ABSTRACT
The oxylipin-dependent quorum-sensing system (ODS) of Pseudomonas aeruginosa relies on the production and sensing of two extracellular oxylipins, 10S-hydroxy-(8E)-octadecenoic acid (10-HOME) and 7S,10S-dihydroxy-(8E)-octadecenoic acid (7,10-DiHOME). Here, we implemented a genetic screen of P. aeruginosa strain PAO1 aimed to identify genes required for 10-HOME and 7,10-DiHOME production. Among the 14 genes identified, four encoded previously known components of the ODS and 10 encoded parts of the Xcp type II secretion system (T2SS). We subsequently created a clean xcpQ deletion mutant, which encodes the necessary outer membrane component of Xcp, and found it recapitulated the impaired functionality of the T2SS transposon mutants. Further studies showed that the ΔxcpQ mutant was unable to secrete the oxylipin synthase enzymes across the outer membrane. Specifically, immunoblotting for OdsA, which is responsible for the generation of 10-HOME from oleic acid, detected the enzyme in supernatants from wild-type PAO1 but not ΔxcpQ cultures. Likewise, chromatography of supernatants found that 10-HOME was not in supernatants collected from the ΔxcpQ mutant. Accordingly, diol synthase activity was increased in the periplasm of ΔxcpQ mutant consistent with a stoppage in its transport. Importantly, after exposure of the ΔxcpQ mutant to exogenous 10-HOME and 7,10-DiHOME, the ODS effector genes become active; thus, the sensing component of the ODS does not involve the T2SS. Finally, we observed that Xcp contributed to robust in vitro and in vivo biofilm formation in oleic acid availability- and ODS-dependent manner. Thus, T2SS-mediated transport of the oxylipin synthase enzymes to outside the bacterial cell is required for ODS functionality.
IMPORTANCE We previously showed that the ODS of P. aeruginosa produces and responds to oxylipins derived from host oleic acid by enhancing biofilm formation and virulence. Here, we developed a genetic screen strategy to explore the molecular basis for oxylipins synthesis and detection. Unexpectedly, we found that the ODS autoinducer synthases cross the outer membrane using the Xcp type 2 secretion system (T2SS) of P. aeruginosa, and so the biosynthesis of oxylipins occurs extracellularly. T2SS promoted biofilm formation in the presence of oleic acid as a result of ODS activation. Our results identify two new T2SS secreted proteins in P. aeruginosa and reveal a new way by which this important opportunistic pathogen interacts with the host environment.
KEYWORDS: Pseudomonas aeruginosa, quorum sensing, oxylipin, oleic acid, autoinducer, type 2 secretion system
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen that can cause disease in plants, animals, and humans with breaches in their mechanical or physiological defense barriers (1). One reason for this is that P. aeruginosa has a versatile battery of extracellular and surface-associated virulence factors and is therefore able to form recalcitrant biofilms that protect it from averse conditions and noxious environmental factors, including the immune system and antibiotics (2). Quorum sensing (QS) is a bacterial cell-to-cell communication system that functions to regulate behavior at the cell community level. QS systems have been shown to play a critical role in the regulation of virulence factors and biofilm formation in P. aeruginosa (3). Indeed, deletion of any of the previously described interconnected QS systems of P. aeruginosa—las, rhl, PQS, and IQS—has been demonstrated to attenuate bacterial virulence in a variety of animal models (4).
Oxylipins are bioactive oxygenated lipids. In mammals, oxylipins are derived from polyunsaturated fatty acids by the action of cyclooxygenase, lipoxygenase, or cytochrome P450 oxygenase enzymes (5). They serve to modulate inflammatory pathways but also have multiple other functions, including antimicrobial properties. Oxylipins are also produced by invertebrates, plants, and fungi (6). Typically, oxylipins are not stored in tissues but are formed on demand from precursor fatty acids of endogenous sources (7). Pertinently, P. aeruginosa oxylipin-dependent quorum-sensing system (ODS) relies on the presence and sensing of the extracellular oxylipins (10S)-hydroxy-(8E)-octadecenoic acid (10-HOME) and 7S,10S-dihydroxy-(8E)-octadecenoic acid (7,10-DiHOME) for virulence (8). We have shown that these are synthesized by P. aeruginosa from exogenous oleic acid (OA) using the fatty acid diol synthase (DS) enzymes that are encoded by the ds operon (Fig. 1A) (9). Specifically, using the prick model of Drosophila infection, we have demonstrated that P. aeruginosa can synthesize 10-HOME and 7,10-DiHOME oxylipins using OA scavenged from damaged tissues in vivo and this contributes to fly death. A P. aeruginosa mutant unable to synthesize oxylipins was significantly less virulent than its parent wild-type (WT) strain. Consistent with this, and when using the feeding model of Drosophila, i.e., OA is not available since the bacteria are restricted to the digestive tract, the effect of the ODS on virulence was only observed when the fly diet was supplemented with OA (10).
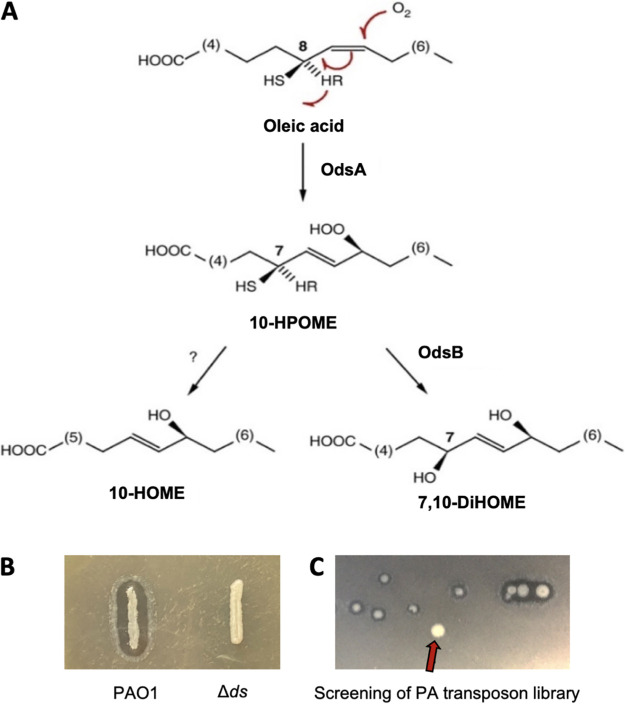
FIG 1.
