ABSTRACT
Ertapenem is one of the carbapenems recommended for treating extended-spectrum β-lactamase (ESBL)-producing Enterobacterales. However, efficacy data are limited. We compared 30-day mortality rates for patients receiving ertapenem and other carbapenems for treatment of ESBL-producing Enterobacterales bacteremia. A multicenter, retrospective study was performed from January 2013 to December 2020 at three hospitals. Patients who received only members of one group of carbapenems (group 1 or group 2) throughout their treatment for ESBL-producing Escherichia coli or Klebsiella pneumoniae bacteremia were enrolled. To compare 30-day all-cause mortality rates in the two groups, propensity score matching was used to control for selection bias. Subgroup analyses were performed for several subgroups. Secondary outcomes included Clostridioides difficile infection (CDI) and the emergence of multidrug-resistant Gram-negative bacteria within 90 days after initiation of carbapenem treatment. One-to-one propensity score matching yielded 162 pairs of patients from the total of 603 patients included. There was no difference in 30-day mortality rates between ertapenem and the other carbapenems in adjusted analyses (hazard ratio, 0.60 [95% confidence interval [CI], 0.29 to 1.22]) of the propensity score-matched cohorts. A similar result was obtained in a subgroup analysis of patients who suffered severe sepsis or septic shock and those who did not (P = 0.54 for interaction). Emergence of CDI (odds ratio [OR], 0.99 [95% CI, 0.44 to 2.20]) and carbapenem-resistant Enterobacterales (OR, 1.31 [95% CI, 0.51 to 3.53]) did not differ between the two groups. Our study suggests that the efficacy of ertapenem may be comparable to that of the other carbapenems in treatment of ESBL-producing E. coli and K. pneumoniae bacteremia.
KEYWORDS: ertapenem, extended-spectrum β-lactamase, carbapenems
INTRODUCTION
Carbapenems are primarily recommended for treatment of extended-spectrum-β-lactamase (ESBL)-producing Enterobacterales infections (1, 2). There are currently two groups of carbapenems; ertapenem is the only group 1 carbapenem, while group 2 includes meropenem, imipenem, and doripenem. Ertapenem was developed later than the other carbapenems and has unique features. It has a longer half-life; therefore, once-daily dosing as an outpatient is possible, thus shortening the hospital stay (3). In addition, it has lower activity against Pseudomonas and Acinetobacter species and thus exerts less selection pressure for resistance to these bacteria than do other carbapenems (4). Because of these advantages, ertapenem is widely used in clinical practice in treatment of infections due to ESBL-producing organisms. Guidelines also list ertapenem as one of the treatment options for ESBL-producing infections (1, 2).
However, there has been concern about the efficacy of ertapenem in patients with severe illness. The pharmacokinetic target attainment of ertapenem seemed to be insufficient in several studies due to changes in pharmacokinetic parameters in critically ill patients (5–7). Furthermore, data evaluating the efficacy of ertapenem, compared to other carbapenems, in the treatment of ESBL producers are limited. Several observational studies have shown that ertapenem is not inferior to group 2 carbapenems, but the existing studies have several limitations, such as small sample size, lack of balancing between the two groups, and contamination between study drug exposures (8–11). The baseline characteristics of the two groups of patients were not well balanced because patients with more severe infections or underlying diseases tended to receive a group 2 carbapenem even after propensity score matching (9–11). Because of this bias, some studies found ertapenem to be more effective than other carbapenems (11, 12).
The largest European multinational cohort study tried to overcome this problem by propensity score matching and showed in a well-balanced cohort that ertapenem was not inferior to group 2 carbapenems as definitive therapy (10). However, the outcome of this definitive treatment cohort was not free from the effects of empirical regimens because some patients received members of both groups of carbapenems. Empirical therapy, especially when initiated in the early period of sepsis, may have a major influence on outcomes. With a comparative study design that includes patients receiving both arms of the treatment, the individual effects of each drug cannot be accurately evaluated. To overcome these limitations, we compared the outcomes of treatment of ESBL-producing Enterobacterales bacteremia in a propensity score-matched cohort of patients who received either group 1 or group 2 carbapenems throughout the course of their treatment.
RESULTS
Study population.
