Abstract
The present study aimed to evaluate the clinical outcomes of magnetic-activated cell sorting (MACS) in sperm preparation for male subjects with a sperm DNA fragmentation index (DFI) ≥30%. A total of 86 patients who had undergone their first long-term long protocol were selected. The protocol involved in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) cycles, and the patients were divided into the MACS or control groups. The MACS group included sperm samples analyzed with MACS that were combined with density gradient centrifugation (DGC) and the swim-up (SU) technique (n = 39), and the control group included sperm samples prepared using standard techniques (DGC and SU; n = 41). No differences were noted with regard to basic clinical characteristics, number of oocytes retrieved, normal fertilization rate, cleavage rate, or transplantable embryo rate between the two groups in IVF/ICSI. In addition, the clinical pregnancy and implantation rates of the first embryo transfer cycles indicated no significant differences between the two groups. However, there was a tendency to improve the live birth rate (LBR) of the first embryo transfer cycle (63.2% vs 53.9%) and the cumulative LBR (79.5% vs 70.7%) in the MACS group compared with the control group. Moreover, the number of transferred embryos (mean ± standard deviation [s.d.]: 1.7 ± 0.7 vs 2.3 ± 1.6) and the transfer number of each retrieved cycle (mean ± s.d.: 1.2 ± 0.5 vs 1.6 ± 0.8) were significantly lower in the MACS group than those in the control group. Thus, the selection of nonapoptotic spermatozoa by MACS for higher sperm DFI could improve assisted reproductive clinical outcomes.
Keywords: cumulative live birth rate, fertility rate, intracytoplasmic sperm injection, sperm DNA fragmentation index, sperm DNA integrity
INTRODUCTION
Semen analysis is essential to measure sperm concentration, motility, and morphology according to the World Health Organization criteria,1 as well as to assess the sperm DNA fragmentation index (DFI). Several controversial tests have been developed to measure sperm DNA integrity, including detection of DNA strand breaks2 and assessment of the sperm chromatin structure.3 Up to 40% of men with unexplained infertility and repeated pregnancy loss have increased sperm DNA fragmentation.4 A strong association between higher sperm DFI and the embryological or clinical outcomes of assisted reproductive technology (ART) cycles has been established.5,6
Patients who exhibit an abnormal increase in sperm DFI are recommended to seek early interventions, such as modifying their lifestyle, controlling their weight, starting nutritional supplements, and undergoing oral antioxidant treatment or varicocele surgery.7,8 In addition, advanced sperm preparations have been developed to select sperm free of DNA damage to be used clinically in ART. However, the improvement of embryological and clinical outcomes for these applications is limited and controversial.9 The application of motile sperm organelle morphological examination has increased the live birth rate (LBR) of patients with recurrent implantation failure.10 Isolation of mature sperm using the Zeta method, electrophoresis or the hyaluronic acid binding site method could not provide the desired improvement in the clinical and embryological outcomes of the in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles.11,12,13
The translocation of phosphatidylserine (PS) to the outer cell membrane is a sign of early apoptosis induction. The detection of PS using flow cytometry can reflect sperm DNA integrity. In recent years, microfluidic sorting of unprocessed semen has been allowed for the selection of clinically usable, highly motile sperm with nearly undetectable levels of DNA fragmentation.14 Sperm sorting has also been performed using magnetic activated cell sorting (MACS), in addition to standard sperm preparation techniques, such as differential density gradient centrifugation (DGC) followed by the swim-up (SU) method. The MACS method has demonstrated positive effects on the embryological and clinical outcomes of IVF/ICSI cycles.15 To select the preferred method of sperm preparation for patients with high DFI, the comparison of MACS combined with standard sperm preparation (MACS-DGC-SU) methods with the conventional method (DGC-SU) was evaluated in Chinese men with high DFI during their IVF/ICSI cycles.
