Abstract
We report the spontaneous formation of a stable mannitol-producing variant of Leuconostoc pseudomesenteroides. The mannitol-producing variant showed mannitol dehydrogenase activity which was absent in the parental strain. It was also able to use fructose and glucose simultaneously, whereas the parental strain showed diauxic growth with these sugars. A possible explanation of these observations is discussed.
Starter cultures of lactic acid bacteria are often prepared by drying processes which impose stress conditions on the cells. Mannitol was shown to have an osmoprotecting effect on several organisms, including lactic acid bacteria, and it also enhances the survival of dried Lactococcus lactis cells (4). In addition, mannitol has an antioxidant effect by scavenging off free hydroxyl radicals, preventing oxidative damage (13). Besides that, mannitol is about half as sweet as sucrose and since it is not metabolizable by humans it is considered to be a low-calorie sweetener (6). The application of mannitol-overproducing lactic acid bacteria may lead to the production of fermented foods with extra nutritional value (functional foods). Up to now, mannitol has been produced by chemical hydrogenation of fructose, resulting in mannitol and its isomer sorbitol in almost equal amounts (8).
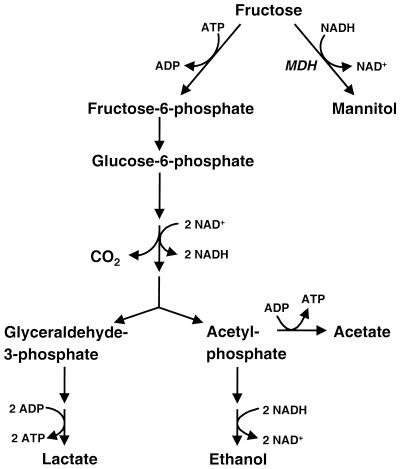
In the presence of fructose or sucrose the heterofermentative lactic acid bacterium Leuconostoc mesenteroides is able to produce high levels of mannitol (14, 15). Heterofermentative lactic acid bacteria normally ferment carbohydrates to equimolar amounts of lactate, carbon dioxide, and ethanol (2). An extra ATP can be gained by the production of acetate instead of ethanol. The regeneration of reducing equivalents can be achieved by reducing fructose to mannitol by the activity of an NADH-linked mannitol dehydrogenase (Fig. 1). Also, some homofermentative lactic acid bacteria are known to produce mannitol, but only in very small amounts (5, 7). Neves et al. (9) showed that a lactate dehydrogenase-deficient mutant of Lactococcus lactis transiently accumulates intracellular mannitol.
FIG. 1.
Overview of the pathway of fructose utilization in mannitol-producing heterofermentative lactic acid bacteria. A more detailed pathway of hexose fermentation by heterofermentative lactic acid bacteria was presented by Axelsson (2). MDH, mannitol dehydrogenase.
In this work, our research focuses on the physiology of the growth and production of mannitol by Leuconostoc pseudomesenteroides.
Growth experiments and analyses.
L. pseudomesenteroides DSM 20193 was routinely cultivated in static N2-flushed sealed bottles at 30°C with a glucose medium derived from the growth medium described by Vandamme et al. (15) with 150 mM glucose at an initial pH of 6.5. Growth experiments with batch and continuous cultures were performed in a glass fermenter with a working volume of 600 ml at 30°C, an agitation rate of 300 rpm, and a N2 atmosphere. All growth experiments were performed in a fructose medium that was similar to the glucose medium mentioned above except that glucose was replaced by 150 mM fructose, unless otherwise described. The initial pH was 6.5, and after a free pH course until pH 4.5, the pH was kept constant at 4.5 using 2 M NaOH. The dilution rate in continuous fermentations was set at 0.1 h−1 and controlled by the pump rate of the medium inlet. Growth was monitored by optical density measurement at 600 nm (OD600). Sugars and fermentation products were analyzed by high-performance liquid chromatography using an InterAction ION-300 column (Alltech, Breda, The Netherlands) at 90°C with a flow rate of 0.4 ml min−1, with 3 mM H2SO4 as the eluent, and were detected by refractive index detection. It was assumed that equimolar amounts of carbon dioxide and lactate were produced. Mannitol-producing cells were distinguished from non-mannitol-producing cells by plating out culture samples on glucose agar plates. Separate colonies were suspended in fructose medium and incubated for 48 h. Hereafter, mannitol production in the culture samples was analyzed by the colorimetric assay of alditols described by Sanchez (11).