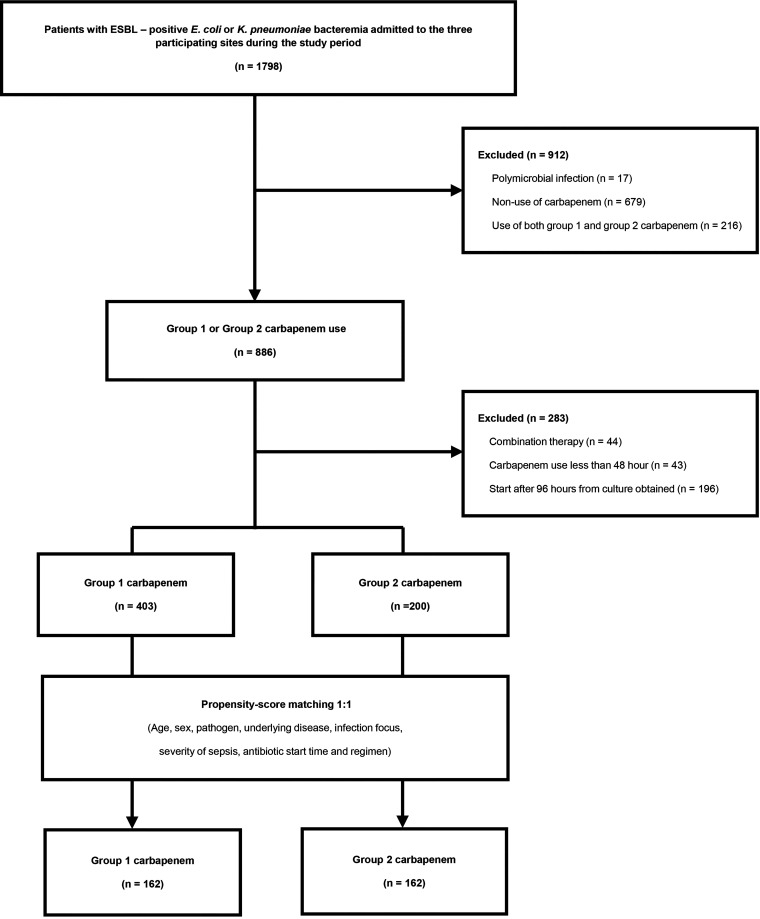
A total of 1,798 unique patients with ESBL-producing Escherichia coli or Klebsiella pneumoniae bacteremia were identified over the study period, of whom 603 fulfilled the eligibility criteria. In the end, 162 pairs of propensity score-matched patients were selected (Fig. 1). The numbers of patients enrolled in each hospital were 124 (Hallym University Sacred Heart Hospital), 119 (Dongtan Sacred Heart Hospital), and 81 (Kangnam Sacred Heart Hospital) patients. The characteristics of the 603 patients and the propensity score-matched cohort (n = 324) are shown in Table 1. In the overall cohort, patients receiving ertapenem were more likely to have E. coli bacteremia (93.1% versus 81.5%; P < 0.001) and less likely to have nosocomial infections (18.9% versus 27.0%; P = 0.022). The sources of bacteremia also differed between the two groups (P < 0.001). Moreover, patients in the ertapenem group were less likely to have severe sepsis or septic shock and to be admitted to the intensive care unit (ICU) than were those in the group 2 carbapenem group. Patients with high Charlson’s comorbidity index (CCI) scores (scores of ≥3) were more common in the group 2 carbapenem group than in the ertapenem group (92.5% versus 79.9%; P < 0.001). Antibiotic prescriptions are shown in Table 1. Patients in the group 2 carbapenem group received an appropriate antibiotic and carbapenem earlier than patients receiving ertapenem in the overall cohort. After propensity score matching, baseline characteristics and antibiotic regimens were well balanced when standardized mean differences (SMDs) were evaluated (see Fig. S1 in the supplemental material). Collinearity between the continuous and categorical variables was not observed. The Hosmer-Lemeshow test to assess the goodness of fit of the regression model was appropriate (P = 0.89), and the C-statistic (area under the curve) was 0.796.
FIG 1.
Study flowchart.
TABLE 1.
Clinical characteristics of patients with ESBL-producing E. coli and K. pneumoniae bacteremia in the overall and propensity score-matched cohorts
| Parametera | Data for: |
|||||
|---|---|---|---|---|---|---|
| Overall cohort |
Propensity score-matched cohort |
|||||
| Ertapenem (n = 403) | Other carbapenems (n = 200) | P | Ertapenem (n =162) | Other carbapenems (n = 162) | P | |
| Age (mean ± SD) (yr) | 71.0 ± 13.9 | 72.7 ± 13.2 | 0.20 | 72.7 ± 12.1 | 72.6 ± 13.6 | 0.80 |
| Male sex (no. [%]) | 133 (33) | 92 (46) | 0.0019 | 60 (37.0) | 70 (43.2) | 0.26 |
| E. coli (no. [%]) | 375 (93.1) | 163 (81.5) | <0.0001 | 143 (88.3) | 137 (84.6) | 0.33 |
| Nosocomial acquisition (no. [%]) | 76 (18.9) | 54 (27) | 0.022 | 39 (24.1) | 40 (24.7) | 0.90 |
| Underlying disease (no. [%]) | ||||||
| Diabetes mellitus | 178 (44.2) | 74 (37.0) | 0.093 | 70 (43.2) | 65 (40.1) | 0.57 |
| Liver cirrhosis | 23 (5.7) | 11 (5.5) | 0.92 | 10 (6.2) | 10 (6.2) | 1.00 |
| ESRD | 7 (1.7) | 3 (1.5) | 1.00 | 2 (1.2) | 2 (1.2) | 1.00 |
| COPD | 2 (0.5) | 9 (4.5) | 0.0012 | 2 (1.2) | 1 (0.6) | 1.00 |
| Solid tumor, localized | 66 (16.4) | 36 (18) | 0.62 | 30 (18.5) | 31 (19.1) | 0.89 |
| Metastatic solid tumor | 21 (5.2) | 27 (13.5) | 0.0004 | 15 (9.3) | 19 (11.7) | 0.47 |
| Hematological malignancy | 0 (0) | 6 (3) | 0.0013 | |||
| Lymphoma | 2 (0.5) | 5 (2.5) | 0.043 | 1 (0.6) | 1 (0.6) | 1.00 |
| Solid organ transplantation | 1 (0.3) | 2 (1) | 0.26 | 1 (0.6) | 1 (0.6) | 1.00 |