Screening assay for the search of genes affecting oxylipin production. (A) Oxylipins biosynthetic pathway of P. aeruginosa. The enzyme 10(S)-dioxygenase (OdsA) transforms host OA into 10S-hydroperoxide-octadecenoic acid (10S-HPOME) by stereospecific oxygenation at position C-10 of the OA alkyl chain. Subsequently, 10S-HPOME could be isomerized by the enzyme (7S,10S)-hydroperoxide isomerase to form 7S,10S-DiHOME or be reduced to 10-HOME by an undefined mechanism. (B) Picture showing the WT phenotype of P. aeruginosa versus that of the Δds deletion mutant when plated on LB agar + OA (1%). (C) Representative picture of a plate section of our transposon screening showing a colony lacking the halo.
Recently, we reported that oxylipin production and sensing by P. aeruginosa has all the traits of a QS system (11). These include the following: (i) autoinducers 10-HOME and 7,10-DiHOME are synthesized by bacterial factors; (ii) 10-HOME and 7,10-DiHOME accumulate outside the cell in cell-density dependent fashion; (iii) the expression of odsA and odsB, which encode the oxylipins synthases are controlled as part of a oxylipin-dependent positive-feedback loop; (iv) this involves meeting a threshold concentration to induce the ODS regulon; and (v) the system regulates bacterial functions that require collective coordination, including twitching, swarming, and flagellum-mediated swimming, which together promote biofilm formation (11) and virulence in both plants and animals (10). We have therefore named this oxylipin quorum-sensing system as the oxylipin dependent quorum-sensing system (ODS).
In most cases, the QS autoinducers are synthesized from endogenously generated precursors, although we and others have demonstrated that bacteria can also use exogenous sources for the synthesis of these signal molecules (11, 12). Accordingly, the biosynthesis of bacterial QS autoinducers canonically occurs intracellularly (13), and these molecules are released to the extracellular space by a variety of means depending on the nature of the autoinducers and the bacterial species (14–17). Importantly, and prior to this report, we worked under the assumption that this held true for the ODS and that imported OA from the bacterial environment was converted to 10-HOME and 7,10-DiHOME within the periplasm of the bacteria by the DS enzymes (18). However, we demonstrate here that this is not the case and that, instead, the DS enzymes are secreted from the periplasmic space via the Xcp type II secretion system (T2SS). Once outside the cell, they use host-derived exogenous OA to synthesize the oxylipin autoinducers. This new QS strategy highlights the versatility by which P. aeruginosa senses and takes advantage of the host environment.
RESULTS
Screening for P. aeruginosa factors involved in the production of the ODS autoinducers.
The DS activity of P. aeruginosa introduces one or two hydroxyl groups into the alkyl chain of OA (Fig. 1A) (8, 19); the oxylipins derived from this activity being more hydrophilic than the OA substrate. In addition, these oxylipins have surfactant emulsifying properties that help to dissolve OA in suspension above the critical micelle concentration. We noticed that these properties of oxylipins enable WT P. aeruginosa colonies to produce a transparent halo when this bacterium is grown on lysogeny broth (LB) agar plates containing 1% OA, which renders the medium opaque. Consequently, P. aeruginosa lacking DS activity, i.e., the Δds mutant, do not from this halo when grown under the same conditions (Fig. 1B).
We took advantage of this characteristic phenotype to identify the bacterial genes required for oxylipin production. To do this, we performed a genetic screen using a sequence-defined transposon (ISphoA/hah or ISlacZ/hah) insertion library created in the model strain PAO1 (20). In total, we screened more than 30,000 independent clones for the inability to form the halo on LB agar supplemented with 1% OA (Fig. 1C). We identified 31 colonies unable to form the transparent halo even after restreaking on fresh plates. We successfully PCR amplified and sequenced the DNA regions flanking the transposon for all the 31 mutants, thereby identifying 15 genes putatively required for oxylipin production (Table 1).
TABLE 1.
Genes identified in the screening, their frequencies, and their functions
| Gene | Name | Frequency | Annotated function |
|---|---|---|---|
| PA1288 | odsT | 2 | Oxylipin transporter |
| PA2076 | odsR | 2 | Transcriptional regulator |
| PA2077 | odsA | 1 | Oleate 10S-dioxygenase |
| PA2078 | odsB | 2 | Oleate (7S,10S)-hydroperoxide isomerase |
| PA4946 | mutL | 1 | DNA mismatch repair protein MutL |
| PA3101 | xcpT | 3 | General secretion pathway protein T |
| PA3104 | xcpP | 1 | General secretion pathway protein P |
| PA3105 | xcpQ | 1 | General secretion pathway protein D |
| PA3103 | xcpR | 4 | General secretion pathway protein E |
| PA3100 | XcpU | 1 | General secretion pathway protein U |
| PA3099 | xcpV | 1 | General secretion pathway protein I |
| PA3098 | xcpW | 3 | General secretion pathway protein J |
| PA3097 | xcpX | 3 | General secretion pathway protein K |
| PA3096 | xcpY | 1 | General secretion pathway protein L |
| PA4528 | pilD | 5 | Prepilin peptidase |
Analysis of the identified clones revealed that three of them carried a transposon insertion in the ds operon, which encodes the DS enzymes OdsA and OdsB that catalyze oxylipin biosynthesis annotated as PA2077 and PA2078, respectively, in the Pseudomonas Genome Database (21). Two other transpositions occurred in odsR (PA2076): the ds operon transcriptional regulator found immediately upstream and one found in an orientation opposite to that of the ds operon. In addition, two transpositions were identified in the outer membrane transporter encoded by PA1288, previously called ExfadLO and here renamed OdsT to comply with conventional gene nomenclature. OdsT was previously identified as an outer membrane oxylipin transporter (18). Based on our previous knowledge on the components required for oxylipin production, the extracellular accumulation of oxylipins, and activation of the ds operon, the above-mentioned transposition events were expected and thus confirmed the validity of the screening strategy.
Transposon insertions providing new insight into the ODS included 19 transpositions in genes encoding nine distinct components of the Xcp T2SS; all of which are found in the Xcp region of PAO1 chromosome (PA3095 to PA3105). Another five transpositions occurred in pilD, the gene encoding a prepilin peptidase, which is vital for the processing of some T2SS components in P. aeruginosa (22). Lastly, one transposition occurred in mutL, which encodes a DNA mismatch repair enzyme which promotes large chromosomal deletions in P. aeruginosa (23). Subsequent studies using a clean deletion mutL mutant failed to corroborate the halo deficient phenotype, suggesting that the colony phenotype of the mutL transposition found was most likely due to a secondary mutation caused by the consequent MutL-deficient hypermutagenic phenotype (23).
Oxylipin synthases cross the outer membrane via Xcp T2SS.