PARTICIPANTS AND METHODS
Experimental design
This prospective study was performed in the Center for Reproductive Medicine of Nanjing Drum Tower Hospital (Nanjing, China) between January 2018 and December 2019. It was approved by the Nanjing Drum Tower Hospital Ethics Committee (approval No. SZ-2018-MASS1). A total of 86 couples who were treated with IVF/ICSI due to female tubal and pelvic factors were included. All the participants signed informed consent. This was the first IVF/ICSI cycle for all the couples included. The indications for IVF/ICSI included unknown factors and mixed factors. Males aged 20–45 years old and sperm DFI ≥30% were included in the study. Males with anatomic abnormalities in the testis, epididymis, or vas deferens, or chromosomal abnormalities were excluded from the study. Females with an age >40 years, decreased ovarian reserve determined by follicle-stimulating hormone (FSH >15 mIU ml−1), thin endometrium (<7 mm on the day of embryo transfer), congenital uterine malformation, polycystic ovary syndrome, chromosomal abnormalities, endometriosis, adenomyosis, or ovulation failure were excluded from the present study. Six couples were excluded because of cycle canceled or no viable reagent (Table 1). The included couples were divided into two groups, namely, the MACS group with MACS combined with DGC and SU as sperm preparation techniques (n = 39) and the control group with DGC and SU as sperm preparation techniques (n = 41). The couples were grouped in the MACS group or control group by random. Randomization was performed through the use of a computer-generated table of random numbers. Randomization was carried out by one of the investigators who did not participate in the inclusion of patients or in the medication treatments. All participants were treated with levocarnitine (L-carnitine, Via Pontina Km, Rome, Italy) at a dose of 1 g per day orally for 1 month before controlled ovarian hyperstimulation. The present study is registered on the Clinical Trials website (No. NCT03968367).
Table 1.
Comparison of clinical baseline data between the MACS and control groups
| Clinical variable | Control group | MACS group | t/Z | P |
|---|---|---|---|---|
| Total number of retrieved oocyte cycles (n) | 41 | 39 | ||
| Female age (year), mean±s.d. | 30.4±3.7 | 30.1±4.5 | 0.36 | 0.71a |
| Male age (year), mean±s.d. | 32.1±5.1 | 31.3±4.9 | 0.75 | 0.45a |
| Infertile duration (year), mean±s.d. | 4.1±2.6 | 3.6±2.5 | 0.87 | 0.39b |
| Female BMI (kg m−2), mean±s.d. | 22.3±2.8 | 21.5±3.1 | 1.13 | 0.26b |
| Basal FSH (IU l−1), mean±s.d. | 7.3±1.7 | 7.6±1.9 | 0.77 | 0.44a |
| Antral follicle count (n), mean±s.d. | 17.3±5.5 | 19.7±6.8 | 1.68 | 0.09b |
| Sperm concentration (×106 ml−1), mean±s.d. | 35.8±36.3 | 28.9±26.1 | 0.98 | 0.32b |
| Sperm motility (PR + NP, %), mean±s.d. | 23.2±21.5 | 17.8±13.8 | 1.34 | 0.18a |
| Normal sperm morphology (%), mean±s.d. | 2.6±2.6 | 2.7±2.8 | 0.13 | 0.90a |
| Sperm DFI (%), mean±s.d. | 38.2±5.1 | 40.1±12.1 | 0.93 | 0.35b |
aStudent’s t-test, bMann–Whitney U test. t/Z value represented the statistical results of Student’s t-test or Mann–Whitney U test. MACS: magnetic activated cell sorting; DFI: DNA fragmentation index; PR: rate of progressively motile sperm; NP: rate of nonprogressively motile sperm; s.d.: standard deviation; BMI: body mass index; FSH: follicle-stimulating hormone
Controlled ovarian hyperstimulation protocols
The women included in the present study were treated with a long-term protocol. The long-term protocol was initiated on day 2 of the menstrual cycle with the administration of the gonadotrophin-releasing hormone analog triptorelin (Decapeptyl, Ferring AG, Barr, Switzerland) at a daily dose of 1 ml (3.75 mg) subcutaneously. Following 4–6 weeks, ovarian stimulation was initiated with a daily dose of 100–225 IU recombinant FSH (Gonal F; Merck Serono, Geneva, Switzerland). Before ovarian stimulation, desensitization was achieved (i.e., estrogen ≤0.05 nmol l−1, follicles ≤5 mm in diameter, and endometrial thickness ≤5 mm). Human chorionic gonadotrophin (HCG) was administered when the diameter of the leading follicle reached 18–20 mm. Oocyte retrieval was performed 36 h following HCG administration.