| Chemotherapy within 6 mo (no. [%]) | 25 (6.2) | 31 (15.5) | 0.0002 | 14 (8.6) | 19 (11.7) | 0.36 |
| Immunosuppressive agent within 1 mo (no. [%]) | 8 (2.0) | 6 (3) | 0.57 | 3 (1.9) | 3 (1.9) | 1.00 |
| CCI (median [IQR]) | 5 (3–6) | 5 (4–7) | <0.0001 | 5 (4–6) | 5 (4–7) | 0.11 |
| CCI of ≥3 (no. [%]) | 322 (79.9) | 185 (92.5) | <0.0001 | 146 (90.1) | 147 (90.7) | 0.85 |
| Source of infection (no. [%]) | <0.0001 | 0.56 | ||||
| Urinary tract | 333 (82.6) | 110 (55) | 113 (69.8) | 104 (64.2) | ||
| Biliary | 26 (6.5) | 38 (19) | 17 (10.5) | 21 (13.0) | ||
| Other | 44b (10.9) | 52c (26) | 32d (19.8) | 37e (22.8) | ||
| Pitt bacteremia score (median [IQR]) | 1 (0–2) | 2.0 (0–3) | <0.0001 | 1 (0–2) | 2.0 (0–3) | 0.023 |
| Severe sepsis or septic shock (no. [%]) | 186 (46.2) | 124 (62) | 0.0002 | 91 (56.2) | 95 (58.6) | 0.65 |
| ICU admission (no. [%]) | 101 (25.1) | 103 (51.5) | <0.0001 | 72 (44.4) | 76 (46.9) | 0.66 |
| Hospital stay (mean ± SD) (days) | 15.7 ± 18.0 | 21.5 ± 19.0 | <0.0001 | 16.9 ± 22.5 | 20.2 ± 14.5 | <0.0001 |
| ICU stay (mean ± SD) (days) | 8.5 ± 13.5 | 10.2 ± 15.1 | 0.0079 | 9.0 ± 15.2 | 8.9 ± 9.1 | 0.017 |
| Antibiotic regimen (no. [%]) | ||||||
| Carbapenem as first antibiotic | 125 (31.0) | 103 (51.5) | <0.0001 | 76 (46.9) | 82 (50.6) | 0.50 |
| Other antibiotics as first antibiotic | <0.0001 | 0.017 | ||||
| Third-generation cephalosporin | 127 (31.5) | 43 (21.5) | 38 (23.5) | 38 (23.5) | ||
| Cefepime | 3 (0.7) | 7 (3.5) | 0 (0) | 6 (3.7) | ||
| Piperacillin plus tazobactam | 53 (13.2) | 30 (15.0) | 25 (15.4) | 21 (13.0) | ||
| Fluoroquinolones | 85 (21.1) | 12 (6) | 22 (13.6) | 10 (6.2) | ||
| Other | 10 (2.5) | 5 (2.5) | 1 (0.6) | 5 (3.1) | ||
| Time to active antibiotic after blood culture (mean ± SD) (h) | 34.4 ± 34.6 | 21.2 ± 27.7 | <0.0001 | 24.8 ± 30.5 | 22.1 ± 28.7 | 0.53 |
| Active antibiotic within 24 h (no. [%]) | 211 (52.4) | 140 (70.0) | <0.0001 | 106 (65.4) | 111 (68.5) | 0.55 |
| Active antibiotic within 48 h (no. [%]) | 257 (63.8) | 160 (80.0) | <0.0001 | 122 (75.3) | 127 (78.4) | 0.51 |
| Active antibiotic within 72 h (no. [%]) | 304 (75.4) | 186 (93.0) | <0.0001 | 142 (87.7) | 149 (92.0) | 0.20 |
| Time to carbapenem after blood culture (mean ± SD) (h) | 44.3 ± 34.2 | 28.8 ± 30.1 | <0.0001 | 33.4 ± 32.4 | 30.6 ± 31.2 | 0.37 |
| Carbapenem within 24 h (no. [%]) | 150 (37.2) | 114 (57.0) | <0.0001 | 83 (51.2) | 88 (54.3) | 0.58 |
| Carbapenem within 48 h (no. [%]) | 207 (51.4) | 142 (71.0) | <0.0001 | 104 (64.2) | 110 (67.9) | 0.48 |
| Carbapenem within 72 h (no. [%]) | 275 (68.2) | 177 (88.5) | <0.0001 | 134 (82.7) | 140 (86.4) | 0.36 |
| Duration of carbapenem treatment (mean ± SD) (days) | 11.04 ± 5 | 13.7 ± 7.5 | <0.0001 | 11.3 ± 5.7 | 13.6 ± 7.2 | 0.002 |
| Total duration of active therapy (mean ± SD) (days) | 13.13 ± 6.67 | 15.3 ± 9.1 | 0.0045 | 13.3 ± 7.4 | 15.4 ± 9.2 | 0.016 |
| Death (no. [%]) | ||||||
| Day 7 | 6 (1.5) | 9 (4.5) | 0.047 | 4 (2.5) | 6 (3.7) | 0.52 |
| Day 14 | 15 (3.7) | 23 (11.5) | 0.0002 | 9 (5.6) | 16 (9.9) | 0.15 |
| Day 30 | 26 (6.5) | 34 (17) | <0.0001 | 15 (9.3) | 25 (15.4) | 0.091 |
ESRD, end-stage renal disease; COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
Intraabdominal infection, 20 patients; pneumonia, 6 patients; skin and soft tissue infection, 4 patients; catheter-related infection, 5 patients; unknown, 9 patients.