T2SSs are involved in the transport of proteins across the outer membrane from the periplasmic space (24). In P. aeruginosa, Xcp is involved in the translocation of multiple proteins, including established virulence factors (25). To confirm the role of the T2SS in OdsA and OdsB secretion and oxylipin production, we made an in-frame deletion of the gene xcpQ (ΔxcpQ), which encodes the function-required outer membrane porin component of the T2SS. As expected, ΔxcpQ colonies failed to produce transparent halos when plated on LB containing 1% OA (Fig. 2A). In addition, using thin-layer chromatography (TLC), we confirmed that ΔxcpQ failed to accumulate any 10-HOME or 7,10-DiHOME in supernatants when grown in LB supplemented with OA (Fig. 2B). Importantly, an ΔxcpQ mutant complemented in trans with a WT xcpQ gene restored the halos and production of oxylipins (Fig. 2A and B). Using mass spectrometry analysis with in-tandem gel filtration and ion exchange, we detected the presence of OdsA and OdsB in the high-pressure liquid chromatography (HPLC)-separated fraction of PAO1 extracellular media that maintained oxylipin synthase activity (see Table S2 in the supplemental material). These were the first and third most abundant proteins detected, respectively. Extracellular accumulation of OdsA was then confirmed using antibody against a purified recombinant version of the protein; immunoblots detected OdsA in the supernatants of WT PAO1 but not of ΔxcpQ mutant (Fig. 2C), reaffirming the importance of the T2SS in its secretion. Accordingly, when OA was added to supernatants collected from the ΔxcpQ mutant, we did not observe the production of oxylipins, whereas supernatants from WT PAO1 demonstrated this activity (Fig. 2D). Furthermore, when PAO1 and ΔxcpQ strains were treated with exogenous 7,10-DiHOME to highly induce DS gene expression, DS enzyme activity was not detected in ΔxcpQ supernatants (Fig. 2E) but was higher in periplasmic extracts of the mutant than in the WT (Fig. 2F). This finding suggests that the enzymes accumulate in this space due to their inability to be secreted. The quality of periplasm fractionation was assessed using subcellular marker enzymes: cytosolic isocitrate dehydrogenase, periplasm-associated alkaline phosphatase, and membrane-bound lactate dehydrogenase (Fig. 2G).
FIG 2.
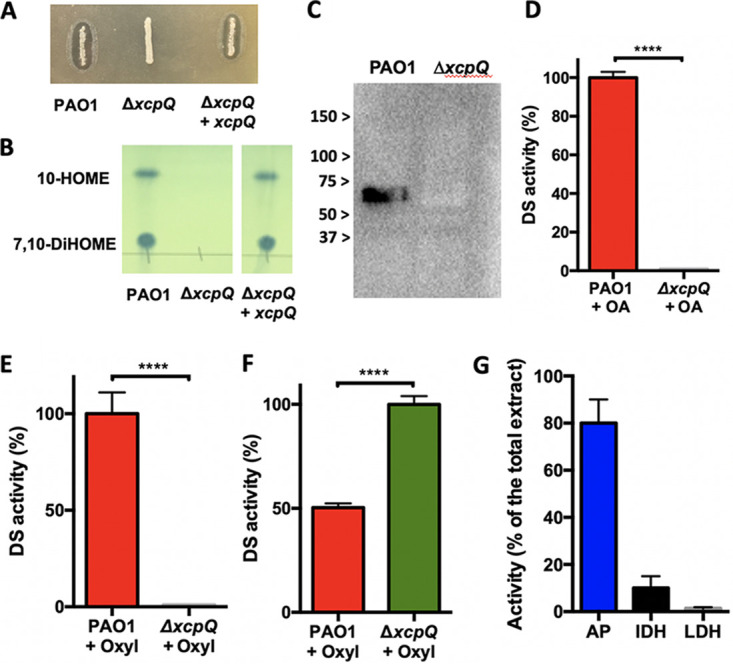
Accumulation of 7,10 DiHOME oxylipin in culture supernatants of PAO1 and its isogenic mutant ΔxcpQ. A) The ΔxcpQ mutant failed to produce 7,10-DiHOME when cultured on LB agar + OA (1%). The production of 7,10-DiHOME was restored by ΔxcpQ when it was complemented with a WT xcpQ gene expressed from a plasmid. B) Thin layer chromatography (TLC) analysis of supernatants from PAO1, ΔxcpQ, and ΔxcpQ complemented in trans detected the oxylipins 10-HOME and 7,10-DiHOME in the supernatant of PAO1 and ΔxcpQ complemented in trans. C) Immunoblot for OdsA in PAO1 and ΔxcpQ supernatants only detects the enzyme in PAO1 media. D) DS enzyme activity, as determined by the ability of cell-free supernatant to convert OA into oxylipins, was only observed for PAO1 and not ΔxcpQ when cultured in the presence of OA or E) oxylipins. F) DS enzymes accumulate in the in the periplasm of ΔxcpQ versus WT as determined by the ability of isolated periplasm fractions to convert OA into oxylipins. G) Activity of specific subcellular maker enzymes, IDH, isocitrate dehydrogenase (cytosolic), LDH, lactate dehydrogenase (membrane) and AP, alkaline phosphatase (periplasmic) was also measured to confirm the accuracy of cell fractionation. Enzyme activity in the periplasm fraction is expressed as a percentage of the total activity produced by crude cell extracts of the same cultures.
ODS functionality requires DS enzyme secretion.
The ODS involves the accumulation of oxylipins in the extracellular medium and subsequent sensing of the oxylipin signal (11). To determine the impact of the T2SS in the expression of genes under the control of the ODS, we monitored the expression kinetic of a representative ODS-regulated gene, PA3427 (11). For this, we made a transcriptional PA3427 promoter reporter fusion with lacZ. As expected, in a ΔxcpQ background, PA3427-lacZ was unresponsive to the presence of OA (Fig. 3A). This is because the secretion of a basal level of DS enzymes is required to produce the extracellular oxylipins that are subsequently incorporated in the bacteria and induce the ds operon, creating a positive regulatory feedback (11). To rule out any collateral effect that a dysfunctional T2SS might have on the expression of PA3427, we corroborated that this gene was expressed normally in the ΔxcpQ mutant at the same level of PAO1 when induced with the purified oxylipin 7,10-DiHOME (Fig. 3B). Thus, Xcp is required for DS secretion, leading to extracellular oxylipin production; however, the T2SS is not involved in the sensing or response to the oxylipin signal.
FIG 3.
