ABSTRACT
Pyrazinamide is one of the first-line antituberculosis drugs. The efficacy of pyrazinamide is associated with the ratio of 24-h area under the concentration–time curve (AUC24) to MIC. The objective of this study was to develop and validate a limited sampling strategy (LSS) based on a population pharmacokinetic (popPK) model to predict AUC24. A popPK model was developed using an iterative two-stage Bayesian procedure and was externally validated. Using data from 20 treatment-naive adult tuberculosis (TB) patients, a one compartment model with transit absorption and first-order elimination best described pyrazinamide pharmacokinetics and fed state was the only significant covariate for absorption rate constant (ka). External validation, using data from 26 TB patients, showed that the popPK model predicted AUC24 with a slight underestimation of 2.1%. LSS were calculated using Monte Carlo simulation (n = 10,000). External validation showed LSS with time points 0 h, 2 h, and 6 h performed best with RMSE of 9.90% and bias of 0.06%. Food slowed absorption of pyrazinamide, but did not affect bioavailability, which may be advantageous in case of nausea or vomiting in which food can be used to diminish these effects. In this study, we successfully developed and validated a popPK model and LSS, using 0 h, 2 h, and 6 h postdose samples, that could be used to perform therapeutic drug monitoring (TDM) of pyrazinamide in TB patients.
KEYWORDS: food, limited sampling strategy, pharmacology, population pharmacokinetics, pyrazinamide, tuberculosis
INTRODUCTION
Despite the high efficacy for drug-susceptible tuberculosis (TB) treatment, TB remains one of the top 10 causes of death worldwide. TB is second only to COVID-19 as a leading cause of death from a single infectious agent, ranking above HIV/AIDS. In 2020, there was a global drop on reported TB cases due to the COVID-19 pandemic. Nevertheless, the WHO estimated that 10 million people suffered and 1.5 million died from TB in 2020 (1). First-line TB treatment consists of isoniazid, rifampicin, pyrazinamide, and ethambutol for the first 2 months, continued with isoniazid and rifampicin for another 4 months (2).
Pyrazinamide is a prodrug that is bioactivated to pyrazinoic acid by the pyrazinamidase enzyme inside M. tuberculosis (3). Although its mechanism of action is not clearly understood, pyrazinamide, with its high sterilizing activity, has been an essential component in the treatment of TB for a long time. The addition of pyrazinamide to the rifampicin-based regimen appeared essential to reduce the duration of treatment by 3 months, from 6 to 9 months. Its use was associated with a reduced relapse rate as well, illustrating its sterilizing properties whereby intracellularly located M. tuberculosis persister organisms with low metabolic and low replication rate appear to be killed effectively (3–6).
The efficacy of pyrazinamide is associated with the ratio of 24-h area under the concentration–time curve (AUC24) to MIC (7). Low pyrazinamide exposure may lead to suboptimal treatment, as shown in two independent cohort studies from South Africa and China (8, 9). A pyrazinamide AUC24/MIC ratio > 2.79 was the predominant predictor for 2-months culture conversion in drug-susceptible TB patients treated with first-line anti-TB drugs (8). Pasipanodya et al. showed that poor treatment outcome was associated with AUC24 ≤363 mg*h/L of pyrazinamide in TB patients treated with first-line anti-TB drugs (9). However, patients received a median dose of 36 mg/kg pyrazinamide, which is higher than the WHO advised dosing of 25 mg/kg and 64% of the population were retreatment patients, indicating a selected patient group (9). A recent study showed that the majority of the patients using 20 to 25 mg/kg pyrazinamide (57/72, 79%) did not achieve this therapeutic target AUC24 >363 mg*h/L (10). Variability in pyrazinamide exposure has been explained by factors like sex, body weight, and comorbidities (10–13). To optimize treatment, therapeutic drug monitoring (TDM) has been suggested for selected patients and drugs (14–16). Precision of TDM may improve when dose adjustment is based on AUC24, that is calculated from a full curve consisting of multiple samples during the day. However, obtaining a full pharmacokinetic (PK) curve to estimate AUC24 is a laborious and expensive procedure and therefore not feasible in clinical practice (17). A sampling strategy using a limited number of appropriately timed blood samples to predict AUC24, may be a solution to overcome this problem (18).
The aim of this study was to develop a population PK (popPK) model of pyrazinamide and a limited sampling strategy (LSS) that can be used to accurately predict AUC24 of pyrazinamide in patients with TB.