Sperm preparation
Sperm DFI measured by a sperm chromatin structure assay (SCSA) was performed 1–2 months before oocyte retrieval for all male patients. On the day of oocyte retrieval, the selected sperm sample was assessed for volume, concentration, motility, and morphology according to the World Health Organization (2010) criteria.1 In the control group, the assessed samples were processed with classic semen preparation techniques, which included DGC followed by SU. The seminal fluid was removed by DGC (SpermGrad; Vitrolife, V.Frölunda, Sweden) followed by centrifugation (KUBOTA 2420, KUBOTA, Osaka, Japan) at 400g for 20 min. The sperm cells were resuspended and washed in 3 ml of SpermRinse (Vitrolife) followed by centrifugation at 300g for 10 min. The pellet was resuspended in 0.2 ml of IVF medium (Vitrolife) and incubated for 0.5 h at 37°C. The nonapoptotic spermatozoa in the sperm samples derived from the MACS group were isolated by MACS before DGC and SU. A total of 1 ml of sperm samples were mixed with 2 ml of MACS ART binding buffer, and the samples were centrifuged at 300g for 4 min. The sperm samples were incubated with 100 μl of MACS ART annexin-V reagent for 15 min at room temperature. MACS ART binding buffer was added to a total volume of 500 μl according to the protocol provided by the manufacturer (Annexin-V MicroBead Kit; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The suspension was transferred to a separation column (MiniMacs; Miltenyi Biotec GmbH). The labeled annexin-V-positive spermatozoa were retained in the column, and the annexin-V-negative viable spermatozoa passed through the column. The latter spermatozoa were further processed by DGC and SU.
Embryo culture, assessment, vitrification, and warming
Fertilized oocytes were cultured in fertilization medium (Cook, Brisbane, Australia) and examined for the presence of pronuclei at 16–18 h following fertilization. The fertilized zygotes (2 pronucleus; 2PN) were cultured in SAGE 1-Step continuous culture medium (Origio, Malov, Denmark) before embryo transfer on day 3 or 5. Day 3 embryo assessment was divided into three grades according to Istanbul consensus: Grade 1 embryos had stage-specific cell-sized mononucleated blastomeres with <10% fragmentation; Grade 2 embryos were the stage-specific cell size for the majority of cells, with no evidence of multinucleation and 10%–25% fragmentation; and Grade 3 embryos had non-stage-specific cell-sized blastomeres, evidence of multinucleation and severe fragmentation (>25%). Grade 1 was considered a high-quality embryo, and Grades 1–2 were considered transferable embryos. The supernumerary embryos were cultured until the 5th or 6th day for the development of blastocyst-stage embryos following embryo transfer on day 3. The blastocyst stage embryos were cryopreserved. The blastocysts were graded according to Gardner et al.16 In brief, blastocyst grading includes a morphological assessment of blastocyst expansion (Grade I–VI), inner cell mass (Grade A–C), and trophectoderm (Grade A–C). Grade I–II embryos were considered high-quality embryos, and Grade I–III transferable embryos were considered high-quality embryos. More than IV BB blastocysts were considered high-quality blastocysts. More than IV CC blastocysts were transferable blastocysts. The cryopreservation and warming of embryos were performed by Vitrification and Thawing KITs (Kitazato BioPharma Co., Ltd., Fuji, Japan). The procedures were performed in strict accordance with the manufacturer's instructions.
Embryo transfer
Embryo transfer was scheduled without inappropriate conditions for transplantation in the fresh cycle. In total, 1–2 embryos were transferred into the uterus by abdominal ultrasound on the 3rd or 5th day following oocyte retrieval. Dydrogesterone tablets (Duffton, Abbott, Weesp, The Netherlands) and progesterone sustained-release vaginal Gel (Crinone, Merck Serono, Switzerland) were used for corpus luteum support.
The patients who were at risk of ovarian hyperstimulation, higher progesterone levels on the day of HCG injection, or other conditions, such as fever, intra-abdominal cavity bleeding, and lack of pregnancy following fresh cycle transplantation, were subjected to frozen-thawed embryo transfer. In the frozen-thawed embryo transfer cycle, the endometrium was prepared by modified natural cycle or hormone replacement therapy as previously described.17 Corpus luteum support with progesterone was continued until the 10th week of pregnancy.
Follow-up and observation indicators
Serum β-HCG was measured 2 weeks following embryo transfer. Serum β-HCG ≥100 IU l−1 was used to define biochemical pregnancy. Transvaginal ultrasound examination was conducted following 4–6 weeks. Clinical pregnancy was defined as the presence of an intrauterine gestational sac and the active fetal heartbeat. Live birth was defined as persistent pregnancy until 28 weeks with at least 1 surviving child during birth. The main observation indicators and their definitions were as follows: normal fertilization rate (%) = number of oocytes with 2PN and two polar bodies on day 1/total number of MII oocytes × 100%; the cleavage rate (%) = number of cleavage-stage embryos on day 2/number of normal fertilized oocytes × 100%; the blastocyst formation rate (%) = number of blastocyst formations on day 5 and day 6/number of cultured cleavage-stage embryos on day 3 × 100%; transplantable embryo rate (%) = available number of transplantable embryos/2PN cleavage-stage embryos × 100%; and cumulative LBR (%) = number of live births in each egg retrieval cycle (fresh and frozen-thawed embryo transfer)/total number of oocyte retrieval cycles × 100%. The follow-up period lasted for 18–36 months from the day of oocyte retrieval. During the follow-up period, all patients had completion of live birth or no available embryos left in the included oocyte retrieval cycle.