Intraabdominal infection, 19 patients; pneumonia, 11 patients; skin and soft tissue infection, 1 patient; bone and joint infection, 1 patient; central nervous system infection, 2 patients; catheter-related infection, 3 patients; unknown, 15 patients.
Intraabdominal infection, 16 patients; pneumonia, 5 patients; skin and soft tissue infection, 2 patients; catheter-related infection, 4 patients; unknown, 5 patients.
Intraabdominal infection, 16 patients; pneumonia, 7 patients; bone and joint infection, 1 patient; central nervous system infection, 2 patients; catheter-related infection, 1 patient; unknown, 10 patients.
Primary outcome.
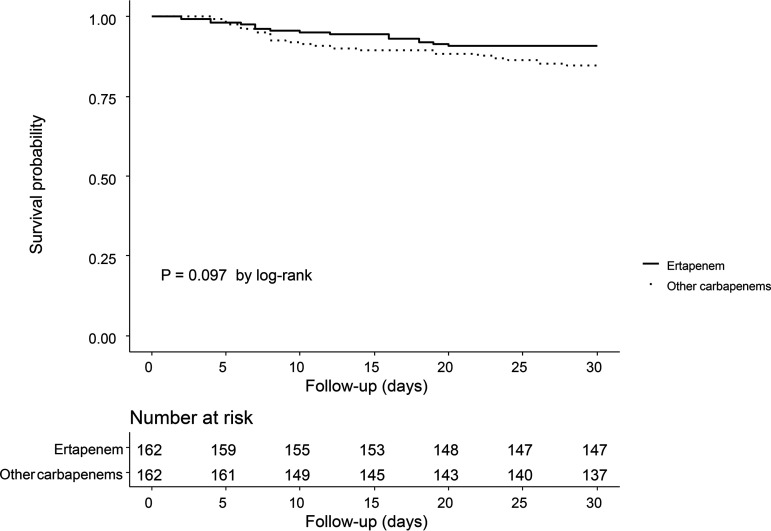
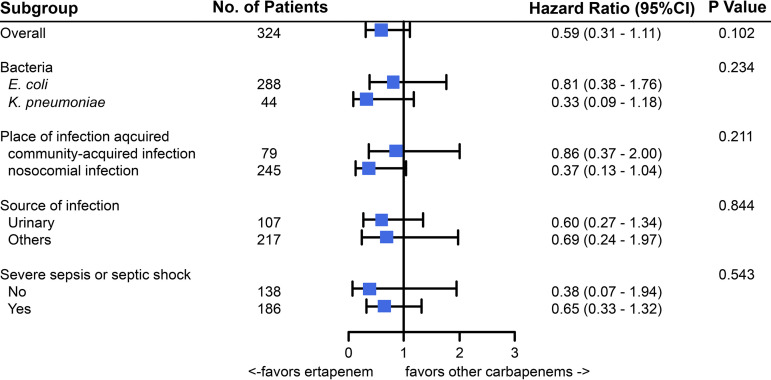
In the propensity score-matched cohort, there were 15 deaths (9.3%) within 30 days in the ertapenem group and 25 (15.4%) in the group 2 carbapenem group. In survival analyses, 30-day mortality rates did not differ between the patients receiving ertapenem and those receiving other carbapenems (P = 0.097 by log-rank test) (Fig. 2). In univariate Cox regression, ertapenem was not associated with 30-day mortality (hazard ratio [HR], 0.59 [95% confidence interval [CI], 0.31 to 1.11]). After adjustment of covariables with P values of <0.2 in the univariate Cox regression analysis, ertapenem was again not associated with 30-day mortality (adjusted HR [aHR], 0.60 [95% CI, 0.29 to 1.22]) (Table 2). In multivariate Cox analysis, nosocomial infection (aHR, 2.68 [95% CI, 1.32 to 5.42]) and metastatic solid tumor (aHR, 3.96 [95% CI, 1.28 to 12.24]) were associated with 30-day mortality. In terms of 7- and 14-day mortality, ertapenem was again not associated with outcomes (Fig. 3; also see Fig. S2 and Tables S1 and S2). In multivariate logistic regression with propensity score-matched cohorts, ertapenem was not associated with 30-day mortality (adjusted odds ratio [aOR], 0.56 [95% CI, 0.23 to 1.37]) (see Table S3). Subgroup analyses for death within 30 days are presented in Fig. 3, and none of the tests for interaction yielded significant results.
FIG 2.
Probability of 30-day survival in the propensity score-matched cohort.
TABLE 2.