RESULTS
The baseline characteristics of both patient cohorts are displayed in Table 1. As can be observed from Table 1, for many patient characteristics, the two cohorts differed significantly (P value <0.05). AUC24 and maximum concentration (Cmax) differed statistically significant between the model and validation patients (Table 2).
TABLE 1.
Patient demographics
| Patient parameter | Model cohort (n = 20) | Validation cohort (n = 26) | P a |
|---|---|---|---|
| Male/female, n (%) | 12/8 (60/40) | 24/2 (92/8) | 0.012b |
| Age, yr | 37.0 (24.0 to 49.3) | 32.5 (22.0 to 40.0) | 0.166 |
| wt, kg | 41.5 (38.8 to 47.3) | 62.4 (55.3 to 67.9) | <0.001 |
| ht, cm | 155 (152 to 161) | 175 (166 to 182) | <0.001 |
| Body mass index (BMI), kg/m2 | 17.0 (16.0 to 18.9) | 20.3 (18.9 to 21.4) | <0.001 |
| Underweight (BMI < 18.5 kg/m2), n (%) | 14 (70) | 3 (11) | <0.001b |
| Dose, mg | 1,200 (1,200 to 1,200) | 1,750 (1,500 to 2,000) | <0.001 |
| Dose/wt, mg/kg | 28.6 (25.4 to 30.2) | 27.8 (26.1 to 29.6) | 0.673 |
| Comorbidity, n (%) HIV Diabetes, type II Hepatitis B Hepatitis C Endstage renal diseasec |
4 (20) 2 (10) 2 (10) 0 0 0 |
10 (38) 0 2 (8) 8 (31) 1 (4) 1 (4) |
0.212b |
| Serum creatinine concn, μmol/L | 75 (64 to 83) | 64 (49 to 71) | 0.012 |
| Estimated glomerular filtration rated, mL/min/1.73m2 | 93 (74 to 119) | 133 (112 to 156) | 0.002 |
| WHO region, n (%) South-East Asia Africa Europe |
20 (100) | 1 (4) 14 (54) 11 (42) |
<0.001b |
| Type of TB, n (%) Pulmonary TB Extrapulmonary TB Pulmonary + Extrapulmonary TB |
20 (100) | 23 (88) 2 (8) 1 (4) |
0.164b |
Continuous data form comparisons of model and validation cohort were tested using the Mann-Whitney U test. Data are presented as median and interquartile range (IQR).
Chi-square test.
Endstage renal disease was defined as glomeral filtration rate < 15 mL/min.
Estimated glomerular filtration rate calculated using MDRD formula.
TABLE 2.
Noncompartmental pharmacokinetic parameters of pyrazinamide geometric mean (range)a
| Pharmacokinetic parameters | Model cohort | Validation cohort | P |
|---|---|---|---|
| Cmax (mg/L) | 41.7 (29.9 to 61.1) | 35.4 (23.6 to 70.3) | 0.006 |
| AUC24 (mg*h/L) | 474 (284 to 645) | 342 (160 to 599) | <0.001 |
| AUC24/dose (h/L) | 0.40 (0.20 to 0.60) | 0.20 (0.11 to 0.34) | <0.001 |
| Tmax (h), median (IQR) | 2.1 (1.7 to 2.8) | 1.6 (0.3 to 5.8) | 0.185 |
Data of model cohort are mean data of the fasted and fed state. Cmax, maximum concentration; AUC24, area under the 24-h concentration–time curve; AUC24/dose, area under the 24-h concentration–time curve divided by pyrazinamide dose in mg; Tmax, time to Cmax; IQR: interquartile range. Mann-Whitney U test was used to compare validation with model cohort.
Population pharmacokinetic model.
The initial model resulted in an Akaike information criterion (AIC) value of 4,809 for one compartment with linear elimination. The one-compartment model with mixed order elimination and two-compartment models were not favorable (AIC 4,956 and 5,079, respectively). The final model was a one-compartment model with transit absorption and first-order elimination and was selected based on AIC (2,149) and goodness-of-fit plots (Fig. 1). The estimated parameters for the final popPK model are shown in Table 3.
FIG 1.