Statistical analyses
The Statistical Package for the Social Sciences (SPSS version 24.0; IBM Corp., Chicago, IL, USA) was used for statistical analysis. The results are expressed as the mean ± standard deviation (s.d.). Student's t-test was conducted to compare the clinical baseline data of continuous variables between the two different groups, and the Mann–Whitney U test was conducted to compare continuous variables with a skewed distribution. The Chi-square test was used for comparisons of the clinical pregnancy rate, implantation rate, and LBR between the groups. P < 0.05 indicated significant differences.
RESULTS
A total of 86 patients consented to participate in the study, and two patients dropped out prior to ovarian stimulation for personal reasons. A total of 42 patients (aged 24–38 years) received MACS-DGC-SU for sperm preparation, and three patients dropped out due to no viable reagent or canceled for personal reasons. The remaining 42 women (aged 24–38 years) received the conventional method (DGC-SU), and one patient dropped out due to a canceled cycle (Figure 1). No patients were lost during follow-up. The cycle cancelation rate was comparable between the MACS and control groups.
Figure 1.
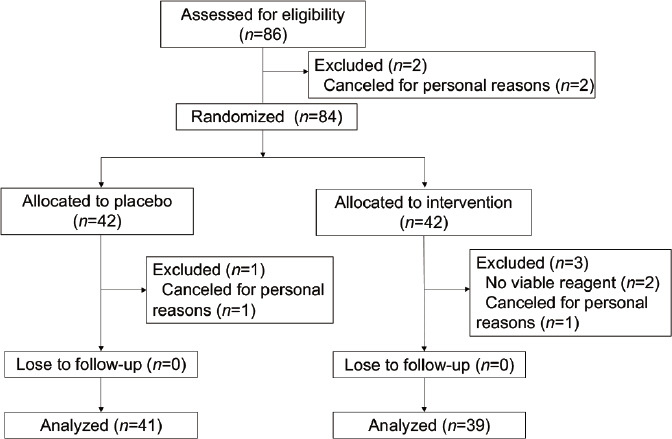
Flow diagram of patient selection for a randomized controlled study examining the effects of magnetic-activated cell sorting of nonapoptotic spermatozoa on IVF/ICSI outcomes. IVF: in vitro fertilization; ICSI: intracytoplasmic sperm injection.
The clinical characteristics of the population are summarized in Table 1. The patients’ baseline characteristics, including age, infertility duration, female body mass index (BMI), basal FSH, and antral follicle count, were comparable between the two groups. No significant differences were noted between the control and MACS groups (all P > 0.05; Table 1). The sperm parameters (concentration, motility, and morphology) on the day of oocyte retrieval were also compared between the MACS and control groups, and there were no significant differences in these parameters (all P > 0.05; Table 1).
In addition, the dosage and duration of gonadotropin used were comparable between the two groups. The number of oocytes retrieved (13.0 ± 5.0 vs 13.7 ± 6.4, P = 0.60) was similar in the two groups. No significant differences were noted with regard to the normal fertilization rate (77.1% vs 76.6%, P = 0.85), cleavage rate (98.3% vs 97.8% P = 0.62), blastocyst formation rate (65.4% vs 66.5%, P = 0.80), transplantable embryo rate (59.7% vs 61.8%, P = 0.59) or lack of available embryos (4.9% vs 2.6%, P = 0.58) between the control and MACS groups (Table 2).
Table 2.