For the preparation of cell extracts, cells were harvested by centrifugation (20,000 × g; 10 min), washed twice in chilled 50 mM potassium phosphate buffer (pH 7.0), and resuspended in the same buffer. Cells were disrupted ultrasonically, and unbroken cells and cell debris were removed by centrifugation (8,000 × g; 10 min). Mannitol dehydrogenase activity was assayed according to the procedure of Sakai and Yamanaka (10). The protein content of the cell extracts was determined by the BCA protein assay (Pierce, Omnilabo International, Breda, The Netherlands) with bovine serum albumin as a standard.
Isolation of a mannitol-producing variant strain.
L. pseudomesenteroides strain DSM 20193 was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). The bacterium was, as indicated by Deutsche Sammlung von Mikroorganismen und Zellkulturen, identical to the strain ATCC 12291 used by Vandamme et al. (15) and Soetaert (14), who showed that the strain produced high levels of mannitol when grown with fructose or sucrose. Surprisingly, strain DSM 20193 did not produce any mannitol when grown in fructose medium, in both batch and continuous cultures. However, when the strain was subcultivated several times in succession, a mannitol-producing variant was spontaneously formed. We observed that the mannitol-producing ability was stable: repeated subcultivation of separate colonies of this variant strain (here designated “mannitol positive”) in liquid or on solid media with glucose medium did not lead to a reappearance of the original strain (here designated “mannitol negative”). Furthermore, the switch from the mannitol-negative to the mannitol-positive phenotype was not found when the strain was grown in the absence of fructose or sucrose. Since strains DSM 20193 and ATCC 12291 are claimed to be identical, we postulate that Vandamme et al. and Soetaert performed their research with a strain that had changed its phenotype in a similar way.
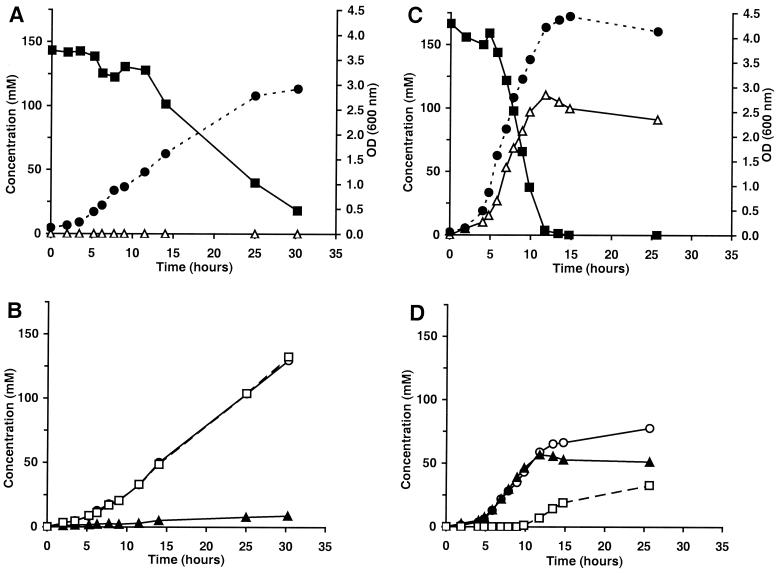
When grown in fructose medium, the mannitol-negative strain showed a maximum growth rate of 0.39 h−1. Equimolar amounts of lactate and ethanol were produced with trace amounts of acetate (Fig. 2A and B). The mannitol-positive variant strain grew faster (maximum growth rate, 0.55 h−1) and produced mannitol with a conversion efficiency of 0.65 mol of mannitol/mol of fructose (Fig. 2C and D). The variant strain produced a high level of acetate, whereas ethanol production was low. When cultivated in a growth medium with both glucose (50 mM) and fructose (100 mM), the mannitol-negative strain showed a diauxic growth pattern. Utilization of fructose started only after glucose depletion, but no mannitol formation was observed. The mannitol-positive variant strain utilized both carbohydrates simultaneously. Glucose was fermented, whereas fructose was converted into mannitol with a conversion efficiency of at most 0.95 mol of mannitol/mol of fructose. The concentrations of mannitol and fermentation products were almost identical compared to those found in the batch culture experiment with fructose only.