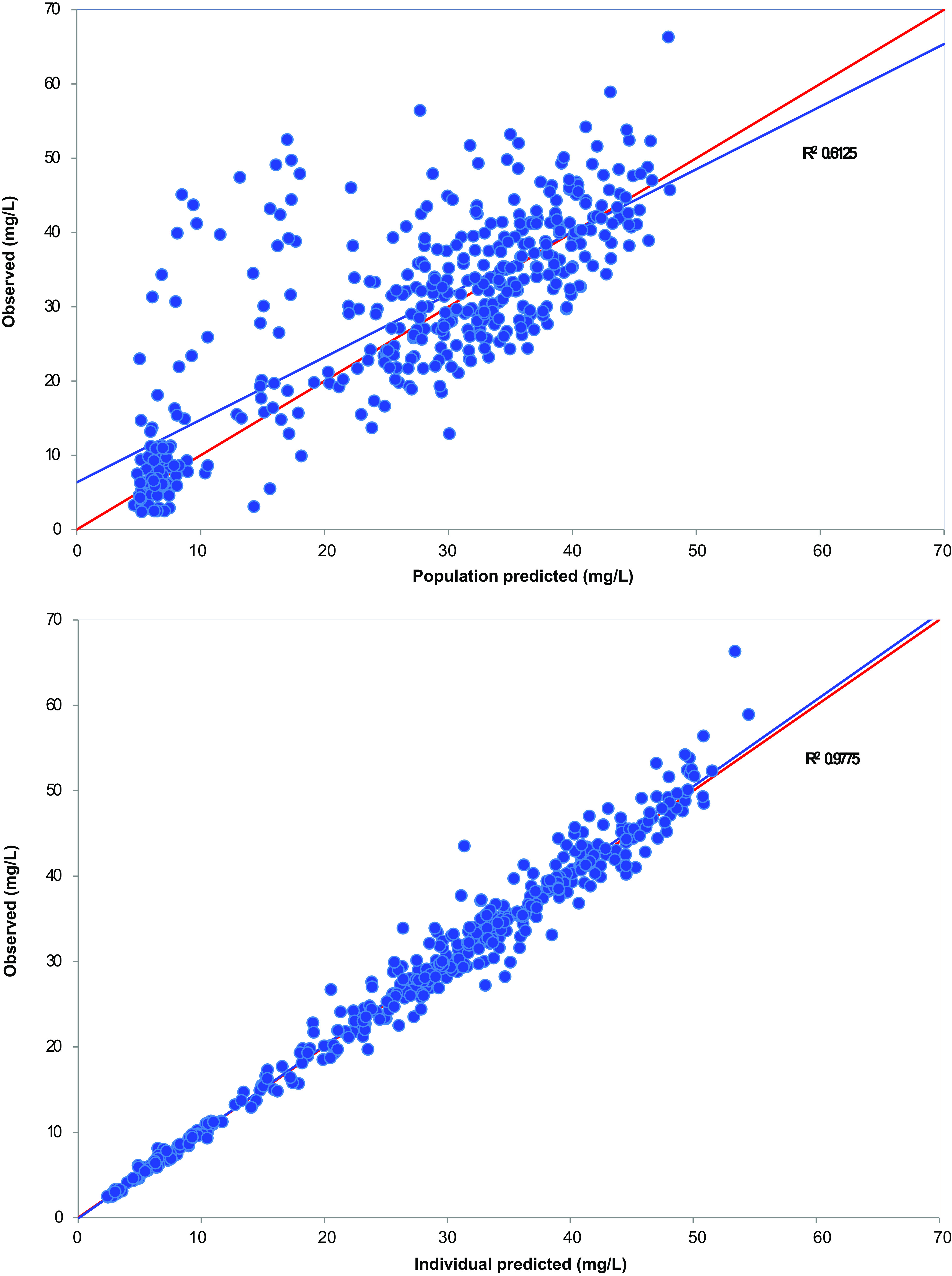
Goodness-of-fit plots for the final population pharmacokinetic model of pyrazinamide.
TABLE 3.