Thirty-day all-cause mortality risks in the propensity score-matched cohort
| Variablea | Unadjusted HR (95% CI) | P | aHRb (95% CI) | P |
|---|---|---|---|---|
| Male sex | 1.71 (0.92–3.18) | 0.090 | 0.91 (0.43–1.91) | 0.80 |
| E. coli | 0.26 (0.13–0.49) | <0.0001 | 0.38 (0.17–0.83) | 0.015 |
| Nosocomial acquisition | 4.26 (2.28–7.94) | <0.0001 | 2.68 (1.32–5.42) | 0.006 |
| Underlying disease | ||||
| Liver cirrhosis | 2.98 (1.25–7.10) | 0.014 | 1.76 (0.58–5.37) | 0.32 |
| ESRD | 4.47 (1.08–18.56) | 0.039 | 1.87 (0.28–12.57) | 0.52 |
| Solid tumor, localized | 2.82 (1.49–5.35) | 0.002 | 1.96 (0.93–4.11) | 0.077 |
| Metastatic solid tumor | 5.42 (2.83–10.39) | <0.0001 | 3.96 (1.28–12.24) | 0.017 |
| Chemotherapy within 6 mo | 3.26 (1.59–6.67) | 0.001 | 1.27 (0.47–3.44) | 0.64 |
| CCI | 1.35 (1.20–1.53) | <0.0001 | 1.03 (0.85–1.25) | 0.75 |
| Source of infection | <0.0001 | 0.45 | ||
| Urinary tract | Reference | Reference | ||
| Biliary | 2.09 (0.75–5.81) | 0.16 | 1.42 (0.45–4.43) | 0.55 |
| Other | 5.30 (2.70–10.44) | <0.0001 | 1.73 (0.74–4.05) | 0.21 |
| Pitt bacteremia score | 1.29 (1.09–1.51) | 0.003 | 1.29 (1.06–1.56) | 0.012 |
| Severe sepsis or septic shock | 3.78 (1.67–8.55) | 0.001 | 3.14 (1.30–7.59) | 0.011 |
| Carbapenem | ||||
| Other carbapenem | Reference | Reference | ||
| Ertapenem | 0.59 (0.31–1.11) | 0.10 | 0.60 (0.29–1.22) | 0.16 |
ESRD, end-stage renal disease.
Variables with P values of <0.20 in univariate analyses were included in adjusted analyses.
FIG 3.
Subgroup analyses.
Secondary outcomes.
Ten patients in the group 2 carbapenem group were already colonized with carbapenem-resistant Enterobacterales (CRE) or carbapenem-resistant Acinetobacter or Pseudomonas species. Therefore, 162 patients in the group 2 carbapenem group and 152 patients in the ertapenem group were compared for acquisition of a multidrug-resistant organism (MDRO) (see Table S4). The incidences of CRE were 6.8% (11/162 patients) and 5.3% (8/152 patients) in the ertapenem group and the other-carbapenem group, respectively, with no significant difference between them (P = 0.37). Carbapenem-resistant Acinetobacter or Pseudomonas species were less frequently acquired in patients receiving ertapenem than in those receiving other carbapenems (odds ratio [OR], 0.36 [95% CI, 0.15 to 0.83]). The rates of Clostridioides difficile infection (CDI) did not differ between the ertapenem group and the other-carbapenem group (8.1% versus 8.0%; P = 0.98).
DISCUSSION
In this study, the 30-day mortality rate for ertapenem was similar to that for other carbapenems for treatment of ESBL-producing E. coli and K. pneumoniae bacteremia. We enrolled patients who were exposed exclusively to either ertapenem or a group 2 carbapenem to reduce contamination effects between the treatment arms. By propensity score matching and further adjustment of confounding factors in multivariate analyses, we found that ertapenem was not inferior to other carbapenems in terms of 30-day mortality rates in ESBL-positive E. coli and K. pneumoniae bacteremia. In addition, the use of ertapenem was not associated with early death (7 and 14 days). In our propensity score-matched cohort, over 85% of patients were infected with E. coli, and over 65% had urinary tract infections. According to our results, patients with urinary tract infections due to ESBL-positive E. coli, even if they were in severe sepsis or septic shock, could be safely treated with ertapenem. However, since only a small number of severe infectious diseases, such as respiratory, central nerve system, or bone and joint infections, were included, it remains to be determined whether the effect of ertapenem in these groups of patients is comparable to that of group 2 carbapenems.
Our findings are consistent with the results of previous observational studies. Lee et al. compared clinical and microbiological outcomes among 244 patients with ESBL-producing E. coli or K. pneumoniae who received ertapenem or other carbapenems, and they found that ertapenem and the other carbapenems had similar efficacies, although they did not adjust other confounding variables (8). Others have reported the same outcomes in propensity score-matched cohorts (9, 11). However, those earlier studies used the carbapenem MIC cutoff used before 2012; therefore, the results need to be interpreted with caution and should not be directly applied in current clinical practice. Until now, there has been only one study that compared ertapenem with other carbapenems using the present carbapenem MIC (10). That study also found that ertapenem appeared to be as effective, in terms of 30-day mortality rates, as the other carbapenems as a targeted therapy against ESBL-producing Enterobacterales bacteremia.
The multinational preregistered cohort study analyzed 88 pairs of propensity score-matched patients in a definitive treatment cohort and concluded that the outcome of definitive treatment with ertapenem was comparable to that with the other carbapenems (10). However, that study suffered from a methodological problem, in that it included patients who were given both groups of carbapenems. In other words, some of the patients receiving ertapenem as definitive treatment received group 2 carbapenems empirically, and vice versa. Although the authors grouped the empirical and definitive treatment cohorts and analyzed them separately, the empirical therapy was not fully under control because those cohorts were not mutually exclusive in terms of the comparators. In study designs that permit counter-drugs to be used interchangeably by the same patients, contamination of drug exposure occurs between groups. As a result, it is difficult to conclude whether the outcome is due to the definitive treatment or the empirical regimen and to measure the extent to which the outcome is influenced by the empirical regimen. Since we aimed to avoid the contamination problem, we excluded patients who received both groups of carbapenems over the course of treatment, even at the risk of reducing the sample size. Despite this, to the best of our knowledge, this is the largest propensity score-matched study that avoids the aforementioned contamination problem. Our results suggest that the efficacy of ertapenem may be comparable to that of the other carbapenems in the treatment of ESBL-producing Enterobacterales.
