Abstract
Background
Interleukin (IL)-7 signaling through CD127 is impaired in lymphocytes in cancers and chronic infections, resulting in CD8+ T cell exhaustion. The mechanisms underlying CD8+ T cell responses to IL-7 in melanoma remain not completely elucidated. We previously showed reduced IL-7 level in melanoma patients. Thus, the aim of this study was to investigate the effect of IL-7 regulation on CD127 expression and CD8+ T cell responses in melanoma.
Methods
Healthy controls and primary cutaneous melanoma patients were enrolled. Membrane-bound CD127 (mCD127) expression on CD8+ T cells was determined by flow cytometry. Soluble CD127 (sCD127) protein level was measured by ELISA. Total CD127 and sCD127 mRNA level was measured by real-time PCR. CD8+ T cells were stimulated with recombinant human IL-7, along with signaling pathway inhibitors. CD8+ T cells were co-cultured with melanoma cell line, and the cytotoxicity of CD8+ T cells was assessed by measurement of lactate dehydrogenase expression.
Results
Plasma sCD127 was lower in melanoma patients compared with controls. The percentage of CD8+ T cells expressing mCD127 was higher, while sCD127 mRNA level was lower in peripheral and tumor-infiltrating CD8+ T cells from melanoma patients. There was no significant difference of total CD127 mRNA expression in CD8+ T cells between groups. IL-7 stimulation enhanced total CD127 and sCD127 mRNA expression and sCD127 release by CD8+ T cells. However, mCD127 mRNA expression on CD8+ T cells was not affected. This process was mainly mediated by phosphatidylinositol 3-kinase (PI3K) pathway. CD8+ T cells from melanoma patients exhibited decreased cytotoxicity. IL-7 stimulation promoted CD8+ T cell cytotoxicity, while inhibition of PI3K dampened IL-7-induced elevation of CD8+ T cell cytotoxicity.
Conclusion
The current data suggested that insufficient IL-7 secretion might contribute to CD8+ T cell exhaustion and CD127 dysregulation in patients with primary cutaneous melanoma.
Keywords: CD127, CD8+ T lymphocytes, Immune regulation, Interleukin-7, Melanoma
Background
There are more than 0.3 million newly diagnosed patients melanoma of skin, which accounts for 0.6% of cancer-related death in 2020 all over the world [1]. The incidence of primary cutaneous melanoma, which is one of the most aggressive malignancies in humans, continues to increase and is responsible for 65% of skin cancer deaths due to the high metastatic ability [2, 3]. The early diagnosis and appropriate therapy for cutaneous melanoma leads to a cure rate of more than 90%. However, advanced stages of cutaneous melanoma always results in poor outcome, which made it a pivotal area of research for development of new therapeutics [4]. Importantly, immune checkpoint inhibitors administration achieves essential clinical benefits in malignant melanoma [5, 6].
Interleukin (IL)-7 is a member of common γ chain receptor cytokine family, and is important for naïve T cell differentiation and survival, as well as memory T cell development and homeostasis [7–9]. Our previous study has been demonstrated that plasma IL-7 was lower in melanoma patients, which was insufficient for maintenance of Th17 cell activation [10]. IL-7 receptor is a heterodimer, and is composed of the common γ chain receptor and IL-7-specific α chain (CD127) [11, 12]. The degree of CD127 expression determines the extent of signal through IL-7/IL-7 receptor complex, which activates two major pathways: Janus kinase/signal transducer and activator of transcription 5 (JAK/STAT5) and Akt/phosphatidylinositol 3-kinase (Akt/PI3K) [13, 14]. Furthermore, IL-7 is known to modulate CD127 expression. There are two forms of CD127 existed, including membrane-bound CD127 (mCD127) on immune cells and soluble CD127 (sCD127) in peripheral blood. Exogenous IL-7 stimulation reduces total CD127 and mCD127 expression in T cells in vitro [15–17] and in vivo [18]. IL-7 also mediates the release of sCD127 from T cells through two potential methods [16]. On the one hand, IL-7 induced an alternative mRNA splicing pathway for CD127. The splicing process leads to the removal of exon 6 encoding the transmembrane domain for CD127, and generates the truncated protein sCD127 [19, 20]. On the other hand, mCD127 could be digested and shed from membrane of immune cells to generate sCD127 mainly by specific matrix metalloproteinases (MMP) [17, 20]. During human immunodeficiency virus (HIV)-1 infection, IL-7 mediates sCD127 release and mCD127 down-regulation in human CD8+ T cells [20]. This process is impaired and results in CD8+ T cell dysfunction [20]. Due to the impairment of CD8+ T cell responsiveness to IL-7 in chronic infections [20, 21] and cancers [22], we sought to test the hypothesis that altered responsiveness to IL-7 in circulating and tumor-infiltrating CD8+ T cells and CD127 expression is one of the probable mechanisms for immune exhaustion in patients with primary cutaneous melanoma.
Methods
Patients and controls
This study was approved in compliance with the Declaration of Helsinki by the Ethics Committee at The First Hospital of Shanxi Medical University (Approval number: 2016-KY-08072 and 2021-K-K102). Written informed consents were obtained from all enrolled patients and controls. The sample size numbers were calculated by Clinical Research Sample Size Calculator. Thirty-eight patients with primary cutaneous melanoma (median age, 43 years; range, 27–68 years; 27 men and 11 women; stage, stage I: 19, stage II: 11; stage III: 8) were enrolled from The First Hospital of Shanxi Medical University between July 2018 and December 2020. The diagnosis of melanoma was made in accordance with the clinical manifestation and was confirmed by pathological examination. All patients were treatment-naïve, who did not receive anti-tumor or immuno-modulatory therapies before sampling. The exclusion criteria were the following: (1) patients with chronic hepatitis virus and HIV-1 infection; (2) patients with autoimmune disorder; (3) patients with other malignancies; (4) patients with severe liver or renal dysfunction; (5) patients with pregnancy. Twenty-two age- and sex-matched healthy individuals (median age, 42 years; range, 22–63 years; 14 men and 8 women) were also enrolled as controls. All enrolled subjects were Chinese Han population.
Isolation of peripheral blood mononuclear cells and tumor-infiltrating lymophocytes
Peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Hypaque density centrifugation reagent Histopaque-1077 (Sigma-Aldrich, St Louis, Missouri, USA). Tumor tissues and para-tumor tissues were obtained from fourteen patients with primary cutaneous melanoma who received surgery. Tumor-infiltrating lymophocytes (TILs) were isolated as previously described [10]. Briefly, 1.3–2 g of tumor or para-tumor tissue was cut into small pieces and passage through 70-µm pore strainers. Cells were treated with collagenase D (500 µg/mL) at 37 °C in 5% CO2 condition for a 30 min digestion. The digested cells were then re-suspended in 44% Percoll (Sigma-Aldrich) in RPMI1640 (vol/vol), and were layed over 56% Percoll in PBS (vol/vol). The gradient was centrifuged at 850×g for 30 min. The interphase containing TILs was collected and washed with RPMI1640. A total of (2.4–8.8) × 105 of TILs were harvested from each tissue sample.