Parameter estimates and bootstrap validation of the final population pharmacokinetic model for pyrazinamidea
| Parameters (unit) | Population |
Bootstrap |
|||
|---|---|---|---|---|---|
| Mean | IIV (%) | RSE (%) | Median | 95% CI | |
| Vd/F (L/43 kg) | 30.3 | 13.3 | 3.1 | 30.4 | 28.4 to 32.2 |
| CL/F (L/h/43kg0.75) | 2.54 | 24.5 | 5.6 | 2.54 | 2.28 to 2.83 |
| ka (1/h) | 8.63 | 104 | 23.8 | 8.68 | 5.50 to 14.3 |
| Effect of fed status on ka | 0.36 | 80.1 | 18.4 | 0.36 | 0.25 to 0.51 |
| F (fixed) Effect of fed status on F |
1.00 1.00 |
6.9 |
1.6 |
1.00 |
0.96 to 1.03 |
| No. of compartments | 5.26 | 107 | 24.4 | 5.31 | 2.96 to 8.61 |
| Proportional error (%) | 6.54 | 3.8 | 6.53 | 5.48 to 7.60 | |
Vd/F, apparent volume of distribution; CL/F, apparent clearance; ka, absorption rate constant; F, bioavailability; IIV, Interindividual variability; RSE, relative standard error; 95% CI, 95% confidence interval.
The only covariate incorporated in the model was a factor for the fed state. This covariate impacted absorption rate constant (ka) and bioavailability (F), with 0.33 ± 85.9% and 1.00 ± 6.8%, respectively (Table 3). Allometric scaling was used to incorporate the effect of body weight on clearance (CL) and volume of distribution (Vd) with the fixed exponents of 0.75 and 1, respectively.
Limited sampling strategy.
LSS, using Bayesian fitting, with one time point between 0 h and 6 h showed acceptable bias and precision (mean percentage prediction error [MPPE] ranged from −0.30% to 0.63%, root mean squared error [RMSE] ranged from 6.43% to 7.86%, r2 ranged from 0.997 to 0.998). LSSs with two or three time points performed even better. LSS, using multiple linear regression, with one to three time points between 0 h and 6 h showed acceptable bias and precision (MPPE ranged from −3.03% to 2.67%, RMSE ranged from 2.74% to 12.72%, r2 ranged from 0.9946 to 0.9996). Again, all LSSs performed within the preset limits of imprecision < 15%, bias < 5%, and r2 > 0.95. As all LSSs, irrespective of time points, performed within the preset limits, we chose LSSs with one, two, and three time points using 0 h, 2 h, and 6 h for external validation because of the historical use for TDM of anti-TB drugs and their clinical suitability (Tables 4 and 5) (19, 20).
TABLE 4.
LSSs of pyrazinamide using the Bayesian approacha
| First sampling time point (h) |
Second sampling time point (h) |
Third sampling time point (h) |
RMSE (%) | MPPE (%) | r2 |
|---|---|---|---|---|---|
| 0 | 7.86 | 0.48 | 0.9970 | ||
| 2 | 6.46 | 0.63 | 0.9980 | ||
| 6 | 7.17 | 0.12 | 0.9975 | ||
| 0 | 2 | 4.95 | –0.57 | 0.9988 | |
| 0 | 6 | 5.12 | –0.13 | 0.9987 | |
| 2 | 6 | 4.63 | –0.65 | 0.9990 | |
| 0 | 2 | 6 | 3.84 | –0.54 | 0.9993 |
RMSE, root mean squared error; MPPE, mean percentage prediction error.
TABLE 5.
LSSs of pyrazinamide using the multiple linear regressiona
| Sampling time point (h) |
AUC0–24, LSS to AUC24, LSS calculation | RMSE (%) | MPPE (%) | r2 | ||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | ||||
| 0 | −0.309 + 6.863*C0 | 12.72 | −3.03 | 0.9946 | ||
| 2 | 0.046 + 5.945*C2 | 6.47 | 0.22 | 0.9979 | ||
| 6 | 0.297 + 5.804*C6 | 8.84 | 2.21 | 0.9969 | ||
| 0 | 2 | −0.147 + 2.384*C0 + 3.899*C2 | 6.14 | −1.33 | 0.9986 | |
| 0 | 6 | −0.073 + 2.996 *C0+ 3.299*C6 | 5.42 | −0.63 | 0.9986 | |
| 2 | 6 | 0.082 + 3.344*C2 + 2.560*C6 | 4.73 | 0.64 | 0.9990 | |
| 0 | 2 | 6 | −0.057 + 1.653*C0 + 2.333*C2+ 2.159*C6 | 3.97 | −0.47 | 0.9993 |
AUC0–24, LSS to AUC24, LSS, area under the 24-h concentration–time curve; C, concentration; RMSE, root mean squared error; MPPE, mean percentage prediction error.
Validation.