As previous studies have reported and as occurs in real clinical practice, patients with severe illness or underlying disease are more likely to receive group 2 carbapenems than ertapenem in order to cover nonfermenters. In addition, due to concerns regarding ertapenem’s efficacy in critically ill patients, clinicians tend to start group 2 carbapenem treatment. In our study, carbapenem and active antibiotic treatments were initiated earlier in patients receiving group 2 carbapenems, which could positively affect the treatment outcomes. Moreover, those severe illnesses or comorbid conditions in the group 2 carbapenem group could negatively influence the treatment outcomes after propensity score matching. In our propensity score-matched cohort, the two groups were well balanced in terms of initiation of active antibiotic and carbapenem treatments, severity of sepsis, and underlying disease (see Fig. S1 in the supplemental material). However, because there was still a 6% difference in mortality rates between the propensity score-matched groups, some imbalance between the groups cannot be excluded, and the generous SMD threshold of 0.2 and/or unmeasured variables might have led to residual confounding. To overcome this, we also performed multivariate analyses in the propensity score-matched cohort.
In our subgroup analyses, we did not find that ertapenem was significantly associated with 30-day mortality in any subgroup (Fig. 3). In contrast with the INCREMENT project, which observed a tendency for ertapenem to be less effective in severe sepsis or septic shock (10), we did not detect any difference in the efficacy of ertapenem in a subgroup analysis of severe sepsis/septic shock (n = 186). This outcome could reduce the concern about the efficacy of ertapenem in critically ill patients. A previous study showed that group 2 carbapenems tended to be favored for patients with isolates other than E. coli (10), and the authors suggested that this might be due to the higher MIC of ertapenem for K. pneumoniae versus E. coli (13), resulting in inadequate pharmacokinetic target attainment for ertapenem. In our study, we were unable to determine whether ertapenem was associated with 30-day mortality in K. pneumoniae bacteremia because we were not able to evaluate the corresponding MIC value and few patients with K. pneumoniae (n = 46) were enrolled; further work is required to see whether ertapenem is associated with a worse outcome in patients with ESBL-producing K. pneumoniae bacteremia.
Ertapenem is expected to cause less acquisition of MDRO or Clostridioides difficile, compared with other carbapenems, because it has no appreciable activity against nonfermenters such as Pseudomonas or Acinetobacter species. Some studies have detected such an effect, although it remains controversial (14–17). In our study, other risk factors for acquiring resistant organisms were not taken into account, although a carbapenem-resistant nonfermenter was more commonly encountered in patients with group 2 carbapenems. Therefore, the result should be interpreted with caution.
Our study has several strengths. First, our strict inclusion criteria were able to avoid the limitations due to antibiotic regimen changes between empirical therapy and definite therapy regimens. Second, we demonstrated comparable efficacy of ertapenem in a large propensity score-matched cohort with well-balanced groups, and we further adjusted other variables to demonstrate ertapenem’s efficacy. Lastly, over one-half of the patients were critically ill (56% of the patients had severe sepsis or septic shock) in the present study, and our study suggests the safety of ertapenem in severe sepsis or septic shock.
This study has some limitations. First, due to its retrospective nature, unmeasured variables could not be adjusted. In addition, despite propensity score matching, the possibility of residual confounding factors existed because we chose a generous SMD value of 0.2 for balancing between the groups. However, in an attempt to reduce residual bias, we further adjusted variables using multivariate analyses, although this was unlikely to eliminate all selection bias. Second, no ESBL confirmation test was performed, and some of the isolates might not be ESBL producers. However, practically all isolates cannot be confirmed for the presence of ESBL, and our result is more likely applicable to real-world practice in treating presumed ESBL-producing Enterobacterales strains. Third, we captured all-cause mortality as the primary outcome, and this might not have been sufficient to capture infection-related outcomes. However, because an objective variable such as death is more reliable for assessing outcomes than are subjective findings such as improving the signs or symptoms of infection and because the specific causes of death could not be accurately established retrospectively, we used all-cause mortality as the primary outcome measure. Lastly, since we did not review the specific dosage of each antibiotic, we cannot exclude the possibility of inappropriate dose administration for some patients; however, underdosing, which could bias our conclusions, is unlikely in current practice.
In conclusion, our study demonstrates that ertapenem has efficacy comparable to that of the other carbapenems in treating ESBL-producing E. coli and K. pneumoniae bacteremia.
MATERIALS AND METHODS
Study population and setting.
We conducted a multicenter retrospective cohort study involving three university-affiliated hospitals in South Korea (Hallym University Sacred Heart Hospital [an 870-bed hospital in Anyang], Kangnam Sacred Heart Hospital [a 570-bed hospital in Seoul], and Dongtan Sacred Heart Hospital [an 880-bed hospital in Hwaseong]). Hospitalized patients (age of >19 years) who had bloodstream infections due to ESBL-positive E. coli or K. pneumoniae between January 2013 and December 2020 were identified retrospectively. Only the initial episode of bacteremia during the period under study was included. Patients who received ertapenem or a group 2 carbapenem (meropenem, doripenem, or imipenem) within 96 h after a positive blood culture was obtained and for at least 48 h were included. Exchange usage among group 2 carbapenems was allowed (e.g., use of doripenem followed meropenem was classified as group 2 carbapenems). Patients meeting the following criteria were excluded: (i) polymicrobial bacteremia, (ii) receiving members of both groups of carbapenems during the course of treatment, or (iii) combination therapy with carbapenem regardless of susceptibility test results. The primary outcome was 30-day all-cause mortality. Secondary outcomes were CDI and the emergence of carbapenem-resistant Gram-negative colonization or infection (CRE or carbapenem-resistant Acinetobacter or Pseudomonas species) within 90 days after carbapenem initiation. The study was approved by the institutional review boards of each of the three participating hospitals (Hallym University Sacred Heart Hospital protocol number 2020-12-009, Dongtan Sacred Heart Hospital protocol number HDT-2020-12-017, and Kangnam Sacred Heart Hospital protocol number HKS 2020-12-011). The requirement for patient consent was waived because the study was retrospective.