Enrichment of CD8+ T cells
CD8+ T cells were purified from PBMCs or TILs by using human CD8+ T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity post enrichment of CD8+ T cells was 95.7 ± 2.4%. Purified CD8+ T cells were cultured in DMEM supplemented with 10% fetal bovine serum, penicillin (100 µg/mL), L-glutamine (2 mmol/L), and anti-CD3/CD28 (1 µg/mL) at 37 °C in 5% CO2 condition.
Cell stimulation and culture
Purified CD8+ T cells were stimulated with recombinant human IL-7 (10 ng/mL; R&D Systems, Minneapolis, Minnesota, USA) [22] for 48 h in the presence of anti-CD3/CD28, along with either JAK inhibitor (10 µmol/L; Sigma-Aldrich, Temecula, California, USA), STAT5 inhibitor (250 µmol/L; Merck Millipore), or PI3K inhibitor (LY294002) (25 µmol/L; Sigma-Aldrich) as previously described [20]. Control cells were only stimulated with anti-CD3/CD28 for maintenance of CD8+ T cell survival. In certain experiments, stimulated CD8+ T cells were washed twice. 104 of CD8+ T cells were co-cultured with 105 of melanoma cell line SK-MEL-5 cells for 48 h.
Enzyme-linked immunosorbent assay
sCD127 level in the plasma and supernatant was measured by Human Soluble Interleukin-7 Receptor, sIL-7R enzyme-linked immunosorbent assay (ELISA) kit (CUSABIO, Wuhan, Hubei Province, China). Interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) level in the supernatants was measured by Human IFN-γ ELISA kit (CUSABIO) and Human TNF-α ELISA kit (CUSABIO), respectively.
Flow cytometry
PBMCs or TILs were stained with anti-human CD8-APC (Clone 3B5; Invitrogen ThermoFisher, Carlsbad, California, USA), and anti-human CD127-PE-Cyanine5 (Clone eBioRDR5; eBioscience, Invitrogen ThermoFisher, San Diego, California, USA) at room temperature for 30 min in the dark. Cells were detected by a FACS Aria II flow cytometer (BD Bioscience, San Jose, California, USA), and analyzed by FlowJo V10 software (FlowJo LCC, TreeStar, Ashland, Oregon, USA).
Real-time PCR
Total RNA was extracted from CD8+ T cells using Trizol reagent (Invitrogen ThermoFisher) according to the instructions from the manufacturer. cDNA was synthesized with random hexamers using PrimeScript RT Master Mix (TaKaRa, Beijing, China). For total CD127 mRNA (including RNA encoding both mCD127 and sCD127) quantification, the primers covered exons 5–7 was designed previously [20]. The PCR was able to amplify full-length CD127 RNA that skipped exon 6. This PCR amplification was performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (TaKaRa). The relative total CD127 mRNA expression to Glyceraldehyde-3-phosphate dehydrpgenase was measured using 2−ΔΔCT method with ABI7500 System Sequence Detection software (Applied Biosystems, Foster, CA, USA). For sCD127 mRNA variant detection, the probe covered nucleotides 777–815 of the variant (lack of exon 6) was previously designed [20]. The PCR amplification was performed using Premix Ex Taq (Probe qPCR) (TaKaRa), and analyzed using ABI7500 System Sequence Detection software (Applied Biosystems).
Cytotoxicity analysis
The cytotoxicity of CD8+ T cells to target cells were calculated by measurement of lactate dehydrogenase (LDH) expression in the supernatants by using LDH cytotoxicity assay kit (Beyotime, Wuhan, Hubei Province, China). LDH level in the supernatant of SK-MEL-5 cells was defined as “low-level control”, while LDH level in the supernatant of Triton X-100 treated SK-MEL-5 cells was defined as “high-level control”. The percentage of target cells = (LDH level in the sample − low-level control)/(high-level control − low-level control) × 100%.
Statistical analysis
Statistical analysis was performed by using SPSS 21.0 (SPSS, Chicago, Illinois, USA). Homogeneity test of variances was firstly performed, and all parameters were followed with normal distribution, which were presented as mean ± standard deviation. Statistical differences between the two groups were determined by Student’s t test. Statistical differences between multi-groups were determined by one-way ANOVA. LSD-t test was used after one-way ANOVA. All tests were two-tailed, and P < 0.05 was considered to indicate a statistically significant difference.
Results
mCD127 expression on CD8+ T cells was higher, while sCD127 was lower in patients with primary cutaneous melanoma
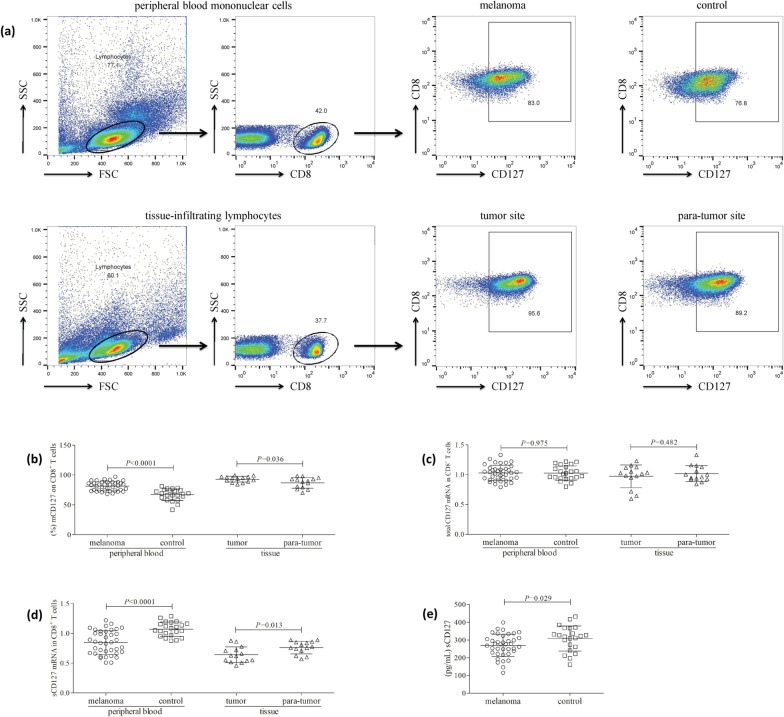
Live lymphocytes were gated according to forward scatter and side scatter. mCD127 expression on CD8+ T cells was analyzed in both PBMCs and TILs (Fig. 1a). The percentage of peripheral CD8+ T cells expressing mCD127 was elevated in patients with primary cutaneous melanoma (81.32 ± 7.68%) when compared with healthy controls (67.05 ± 9.72%; P < 0.0001, Fig. 1b). mCD127 expression on CD8+ T cells in tumor and para-tumor tissue was investigated in fourteen patients with primary cutaneous melanoma. The proportion of tissue-infiltrating CD8+ T cells expressing mCD127 was also increased in tumor tissues (92.94 ± 4.86%) when compared with para-tumor tissues (86.89 ± 9.00%; P = 0.036, Fig. 1b). There was no significant difference of total CD127 mRNA either in peripheral CD8+ T cells between melanoma patients and controls (1.03 ± 0.13 vs. 1.03 ± 0.12; P = 0.975, Fig. 1c), or in tissue-infiltrating CD8+ T cells between tumor tissues and para-tumor tissues (0.97 ± 0.19 vs. 1.02 ± 0.14; P = 0.482, Fig. 1c). sCD127 mRNA level in peripheral CD8+ T cells was lower in melanoma patients (0.85 ± 0.20) when compared with controls (1.07 ± 0.12; P < 0.0001, Fig. 1d). sCD127 mRNA level in tissue-infiltrating CD8+ T cells was also reduced in tumor tissues (0.64 ± 0.13) when compared with para-tumor tissues (0.76 ± 0.11; P = 0.013, Fig. 1d). Plasma sCD127 expression was lower in melanoma patients (269.2 ± 63.24 pg/mL) when compared with controls (308.7 ± 70.58 pg/mL; P = 0.029, Fig. 1e).