Comparison of clinical and laboratory indicators between the two different DNA fragmentation index groups
| Indicator | Control group | MACS group | Z/χ2 | P |
|---|---|---|---|---|
| Total number of oocyte-retrieved cycles (n) | 41 | 39 | ||
| Dosage of Gn used (IU), mean±s.d. | 1914.0±557.4 | 1986.2±642.2 | 0.54 | 0.59a |
| Duration of Gn used (day), mean±s.d. | 10.8±2.0 | 11.5±2.3 | 1.55 | 0.12a |
| Number of oocytes retrieved (n), mean±s.d. | 13.0±5.0 | 13.7±6.4 | 0.52 | 0.60a |
| Normal fertilization rate, % (n/total) | 77.1 (361/468) | 76.6 (321/419) | 0.03 | 0.85 |
| Cleavage rate, % (n/total) | 98.3 (355/361) | 97.8 (314/321) | 0.24 | 0.62 |
| Blastocyst formation rate, % (n/total) | 65.4 (151/231) | 66.5 (131/197) | 0.06 | 0.80 |
| Transplantable embryo rate, % (n/total) | 59.7 (212/355) | 61.8 (194/314) | 0.29 | 0.59 |
| No available embryo rate, % (n/total) | 4.9 (2/41) | 2.6 (1/39) | 0.29 | 0.58 |
| Number of good quality embryos transferred of first embryo transfer cycles (n), mean±s.d. | 1.3±0.6 | 1.5±0.5 | 1.16 | 0.25a |
| Clinical pregnancy rate of first embryo transfer cycles, % (n/total) | 59.0 (23/39) | 65.8 (25/38) | 0.38 | 0.54 |
| Implantation rate of first embryo transfer cycles, % (n/total) | 55.6 (30/54) | 53.6 (30/56) | 0.04 | 0.83 |
| Early miscarriage rate of first embryo transfer cycles, % (n/total) | 5.1 (2/39) | 2.6 (1/38) | 0.32 | 0.57 |
| Twin pregnancy rate of first embryo transfer cycles, % (n/total) | 30.4 (7/23) | 20.0 (5/25) | 0.70 | 0.40 |
| LBR of first embryo transfer cycles, % (n/total) | 53.9 (21/39) | 63.2 (24/38) | 0.68 | 0.41 |
| Mean transfer number of each retrieved cycle (n), mean±s.d. | 1.6±0.8 | 1.2±0.5 | 2.25 | 0.02*,a |
| Mean transfer embryo number of each retrieved cycle (n), mean±s.d. | 2.3±1.6 | 1.7±0.7 | 2.14 | 0.03*,a |
| Cumulative LBR, % (n/total) | 70.7 (29/41) | 79.5 (31/39) | 0.82 | 0.37 |
*P<0.05, aMann–Whitney U test, Z/χ2 value represented the statistical results of Mann–Whitney U test or Chi-square test. Cleavage rate (%)=number of cleavage-stage embryos on day 2/number of normal fertilized oocytes ×100%; blastocyst formation rate (%)=number of blastocyst formation on day 5 and day 6/number of cultured cleavage-stage embryos on day 3 ×100%; transplantable embryo rate (%)=available number of transplantable embryos/2PN cleavage-stage embryos ×100%; cumulative LBR (%)=number of live births in each egg retrieval cycle (fresh and frozen-thawed embryos transfer)/total number of oocyte retrieval cycles ×100% (the follow-up lasted for 18–36 months from the day of oocyte retrieval; and during the follow-up period, frozen embryos were not collected from patients without live births). LBR: live birth rate; 2PN: 2 pronucleus; MACS: magnetic activated cell sorting; s.d.: standard deviation
The IVF/ICSI clinical outcomes of the first embryo transfer cycles were compared between the two groups. The clinical pregnancy rate (59.0% vs 65.8%, P = 0.54), implantation rate (55.6% vs 53.6%, P = 0.83), early miscarriage rate (5.1% vs 2.6%, P = 0.57), and twin rate (30.4% vs 20.0%, P = 0.40) of the first embryo transfer cycles were not significantly different between the control and MACS groups (Table 2). In addition, in the first fresh embryo transfer cycle, there was no significant difference in the proportion of day 3 or day 5 fresh embryo transfer between the two groups. There was no significant difference in clinical results on day 3 or day 5 fresh embryo transfer between the control and MACS groups (data not shown here). It is worth noting that the LBR of the first embryo transfer cycles (63.2% vs 53.9%) and the cumulative LBR were higher in the MACS group (79.5% vs 70.7%) than those in the control group, although no significant differences were observed (both P > 0.05). In addition, the mean number of transferred embryos (1.7 ± 0.7 vs 2.3 ± 1.6, P = 0.03) and the mean transfer number (1.2 ± 0.5 vs 1.6 ± 0.8, P = 0.02) of each retrieved cycle until live birth were significantly lower in the MACS group than those in the control group.