Variables and definitions.
Patients’ medical records were reviewed by infectious diseases specialists in each center. Demographic information, CCI values, and data on preexisting medical conditions, the source of bacteremia, the severity of sepsis, the antimicrobial regimen and duration, and death were collected (18, 19). Pitt bacteremia scores were collected within 24 h before and 24 h after collection of blood cultures, and the worst readings were recorded. (20). When mortality data were unavailable in the hospital electronic medical charts, we collected them from the National Statistical Office (Statistic Korea, KOSTA). Nosocomial infection was defined as the onset of symptoms 48 h or more after admission or within 48 h after hospital discharge. Active antibiotic was defined as any antibiotic with in vitro susceptibility. Times to initiation of active antibiotic were captured in hours from the time of blood culture collection. Times to initiation of the study antibiotic (carbapenem) were calculated from the time of blood culture collection. CDI was defined as the presence of C. difficile toxin A or B by enzyme immunoassay or PCR test within 90 days after the initiation of carbapenem treatment. Carbapenem-resistant Gram-negative colonization or infection was defined as recovery of Enterobacterales, Pseudomonas, or Acinetobacter species resistant to carbapenem in subsequent clinical or surveillance cultures within 90 days after initiation of carbapenem treatment. Patients who were colonized with such resistant organisms and had CDI within 1 year were excluded from the analysis. Day 1 was defined as the first day of blood culture collection.
Microbiological methods.
E. coli and K. pneumoniae were identified in each center using automated microbiology systems, and ESBL was detected with a Vitek 2 (bioMérieux) or MicroScan (Siemens Healthcare) system. ESBL-positive isolates from the automated systems were selected. All isolates were ceftriaxone or cefotaxime nonsusceptible. Then, all carbapenem-nonsusceptible isolates were screened for the presence of carbapenemase and excluded from the study regardless of the presence of carbapenemase. Current Clinical and Laboratory Standards Institute breakpoints were used to define susceptibility to the administered antibiotics (21). The MIC cutoff values for each carbapenem were followed. The MIC cutoff for ertapenem is ≤0.5 mg/L, and those for meropenem, imipenem, and doripenem are 1 mg/L.
Statistical analyses.
Since differences in baseline characteristics between patients receiving ertapenem and those receiving other carbapenems were frequently observed in previous studies, we used propensity score matching to balance the groups (22). For this purpose, we developed a multivariate logistic regression model to estimate a propensity score for the likelihood of each patient receiving ertapenem. The covariates included for generating propensity scores were the following: age, sex, bacterial species, nosocomial infection, underlying disease, CCI, use of immunosuppressive agent, chemotherapy, source of infection, severity of sepsis, ICU admission, and antibiotic start time. Collinearity between the variables used to calculate the propensity scores was evaluated. One-to-one greedy matching was used with a caliper width of 0.20. SMDs were tested to ensure balance between ertapenem and other carbapenems after propensity score matching. Baseline characteristics were considered balanced if the SMD values were <20%.
The chi square or Fisher’s exact test was used to compare baseline categorical variables between groups as appropriate. The Mann-Whitney U test was used to compare the baseline medians of continuous variables. Mortality rates were plotted using Kaplan-Meier curves and compared using the log-rank test. A Cox proportional hazard model was used to estimate HRs and associated 95% CIs for potential risk factors for 30-day mortality. Variables with P values of <0.2 in univariate analyses were included in multivariate models. Subgroup analyses for 30-day mortality were also performed. In addition, univariate and multivariate logistic regressions were performed for 30-day mortality in propensity score-matched cohorts. For secondary outcomes, only univariate logistic analyses were performed. The Hosmer-Lemeshow test was used to assess the goodness of fit of regression models. All statistical analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC).
ACKNOWLEDGMENTS
This study was supported in part by a research grant (NRF-2020R1G1A1099882) from the National Research Foundation (NRF) of Korea.