Fig. 1.
Membrane-bound CD127 (mCD127) on CD8+ T cells was higher, while soluble (sCD127) was lower in patients with primary cutaneous melanoma. Peripheral blood mononuclear cells were isolated from all enrolled subjects (38 patients with primary cutaneous melanoma and 22 healthy controls), while tissue-infiltrating lymphocytes were isolated from tumor and para-tumor tissues of 14 melanoma patients with surgical operation. Cells were stained with anti-CD8 and anti-CD127, and were analyzed by flow cytometry. a The representative flow cytometry analyses for peripheral blood mononuclear cells and tissue-infiltrating lymphocytes are shown. Live lymphocytes were gated according to forward scatter (FSC) and side scatter (SSC). mCD127 expression within CD8+ T cells were assessed. b The percentage of peripheral CD8+ T cells expressing mCD127 was elevated in melanoma patients compared with controls. The percentage of tissue-infiltrating CD8+ T cells expressing mCD127 was also increased in tumor tissues compared with para-tumor tissues. CD8+ T cells were purified from peripheral bloods and tissue-infiltrating lymphocytes. Total CD127 mRNA and sCD127 mRNA variant in CD8+ T cells was measured by real-time PCR. c Total CD127 mRNA level in peripheral CD8+ T cells was comparable between melanoma patients and controls. Total CD127 mRNA level in tissue-infiltrating CD8+ T cells was also comparable between tumor tissues and para-tumor tissues. d sCD127 mRNA level in peripheral CD8+ T cells was lower in melanoma patients compared with controls. sCD127 mRNA level in tissue-infiltrating CD8+ T cells was also lower in tumor tissues compared with para-tumor tissues. e sCD127 expression in the plasma was measured by ELISA. Plasma sCD127 level was lower in melanoma patients compared with controls. Individual level of each subject is shown. Statistical analysis was performed using Student’s t test
IL-7 stimulation induced sCD127 release without affecting mCD127 expression on CD8+ T cells in patients with primary cutaneous melanoma and controls
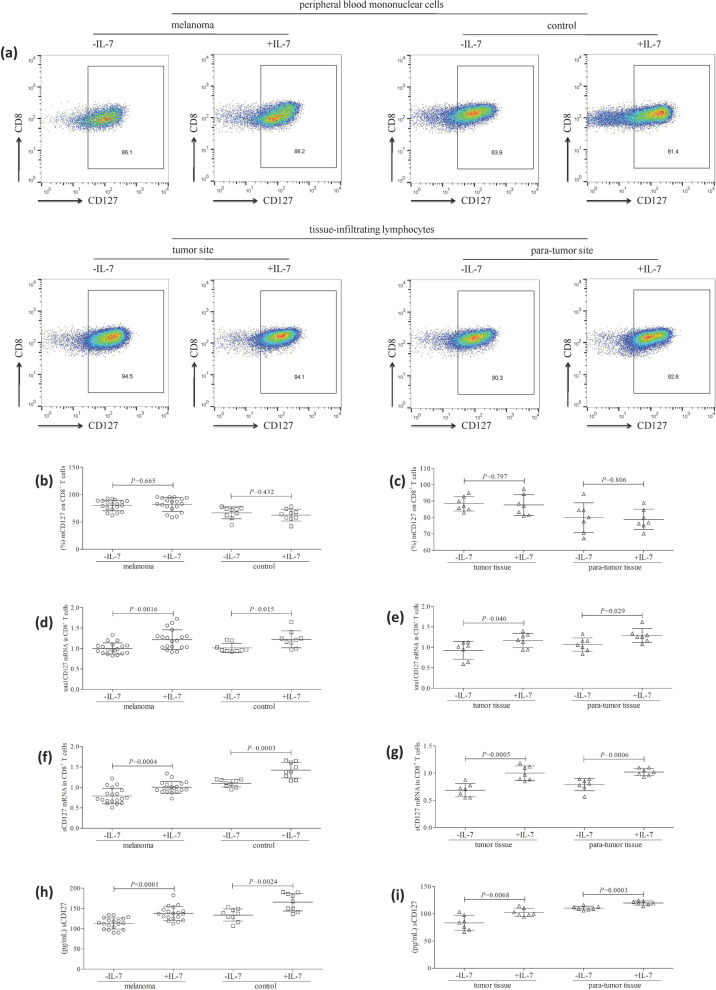
104 of purified CD8+ T cells from patient with primary cutaneous melanoma and controls were stimulated with recombinant human IL-7 (10 ng/mL) for 48 h in the presence of anti-CD3/CD28. Control cells were cultured with anti-CD3/CD28 only. The representative flow dots for mCD127 expression in peripheral and tissue-infiltrating CD8+ T cells are shown in Fig. 2a. There was no significant difference of the percentage of peripheral CD8+ T cells expressing mCD127 between cells with and without IL-7 stimulation in either melanoma patients or controls (P > 0.05, Fig. 2b). Similarly, IL-7 stimulation did not affect the percentage of tissue-infiltrating CD8+ T cells expressing mCD127 in either tumor or para-tumor tissues (P > 0.05, Fig. 2c). IL-7 stimulation enhanced total CD127 mRNA level both in peripheral CD8+ T cells from melanoma patients (1.22 ± 0.24 vs. 1.00 ± 0.14; P = 0.0016, Fig. 2d) and controls (1.23 ± 0.21 vs. 1.01 ± 0.11; P = 0.015, Fig. 2d), as well as in tissue-infiltrating CD8+ T cells from tumor tissues (1.17 ± 0.18 vs. 0.93 ± 0.22; P = 0.040, Fig. 2e) and para-tumor tissues (1.29 ± 0.17 vs. 1.07 ± 0.16; P = 0.029, Fig. 2e). Similarly, IL-7 stimulation also promoted sCD127 mRNA level both in peripheral CD8+ T cells from melanoma patients (1.00 ± 0.14 vs. 0.79 ± 0.19; P = 0.0004, Fig. 2f) and controls (1.42 ± 0.19 vs. 1.10 ± 0.09; P = 0.0003, Fig. 2f), as well as in tissue-infiltrating CD8+ T cells from tumor tissues (1.00 ± 0.13 vs. 0.69 ± 0.12; P = 0.0005, Fig. 2g) and para-tumor tissues (1.02 ± 0.07 vs. 0.79 ± 0.11; P = 0.0006, Fig. 2g). IL-7 mediated the elevation of sCD127 secretion by peripheral CD8+ T cells from both melanoma patients (137.6 ± 17.09 pg/mL vs. 112.9 ± 13.84 pg/mL; P < 0.0001, Fig. 2h) and controls (165.7 ± 21.91 pg/mL vs. 133.8 ± 15.10 pg/mL; P = 0.0024, Fig. 2h). IL-7 also induced sCD127 secretion by tissue-infiltrating CD8+ T cells from both tumor tissues (83.44 ± 13.59 pg/mL vs. 102.5 ± 7.47 pg/mL; P = 0.0068, Fig. 2i) and para-tumor tissues (119.9 ± 3.67 pg/mL vs. 110/5 ± 3.45 pg/mL; P = 0.0003, Fig. 2i).
