40:37–40. doi: 10.1016/j.coviro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Accili D. Can COVID-19 cause diabetes? Nat. Metab. 2021;3:123–125. doi: 10.1038/s42255-020-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denson J.L., et al. Metabolic syndrome and acute respiratory distress syndrome in hospitalized patients With COVID-19. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.40568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta A., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sardu C., et al. Hypertension, thrombosis, kidney failure, and diabetes: Is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9:1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santulli G., Morelli M., Gambardella J. Is endothelial dysfunction the concealed cornerstone of COVID-19? BMJ. 2020;368 [Google Scholar]
- 30.Qin Z., et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics. 2021;11:8076–8091. doi: 10.7150/thno.61810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu S.X., et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2020 doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perea Polak A., et al. Complement-mediated thrombogenic vasculopathy in COVID-19. Int J. Dermatol. 2020 doi: 10.1111/ijd.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesquida J., et al. Peripheral microcirculatory alterations are associated with the severity of acute respiratory distress syndrome in COVID-19 patients admitted to intermediate respiratory and intensive care units. Crit. Care. 2021;25:381. doi: 10.1186/s13054-021-03803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmaier A.A., et al. Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19. JCI Insight. 2021;6 doi: 10.1172/jci.insight.151527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otifi H.M., Adiga B.K. Endothelial dysfunction in Covid-19. Am. J. Med. Sci. 2022 doi: 10.1016/j.amjms.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelliher S., et al. Non-severe COVID-19 is associated with endothelial damage and hypercoagulability despite pharmacological thromboprophylaxis. J. Thromb. Haemost. 2022 doi: 10.1111/jth.15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amraei R., et al. Extracellular vimentin is an attachment factor that facilitates SARS-CoV-2 entry into human endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2022;119 doi: 10.1073/pnas.2113874119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gambardella, J. & Santulli, G. What is linking COVID-19 and endothelial dysfunction? Updates on nanomedicine and bioengineering from the 2020 AHA Scientific Sessions. European Heart Journal (Cardiovascular Pharmacotherapy), In press 7 (2021) e2-e3, 10.1093/ehjcvp/pvaa145. [DOI] [PMC free article] [PubMed]
- 41.Kaur S., Tripathi D.M., Yadav A. The enigma of endothelium in COVID-19. Front Physiol. 2020;11:989. doi: 10.3389/fphys.2020.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matarese A., Gambardella J., Sardu C., Santulli G. miR-98 Regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines. 2020;8:462. doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmetaj-Shala B., et al. Cardiorenal tissues express SARS-CoV-2 entry genes and basigin (BSG/CD147) increases with age in endothelial cells. JACC Basic Transl. Sci. 2020;5:1111–1123. doi: 10.1016/j.jacbts.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mone P., et al. miR-24 targets the transmembrane glycoprotein neuropilin-1 in human brain microvascular endothelial cells. Noncoding RNA. 2021;7:9. doi: 10.3390/ncrna7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts K.A., Colley L., Agbaedeng T.A., Ellison-Hughes G.M., Ross M.D. Vascular manifestations of COVID-19 - thromboembolism and microvascular dysfunction. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.598400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortiz M.E., et al. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.102976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelesidis T., et al. The ApoA-I mimetic peptide 4F attenuates in vitro replication of SARS-CoV-2, associated apoptosis, oxidative stress and inflammation in epithelial cells. Virulence. 2021;12:2214–2227. doi: 10.1080/21505594.2021.1964329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varzideh F., et al. Sortilin drives hypertension by modulating sphingolipid/ceramide homeostasis and by triggering oxidative stress. J. Clin. Invest. 2022;132 doi: 10.1172/JCI156624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gambardella J., et al. Role of endothelial G protein-coupled receptor kinase 2 in angioedema. Hypertension. 2020;76:1625–1636. doi: 10.1161/HYPERTENSIONAHA.120.15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mone P., et al. SGLT2 inhibition via empagliflozin Improves endothelial function and reduces mitochondrial oxidative stress: Insights from frail hypertensive and diabetic patients. Hypertension. 