FIG. 2.
Growth, fructose utilization, and product formation of the wild-type L. pseudomesenteroides strain DSM 20193 (A, B) and the spontaneously formed mannitol-producing variant strain (C and D) in batch culture in fructose medium. (A and C) ●, growth (OD600); ■, fructose; ▵, mannitol. (B and D) ○, lactate; ▴, acetate; □, ethanol. Each value represents the mean of duplicate measurements, which varied by not more than 5%. Carbon recovery varied between 95 and 105%.
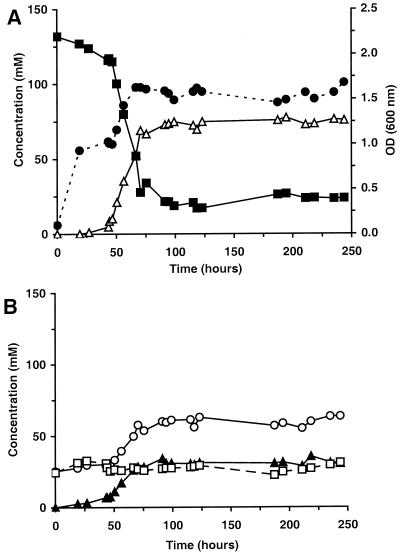
The same switch from the mannitol-negative phenotype to the mannitol-positive phenotype was found in continuous culture experiments. Initially no mannitol was produced, but after at least 40 h of incubation, mannitol production started (Fig. 3). During this change in metabolism, the amount of mannitol produced was found to be proportional to the relative number of mannitol-positive cells in the fermenter. From the moment mannitol production had stabilized, the mannitol-negative strain was completely outgrown by the mannitol-positive variant.
FIG. 3.
Growth, fructose utilization, and product formation of L. pseudomesenteroides strain DSM 20193 in continuous culture in fructose medium at 30°C, pH 4.5, and at a dilution rate of 0.1 h−1 with 150 mM fructose. (A) ●, growth (OD600); ■, fructose; ▵, mannitol. (B) ○, lactate; ▴, acetate; □, ethanol. Carbon recovery varied between 95 and 105%.
Mannitol dehydrogenase activity.
The mannitol-negative strain did not show any mannitol dehydrogenase activity, but activities of up to 6.4 μmol min−1 mg of protein−1 were observed in the mannitol-positive variant. The mannitol dehydrogenase activity in the cell extracts of the mannitol-positive strain was independent of the carbohydrate source in the growth medium, indicating that the enzyme was constitutively present. The maximal reduction rate of fructose was approximately four times higher than the maximal oxidation rate of mannitol (6.4 and 1.7 μmol min−1 mg of protein−1, respectively). No activities were found with sorbitol, fructose-6-phosphate, fructose-1-phosphate, or mannitol-1-phosphate, indicating that the enzyme has a high substrate specificity. Protein pattern analysis of cell extracts by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) showed that the mannitol-positive strain contains an enzyme with a molecular size of 43 kDa, whereas the mannitol-negative strain does not. Using the enzyme activity staining procedure of Selander et al. (12), mannitol dehydrogenase activity in cell extracts of mannitol-positive cells was made visible on a native PAGE gel. The intense protein band was not observed in cell extracts of mannitol-negative cells. Analysis of the stained protein band by sodium dodecyl sulfate-PAGE confirmed the molecular size of 43 kDa.
Conclusions.
We isolated a mannitol-producing variant strain of L. pseudomesenteroides DSM 20193 that has a growth advantage over the mannitol-negative parental strain due to a higher energy production rate from the utilization of fructose, and it quickly predominated in the growth culture. The mannitol-producing variant differs from the mannitol-negative original strain in two physiological aspects: the presence of mannitol dehydrogenase activity and the simultaneous utilization of fructose and glucose. The described change in phenotype was not observed before in mannitol-producing organisms, and the mechanism of the change in phenotype is also not yet clear. The presence of mannitol dehydrogenase is clearly a prerequisite for mannitol production. However, it is unlikely that the presence of mannitol dehydrogenase could enable the simultaneous utilization of fructose and glucose. Since the mannitol-negative strain does not utilize glucose and fructose at the same time, a repression of the uptake of fructose by glucose is plausible. This repression was not observed in the mannitol-positive variant strain, indicating that in the latter strain there might be a fructose transporter that is insensitive for repression by glucose. The change in phenotype might be caused by a spontaneously formed mutation on the regulatory level of a gene cluster coding for mannitol dehydrogenase plus possibly a second fructose transporter. Spontaneous mutations in lactic acid bacteria, often leading to growth advantages, have been reported before (1, 3). Further research on the occurrence of this mechanism is in progress.