Fig. 2.
Recombinant human IL-7 stimulation did not affect mCD127 expression on CD8+ T cells, but induced sCD127 release in patients with primary cutaneous melanoma and controls. 104 of peripheral CD8+ T cells (nineteen patients with primary cutaneous melanoma and nine healthy controls) and tissue-infiltrating CD8+ T cells (tumor and para-tumor tissues of seven melanoma patients) were stimulated with recombinant human IL-7 (10 ng/mL) for 48 h in the presence of anti-CD3/CD28. Control cells were cultured with anti-CD3/CD28 only. Cells and supernatants were harvested. a mCD127 expression on CD8+ T cells was investigated by flow cytometry, and the representative flow dots are shown. b There was no significant difference of the percentage of peripheral CD8+ T cells expressing mCD127 between cells with and without IL-7 stimulation in either melanoma patients or controls. c There was no remarkable difference of the percentage of tissue-infiltrating CD8+ T cells expressing mCD127 between cells with and without IL-7 stimulation in either tumor or para-tumor tissues. d IL-7 stimulation enhanced total CD127 mRNA level in peripheral CD8+ T cells in both melanoma patients and controls. e IL-7 stimulation enhanced total CD127 mRNA level in tissue-infiltrating CD8+ T cells in both tumor tissues and para-tumor tissues. f IL-7 stimulation promoted sCD127 mRNA level in peripheral CD8+ T cells in both melanoma patients and controls. g IL-7 stimulation promoted sCD127 mRNA level in tissue-infiltrating CD8+ T cells in both tumor tissues and para-tumor tissues. h IL-7 stimulation promoted sCD127 secretion by peripheral CD8+ T cells in both melanoma patients and controls. i IL-7 stimulation promoted sCD127 secretion by tissue-infiltrating CD8+ T cells in both tumor tissues and para-tumor tissues. Individual level of each subject is shown. Statistical analysis was performed using Student’s t test
IL-7-induced sCD127 release by CD8+ T cells could be inhibited by PI3K blockade
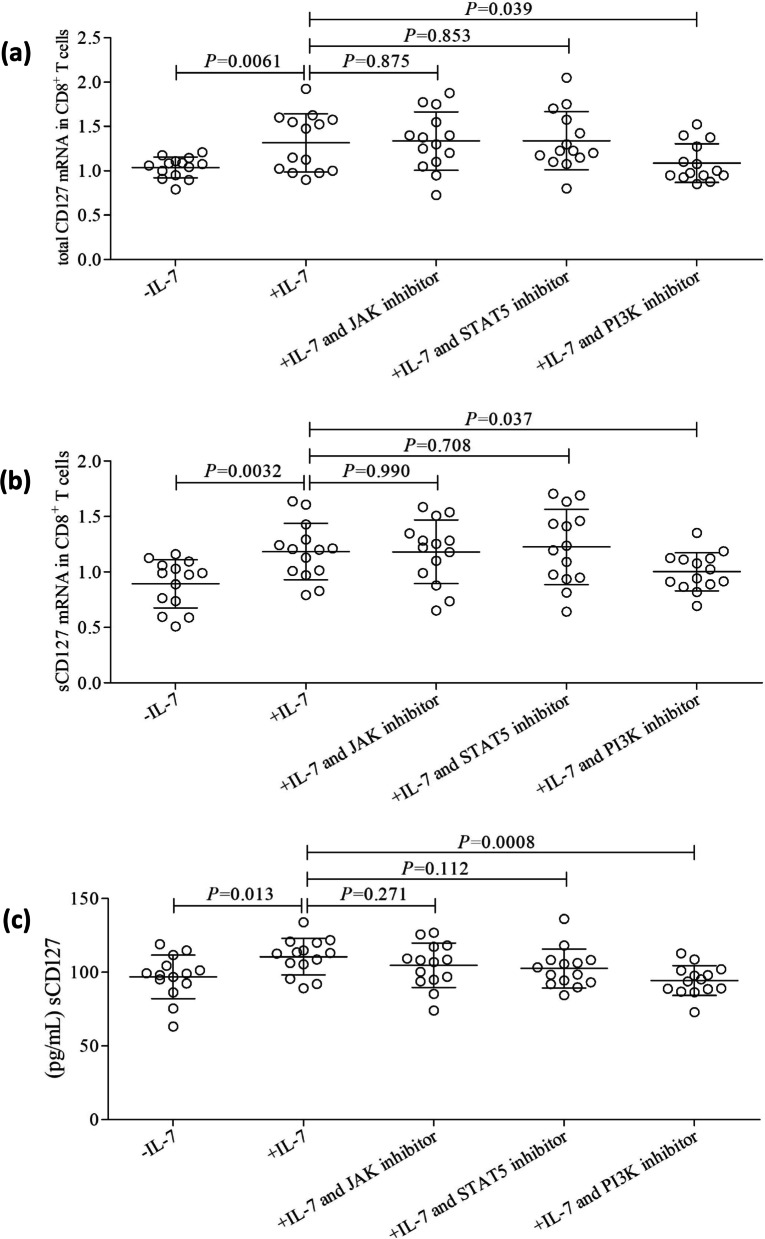
JAK, STAT5, and PI3K are primary activated signaling pathways following the binding of IL-7 to IL-7 receptor complex [13, 14]. To investigate the mechanisms by which IL-7 modulates the CD127 secretion by CD8+ T cells, 104 of purified peripheral CD8+ T cells from fourteen patient with primary cutaneous melanoma were stimulated with recombinant human IL-7 (10 ng/mL) for 48 h in the presence of anti-CD3/CD28, along with either JAK inhibitor (10 µmol/L), STAT5 inhibitor (250 µmol/L), or PI3K inhibitor (25 µmol/L). Control cells were cultured with anti-CD3/CD28 only. Inhibition of either JAK or STAT5 did not affect IL-7-mediated elevation of total CD127 mRNA expression (Fig. 3a), sCD127 mRNA level (Fig. 3b), or sCD127 production by CD8+ T cells (Fig. 3c) (P > 0.05). Importantly, PI3K inhibitor abrogated the effect of IL-7 on total CD127 mRNA (Fig. 3a), sCD127 mRNA expression (Fig. 3b), or sCD127 secretion by CD8+ T cells (Fig. 3c) (P < 0.05).
Fig. 3.