DISCUSSION
In assisted reproduction cycles, the quality of sperm is vital for optimal clinical outcomes. In addition to the motility and deformity of the sperm, its DFI indicates its ability to deliver an integral nucleus. This participates in maintaining a healthy pregnancy.18 It is clear that sperm DNA damage causes adverse effects on reproductive outcomes. Since effective treatment modalities to improve sperm DNA damage are limited, the noninvasive sperm optimization technology has been applied to select sperm free of DNA damage before IVF-ET in addition to the standard sperm preparation-DGC and SU methods.
MACS is a procedure that utilizes the annexin V-conjugated microbead technique to remove spermatozoa with externalized PS during sperm preparation. The first clinical trial of the MACS-DGC procedure was reported by Dirican et al.19 in patients with oligoasthenozoospermia in 2008. Furthermore, Rawe et al.20 reported live birth for the first time in 2010 using the ICSI procedure following MACS. Nonspecific binding of the eluted spermatozoa was excluded, and the safety of this novel technique was confirmed by preliminary observations.21,22 Previous results indicated that MACS coupled with DGC can lead to a significant reduction in sperm DNA fragmentation and higher sperm viability.23 However, whether the use of MACS exerts positive effects on clinical outcomes in IVF/ICSI remains controversial. In the present study, MACS coupled with DGC and SU was performed in 39 semen samples (MACS group), whereas the DGC and SU methods were performed on a separate set of 41 semen samples (control group) during sperm preparation. Nonapoptotic spermatozoa were isolated by the MACS procedure in the study group. The parameters of cleavage, fertilization, pregnancy, and early miscarriage rate of first embryo transfer cycles were compared between the two methods of sperm preparation. The majority of the studies conducted previously evaluated a single embryo transfer cycle. However, in the present study, the cumulative pregnancy rate was estimated for the entire IVF/ICSI cycle.
Initially, the potential of MACS to improve the status of the embryo was assessed. The results suggested that MACS sperm sorting could not affect the normal fertilization rate or the rate of transferable embryos for patients with high DFI. Romany et al.24 indicated that MACS could not improve the fertilization rates and embryo quality rates in the ICSI procedure performed on unselected male subjects. However, it has been found that the elimination of apoptotic spermatozoa by MACS could increase the fertilization rate and embryo quality in couples with unexplained infertility (UI)25 or severe male factor infertility26 treated with ICSI. The possible reason for these contradictory results may be the differences in the selected population. It has been shown that unselected males cannot benefit from MACS. However, in couples with UI and severe male factor infertility, who tend to have low fertilization rates, the use of current IVF procedures may result in improved embryological outcomes. In the present study, the enrolled women were those with normal ovarian reserve, and the retrieved oocytes exhibited optimal quality. Fertilized high-quality oocytes may partially repair the DNA integrity of the sperm, thereby forming a higher-quality embryo. However, the mechanism of sperm DNA repair in fertilized oocytes is still unclear.27 The repair effect of fertilized oocytes on damaged sperm DNA is associated with the extent and type of DNA damage and the capacity of the oocyte to repair this damage.28 The fertility method (IVF/ICSI) could also explain the contradictory results. Whether men with normal semen and increased DFI should choose ICSI to improve their clinical outcome remains unknown. The current evidence is insufficient and debatable.29 These findings have been attributed to the preference of ICSI in men with high sperm DFI. The selection of morphologically normal motile sperm may in part result in the improvement of clinical outcomes.27 Although relatively normal-looking sperm cells have been selected in ICSI insemination, it is impossible to confirm the DNA integrity and intrinsic quality of the selected sperm cells. By comparison, IVF is a more natural method of insemination. However, insufficient data are present in the literature to confirm significant differences in the repair ability of oocytes between ICSI and IVF. Therefore, IVF/ICSI was selected based on the density and mobility of the sperm cells but not on DFI. Therefore, it can be concluded that DFI is not an independent indicator that can be solely used to predict the embryological outcome during IVF/ICSI.30,31
Furthermore, the present study aimed to identify the clinical outcomes of the first embryo transfer cycles following MACS application. The data suggest no significant differences at the clinical pregnancy, implantation, and early miscarriage rates of the first embryo transfer cycles between MACS group and control group. However, there was a tendency to improve the LBR with MACS (63.2%) compared with control samples (53.9%). Dirican et al.19 reported significantly lower pregnancy and implantation rates in the study group. No significant differences were noted in the pregnancy rates when the MACS-ICSI procedure was applied in oocyte donation cycles.24 Herrero et al.32 also reported an increase in the LBR by using DGC-MACS. Significantly higher LBR was noted following IVF in men with low sperm DNA fragmentation compared with that noted in men with high sperm DNA fragmentation when a combination treatment of IVF and ICSI was used.33 The use of the first embryo transfer cycles as a parameter in evaluating the efficiency of MACS in ART exhibits certain limitations. Therefore, the cumulative LBR, the mean number of transferred embryos and the mean transfer number of each retrieved cycle until live birth were compared between the control and study groups. The results suggested that the mean number of transferred embryos and mean transfer number of the MACS group were significantly decreased compared with those of the control group. The cumulative LBR of the MACS-DGC-SU group was increased compared with that of the DGC-SU group, although the difference was not statistically significant. These results suggested that sperm DNA integrity was closely associated with overall embryonic development and quality. MACS sperm sorting could reduce the number of transplanted embryos and the time to pregnancy. It is not currently known whether specific populations can particularly benefit from MACS sorting, such as women who are older or have poor ovarian function.34 These findings have to be investigated in future trials with larger and more stratified sample groups.