We declare no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2021. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P aeruginosa). Clin Infect Dis 72:e169–e183. 10.1093/cid/ciaa1478. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Bano J, Gutierrez-Gutierrez B, Machuca I, Pascual A. 2018. Treatment of infections caused by extended-spectrum-beta-lactamase-, AmpC-, and carbapenemase-producing Enterobacteriaceae. Clin Microbiol Rev 31:e00079-17. 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhanel GG, Johanson C, Embil JM, Noreddin A, Gin A, Vercaigne L, Hoban DJ. 2005. Ertapenem: review of a new carbapenem. Expert Rev Anti Infect Ther 3:23–39. 10.1586/14787210.3.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Shah PM. 2008. Parenteral carbapenems. Clin Microbiol Infect 14(Suppl 1):175–180. 10.1111/j.1469-0691.2007.01868.x. [DOI] [PubMed] [Google Scholar]
- 5.Brink AJ, Richards GA, Schillack V, Kiem S, Schentag J. 2009. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int J Antimicrob Agents 33:432–436. 10.1016/j.ijantimicag.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Burkhardt O, Kumar V, Katterwe D, Majcher-Peszynska J, Drewelow B, Derendorf H, Welte T. 2007. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. J Antimicrob Chemother 59:277–284. 10.1093/jac/dkl485. [DOI] [PubMed] [Google Scholar]
- 7.Burkhardt O, Kumar V, Schmidt S, Kielstein JT, Welte T, Derendorf H. 2010. Underdosing of ertapenem in critically ill patients with pneumonia confirmed by Monte Carlo simulations. Int J Antimicrob Agents 35:96–97. 10.1016/j.ijantimicag.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Lee NY, Huang WH, Tsui KC, Hsueh PR, Ko WC. 2011. Carbapenem therapy for bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. Diagn Microbiol Infect Dis 70:150–153. 10.1016/j.diagmicrobio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Collins VL, Marchaim D, Pogue JM, Moshos J, Bheemreddy S, Sunkara B, Shallal A, Chugh N, Eiseler S, Bhargava P, Blunden C, Lephart PR, Memon BI, Hayakawa K, Abreu-Lanfranco O, Chopra T, Munoz-Price LS, Carmeli Y, Kaye KS. 2012. Efficacy of ertapenem for treatment of bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 56:2173–2177. 10.1128/AAC.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutierrez-Gutierrez B, Bonomo RA, Carmeli Y, Paterson DL, Almirante B, Martinez-Martinez L, Oliver A, Calbo E, Pena C, Akova M, Pitout J, Origuen J, Pintado V, Garcia-Vazquez E, Gasch O, Hamprecht A, Prim N, Tumbarello M, Bou G, Viale P, Tacconelli E, Almela M, Perez F, Giamarellou H, Cisneros JM, Schwaber MJ, Venditti M, Lowman W, Bermejo J, Hsueh PR, Mora-Rillo M, Gracia-Ahulfinger I, Pascual A, Rodriguez-Bano J. 2016. Ertapenem for the treatment of bloodstream infections due to ESBL-producing Enterobacteriaceae: a multinational pre-registered cohort study. J Antimicrob Chemother 71:1672–1680. 10.1093/jac/dkv502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu UI, Chen WC, Yang CS, Wang JL, Hu FC, Chang SC, Chen YC. 2012. Ertapenem in the treatment of bacteremia caused by extended-spectrum beta-lactamase-producing Escherichia coli: a propensity score analysis. Int J Infect Dis 16:e47–e52. 10.1016/j.ijid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Rattanaumpawan P, Werarak P, Jitmuang A, Kiratisin P, Thamlikitkul V. 2017. Efficacy and safety of de-escalation therapy to ertapenem for treatment of infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae: an open-label randomized controlled trial. BMC Infect Dis 17:183. 10.1186/s12879-017-2284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CC, Chen YS, Toh HS, Lee YL, Liu YM, Ho CM, Lu PL, Liu CE, Chen YH, Wang JH, Tang HJ, Yu KW, Liu YC, Chuang YC, Xu Y, Ni Y, Ko WC, Hsueh PR. 2012. Impact of revised CLSI breakpoints for susceptibility to third-generation cephalosporins and carbapenems among Enterobacteriaceae isolates in the Asia-Pacific region: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2002–2010. Int J Antimicrob Agents 40(Suppl):S4–S10. 10.1016/S0924-8579(12)70003-1. [DOI] [PubMed] [Google Scholar]
- 14.Yoon YK, Yang KS, Lee SE, Kim HJ, Sohn JW, Kim MJ. 2014. Effects of group 1 versus group 2 carbapenems on the susceptibility of Acinetobacter baumannii to carbapenems: a before and after intervention study of carbapenem-use stewardship. PLoS One 9:e99101. 10.1371/journal.pone.0099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sousa D, Castelo-Corral L, Gutierrez-Urbon JM, Molina F, Lopez-Calvino B, Bou G, Llinares P. 2013. Impact of ertapenem use on Pseudomonas aeruginosa and Acinetobacter baumannii imipenem susceptibility rates: collateral damage or positive effect on hospital ecology? J Antimicrob Chemother 68:1917–1925. 10.1093/jac/dkt091. [DOI] [PubMed] [Google Scholar]
- 16.Falagas ME, Tansarli GS, Kapaskelis A, Vardakas KZ. 2013. Ertapenem use and antimicrobial resistance to group 2 carbapenems in Gram-negative infections: a systematic review. Expert Rev Anti Infect Ther 11:69–78. 10.1586/eri.12.149. [DOI] [PubMed] [Google Scholar]
- 17.Nicolau DP, Carmeli Y, Crank CW, Goff DA, Graber CJ, Lima AL, Goldstein EJ. 2012. Carbapenem stewardship: does ertapenem affect Pseudomonas susceptibility to other carbapenems? A review of the evidence. Int J Antimicrob Agents 39:11–15. 10.1016/j.ijantimicag.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup . 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 20.Chow JW, Yu VL. 1999. Combination antibiotic therapy versus monotherapy for Gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 11:7–12. 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing, 27th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Amoah J, Stuart EA, Cosgrove SE, Harris AD, Han JH, Lautenbach E, Tamma PD. 2020. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of Gram-negative bloodstream infections. Clin Infect Dis 71:e497–e505. 10.1093/cid/ciaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S3 and Tables S1 to S4. Download aac.00287-22-s0001.pdf, PDF file, 0.4 MB (375.5KB, pdf)