Influence of JAK, STAT5, and PI3K inhibitor to IL-7-mediated CD127 expression in CD8+ T cells in patients with primary cutaneous melanoma. 104 of purified peripheral CD8+ T cells from fourteen patient with primary cutaneous melanoma were stimulated with recombinant human IL-7 (10 ng/mL) for 48 h in the presence of anti-CD3/CD28, along with either JAK inhibitor (10 µmol/L), STAT5 inhibitor (250 µmol/L), or PI3K inhibitor (25 µmol/L). Control cells were cultured with anti-CD3/CD28 only. Cells and supernatants were harvested. Total CD127 mRNA and sCD127 mRNA level in CD8+ T cells were measured by real-time PCR. a IL-7 stimulation promoted total CD127 mRNA expression in CD8+ T cells. Either JAK inhibitor or STAT5 inhibitor did not affect IL-7-mediated elevation of total CD127 mRNA expression, while PI3K inhibitor abrogated the effect of IL-7 on total CD127 mRNA induction in CD8+ T cells. b IL-7 stimulation also enhanced sCD127 mRNA level in CD8+ T cells. Either JAK inhibitor or STAT5 inhibitor did not influence IL-7-induced elevation of sCD127 mRNA expression, while PI3K inhibitor abrogated the effect of IL-7 on sCD127 mRNA induction in CD8+ T cells. c sCD127 level in the supernatants was measured by ELISA. IL-7 stimulation promoted sCD127 level in the cultured supernatant of CD8+ T cells. Either JAK inhibitor or STAT5 inhibitor did not influence IL-7-induced sCD127 secretion, while PI3K inhibitor abrogated the effect of IL-7 on sCD127 production by CD8+ T cells. Individual level of each subject is shown. Statistical analysis was performed using one-way ANOVA and LSD-t test
IL-7-induced enhancement of CD8+ T cell cytotoxicity could be suppressed by PI3K blockade
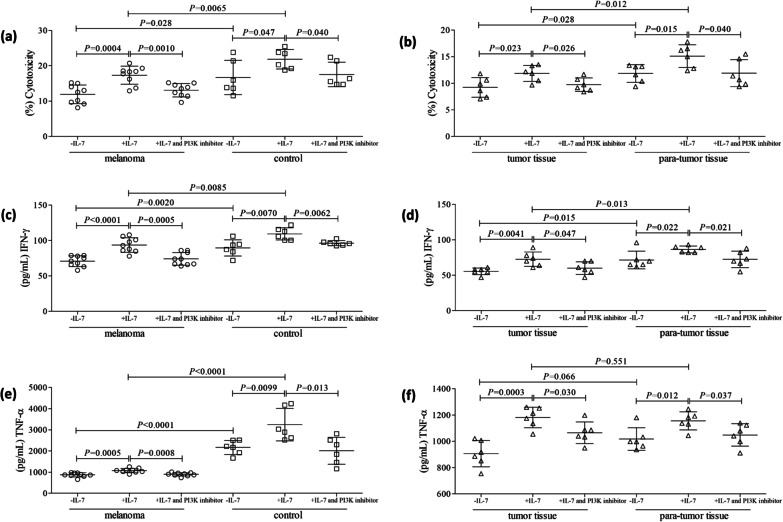
Purified CD8+ T cells from HLA-A02 restricted patient with primary cutaneous melanoma and HLA-A02 restricted healthy individuals were stimulated with recombinant human IL-7 (10 ng/mL) and PI3K inhibitor (25 µmol/L) for 48 h in the presence of anti-CD3/CD28. Cells were washed twice, and 104 of stimulated CD8+ T cells were co-cultured with 105 of SK-MEL-5 cells for 48 h. Peripheral CD8+ T cells presented decreased cytotoxicity from melanoma patients, which mediated reduced target cell death compared with healthy individuals (11.88 ± 2.64% vs. 16.67 ± 4.86%; P = 0.028, Fig. 4a). Similarly, tissue-infiltrating CD8+ T cells also showed reduced cytotoxicity from tumor tissues compared with para-tumor tissues (11.85 ± 1.67% vs. 9.23 ± 1.85%; P = 0.028, Fig. 4b). IL-7 promoted the cytotoxicity of peripheral CD8+ T cells from melanoma patients (P = 0.0004, Fig. 4a) and controls (P = 0.047, Fig. 4a), as well as tissue-infiltrating CD8+ T cells from tumor tissues (P = 0.023, Fig. 4b) and para-tumor tissues (P = 0.015, Fig. 4b). The cytotxocity of CD8+ T cells was still lower in peripheral blood and tumor tissues in melanoma patients (P < 0.05, Fig. 4a, b). PI3K inhibitor abrogated IL-7-induced elevation of peripheral and tissue-infiltrating CD8+ T cells in vitro (P < 0.05, Fig. 4a, b). IFN-γ and TNF-α level in the supernatants was measured by ELISA. IL-7 enhanced both IFN-γ (P < 0.05, Fig. 4c, d) and TNF-α (P < 0.05, Fig. 4e,f) expression in the cultured supernatants of peripheral and tissue-infiltrating CD8+ T cells co-culture system. PI3K inhibitor also abrogated IL-7-induced IFN-γ (P < 0.05, Fig. 4c, d) and TNF-α (P < 0.05, Fig. 4e, f) secretion by peripheral and tissue-infiltrating CD8+ T cells.
Fig. 4.
Influence of PI3K inhibitor to IL-7-mediated CD8+ T cell cytotoxicity in patients with primary cutaneous melanoma. Purified peripheral CD8+ T cells (nine HLA-A02 restricted patient with primary cutaneous melanoma and six HLA-A02 restricted healthy individuals) and tissue-infiltrating CD8+ T cells (tumor and para-tumor tissues of six HLA-A02 restricted melanoma patients) were stimulated with recombinant human IL-7 (10 ng/mL) and PI3K inhibitor (25 µmol/L) for 48 h in the presence of anti-CD3/CD28. Cells were washed twice, and 104 of stimulated CD8+ T cells were co-cultured with 105 of SK-MEL-5 cells for another 48 h. The percentage of target cell death was calculated by measurement of LDH level in the supernatants. IFN-γ and TNF-α level in the supernatants was measured by ELISA. Inhibition of PI3K dampened the cytotoxicity of a peripheral CD8+ T cells from melanoma patients and controls, as well as b tissue-infiltrating CD8+ T cells from tumor tissues and para-tumor tissues. Inhibition of PI3K inhibitor suppressed IFN-γ secretion by c peripheral CD8+ T cells from melanoma patients and controls, as well as d tissue-infiltrating CD8+ T cells from tumor tissues and para-tumor tissues. Inhibition of PI3K inhibitor also suppressed TNF-α secretion by e peripheral CD8+ T cells from melanoma patients and controls, as well as f tissue-infiltrating CD8+ T cells from tumor tissues and para-tumor tissues. Individual level of each subject is shown. Statistical analysis was performed using one-way ANOVA and LSD-t test
Discussion
In the present study, we firstly screened the expression profile of sCD127 and mCD127 expression in CD8+ T cells in patients with primary cutaneous melanoma. Circulating sCD127 was lower, while mCD127 expression on CD8+ T cells was higher in melanoma patients. Meanwhile, sCD127 mRNA variant was also lower in melanoma patients, although there was no significant difference of total CD127 mRNA variant between melanoma patients and healthy individuals. Moreover, IL-7 is known to regulate CD127 expression in CD8+ T cells through multiple mechanisms in vitro [17, 19, 20], and we previously reported the decreased expression of peripheral IL-7 in melanoma patients [10]. Thus, we further investigated the modulatory activity of exogenous IL-7 to CD127 expression and cytotoxicity of circulating and tissue-infiltrating CD8+ T cells in melanoma patients. We found that recombinant human IL-7 stimulation promoted sCD127 release by CD8+ T cells through PI3K signaling pathway. This process was accompanied by the elevation of both sCD127 mRNA and total CD127 mRNA in CD8+ T cells, but mCD127 expression on CD8+ T cells was not affected. Inhibition of PI3K abrogated IL-7-induced enhancement of CD8+ T cell cytotoxicity in both melanoma patients and healthy individuals. The current data revealed that IL-7 contributed to cytotoxicity of CD8+ T cells and regulation of CD127 expression.