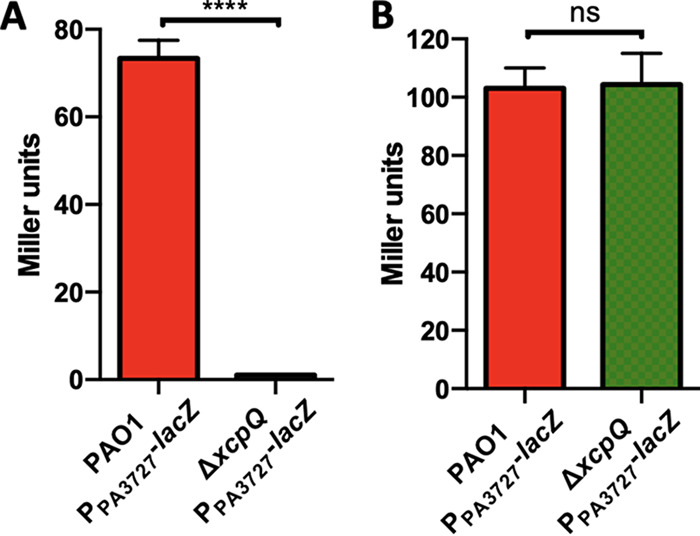
β-Galactosidase activity of PPA3427-lacZ fusion in PAO1 and ΔxcpQ genetic backgrounds. (A) The ΔxcpQ mutant showed a negligible PA3727-lacZ expression in the presence of OA. (B) The ΔxcpQ mutant showed the same level of expression as PAO1 when induced with 7,10-DiHOME.
The T2SS is linked to biofilm formation in P. aeruginosa.
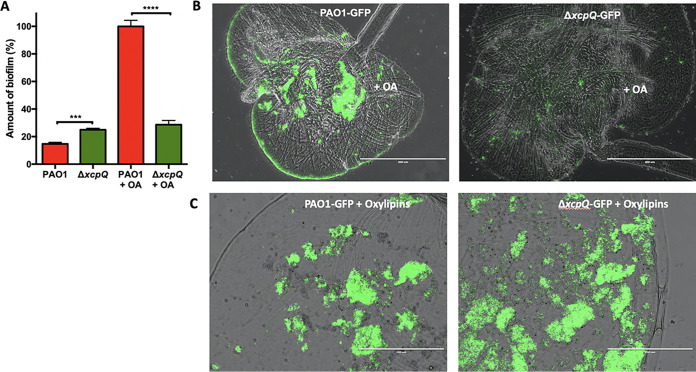
The Xcp system of P. aeruginosa secretes biofilm matrix degradation enzymes that make this system detrimental for biofilm formation in common lab biofilm testing conditions (26). However, considering that we showed here that Xcp is required for ODS functionality and that ODS promotes biofilm formation in the presence of OA, we hypothesized that the specific affect of the Xcp system on biofilm might depend on the testing conditions. Here, we show using a microtiter plate model that, in agreement with previous studies, the ΔxcpQ mutant demonstrated an increase in the amount of biofilm compared to the WT PAO1 strain when both strains were grown in the absence of OA (Fig. 4A). However, in the presence of OA, the amount of biofilm formed by PAO1 was significantly greater than that of the ΔxcpQ strain (Fig. 4A). This result agreed with our previous study reporting that the ODS promoted biofilm formation in vitro (10). We also recapitulated this result in vivo using Drosophila melanogaster fed with OA-supplemented food. Imaging of fly crops conclusively showed reduced biofilm formation for ΔxcpQ versus the PAO1 control (Fig. 4B). To confirm that the reduced amount of biofilm observed in the crops of flies infected with the ΔxcpQ mutant was due to the deficiency of the ΔxcpQ mutant in producing oxylipins and not to other T2SS function, we tested ΔxcpQ biofilm formation in D. melanogaster fed with P. aeruginosa oxylipins. Under this condition, the ΔxcpQ mutant produced an amount of biofilm similar to that produced by PAO1 (Fig. 4C). Thus, the P. aeruginosa T2SS promotes biofilm formation, provided that OA is available and a functional ODS is present.
FIG 4.
Biofilm formation by PAO1 and its isogenic ΔxcpQ mutant. (A) The ΔxcpQ mutant produces more biofilm than does WT PAO1 in vitro, as shown using a microtiter plate model. In contrast, WT produces significantly more biofilm when in media supplemented with OA. (B) Fluorescence microscopy analysis of Drosophila melanogaster crops infected with PAO1 expressing GFP (PAO-GFP) or ΔxcpQ mutant expressing GFP (ΔxcpQ-GFP). PAO1 formed more biofilm than the ΔxcpQ mutant. (C) PAO1 formed an amount of biofilm to similar to that formed by the ΔxcpQ mutant when fly food was supplemented with P. aeruginosa oxylipins. Scale bars, 400 or 200 μm. The size/resolution for each panel was adjusted to 2.125 × 1.587 in./600 dpi from 17.770 × 13.333 in./72 dpi of the originals. Pictures are representative of three independent experiments.
DISCUSSION
We report here that the P. aeruginosa oxylipin synthases OdsA and OdsB are exported to the extracellular space through the Xcp T2SS. Once in the extracellular space, the DS enzymes use exogenous OA as a substrate to synthetize the oxylipin inducers 10-HOME and 7,10-diHOME, which in turn enter the bacterial cells, presumably via the OdsT transporter, to activate the ODS (see proposed model in Fig. 5). Importantly, this study establishes a new link between the T2SS, QS, and biofilm formation in the relevant opportunistic pathogen P. aeruginosa.
FIG 5.