2022;79:1633–1643. doi: 10.1161/HYPERTENSIONAHA.122.19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L., et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 52.Nagashima S., et al. Endothelial dysfunction and thrombosis in patients with COVID-19-brief report. Arterioscler. Thromb. Vasc. Biol. 2020;40:2404–2407. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burkhard-Koren N.M., et al. Higher prevalence of pulmonary macrothrombi in SARS-CoV-2 than in influenza A: autopsy results from ‘Spanish flu’ 1918/1919 in Switzerland to Coronavirus disease 2019. J. Pathol. Clin. Res. 2020 doi: 10.1002/cjp2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Izcovich A., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiorentino G., et al. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansson P.I., et al. The effect of prostacyclin (Iloprost) infusion at a dose of 1 ng/kg/min for 72 h compared to placebo in mechanically ventilated patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:746. doi: 10.1186/s13063-020-04696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu F., Zhu Y., Zhang J., Li Y., Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adebayo A., et al. l-Arginine and COVID-19: an update. Nutrients. 2021;13 doi: 10.3390/nu13113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leucker T.M., et al. Effect of crizanlizumab, a P-selectin inhibitor, in COVID-19: a placebo-controlled, randomized trial. JACC Basic Transl. Sci. 2021;6:935–945. doi: 10.1016/j.jacbts.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Z., Yang K.Y., Huang Y., Lui K.O. Endothelial contribution to COVID-19: an update on mechanisms and therapeutic implications. J. Mol. Cell Cardiol. 2021;164:69–82. doi: 10.1016/j.yjmcc.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma A., et al. A randomized open-label trial to evaluate the efficacy and safety of triple therapy with aspirin, atorvastatin, and nicorandil in hospitalised patients with SARS Cov-2 infection: a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22:451. doi: 10.1186/s13063-021-05361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava S., Garg I., Hembrom A.A., Kumar B. Assessment of nitric oxide (NO) potential to mitigate COVID-19 severity. Virusdisease. 2021;32:589–594. doi: 10.1007/s13337-021-00702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isidori A.M., et al. Targeting the NO-cGMP-PDE5 pathway in COVID-19 infection. The DEDALO project. Andrology. 2021;9:33–38. doi: 10.1111/andr.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brosnan M.E., Brosnan J.T. Renal arginine metabolism. J. Nutr. 2004;134(2791S–2795S):2796S–2797S. doi: 10.1093/jn/134.10.2791S. (discussion) [DOI] [PubMed] [Google Scholar]
- 65.Wu G., Meininger C.J., McNeal C.J., Bazer F.W., Rhoads J.M. Role of L-Arginine in nitric oxide synthesis and health in humans. Adv. Exp. Med. Biol. 2021;1332:167–187. doi: 10.1007/978-3-030-74180-8_10. [DOI] [PubMed] [Google Scholar]
- 66.Rath M., Muller I., Kropf P., Closs E.I., Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. 2014;5:532. doi: 10.3389/fimmu.2014.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gambardella J., et al. Arginine and endothelial function. Biomedicines. 2020;8:277. doi: 10.3390/biomedicines8080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrew P.J., Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 69.Jorens P.G., Vermeire P.A., Herman A.G. L-Arginine-dependent nitric oxide synthase: a new metabolic pathway in the lung and airways. Eur. Respir. J. 1993;6:258–266. [PubMed] [Google Scholar]
- 70.Tousoulis D., Kampoli A.M., Tentolouris C., Papageorgiou N., Stefanadis C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharm. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 71.Dormanns K., Brown R.G., David T. The role of nitric oxide in neurovascular coupling. J. Theor. Biol. 2016;394:1–17. doi: 10.1016/j.jtbi.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Ströhle A., von Bibra H., Hahn A. L-Arginine and vascular health. Med Mon. Pharm. 2016;39:515–520. [PubMed] [Google Scholar]
