The anti-geronic protein α-Klotho (referred to as Klotho), plays a potential role in delaying age-related declines in cellular and tissue function, which has generated considerable interest in targeting this protein for the treatment of age-related neurodegenerative diseases.1 Systemic exogenous elevation of Klotho enhances cognition and neural resilience in young, aging, and in vivo models of Parkinson’s disease (PD).2–4 Interestingly, in addition to age-related abnormalities, cognitive decline, and premature death, Klotho-deficient mice display nigrostriatal neurodegeneration, a hallmark of PD.5,6 We recently identified a role for Klotho in the regulation of mitochondrial DNA damage and bioenergetics.7 Given increased mitochondrial DNA damage and mitochondrial dysfunction in PD, we explored circulating levels of Klotho in human PD for the first time.8–10
To determine whether circulating plasma Klotho levels were associated with PD, we analyzed 122 plasma samples from the Fox Investigation for New Discovery of Biomarkers (BioFIND) cohort, a cross-sectional, observational study of moderate to advanced PD patients and age- and sex-matched healthy controls in which biospecimens were collected with standardized clinical and sample acquisition protocols.11 Education, race, and co-morbidities did not differ significantly between PD and healthy control groups. Total full-length Klotho levels were determined in the BioFIND plasma samples using a modified immunoprecipitation-immunoblot method, similar to our published studies.12,13 When analyzed dichotomously, plasma Klotho levels were not different between healthy controls and PD patients (Fig. 1A). However, levels of Klotho tended to be higher in women compared to men in the PD group; this difference between men and women with regards to Klotho levels was not observed in the healthy control group (Fig. 1A). Biological sex is a critical factor in the development and phenotypic expression of PD.14 These results are in contrast to the most common form of dementia, Alzheimer’s disease, in which Klotho levels were decreased in the cerebrospinal fluid and plasma, perhaps highlighting differences in underlying mechanisms of these two neurodegenerative diseases.15,16 However, it is important to note that the immunoprecipitation–immunoblotting assay used in this study is more efficient in capturing soluble Klotho13 and selectively measures the full-length Klotho,12 as compared to the commercial ELISA commonly used. Therefore, caution needs to be exercised in directly comparing the two different methodologies for measures of Klotho. Further, it was recently reported that Klotho haplotypes interact with the APOE genotype to modify Alzheimer’s disease risk.17 Correlating Klotho levels with Klotho haplotype information is an important consideration for a future study.
FIG. 1.
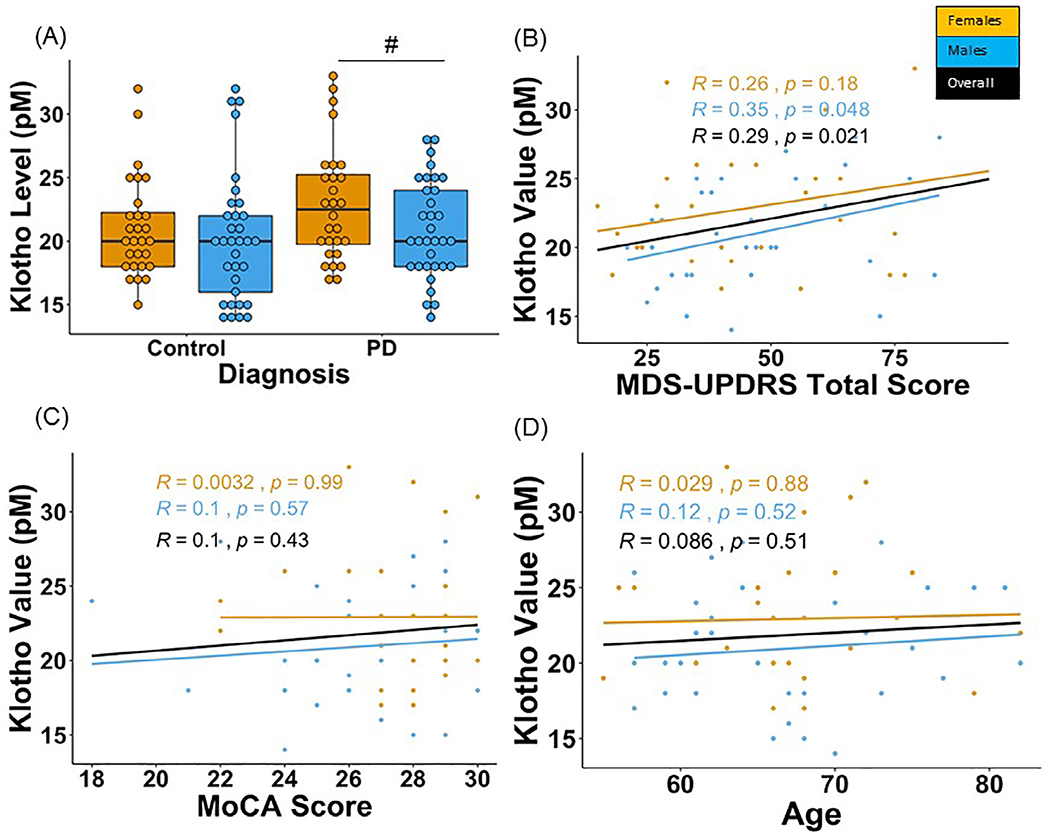