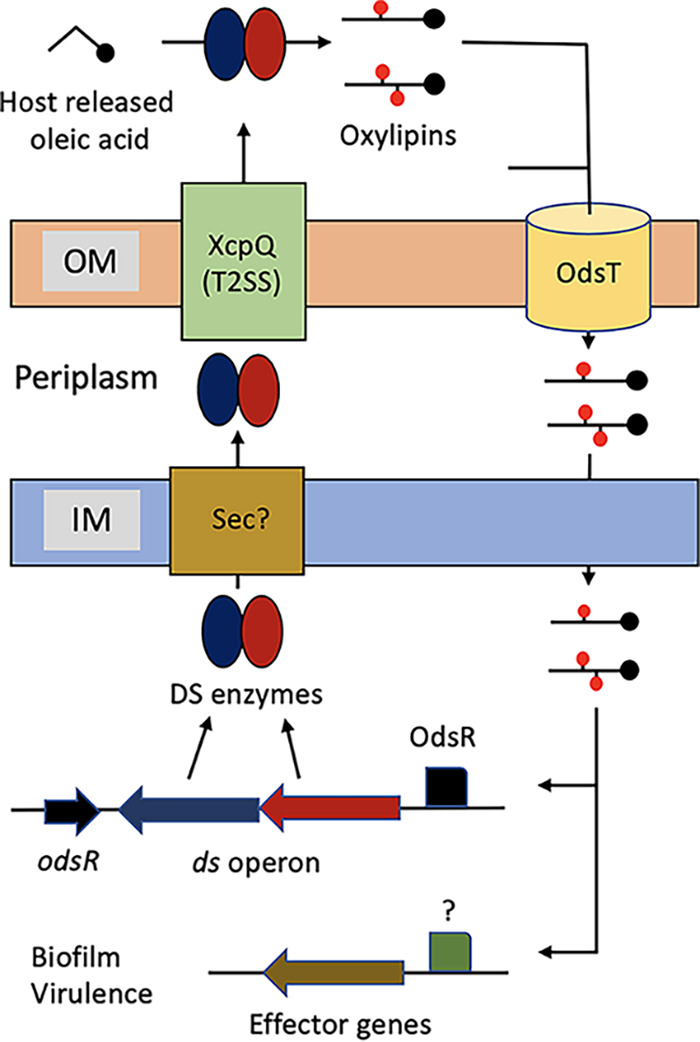
Proposed model of ODS. At low cell density, the ds operon is weakly expressed. When the cell density increases and host OA is present, the DS enzymes start to produce oxylipins. Subsequently, the oxylipins enter the cells through OdsT and bind OdsR, which in turn induces the ds operon. Induced DS enzymes cross the inner membrane through the Sec secretory pathway to reach the periplasm. Then, the enzymes cross the outer membrane via Xcp T2SS. This causes a sudden accumulation of extracellular DS enzymes and therefore of oxylipins, which further activates the ODS and induces the effector genes. The as-yet-undetermined parts of the model, i.e., the sec pathway and the transcriptional activator(s) of the effector genes, are marked with a question mark.
In P. aeruginosa, Xcp is involved in the secretion of several virulence factors, such as the elastase LasB, the lipase LipA, and the alkaline phosphatase PhoA (27). P. aeruginosa has a second T2SS known as Hxc (28), but this is thought to have a considerably restricted role, and we observed no evidence of it being involved with the ODS. Our finding that the Xcp also exports OdsA and OdsB, increases the repertoire of virulence factors known to be secreted by this general secretory pathway in P. aeruginosa. All described T2SSs in Gram-negative bacteria employ a two-step process to secrete proteins from the cytoplasm to the extracellular space through a transient periplasmic intermediate. The first step of translocation through the inner membrane is commonly carried out by the Sec or Tat systems (29, 30). Analysis of the amino acid sequence identified a putative N-terminal signal peptide in both OdsA and OdsB, suggesting that both use the Sec secretory pathway for translocation through the inner membrane, although this remains to be demonstrated (31). Interestingly, the fact that we did not detect transposition events in genes encoding components of the Sec secretory pathway is likely due to the essential role for the viability of this system in most bacteria (32, 33).
In our genetic screen we identified two independent transposition events in odsT, which confirms that the encoded transporter is essential for the normal functioning of the ODS. The fatty acid movement through FadL-like transporters is bidirectional. Thus, prior to this report, we postulated that OdsT was responsible for the export of oxylipins synthesized in the periplasm. In light of our finding that oxylipins are synthesized extracellularly as a result of the Xcp-dependent secretion of DS enzymes, we instead speculate that the main function of OdsT is to import extracellular oxylipins. Ongoing studies in our laboratory are testing this hypothesis. Note that odsT disruption can also alter oxylipin accumulation in the extracellular media, since oxylipin import is required to activate the positive regulatory loop that induces the ds operon (11).
Biofilm formation is an important mechanism by which bacteria establish themselves within a host, as well as a mechanism of defense from host factors. OA is an abundant fatty acid in host tissues (34), and we previously demonstrated in Drosophila melanogaster that Pseudomonas aeruginosa scavenges OA from the host to produce oxylipins, which promotes biofilm formation in vivo (10). In humans, OA is the most abundant fatty acid present in the adipose tissue and the second in all tissues (34). Interestingly, the amount of free OA in tissues and plasma of patients with severe burn injuries is particularly high, and under this condition P. aeruginosa infections are frequent (35). Here, we show that the Xcp T2SS promotes biofilm formation, both in vitro and in vivo, in the presence of OA in an ODS-dependent fashion. The convergence of these traits makes sense since OA is a host-derived signal, especially from wounded hosts, and the formation of biofilm as a result of ODS activation would be a means to adapt to and persist in the hostile host environment.
In summary, we developed a simple screening strategy that allowed the identification of bacterial genes required for ODS functioning in P. aeruginosa. As a result, we found that the Xcp T2SS of P. aeruginosa translocates the DS enzymes, which synthesize the ODS autoinducers, through the outer membrane. ODS is an environment-specific QS system, which depends on the presence of exogenous OA. Thus, we propose that the translocation of the DS enzymes to the extracellular medium, which is peculiar among autoinducer synthases, is a way for this QS to be fine-tuned to the host environment, allowing P. aeruginosa to respond quickly to this condition.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains, plasmids, and oligonucleotides used in this study are described in Table 2. Used strains were routinely grown in lysogeny broth (LB) medium at 37°C, to which agar was added when solid medium was required. When required, P. aeruginosa was grown in M63 media supplemented with 0.2% glucose, 0.1% Casamino Acids, and 1 mM MgSO4 (M63 complete). Antibiotics were added, when necessary, at the following concentrations: ampicillin at 100 μg/mL and carbenicillin at 300 μg/mL for P. aeruginosa, chloramphenicol at 25 μg/mL for Escherichia coli and 200 μg/mL for P. aeruginosa, and kanamycin at 25 μg/mL. We added 90% OA (Sigma) to cultures for oxylipin production and purification. M63 complete or LB media were supplemented with 99% OA (Sigma) or purified oxylipins at the specified concentrations when required. LB agar without NaCl plus 15% sucrose was used to segregate suicide plasmids from merodiploids during construction of xcpQ deletion mutant (ΔxcpQ) strain by allelic exchange (see below).
TABLE 2.
Strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Description | Source or reference |
|---|---|---|
| Strains | ||
| PAO1 | P. aeruginosa wild-type model strain | WTa |
| Δds | PAO1 containing an in-frame deletion of the ds operon | 10 |
| ΔxcpQ | PAO1 containing an in-frame deletion of the xcpQ gene | This study |
| ΔxcpQ(pBB-xcpQ) | ΔxcpQ strain containing plasmid pBB-xcpQ | This study |
| PAO1(pBB-PA3727-lacZ) | PAO1 strain containing plasmid pBB-PA3727-lacZ | 11 |
| ΔxcpQ(pBB-P3727-lacZ) | ΔxcpQ strain containing plasmid pBB-PA3727-lacZ | This study |
| PAO1-GFP | PAO1 strain expressing GFP | 10 |
| ΔxcpQ-GFP | ΔxcpQ strain expressing GFP | This study |
| OneShot TOP10 | E. coli used for plasmid propagation | Invitrogen |
| S17-1λpir | E. coli used as donor strain for the introduction of suicide plasmids into P. aeruginosa | 41 |
| Plasmids | ||
| pBBR1MCS | Conjugative multipurpose cloning vector able to replicate in P. aeruginosa | 42 |
| pEX100Tlink | Suicide vector used for allelic replacement in P. aeruginosa | 43 |
| pEX-xcpQ | pEX100Tlink containing the xcpQ gene of PAO1 | This study |
| pEX-DxcpQ | pEX-PA2076 containing an in-frame deletion of xcpQ | This study |
| pBB-xcpQ | pBBR1MCS expressing xcpQ | This study |
| pBB-PA3727-lacZ | pBBR1MCS expressing lacZ under the xcpQ promotor | 11 |
| Oligonucleotides | ||
| xcpQ-BamHI-FW | For internal deletion of xcpQ/gatcggatcccgaacgactggaaggggc | This study |
| xcpQ-BamHI-RV | For internal deletion of xcpQ/gtcattataaaacgccaaccagttgttcgat | This study |
| xcpQ-HindIII-RV | For cloning xcpQ in pEX100Tlink and pBBR1MCS/gtcaaagcttaccggtccgatgctgctgg | This study |
| xcpQ-SacI-FW | For cloning xcpQ in pEX100Tlink and pBBR1MCS/tcaggagctcccacgagtcgatccgcag | This study |
Source: Washington University, Manoil lab.
Transposon library screening and mutant characterization.
A random mariner transposon library of P. aeruginosa was acquired from the University of Washington (ref). The transposon library was amplified by growing the bacteria in LB broth up to an optical density at 600 nm (OD600) of 1 (exponential phase), and proper dilutions of the bacterial suspension were plated on LB agar supplemented with 1% OA to obtain separate clones. More than 30,000 independent colonies were obtained and, from these, clones lacking a transparent halo were selected for further analysis. The gene mutation of each selected clone was identified by DNA sequencing, as previously described (20).
Genetic constructions.
A region of P. aeruginosa PAO1 genome comprising the xcpQ gene and its flanking regions (~550 bp of each flank) was amplified using primers xcpQ-F-SacI and xcpQ-R-HindIII (Table 1). The primers introduced SacI and HindIII restriction sites at the extremes of the amplified fragment, which were used to insert the fragment into the pEX100Tlink Suicide Vector digested with the same enzymes to obtain pEX-xcpQ plasmid. Subsequently, an internal fragment of 1,867 bp was deleted from the xcpQ gene by doing a reverse PCR amplification using pEX-xcpQ as the template and the primers ΔxcpQ-F-BamHI and ΔxcpQ-R-BamHI. The amplified fragment was digested with BamHI and self-ligated. The obtained plasmid, which was named pEX-ΔxcpQ, contains the xcpQ gene with an internal in-frame deletion flanked by ~550 bp by each side (required for homologous recombination). This suicide plasmid was used to delete xcpQ from PAO1 chromosome by allelic replacement. As an ODS reporter strain, we used the transcriptional promoter reporter fusion of the ODS downstream regulated gene PA3427 with lacZ constructed for a previous study (11).
Periplasm extraction.
The periplasm was extracted following the method of Wood (36) with modifications by Robles-Price et al. (37). Briefly, cells were collected by centrifugation (4,000 × g, 10 min, 4°C), washed twice in 30 mM Tris-HCl–150 mM NaCl (pH 7.1), and kept in ice for no longer than 1 h. The periplasm was further obtained by suspending the cells in 6 mL of 30 mM Tris-HCl, 20% sucrose, 4 mM EDTA, 0.5 mg/mL lysozyme, and 1 mM phenylmethylsulfonyl fluoride (pH 8), followed by incubation for 60 min at 30°C with gentle shaking. MgCl2 was added at 10 mM final concentration as soon as the suspension reached 30°C. Finally, the suspension was centrifuged (11,000 × g, 15 min, 4°C), and the supernatant containing the periplasmic fraction was collected. Alkaline phosphatase (AP), isocitrate dehydrogenase (IDH), and lactate dehydrogenase (LDH) activities were assayed as described previously to determine the relative purity of our periplasmic cell fraction (18).
β-Galactosidase activity assay.
P. aeruginosa strains to be assayed were grown overnight in LB agar plates, and then bacterial suspensions were prepared in fresh M63 to an OD600 of 0.5 with or without oxylipins or OA (0.1 mg/mL). Cultures were incubated at 30°C for 2 h, and then 250 μL of each culture was mixed with 250 μL of Z buffer (Na2HPO4⋅7H2O [0.06 M], NaH2PO4⋅H2O [0.04 M], KCl [0.01 M], MgSO4 [0.001 M], β-mercaptoethanol [0.05 M]; pH to 7.0), 50 μL of 0.1% sodium dodecyl sulfate (SDS), and 100 μL of chloroform, and the mix was vortexed for 20 s. The tubes were incubated at 30°C for 5 min, and the reaction started by adding 100 μL of o-nitrophenyl-β-d-galactoside (ONPG; 4 mg/mL), followed by brief vortex mixing. Reaction mixtures were incubated at 30°C for 1 h and stopped by adding 250 μL of 1 M Na2CO3. The OD420 and OD550 were measured for each tube. Finally, the β-galactosidase activity was calculated using the following equation: Miller U = 1,000 × [OD420 – (1.75 × OD550)]/(T × V × OD600), where OD420 and OD550 are the final reads from the reaction mixture, OD600 is the initial cell density of the cultures, T is the time of the reaction in minutes, and V is the volume of culture used in the assay in mL.
Thin-layer chromatography.
TLC analyses were run on 60-Å silica gel plates (20 × 10 cm, 200-μm thickness; Whatman). The mobile-phase solvent was a mix of hexane, ether, and acetic acid (80/20/5). TLC plates were visualized with 10% phosphomolybdic acid in ethanol.
Quantification of diol synthase activity.
The DS activity was determined by incubating the samples with 1 mg/mL of OA for 1 h at 30°C. The reaction was stopped by acidifying the sample to pH 2 with hydrochloride acid. Then, the oxylipins were extracted with an equal volume of ethyl acetate and analyzed by TLC. The relative amount of oxylipin 7,10-DiHOME was quantitated by densitometry of the TLC spots using ImageJ software.
Purification of 7,10-DiHOME oxylipin.