A mannitol-producing Leuconostoc strain would be directly applicable for use in food products. However, Leuconostoc strains are only utilized in a few fields of the food industry and the requirement for fructose or sucrose may also limit the variety of applications. On the other hand, homofermentative dairy strains such as Lactococcus lactis do not produce mannitol. Mannitol formation in such strains is obviously strongly regulated, so much more research is required in order to achieve a mannitol-overproducing dairy lactic acid bacterium. The results obtained in our work may contribute to an understanding of the regulatory mechanisms involved in mannitol production and may eventually be used to induce mannitol production in other lactic acid bacteria.
Acknowledgments
We thank Dirk Martens for valuable discussions concerning this work.
REFERENCES
- 1.Ahrné S, Molin G. Spontaneous mutations changing the raffinose metabolism of Lactobacillus plantarum. Antonie Leeuwenhoek. 1991;60:87–93. doi: 10.1007/BF00572697. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson L T. Lactic acid bacteria: classification and physiology. In: Salminen S, von Wright A, editors. Lactic acid bacteria. New York, N.Y: Marcel Dekker Inc.; 1993. pp. 1–63. [Google Scholar]
- 3.Curragh H J, Collins M A. High levels of spontaneous drug resistance in Lactobacillus. J Appl Bacteriol. 1992;73:31–36. [Google Scholar]
- 4.Efiuvwevwere B J O, Gorris L G M, Smid E J, Kets E P W. Mannitol-enhanced survival of Lactococcus lactis subjected to drying. Appl Microbiol Biotechnol. 1999;51:100–104. [Google Scholar]
- 5.Ferain T, Schanck A N, Delcour J. 13C nuclear magnetic resonance analysis of glucose and citrate end products in an ldhL-ldhD double-knockout strain of Lactobacillus plantarum. J Bacteriol. 1996;178:7311–7315. doi: 10.1128/jb.178.24.7311-7315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furia T E. Handbook of food additives. 2nd ed. West Palm Beach, Fla: CRC Press; 1972. [Google Scholar]
- 7.Loesche W J, Kornman K S. Production of mannitol by Streptococcus mutans. Arch Oral Biol. 1976;21:551–553. doi: 10.1016/0003-9969(76)90021-2. [DOI] [PubMed] [Google Scholar]
- 8.Makkee M, Kieboom A P G, van Bekkum H. Production methods of d-mannitol. Starch. 1985;37:136–141. [Google Scholar]
- 9.Neves A R, Ramos A, Shearman C, Gasson M J, Almeida J S, Santos H. Metabolic characterization of Lactococcus lactis deficient in lactate dehydrogenase using in vivo13C-NMR. Eur J Biochem. 2000;267:3859–3868. doi: 10.1046/j.1432-1327.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- 10.Sakai S, Yamanaka K. Crystalline d-mannitol:NAD+ oxidoreductase from Leuconostoc mesenteroides. Biochim Biophys Acta. 1968;151:684–686. doi: 10.1016/0005-2744(68)90017-x. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez J. Colorimetric assay of alditols in complex biological samples. J Agric Food Chem. 1998;46:157–160. doi: 10.1021/jf970619t. [DOI] [PubMed] [Google Scholar]
- 12.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen B, Jensen R G, Bohnert H J. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol. 1997;115:527–532. doi: 10.1104/pp.115.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soetaert W. Synthesis of d-mannitol and l-sorbose by microbial hydrogenation and dehydrogenation of monosaccharides. Ph.D. thesis. Ghent, Belgium: University of Ghent; 1991. [Google Scholar]
- 15.Vandamme E J, Van Loo J, De Laporte A. Dynamics and regulation of sucrose phosphorylase formation in Leuconostoc mesenteroides fermentations. Biotechnol Bioeng. 1987;29:8–15. doi: 10.1002/bit.260290103. [DOI] [PubMed] [Google Scholar]