Klotho levels in PD patients and healthy controls in the BioFIND cohort. A pilot study was performed with four BioFIND subject plasma samples to confirm assay quality control. Klotho levels were measured in duplicate and compared against the standard range for healthy adults (22–40 pM). Plasma samples used to confirm assay quality fell in the range: 20.7–37.2 pM (mean = 29.7 ± 7.2 pM). The duplicate values for the samples in the pilot had an acceptable coefficient of variation (7%). Therefore, we proceeded to analyze the full cohort of plasma samples. A total of 122 plasma samples from baseline (V1) were secured from the BioFIND11 cohort database; 61 PD samples and 61 age- and sex-matched healthy controls. Samples were selected such that each age decade (50–90 years) was represented and matched between the two groups. Important clinical characteristics of the PD patients analyzed include average age = 66.9 ± 6.8 years, mean = 51.7 ± 20.9 MDS-UPDRS score, the H&Y score ranged from 1–4, and the average MoCA and RBD score was 26.8 ± 2.5, 5.5 ± 3.4, respectively. The healthy control demographics include average age = 66.8 ± 6.9 years, MoCa and RBD scores are 27.8 ± 1.4, 2.1 ± 1.6, respectively. The analysis for Klotho levels was performed blinded. (A) Female PD patients tended to have higher Klotho levels compared to male PD patients. Levels of Klotho were similar in men and women in healthy controls. (B) No correlation was found between Klotho levels and total MDS-UPDRS scores in PD patients. (C) A lack of an association between Klotho and MoCA scores and (D) age was observed. #Denotes tendency for statistical significance (P = 0.05–0.10). Raw data were sorted by patient number and compiled together using R version 1.1.463. Group comparisons between PD and healthy control groups for Klotho levels were performed using a one-way ANOVA. A two-way ANOVA was performed to understand the interaction between disease (PD and control) and biological sex (men and women). Exploratory post-hoc using one-way ANOVAs were performed for PD and control groups separately. Separate univariate ANOVAs were performed on the PD group for comparing Klotho levels in groups stratified based on years since diagnosis and those based on H&Y Scores (P < 0.05). Bonferroni corrections were performed for suitable comparisons to account for family-wise errors. Finally, Pearson product–moment correlation coefficients (r2) were calculated (R program-library ggscatter) for levels of Klotho and clinical scores from the MDS-UPDRS. The statistical significance thresholds were adjusted for multiple comparisons and set at P < 0.01 for all correlations.
We next tested the correlation between Klotho levels and PD-relevant clinical measures and whether sex modified these relationships. A lack of an association was observed between total MDS-UPDRS scores and Klotho values in PD patients (Fig. 1B). Additionally, Klotho levels did not correlate with MoCA scores in PD patients; stratifying by sex yielded similar results (Fig. 1C). We also did not find correlations between Klotho levels and advancing age in the PD group (Fig. 1D). Despite a link between pathological aging and PD-associated neurodegeneration, the role of Klotho in the pathophysiology of PD is largely unexplored. Although, in this study, we did not detect peripheral changes in plasma Klotho levels with PD thereby limiting its use as a diagnostic biomarker, this does not exclude the possibility of alterations of Klotho in post-mortem PD brains or CSF derived from PD patients in a biofluid that may reflect more closely central nervous system changes. Overall, our findings may shed light on underlying Klotho pathology in neurodegenerative diseases and has implications for therapeutically targeting the Klotho pathway in PD.