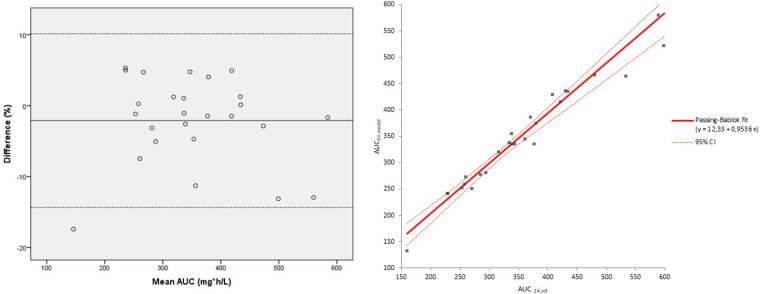
The external validation of the popPK model using validation cohort data indicated a mean underestimation of 2.1% (standard deviation [SD] 6.1%) of AUC24, model compared with AUC24, ref (Fig. 2A). One patient had a result below the lower line of agreement, the underestimation was 17.4% which could be explained by a low AUC24 (both AUC24, ref and AUC24, model of 160 and 132 mg*h/L, respectively). This indicates that if a patient shows a low AUC24 using the model, the real AUC24 might be higher, but would still be too low. So, a large dose increase, and a subsequent TDM cycle, could be considered. The Passing-Bablok regression (Fig. 2B) showed no constant error (intercept 12.33; 95% CI = −27.35 to 52.29), nor a proportional error (slope 0.95; 95% CI = 0.82 to 1.1). A strong linear relationship was found between AUC24, ref and AUC24, model with a correlation coefficient r2 = 0.94.
FIG 2.
External validation of the popPK model (n = 26). Bland-Altman plot of mean of AUC24, ref and AUC24, model (mg*h/L) versus the difference in % of the mean AUC24. The solid line indicates the mean difference. The corresponding limits of agreement (mean difference ± 2 SD difference) are depicted as dashed lines. Passing-Bablok plot.
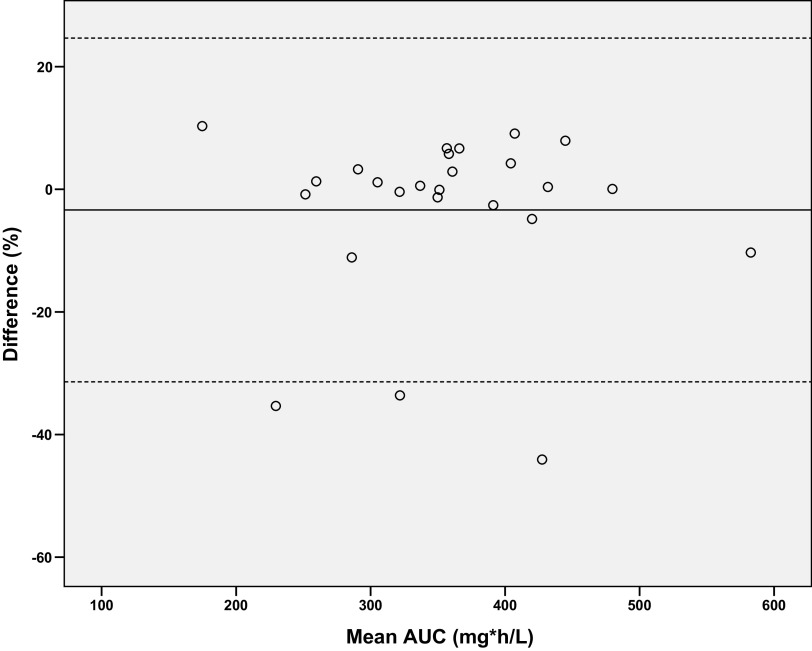
LSSs with one to three time points using 0 h, 2 h, and 6 h were externally validated with validation patients; however, the acceptance criteria (RMSE < 15%, median absolute percentage prediction error [MAPE] < 15% and bias < 5%) were not met for the majority of the LSSs (Table 6). Although RMSE was considered too high, LSS0 showed acceptable performance with 81% of the patients with PPE < 15% and was able to discriminate between AUC ≤ or > 363 mg*h/L in 81% of patients. The LSS0-2-6 showed the best performance in the external validation, with RMSE of 9.90% and bias of 0.06%, and it was the only LSS meeting the preset criteria, so we used it for further evaluation. The Bland-Altman plot of LSS0-2-6 indicated a mean underestimation of 3.4% (SD 14.0%, Fig. 3). The LSS0-2-6 did not completely fit the curves of three patients correctly, resulting in an underestimation of 34%, 35% and 44%, respectively. All three patients showed slow drug absorption (with time to maximum concentration Tmax ranging from 3.5 h to 5.8 h) that resulted in difficulties in fitting.