The expression of IL-7 receptor complex on the immune cells not only determines the responsiveness of the cells to IL-7, but also showed the consuming efficiency of cell to IL-7 [23]. Thus, chronic infection and cancers might induce the changes in CD127 expression profile, leading to the immune tolerance or evasion for viral persistence or tumor metastasis. However, CD127 expression profile was divergently reported in various diseases. Chronic hepatitis C patients had lower mCD127 level on circulating CD8+ T cells [21]. Similarly, reduced mCD127 expression on T cells was closely associated with decreased CD4+ T cell counts and increased viral replication in HIV-1-infected patients [24]. There was no significant difference of mCD127 expression on peripheral or liver-infiltrating CD8+ T cells between hepatocellular carcinoma patients and controls [22]. However, number of T cells expressing CD127 was reduced in peripheral blood of patients with breast cancer, resulting in IL-7 signaling defects [25]. Herein, we showed that there was imbalance between sCD127 and mCD127 expression on CD8+ T cells in melanoma patients. In contrast to previous findings, our results revealed lower level of circulating sCD127 and higher expression of mCD127 on CD8+ T cells in melanoma patients. Meanwhile, sCD127 mRNA, but not total RNA, was also reduced in CD8+ T cells in melanoma patients, indicating that melanoma might mainly suppress sCD127 release from CD8+ T cells, resulting in the elevation of mCD127 expression on CD8+ T cells without impacting total CD127 level. However, the role of alternative expression profile of CD127 in melanoma is still need further elucidated.
The mechanisms involved in the regulation of sCD127/mCD127 expression are not fully understood. In vitro IL-4 stimulation suppressed IL-7-mediated STAT5 phosphorylation, leading to reduction in mCD127 expression and mature CD8+ T cells proliferation [26]. sCD127 secretion by purified CD8+ T cells was also dependent on MMP-9 activity, which did not affect mCD127 expression on the cell surface in HIV-infected patients [20]. IL-7 could mediate alternative CD127 expression profile in various diseases. In this study, the regulatory function of IL-7 to CD127 expression in melanoma patients was investigated. In the previous study on IL-7 regulation to CD8+ T cells in HCC patients, 10 ng/ml of IL-7 was used in vitro stimulation [22]. Similarly, Hou et al. used 5 ng/ml of IL-7 for CD8+ T cells stimulation in vitro in chronic hepatitis C virus-infected patients [21]. The higher concentration of exogenous IL-7 might be sufficient for cellular stimulation in cancers. Thus, we chose 10 ng/ml of recombinant IL-7 for CD8+ T cells stimulation in melanoma patients. Our present study revealed that exogenous IL-7 promoted the release of sCD127 by CD8+ T cells, but mCD127 expression on CD8+ T cells was not affected. This was controversial with the previous reports showing that in vitro human T cells expressed reduced mCD127 in the presence of exogenous IL-7 [16, 17, 20] and in vivo IL-7 administration led to the reduction of mCD127 expression on T cells [18, 27]. Most the above studies were performed in HIV-infected individuals. However, the regulatory role of IL-7 might be in a context-specific manner, and might be different in cancers. Thus, our current data suggested that IL-7-induced sCD127 release by CD8+ T cells might be not related to proteolytic cleavage and shedding of the membrane receptor as described with other cytokine receptors [28, 29], which was not consistent with the in vitro and in vivo regulatory mechanism between mCD100 and sCD100 balance in lung cancer [30] and chronic viral infections [31, 32]. Moreover, IL-7 also induced the pathway favoring the alternatively spliced version of CD127 gene, leading to the direct secretion of truncated sCD127. Meanwhile, total CD127 RNA level in CD8+ T cells was also increased in response to IL-7 stimulation. This indicated that IL-7 could also mediated sCD127 production via increased gene expression, resulting in transcriptional regulation of sCD127 and total CD127 gene. Previous studies also demonstrated post-transcriptional and translational modulation to mCD127 in mice [33]. Thus, it was likely that IL-7 might induce further pathways following translation of spliced sCD127 mRNA variant to elevate sCD127 release by CD8+ T cells. Furthermore, IL-7-induced sCD127 release by CD8+ T cells was mainly dependent on PI3K activity, but was independent of JAK/STAT5 signaling pathway. This was not consistent with our previous finding that in vitro regulation of Th17 response to IL-7 was STAT5 dependent in melanoma patients [10]. IL-7 receptor complex could activate PI3K/Akt/mTOR signaling pathway in lung cancer cell line and leukemia transformation cell line [34, 35]. IL-7 receptor-dependent PI3K also competed with STAT5 signal to regulate T cell development and homeostasis in mice [36]. Taken together, IL-7 induced an increase in sCD27 mRNA variant in CD8+ T cells and release of sCD127 by CD8+ T cells probably through PI3K signaling pathway.
CD8+ T cell exhaustion was one of the hallmarks in the immunopathogenesis of chronic viral infection and malignancies [37]. Our present data revealed the decreased cytotoxicity of both peripheral and tumor-infiltrating CD8+ T cells to target melanoma cell line in vitro, confirming the CD8+ T cell dysfunction or exhaustion in melanoma patients. Consistent with the previous finding in viral infection and cancers [22, 38–40], we found that IL-7 promoted peripheral and tissue-infiltrating CD8+ T cell activity in melanoma patients. CD8+ T cells exhibited cytotoxicity via two independent pathways. CD8+ T cells not only revealed direct cytolytic activity to target cells through perforin/granzyme secretion and Fas/FasL interaction, but also secreted pro-inflammatory cytokines, including IFN-γ and TNF-α, inducing cytokine-mediated cell necrosis or apoptosis [41, 42]. IL-7 stimulation to CD8+ T cells from melanoma patients not only enhanced direct cytolytic function of CD8+ T cells to target cells, but also promoted IFN-γ and TNF-α secretion, indicating that IL-7 increased cytolytic and non-cytolytic activity of CD8+ T cells in melanoma patients. Importantly, CD8+ T cell-induced cytotoxicity or IFN-γ production in response to IL-7 stimulation was still lower in peripheral blood and tumor tissue of melanoma patients. This might be due to the decreased responsiveness of CD8+ T cells to IL-7 in cancers, As previous findings showed that IL-7 did not affect tumor patients derived CD8+ T cell proliferation in vitro [22]. Furthermore, administration of PI3K inhibitor dampen IL-7-induced enhancement of CD8+ T cell cytotoxicity, which was closely related to CD127 regulation. TNF-α secretion by IL-7 stimulation was increased by in CD8+ cells from tumor and para-tumor tissue. However, PI3K inhibitor administration did not reduce the TNF-α level to untreated level. On the one hand, CD8+ T cell-induced target cells was significantly decreased to untreated level with IL-7 stimulation in combination with PI3K blockade, indicating that TNF-α might not be a key mediator for cytotoxicity of CD8+ T cells. On the other hand, IL-7 treatment increased α4β7 integrin expression on naïve human T cells [43, 44], resulting in the amplification of inflammation induced by other cytokines, primarily TNF-α [45]. IL-7 signaling pathway influence anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease [43]. Transcription factor FoxO1, which ameliorated TNF-α-induced tissue damage [46], also served as the downstream of IL-7 signaling pathway and contributed to adenosine-mediated CD8+ T cells immunosuppression [47]. Thus, there might be another signaling pathway, which was activated by IL-7, leading to TNF-α production. Moreover, PI3K plays multiple activities both physiologically and pathologically. Thus, PI3K might impact sCD127 expression, while separately having an impact on the cytotoxic potential of CD8+ T cells. Taken together, the current data indicated that IL-7 promoted CD127 expression and the cytotoxic potential of CD8+ T cell in melanoma patients. However, the major limitation of the present work was the small sample size, which could be further addressed by in vivo experiments.