7,10-Di-HOME was purified as previously described (10). Briefly, PAO1 was plated in LB agar and incubated overnight at 30°C. The bacterial biomass was scraped from the plate and used to inoculate 200 mL of M63 complete supplemented with 1% OA. The culture was allowed to produce oxylipins and then centrifuged at 8,000 × g for 15 min to remove bacterial cells. The supernatant was recovered and acidified (pH 2) with acetic acid glacial. Then, a 1 vol/vol organic extraction with ethyl acetate was carried out, and the organic phase was evaporated. The dried mixture obtained was dissolved in 3 mL of ethyl acetate and used to purify 7,10-DiHOME using an Isco Teledyne Combiflash Rf 200 with four channels with 340CF ELSD (evaporative light scattering detector). A Universal RediSep solid-sample loading prepacked cartridges (5.0 g silica) were used to absorb the crude product and purified on 24 g of silica RediSep Rf Gold Silica (20- to 40-μm spherical silica) columns using an increasing gradient of ethyl acetate (solvent B) over hexane (solvent A). Fractions collected for each detected peak were combined, evaporated, and then dissolved in ethanol. The purity of the 7,10-DiHOME was checked by HPLC/MS analysis, as previously described (8).
Biofilm assay inside Drosophila crops.
P. aeruginosa colonization of a D. melanogaster crop was performed as previously described by Mulcahy et al. (38). All experiments were performed with 3-day-old D. melanogaster from both sexes of WT Oregon R (acquired from Carolina Biologicals). P. aeruginosa strains constitutively expressing green fluorescent protein (GFP) were cultured on LB agar plates. Bacteria were resuspended in LB to an OD600 of 1. Then, 100 μL of the suspension was spotted onto a sterile filter (Whatman) that was placed on the surface of 5 mL of LB agar supplemented with 5% sucrose and 1% OA. Flies were allowed to grow under this condition for 20 h and then killed. Crops were placed on a drop of phosphate-buffered saline on a microscope slide, sealed with a coverslip, and observed using an EVOS FL cell imaging system. Pictures were obtained using the same settings for each picture.
Mass spectrometry.
P. aeruginosa PAO1 strain was grown in M63 medium supplemented with OA up to an OD600 of 0.6 at 30°C and shaking (240 rpm). Bacteria were removed by centrifugation (6,000 rpm, 5 min), and the supernatant was filtered using a 0.22-μm-pore-size membrane filter (Corning). The supernatant was reduced, denatured, and separated by one-dimensional polyacrylamide gel electrophoresis (1D PAGE) as previously referenced (39). This step was carried out on a 10% SDS Bis-Tris gel (Invitrogen) according to the manufacturer’s instructions. The gels were stained, the entire lanes for each sample were partitioned into 3-MW fractions, and each gel plug was equilibrated in 100 mM ammonium bicarbonate. Each gel plug was then digested with Trypsin Gold (Promega) according to the manufacturer’s instructions, and peptide extracts were reconstituted in 0.1% formic acid–ddH2O at ~0.1 μg μL−1. Mass spectrometry runs were carried out, and the data were processed, searched, filtered, grouped, and quantified, as previously reported in detail by Ludwig et al. (40) (that is, under section 2.5 nLC-ESI-MS2 and Protein IDs for GeLC).
Western blotting.
OdsA was identified in the supernatant of P. aeruginosa by Western blotting with a mouse anti-OdsA polyclonal antibody. The supernatant was electrophoresed in 4 to 15% Mini-Protein TGX gels according to the manufacturer’s instructions (Bio-Rad), and the resolved proteins were transferred to a polyvinylidene difluoride membrane by electroblotting using a Trans-Blot Turbo apparatus (Bio-Rad). The membrane was transferred to an iBind apparatus (Invitrogen), where all remaining immunoblotting steps were carried out. After a final wash, the membrane was developed using SuperSignal West Pico (Thermo Scientific).
Statistical analysis.
Data are representative of three technical replicates and three biological replicates for each condition. Means were plotted, and a Student unpaired t test (two-tailed) was used to determine differences between the means of various conditions after it was determined that the variance was similar between groups. All statistical analyses were performed using GraphPad Prism 8.3.1 software.
Data availability.
The data supporting the findings of this study are available within the article and its supplementary information files or from the corresponding author upon request.
ACKNOWLEDGMENTS
J.C.-G. was supported by Award Number P30DK072482 from the National Institute Of Diabetes And Digestive And Kidney Diseases and Award Number PHILLI20G0 from the Cystic Fibrosis Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health or the Cystic Fibrosis Foundation.
Footnotes
Supplemental material is available online only.
Contributor Information
Eriel Martínez, Email: emartz@uab.edu.
Anke Becker, Philipps University Marburg.
REFERENCES
- 1.Kerr KG, Snelling AM. 2009. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect 73:338–344. 10.1016/j.jhin.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Jurado-Martin I, Sainz-Mejias M, McClean S. 2021. Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int J Mol Sci 22:3128. 10.3390/ijms22063128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulcahy LR, Isabella VM, Lewis K. 2014. Pseudomonas aeruginosa biofilms in disease. Microb Ecol 68:1–12. 10.1007/s00248-013-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams P, Camara M. 2009. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191. 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Funk CD. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294:1871–1875. 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 6.Andreou A, Brodhun F, Feussner I. 2009. Biosynthesis of oxylipins in non-mammals. Prog Lipid Res 48:148–170. 10.1016/j.plipres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Hajeyah AA, Griffiths WJ, Wang Y, Finch AJ, O’Donnell VB. 2020. The biosynthesis of enzymatically oxidized lipids. Front Endocrinol (Lausanne) 11:591819. 10.3389/fendo.2020.591819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez E, Hamberg M, Busquets M, Diaz P, Manresa A, Oliw EH. 2010. Biochemical characterization of the oxygenation of unsaturated fatty acids by the dioxygenase and hydroperoxide isomerase of Pseudomonas aeruginosa 42A2. J Biol Chem 285:9339–9345. 10.1074/jbc.M109.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estupinan M, Diaz P, Manresa A. 2014. Unveiling the genes responsible for the unique Pseudomonas aeruginosa oleate-diol synthase activity. Biochim Biophys Acta 1842:1360–1371. 10.1016/j.bbalip.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Martinez E, Campos-Gomez J. 2016. Oxylipins produced by Pseudomonas aeruginosa promote biofilm formation and virulence. Nat Commun 7:13823. 10.1038/ncomms13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez E, Cosnahan RK, Wu M, Gadila SK, Quick EB, Mobley JA, Campos-Gomez J. 2019. Oxylipins mediate cell-to-cell communication in Pseudomonas aeruginosa. Commun Biol 2:66. 