CONCLUSION
In summary, the application of the combination of MACS and standard sperm preparation in ART could be a reliable strategy to enrich sperm with higher chromatin quality spermatozoa and improve assisted reproductive outcomes. The mean number of transferred embryos and the mean transfer number of each retrieved cycle of the MACS group were decreased compared with those of the control group. In addition, the LBR of the first transfer cycle and cumulative LBR were both elevated. However, the comparison of the cumulative LBR may yield significant differences between the two groups if a higher number of patients with high DFI are enrolled.
AUTHOR CONTRIBUTIONS
JXW and LJD contributed to the clinical trial design, patient recruitment, and manuscript review. JM contributed to data acquisition, data interpretation and drafted the manuscript. XXZ contributed to data acquisition. LJC contributed to patient recruitment, screening and data analysis. WY, and QQG contributed to clinical treatment, sperm preparation, and embryo manipulation. HXS developed the original content and performed critical revisions of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81801518 and No. 82071646).
REFERENCES
- 1.5th ed. Geneva: World Health Organization; 2010. World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 2.Simon L, Proutski I, Stevenson M, Jennings D, McManus J, et al. Sperm DNA damage has a negative association with live birth rates after IVF. Reprod Biomed Online. 2013;26:68–78. doi: 10.1016/j.rbmo.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–95. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 4.Sun Q, Huang GN, Sun HX, Fan LQ, Feng Y, et al. CSRM consensus on key indicators for quality control in IVF laboratory. Reprod Med. 2018;27:836–51. [Google Scholar]
- 5.Zinaman MJ, Brown CC, Selevan SG, Clegg ED. Semen quality and human fertility: a prospective study with healthy couples. J Androl. 2000;21:145–53. [PubMed] [Google Scholar]
- 6.Bjorndahl L. Methods for sperm concentration determination. Methods Mol Biol. 2013;927:3–12. doi: 10.1007/978-1-62703-038-0_1. [DOI] [PubMed] [Google Scholar]
- 7.Abad C, Amengual MJ, Gosálvez J, Coward K, Hannaoui N, et al. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia. 2013;45:211–6. doi: 10.1111/and.12003. [DOI] [PubMed] [Google Scholar]
- 8.Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Henkel R. Sperm preparation: state-of-the-art–physiological aspects and application of advanced sperm preparation methods. Asian J Androl. 2012;14:260–9. doi: 10.1038/aja.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boitrelle F, Guthauser B, Alter L, Bailly M, Bergere M, et al. High-magnification selection of spermatozoa prior to oocyte injection: confirmed and potential indications. Reprod Biomed Online. 2014;28:6–13. doi: 10.1016/j.rbmo.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Kheirollahi-Kouhestani M, Razavi S, Tavalaee M, Deemeh MR, Mardani M, et al. Selection of sperm based on combined density gradient and may improve ICSI outcome. Hum Reprod. 2009;24:2409–16. doi: 10.1093/humrep/dep088. [DOI] [PubMed] [Google Scholar]
- 12.Fleming SD, Ilad RS, Griffin AM, Wu Y, Ong KJ, et al. Prospective controlled trial of an electrophoretic method of sperm preparation for assisted reproduction: comparison with density gradient centrifugation. Hum Reprod. 2008;23:2646–51. doi: 10.1093/humrep/den330. [DOI] [PubMed] [Google Scholar]
- 13.Tarozzi N, Nadalini M, Bizzaro D, Serrao L, Fava L, et al. Sperm-hyaluronan-binding assay: clinical value in conventional IVF under Italian law. Reprod Biomed Online. 2009;19(Suppl 3):35–43. doi: 10.1016/s1472-6483(10)60282-9. [DOI] [PubMed] [Google Scholar]
- 14.Quinn MM, Jalalian L, Ribeiro S, Ona K, Demirci U, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33:1388–93. doi: 10.1093/humrep/dey239. [DOI] [PubMed] [Google Scholar]