Conclusion
In summary, insufficient IL-7 secretion might contribute to CD8+ T cell exhaustion and CD127 dysregulation in patients with primary cutaneous melanoma. IL-7 signaling through CD127 and PI3K pathway might be one of the potential therapeutic approaches to promote immune response for treatment of melanoma.
Acknowledgements
We thank the volunteers for their participation in this study.
Abbreviations
- ELISA
Enzyme-linked immunosorbent assay
- HIV
Human immunodeficiency virus
- IFN-γ
Interferon-γ
- IL
Interleukin
- JAK/STAT5
Janus kinase/signal transducer and activator of transcription 5
- LDH
Lactate dehydrogenase
- mCD127
Membrane-bound CD127
- MMP
Matrix metalloproteinases
- PBMCs
Peripheral blood mononuclear cells
- PI3K
Phosphatidylinositol 3-kinase
- sCD127
Soluble CD127
- TILs
Tumor-infiltrating lymophocytes
- TNF-α
Tumor necrosis factor-α
Author contributions
HH designed and supervised the study. HH, BQ, SG, HC, ZZ, and JQ carried out the experiments, analyzed and interpreted the data. HH and BQ drafted the manuscript. JQ revised the manuscript. All authors have read and approved the manuscript.
Funding
None.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to limitations of ethical approval involving the patient data and anonymity but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved in compliance with the Declaration of Helsinki by the Ethics Committee at The First Hospital of Shanxi Medical University (Approval number: 2016-KY-08072 and 2021-K-K102). Written informed consent was obtained from all enrolled patients and controls.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongxia He and Binjun Qiao contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28(6):1005–1011. [PubMed] [Google Scholar]
- 3.Knackstedt T, Knackstedt RW, Couto R, Gastman B. Malignant melanoma: diagnostic and management update. Plast Reconstr Surg. 2018;142(2):202e–216e. doi: 10.1097/PRS.0000000000004571. [DOI] [PubMed] [Google Scholar]
- 4.Dowling J, McGregor SP, Williford P. Update on current treatment recommendations for primary cutaneous melanoma. Dermatol Clin. 2019;37(4):397–407. doi: 10.1016/j.det.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Xu W, Lian B, Cui C, Guo J. The combination therapy with the cytotoxic T lymphocyte-associated antigen-4 and programmed death 1 antibody-induced asthma in a patient with advanced melanoma. J Cancer Res Ther. 2021;17(3):808–810. doi: 10.4103/jcrt.jcrt_419_21. [DOI] [PubMed] [Google Scholar]
- 6.Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, Rapisuwon S, Eroglu Z, Sullivan RJ, Luke JJ, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122(21):3354–3362. doi: 10.1002/cncr.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J, Zhu Z, Xiao H, Wakefield MR, Ding VA, Bai Q, Fang Y. The role of IL-7 in immunity and cancer. Anticancer Res. 2017;37(3):963–967. doi: 10.21873/anticanres.11405. [DOI] [PubMed] [Google Scholar]
- 8.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11(5):330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen V, Mendelsohn A, Larrick JW. Interleukin-7 and Immunosenescence. J Immunol Res. 2017;2017:4807853. doi: 10.1155/2017/4807853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He H, Qiao B, Guo S, Cui H, Li N, Liu H, Qin J, He J, Yang X, Xue W, Wang Y. Induction of T helper 17 cell response by interleukin-7 in patients with primary cutaneous melanoma. Melanoma Res. 2021;31(4):328–337. doi: 10.1097/CMR.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 11.Barata JT, Durum SK, Seddon B. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol. 2019;20(12):1584–1593. doi: 10.1038/s41590-019-0479-x. [DOI] [PubMed] [Google Scholar]
- 12.Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24(3):209–217. doi: 10.1016/j.smim.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallard C, Stegmann AP, van Kleffens T, Smart F, Venkitaraman A, Spits H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10(5):525–535. doi: 10.1016/S1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 14.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200(5):659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J Immunol. 2008;180(8):5201–5210. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- 16.Ghazawi FM, Faller EM, Sugden SM, Kakal JA, MacPherson PA. IL-7 downregulates IL-7Ralpha expression in human CD8 T cells by two independent mechanisms. Immunol Cell Biol. 2013;91(2):149–158. doi: 10.1038/icb.2012.69. [DOI] [PubMed] [Google Scholar]
- 17.Vranjkovic A, Crawley AM, Gee K, Kumar A, Angel JB. IL-7 decreases IL-7 receptor alpha (CD127) expression and induces the shedding of CD127 by human CD8+ T cells. Int Immunol. 2007;19(12):1329–1339. doi: 10.1093/intimm/dxm102. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Rose T, Lambotte O, Pallier C, Delfraissy JF, Colle JH. Identification and biochemical characterization of human plasma soluble IL-7R: lower concentrations in HIV-1-infected patients. J Immunol. 2009;182(12):7389–7397. doi: 10.4049/jimmunol.0900190. [DOI] [PubMed] [Google Scholar]
- 20.Cote SC, Burke Schinkel SC, Berthoud TK, Barros PO, Sanchez-Vidales M, Davidson AM, Crawley AM, Angel JB. IL-7 induces sCD127 release and mCD127 downregulation in human CD8(+) T cells by distinct yet overlapping mechanisms, both of which are impaired in HIV infection. Eur J Immunol. 2020;50(10):1537–1549. doi: 10.1002/eji.201948453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou H, Kang Y, Zeng Y, Li Y, Shang J. Interleukin-7 augments CD8(+) T cells function and promotes viral clearance in chronic hepatitis C virus infection. Cytokine. 2018;102:26–33. doi: 10.1016/j.cyto.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Teng D, Ding L, Cai B, Luo Q, Wang H. Interleukin-7 enhances anti-tumor activity of CD8(+) T cells in patients with hepatocellular carcinoma. Cytokine. 2019;118:115–123. doi: 10.1016/j.cyto.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7(2):144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 24.Crawley AM, Angel JB. The influence of HIV on CD127 expression and its potential implications for IL-7 therapy. Semin Immunol. 2012;24(3):231–240. doi: 10.1016/j.smim.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Vudattu NK, Magalhaes I, Schmidt M, Seyfert-Margolis V, Maeurer MJ. Reduced numbers of IL-7 receptor (CD127) expressing immune cells and IL-7-signaling defects in peripheral blood from patients with breast cancer. Int J Cancer. 2007;121(7):1512–1519. doi: 10.1002/ijc.22854. [DOI] [PubMed] [Google Scholar]