10.1038/s42003-019-0310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer AL, Greenberg EP, Oliver CM, Oda Y, Huang JJ, Bittan-Banin G, Peres CM, Schmidt S, Juhaszova K, Sufrin JR, Harwood CS. 2008. A new class of homoserine lactone quorum-sensing signals. Nature 454:595–599. 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 13.Whiteley M, Diggle SP, Greenberg EP. 2017. Progress in and promise of bacterial quorum sensing research. Nature 551:313–320. 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papenfort K, Bassler BL. 2016. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol 14:576–588. 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 96:11229–11234. 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. 2013. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol 67:43–63. 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 18.Martinez E, Estupinan M, Pastor FI, Busquets M, Diaz P, Manresa A. 2013. Functional characterization of ExFadLO, an outer membrane protein required for exporting oxygenated long-chain fatty acids in Pseudomonas aeruginosa. Biochimie 95:290–298. 10.1016/j.biochi.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson T, Martinez E, Manresa A, Oliw EH. 2010. Liquid chromatography/tandem mass spectrometric analysis of 7,10-dihydroxyoctadecenoic acid, its isotopomers, and other 7,10-dihydroxy fatty acids formed by Pseudomonas aeruginosa 42A2. Rapid Commun Mass Spectrom 24:777–783. 10.1002/rcm.4446. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 100:14339–14344. 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FS. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44:D646–D653. 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunn DN, Lory S. 1991. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc Natl Acad Sci USA 88:3281–3285. 10.1073/pnas.88.8.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen M, Zhang H, Shen W, Zou Z, Lu S, Li G, He X, Agnello M, Shi W, Hu F, Le S. 2018. Pseudomonas aeruginosa MutL promotes large chromosomal deletions through non-homologous end joining to prevent bacteriophage predation. Nucleic Acids Res 46:4505–4514. 10.1093/nar/gky160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitter W, Koster M, Latijnhouwers M, de Cock H, Tommassen J. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol 27:209–219. 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren V, Wretlind B. 1987. Characterization of a Pseudomonas aeruginosa transposon insertion mutant with defective release of exoenzymes. J Gen Microbiol 133:675–681. 10.1099/00221287-133-3-675. [DOI] [PubMed] [Google Scholar]
- 26.Suriyanarayanan T, Periasamy S, Lin MH, Ishihama Y, Swarup S. 2016. Flagellin FliC phosphorylation affects type 2 protease secretion and biofilm dispersal in Pseudomonas aeruginosa PAO1. PLoS One 11:e0164155. 10.1371/journal.pone.0164155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douzi B, Ball G, Cambillau C, Tegoni M, Voulhoux R. 2011. Deciphering the Xcp Pseudomonas aeruginosa type II secretion machinery through multiple interactions with substrates. J Biol Chem 286:40792–40801. 10.1074/jbc.M111.294843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ball G, Durand E, Lazdunski A, Filloux A. 2002. A novel type II secretion system in Pseudomonas aeruginosa. Mol Microbiol 43:475–485. 10.1046/j.1365-2958.2002.02759.x. [DOI] [PubMed] [Google Scholar]
- 29.Papanikou E, Karamanou S, Economou A. 2007. Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol 5:839–851. 10.1038/nrmicro1771. [DOI] [PubMed] [Google Scholar]
- 30.Sargent F. 2007. The twin-arginine transport system: moving folded proteins across membranes. Biochem Soc Trans 35:835–847. 10.1042/BST0350835. [DOI] [PubMed] [Google Scholar]
- 31.Estupinan M, Alvarez-Garcia D, Barril X, Diaz P, Manresa A. 2015. In silico/in vivo insights into the functional and evolutionary pathway of Pseudomonas aeruginosa oleate-diol synthase: discovery of a new bacterial di-heme cytochrome c peroxidase subfamily. PLoS One 10:e0131462. 10.1371/journal.pone.0131462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsirigotaki A, De Geyter J, Sostaric N, Economou A, Karamanou S. 2017. Protein export through the bacterial Sec pathway. Nat Rev Microbiol 15:21–36. 10.1038/nrmicro.2016.161. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berg B, Clemons WM, Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. 2004. X-ray structure of a protein-conducting channel. Nature 427:36–44. 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 34.Hegsted DM, Jack CW, Stare FJ. 1962. The composition of human adipose tissue from several parts of the world. Am J Clin Nutr 10:11–18. 10.1093/ajcn/10.1.11. [DOI] [PubMed] [Google Scholar]
- 35.Cetinkale O, Yazici Z. 1997. Early postburn fatty acid profile in burn patients. Burns 23:392–399. 10.1016/S0305-4179(97)89764-1. [DOI] [PubMed] [Google Scholar]
- 36.Wood PM. 1978. Periplasmic location of the terminal reductase in nitrite respiration. FEBS Lett 92:214–218. 10.1016/0014-5793(78)80757-1. [DOI] [PubMed] [Google Scholar]
- 37.Robles-Price A, Wong TY, Sletta H, Valla S, Schiller NL. 2004. AlgX is a periplasmic protein required for alginate biosynthesis in Pseudomonas aeruginosa. J Bacteriol 186:7369–7377. 10.1128/JB.186.21.7369-7377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulcahy H, Sibley CD, Surette MG, Lewenza S. 2011. Drosophila melanogaster as an animal model for the study of Pseudomonas aeruginosa biofilm infections in vivo. PLoS Pathog 7:e1002299. 10.1371/journal.ppat.1002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galloway JR, Bethea M, Liu Y, Underwood R, Mobley JA, Hunter CS. 2015. SSBP3 interacts with Islet-1 and Ldb1 to impact pancreatic beta-cell target genes. Mol Endocrinol 29:1774–1786. 10.1210/me.2015-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwig MR, Kojima K, Bowersock GJ, Chen D, Jhala NC, Buchsbaum DJ, Grizzle WE, Klug CA, Mobley JA. 2016. Surveying the serologic proteome in a tissue-specific kras(G12D) knockin mouse model of pancreatic cancer. Proteomics 16:516–531. 10.1002/pmic.201500133. [DOI] [PubMed] [Google Scholar]
- 41.de Lorenzo V, Timmis KN. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol 235:386–405. 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 42.Kovach ME, Phillips RW, Elzer PH, Roop RM, II, Peterson KM. 1994. pBBR1MCS: a broad-host-range cloning vector. Biotechniques 16:800–802. [PubMed] [Google Scholar]
- 43.Hmelo LR, Borlee BR, Almblad H, Love ME, Randall TE, Tseng BS, Lin C, Irie Y, Storek KM, Yang JJ, Siehnel RJ, Howell PL, Singh PK, Tolker-Nielsen T, Parsek MR, Schweizer HP, Harrison JJ. 2015. Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat Protoc 10:1820–1841. 10.1038/nprot.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jb.00114-22-s0001.pdf, PDF file, 0.02 MB (23KB, pdf)
Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary information files or from the corresponding author upon request.