- 15.Bucar S, Gonçalves A, Rocha E, Barros A, Sousa M, et al. DNA fragmentation in human sperm after magnetic-activated cell sorting. J Assist Reprod Genet. 2015;32:147–54. doi: 10.1007/s10815-014-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 17.Weissman A, Horowitz E, Ravhon A, Steinfeld Z, Mutzafi R, et al. Spontaneous ovulation versus HCG triggering for timing natural-cycle frozen-thawed embryo transfer: a randomized study. Reprod Biomed Online. 2011;23:484–9. doi: 10.1016/j.rbmo.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Aitken RJ. Sperm function tests and fertility. Int J Androl. 2006;29:69–75. doi: 10.1111/j.1365-2605.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 19.Dirican EK, Ozgun OD, Akarsu S, Akin KO, Ercan O, et al. Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J Assist Reprod Genet. 2008;25:375–81. doi: 10.1007/s10815-008-9250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawe VY, Boudri HU, Alvarez Sedo C, Carro M, Papier S, et al. Healthy baby born after reduction of sperm DNA fragmentation using cell sorting before ICSI. Reprod Biomed Online. 2010;20:320–3. doi: 10.1016/j.rbmo.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Paasch U, Grunewald S, Fitzl G, Glander HJ. Deterioration of plasma membrane is associated with active caspases in human spermatozoa. J Androl. 2003;24:246–52. doi: 10.1002/j.1939-4640.2003.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 22.Lukaszuk K, Wcislo M, Liss J, Stachowicz A, Jakiel G, et al. First pregnancy, somatic and psychological status of a 4-year-old child born following annexin V TESA sperm separation. AJP Rep. 2015;5:e105–8. doi: 10.1055/s-0035-1548726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Said TM, Grunewald S, Paasch U, Glander HJ, Baumann T, et al. Advantage of combiningmagnetic cell separationwith sperm preparation techniques. Reprod Biomed Online. 2005;10:740–6. doi: 10.1016/s1472-6483(10)61118-2. [DOI] [PubMed] [Google Scholar]
- 24.Romany L, Garrido N, Motato Y, Aparicio B, Remohí J, et al. Removal of annexin V-positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: a controlled and randomized trial in unselected males. Fertil Steril. 2014;102:1567–75.e1. doi: 10.1016/j.fertnstert.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Sheikhi A, Jalali M, Gholamian M, Jafarzadeh A, Jannati S, et al. Elimination of apoptotic spermatozoa by magnetic activated cell sorting improves the fertilization rate of couples treated with ICSI procedure. Andrology. 2013;1:845–9. doi: 10.1111/j.2047-2927.2013.00140.x. [DOI] [PubMed] [Google Scholar]
- 26.Ziarati N, Tavalaee M, Bahadorani M, Nasr Esfahani MH. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum Fertil (Camb) 2019;22:118–25. doi: 10.1080/14647273.2018.1424354. [DOI] [PubMed] [Google Scholar]
- 27.Lewis SE, John Aitken R, Conner SJ, Iuliis GD, Evenson DP, et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod Biomed Online. 2013;27:325–37. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 28.González-Marín C, Gosálvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13:14026–52. doi: 10.3390/ijms131114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 30.Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, et al. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95:124–8. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 31.Braga DP, Setti AS, Figueira RC, Iaconelli A, Jr, Borges E., Jr The negative influence of sperm cryopreservation on the quality and development of the embryo depends on the morphology of the oocyte. Andrology. 2015;3:723–8. doi: 10.1111/andr.12049. [DOI] [PubMed] [Google Scholar]
- 32.Herrero MB, Delbes G, Chung JT, Son WY, Holzer H, et al. Case report: the use of annexin V coupled with magnetic activated cell sorting in cryopreserved spermatozoa from a male cancer survivor: healthy twin newborns after two previous ICSI failures. J Assist Reprod Genet. 2013;30:1415–9. doi: 10.1007/s10815-013-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 2015;30:120–7. doi: 10.1016/j.rbmo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Stimpfel M, Verdenik I, Zorn B, Virant-Klun I. Magnetic-activated cell sorting of non-apoptotic spermatozoa improves the quality of embryos according to female age: a prospective sibling oocyte study. J Assist Reprod Genet. 2018;35:1665–74. doi: 10.1007/s10815-018-1242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