- 26.Crawley AM, Vranjkovic A, Young C, Angel JB. Interleukin-4 downregulates CD127 expression and activity on human thymocytes and mature CD8+ T cells. Eur J Immunol. 2010;40(5):1396–1407. doi: 10.1002/eji.200940093. [DOI] [PubMed] [Google Scholar]
- 27.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113(25):6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher N, Meyer D, Mauermann A, von der Heyde J, Wolf J, Schwarz J, Knittler K, Murphy G, Michalek M, Garbers C, et al. Shedding of endogenous interleukin-6 receptor (IL-6R) is governed by a disintegrin and metalloproteinase (ADAM) proteases while a full-length IL-6R isoform localizes to circulating microvesicles. J Biol Chem. 2015;290(43):26059–26071. doi: 10.1074/jbc.M115.649509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chanthaphavong RS, Loughran PA, Lee TY, Scott MJ, Billiar TR. A role for cGMP in inducible nitric-oxide synthase (iNOS)-induced tumor necrosis factor (TNF) alpha-converting enzyme (TACE/ADAM17) activation, translocation, and TNF receptor 1 (TNFR1) shedding in hepatocytes. J Biol Chem. 2012;287(43):35887–35898. doi: 10.1074/jbc.M112.365171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HM, Zhang XH, Ye LQ, Zhang K, Yang NN, Geng S, Chen J, Zhao SX, Yang KL, Fan FF. Insufficient CD100 shedding contributes to suppression of CD8(+) T-cell activity in non-small cell lung cancer. Immunology. 2020;160(2):209–219. doi: 10.1111/imm.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S, Wang L, Pan W, Bayer W, Thoens C, Heim K, Dittmer U, Timm J, Wang Q, Yu Q, et al. MMP2/MMP9-mediated CD100 shedding is crucial for inducing intrahepatic anti-HBV CD8 T cell responses and HBV clearance. J Hepatol. 2019;71(4):685–698. doi: 10.1016/j.jhep.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Zhang DN, Liu Y, Li X, Gao Y, Xi FY, Li Y, Zhu GZ. Imbalance between soluble and membrane-bound CD100 regulates monocytes activity in hepatitis B virus-associated acute-on-chronic liver failure. Viral Immunol. 2021;34(4):273–283. doi: 10.1089/vim.2020.0311. [DOI] [PubMed] [Google Scholar]
- 33.Luo H, Wu Z, Qi S, Jin W, Han B, Wu J. Ephrinb1 and Ephrinb2 are associated with interleukin-7 receptor alpha and retard its internalization from the cell surface. J Biol Chem. 2011;286(52):44976–44987. doi: 10.1074/jbc.M111.316414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jian M, Yunjia Z, Zhiying D, Yanduo J, Guocheng J. Interleukin 7 receptor activates PI3K/Akt/mTOR signaling pathway via downregulation of Beclin-1 in lung cancer. Mol Carcinog. 2019;58(3):358–365. doi: 10.1002/mc.22933. [DOI] [PubMed] [Google Scholar]
- 35.Cante-Barrett K, Spijkers-Hagelstein JA, Buijs-Gladdines JG, Uitdehaag JC, Smits WK, van der Zwet J, Buijsman RC, Zaman GJ, Pieters R, Meijerink JP. MEK and PI3K-AKT inhibitors synergistically block activated IL7 receptor signaling in T-cell acute lymphoblastic leukemia. Leukemia. 2016;30(9):1832–1843. doi: 10.1038/leu.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui G, Shimba A, Ma G, Takahara K, Tani-Ichi S, Zhu Y, Asahi T, Abe A, Miyachi H, Kitano S, et al. IL-7R-Dependent phosphatidylinositol 3-kinase competes with the STAT5 signal to modulate T cell development and homeostasis. J Immunol. 2020;204(4):844–857. doi: 10.4049/jimmunol.1900456. [DOI] [PubMed] [Google Scholar]
- 37.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 38.Tian X, Su Z, Guo S, Wang C, Zhang X, Du J. Interleukin-7 promotes CD8(+) T cell activity in patients with enterovirus 71 associated encephalitis. Int Immunopharmacol. 2019;75:105773. doi: 10.1016/j.intimp.2019.105773. [DOI] [PubMed] [Google Scholar]
- 39.Cote S, Matte J, Sad S, Angel JB, Crawley AM. Complexed soluble IL-7 receptor alpha and IL-7 increase IL-7-mediated proliferation and viability of CD8(+) T-cells in vitro. Cell Immunol. 2015;293(2):122–125. doi: 10.1016/j.cellimm.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Colombetti S, Levy F, Chapatte L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T-cell responses after immunization with recombinant lentivector. Blood. 2009;113(26):6629–6637. doi: 10.1182/blood-2008-05-155309. [DOI] [PubMed] [Google Scholar]
- 41.Saeidi A, Buggert M, Che KF, Kong YY, Velu V, Larsson M, Shankar EM. Regulation of CD8+ T-cell cytotoxicity in HIV-1 infection. Cell Immunol. 2015;298(1–2):126–133. doi: 10.1016/j.cellimm.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065X.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 43.Belarif L, Danger R, Kermarrec L, Nerriere-Daguin V, Pengam S, Durand T, Mary C, Kerdreux E, Gauttier V, Kucik A, et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J Clin Invest. 2019;129(5):1910–1925. doi: 10.1172/JCI121668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cimbro R, Vassena L, Arthos J, Cicala C, Kehrl JH, Park C, Sereti I, Lederman MM, Fauci AS, Lusso P. IL-7 induces expression and activation of integrin alpha4beta7 promoting naive T-cell homing to the intestinal mucosa. Blood. 2012;120(13):2610–2619. doi: 10.1182/blood-2012-06-434779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pathak K. The expanding role of IL-7 and thymic stromal lymphopoietin as therapeutic target for rheumatoid arthritis. Expert Opin Ther Targets. 2014;18(5):581–594. doi: 10.1517/14728222.2014.893295. [DOI] [PubMed] [Google Scholar]
- 46.Huang X, Chen H, Xie Y, Cao Z, Lin X, Wang Y. FoxO1 overexpression ameliorates TNF-alpha-induced oxidative damage and promotes osteogenesis of human periodontal ligament stem cells via antioxidant Defense activation. Stem Cells Int. 2019;2019:2120453. doi: 10.1155/2019/2120453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyas A, Tucer S, Kayhan M, Savas AC, Akdemir I, Cekic C. Interleukin-7 protects CD8(+) T cells from adenosine-mediated immunosuppression. Sci Signal. 2021;14(674):1269. doi: 10.1126/scisignal.abb1269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to limitations of ethical approval involving the patient data and anonymity but are available from the corresponding author on reasonable request.