Acknowledgments:
Data used in the preparation of this article were obtained from the Fox Investigation for New Discovery of Biomarkers (“BioFIND”) database (http://biofind.loni.usc.edu/). For up-to-date information on the study, visit www.michaeljfox.org/biofind, BioFIND is sponsored by The Michael J. Fox Foundation for Parkinson’s Research (MJFF) with support from the National Institute for Neurological Disorders and Stroke (NINDS).
Funding agencies:
This work was supported by grants from the O’Brien Kidney Research Center P30 DK-079328 (O.W.M.), R01 DK091392 (O.W.M.), R01 DK092461 (O.W.M.), Charles Pak Foundation (O.W.M.), and 1R01NS119528 (L.H.S.).
Footnotes
Relevant conflicts of interest/financial disclosures: All authors report no financial disclosures or conflicts of interest relevant to this manuscript.
References
- 1.Cheikhi A, Barchowsky A, Sahu A, et al. Klotho: an elephant in aging research. J Gerontol Ser A Biol Sci Med Sci 2019;74(7):1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leon J, Moreno AJ, Garay BI, et al. Peripheral elevation of a Klotho fragment enhances brain function and resilience in young, aging, and alpha-synuclein transgenic mice. Cell Rep 2017;20(6):1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 2005;280(45):38029–38034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baluchnejadmojarad T, Eftekhari SM, Jamali-Raeufy N, Haghani S, Zeinali H, Roghani M. The anti-aging protein klotho alleviates injury of nigrostriatal dopaminergic pathway in 6-hydroxydopamine rat model of Parkinson’s disease: involvement of PKA/CaMKII/CREB signaling. Exp Gerontol 2017;100:70–76. [DOI] [PubMed] [Google Scholar]
- 5.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997;390(6655):45–51. [DOI] [PubMed] [Google Scholar]
- 6.Kosakai A, Ito D, Nihei Y, et al. Degeneration of mesencephalic dopaminergic neurons in klotho mouse related to vitamin D exposure. Brain Res 2011;1382:109–117. [DOI] [PubMed] [Google Scholar]
- 7.Sahu A, Mamiya H, Shinde SN, et al. Age-related declines in alphaKlotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun 2018;9(1):4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders LH, Laganiere J, Cooper O, et al. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: reversal by gene correction. Neurobiol Dis 2014;62:381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders LH, McCoy J, Hu X, et al. Mitochondrial DNA damage: molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol Dis 2014;70:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsche M, Pereira SL, Klein C, Grunewald A. Mitochondria and Parkinson’s disease: clinical, molecular, and translational aspects. J Parkinsons Dis 2021;11(1):45–60. 10.3233/JPD-201981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang UJ, Goldman JG, Alcalay RN, et al. The BioFIND study: characteristics of a clinically typical Parkinson’s disease biomarker cohort. Mov Disord 2016;31(6):924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker SL, Pastor J, Carranza D, et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant 2015;30(2):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neyra JA, Moe OW, Pastor J, et al. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin Kidney J 2020;13(2):235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerri S, Mus L, Blandini F. Parkinson’s disease in women and men: what’s the difference? J Parkinsons Dis 2019;9(3):501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semba RD, Moghekar AR, Hu J, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci Lett 2014;558:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedighi M, Baluchnejadmojarad T, Fallah S, Moradi N, Afshin-Majd S, Roghani M. The association between circulating Klotho and dipeptidyl peptidase-4 activity and inflammatory cytokines in elderly patients with Alzheimer disease. Basic Clin Neurosci 2020;11(3):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belloy ME, Napolioni V, Han SS, Le Guen Y, Greicius MD. Alzheimer’s disease neuroimaging I. Association of Klotho-VS heterozygosity with risk of Alzheimer disease in individuals who carry APOE4. JAMA Neurol 2020;77(7):849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]