- 73.Bronte V., Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 74.Wijnands K.A., Castermans T.M., Hommen M.P., Meesters D.M., Poeze M. Arginine and citrulline and the immune response in sepsis. Nutrients. 2015;7:1426–1463. doi: 10.3390/nu7031426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morelli M.B., Gambardella J., Castellanos V., Trimarco V., Santulli G. Vitamin C and cardiovascular disease: an update. Antioxid. (Basel) 2020;9:1227. doi: 10.3390/antiox9121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soto M.E., Guarner-Lans V., Soria-Castro E., Manzano Pech L., Perez-Torres I. Is antioxidant therapy a useful complementary measure for Covid-19 Treatment? An algorithm for its application. Med. (Kaunas.) 2020;56 doi: 10.3390/medicina56080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montel-Hagen A., et al. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–1048. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y., et al. Ascorbate recycling in human neutrophils: induction by bacteria. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13816–13819. doi: 10.1073/pnas.94.25.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bauer S.R., Kapoor A., Rath M., Thomas S.A. What is the role of supplementation with ascorbic acid, zinc, vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19? Cleve Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc046. [DOI] [PubMed] [Google Scholar]
- 80.Chavarria A.P., et al. Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput. Struct. Biotechnol. J. 2021;19:1379–1390. doi: 10.1016/j.csbj.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carr A.C., Rowe S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients. 2020;12 doi: 10.3390/nu12113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan J., Tie G., Messina L.M. Tetrahydrobiopterin, L-arginine and vitamin C act synergistically to decrease oxidative stress, increase nitricoxide and improve blood flow after induction of hindlimbischemia in the rat. Mol. Med. 2012;18:676–684. doi: 10.2119/molmed.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuck J.L., et al. Ascorbic acid attenuates endothelial permeability triggered by cell-free hemoglobin. Biochem Biophys. Res. Commun. 2018;495:433–437. doi: 10.1016/j.bbrc.2017.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lundblad C., Bentzer P. Effects of L-arginine on cerebral blood flow, microvascular permeability, number of perfused capillaries, and brain water content in the traumatized mouse brain. Micro Res. 2007;74:1–8. doi: 10.1016/j.mvr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 85.May J.M., Qu Z.C. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys. Res. Commun. 2011;404:701–705. doi: 10.1016/j.bbrc.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9 doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang C., et al. Long COVID: the nature of thrombotic sequelae determines the necessity of early anticoagulation. Front Cell Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.861703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez-Salazar B., et al. COVID-19 and the vasculature: current aspects and long-term consequences. Front Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.824851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sudre C.H., et al. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mone P., et al. L-Arginine enhances the effects of cardiac rehabilitation on physical performance: new insights for managing cardiovascular patients during the COVID-19 pandemic. J. Pharm. Exp. Ther. 2022;381:197–203. doi: 10.1124/jpet.122.001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bustin S.A., Nolan T. RT-qPCR testing of SARS-CoV-2: a primer. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21083004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zha D., Fu M., Qian Y. Vascular endothelial glycocalyx damage and potentialtargeted therapy in COVID-19. Cells. 2022;11:1972. doi: 10.3390/cells11121972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gambardella J., et al. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit. Care. 2021;25:306. doi: 10.1186/s13054-021-03731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ambrosino P., et al. Endothelial dysfunction in COVID-19: a unifying mechanism and a potential therapeutic target. Biomedicines. 2022;10 doi: 10.3390/biomedicines10040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gambardella J., et al. Exosomal miR-145 and miR-885 regulate thrombosis in COVID-19. J. Pharm. Exp. Ther. 2022 doi: 10.1124/jpet.122.001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seitz A., Ong P. Endothelial dysfunction in COVID-19: a potential predictor of long-COVID. Int. J. Cardiol. 2022;349:155–156. doi: 10.1016/j.ijcard.2021.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oikonomou E., et al. Endothelial dysfunction in acute and long standing COVID-19: a prospective cohort study. Vasc. Pharm. 2022;144 doi: 10.1016/j.vph.2022.106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blann A.D., et al. Circulating endothelial cells. Biomark. Vasc. Dis. Thromb. Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 99.Guervilly C., et al. Circulating endothelial cells as a marker of endothelial injury in severe COVID -19. J. Infect. Dis. 2020;222:1789–1793. doi: 10.1093/infdis/jiaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chioh F.W., et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife. 2021;10 doi: 10.7554/eLife.64909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vollenberg R., et al. Indications of persistent glycocalyx damage in convalescent COVID-19 patients: a prospective multicenter study and hypothesis. Viruses. 2021;13 doi: 10.3390/v13112324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fogarty H., et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021;19:2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Charfeddine S., et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Datta S.D., Talwar A., Lee J.T. A proposed framework and timeline of the spectrum of disease due to SARS-CoV-2 infection: illness beyond acute infection and public health implications. JAMA. 2020;324:2251–2252. doi: 10.1001/jama.2020.22717. [DOI] [PubMed] [Google Scholar]
- 105.Taboada M., Caruezo V., Naveira A., Atanassoff P.G. Corticosteroids and the hyper-inflammatory phase of the COVID-19 disease. J. Clin. Anesth. 2020;66 doi: 10.1016/j.jclinane.2020.109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manson J.J., et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheuma. 2020;2:e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Budinger G.R.S., Misharin A.V., Ridge K.M., Singer B.D., Wunderink R.G. Distinctive features of severe SARS-CoV-2 pneumonia. J. Clin. Invest. 2021;131 doi: 10.1172/JCI149412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lavine J.S., Bjornstad O.N., Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371:741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lucas R., et al. Arginase 1: an unexpected mediator of pulmonary capillary barrier dysfunction in models of acute lung injury. Front Immunol. 2013;4:228. doi: 10.3389/fimmu.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reizine F., et al. SARS-CoV-2-induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J. Clin. Immunol. 2021;41:515–525. doi: 10.1007/s10875-020-00920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dean M.J., et al. Transcriptome and functions of granulocytic myeloid-derived suppressor cells determine their association with disease severity of COVID-19. medRxiv. 2021 doi: 10.1101/2021.03.26.21254441. [DOI] [Google Scholar]
- 114.Sacchi A., et al. Expansion of myeloid derived suppressor cells contributes to platelet activation by L-Arginine deprivation during SARS-CoV-2 infection. Cells. 2021;10 doi: 10.3390/cells10082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gelzo M., et al. Inducible Nitric Oxide Synthase (iNOS): why a different production in COVID-19 patients of the two waves. Viruses. 2022;14 doi: 10.3390/v14030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cinar R., Iyer M.R., Kunos G. Dual inhibition of CB1 receptors and iNOS, as a potential novel approach to the pharmacological management of acute and long COVID-19. Br. J. Pharm. 2022;179:2121–2127. doi: 10.1111/bph.15461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 118.Azizi S.A., Azizi S.A. Neurological injuries in COVID-19 patients: direct viral invasion or a bystander injury after infection of epithelial/endothelial cells. J. Neurovirol. 2020;26:631–641. doi: 10.1007/s13365-020-00903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castanares-Zapatero D., et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann. Med. 2022;54:1473–1487. doi: 10.1080/07853890.2022.2076901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rutkai I., et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 2022;13:1745. doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prasannan N., et al. Impaired exercise capacity in post-COVID syndrome: the role of VWF-ADAMTS13 axis. Blood Adv. 2022 doi: 10.1182/bloodadvances.2021006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suh S.Y., et al. Intravenous vitamin C administration reduces fatigue in office workers: a double-blind randomized controlled trial. Nutr. J. 2012;11:7. doi: 10.1186/1475-2891-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stephenson C.M., Levin R.D., Spector T., Lis C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother. Pharm. 2013;72:139–146. doi: 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ceriello A., et al. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care. 2013;36:4104–4108. doi: 10.2337/dc13-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gillani S.W., Sulaiman S.A.S., Abdul M.I.M., Baig M.R. Combined effect of metformin with ascorbic acid versus acetyl salicylic acid on diabetes-related cardiovascular complication; a 12-month single blind multicenter randomized control trial. Cardiovasc. Diabetol. 2017;16:103. doi: 10.1186/s12933-017-0584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.