TABLE 6.
The external validation of the selected LSSsa
| Bias and precision |
Passing-Bablok |
||||||
|---|---|---|---|---|---|---|---|
| LSS | MPPE (%) | RMSE (%) | MAPE (%) | No. of patients with PPE < 15% (%) |
Slope [95%CI] | Intercept [95%CI] |
r2 (Pearson correlation) |
| LSS0 | 2.78 | 22.76 | 6.79 | 17/21 (81) | 0.96 [0.73, 1.27] | 33.86 [–68.24, 128.30] | 0.90 |
| LSS2 | 14.93 | 76.02 | 25.11 | 10/26 (38) | 0.82 [0.52, 1.55] | 119.90 [–166.72, 221.75] | 0.46 |
| LSS6 | 12.38 | 42.94 | 13.40 | 17/26 (65) | 0.83 [0.63, 1.00] | 97.05 [43.07, 168.79] | 0.93 |
| LSS0-2 | −2.69 | 21.83 | 7.06 | 18/23 (78) | 1.07 [0.86, 1.43] | –28.80 [–142.09, 42.29] | 0.75 |
| LSS0-6 | 3.27 | 16.38 | 4.77 | 21/23 (91) | 1.03 [0.79, 1.22] | 1.09 [–48.61, 75.71] | 0.95 |
| LSS2-6 | 9.68 | 37.50 | 12.27 | 18/26 (69) | 0.78 [0.57, 0.94] | 104.60 [45.10, 179.59] | 0.77 |
| LSS0-2-6 | 0.06 | 9.90 | 3.07 | 20/23 (87) | 1.03 [0.89, 1.22] | –9.53 [–69.66, 33.49] | 0.82 |
MPPE, mean percentage prediction error; RMSE, root mean squared error; MAPE, median absolute percentage prediction error; PPE, percentage prediction error; 95% CI, 95% confidence interval; LSSx, limited sampling strategy at time point x h.
FIG 3.
Bland-Altman plot of mean of AUC24, ref and AUC24, LSS 0–2-6 (mg*h/L) versus the difference in % of the mean AUC24. The solid line indicates the mean difference. The corresponding limits of agreement (mean difference ± 2 SD difference) are depicted as dashed lines.
DISCUSSION
In this study, we developed and validated a one-compartmental model with transit compartment and LSS that accurately predicts AUC24 of pyrazinamide in TB patients. The one-compartmental popPK model is in accordance with the clinical observations, as pyrazinamide distributes rapidly and homogeneously over the body (21).
The pharmacokinetics of pyrazinamide were not affected by sex or age. This is in accordance with some literature (11, 22) and contrary to other (12, 23). We adjusted for the effect of body size and included weight using allometric scaling which may explain this difference. The covariate factor fed state showed a significant effect on the absorption rate (0.33 ± 85.9%), indicating that Tmax increases considerably. As a consequence of this, Cmax decreases, which was indeed observed in the original study by Saktiawati et al. (24). The relative effect of the covariate on fixed bioavailability (1.00% ± 6.8%) indicates that bioavailability is not affected by the fed state. This confirms previous studies, reporting that food delayed the absorption rate but not the extent of the absorption (25–27).
Comparing our popPK model, Vd (30.3 L/43 kg) value is in the same range as those reported in literature (median Vd 43 L, range 28 to 62 L) (10–13, 23, 28, 29). Our estimation of CL (2.54 L/h/43°0.75 kg) is somewhat lower than the median reported CL of 4.1 L/h (range 3.3 to 5.1 L/h) (10–13, 22, 23, 28, 29). Partly, this is explained by the fact that we sampled early in treatment and that clearance of pyrazinamide appears to increase over time (13). Indeed the validation cohort, consisting of patients who were on treatment for at least 2 weeks, showed a 42% higher clearance (3.62 L/h/43kg0.75) than our model cohort. Possibly the overall improving condition of the TB patients on effective drug treatment adds to the increased clearance (29). The absorption rate from our model (8.6 1/h) is high compared with others (median 3.5 1/h, range 1.3 to 3.5 1/h) and shows considerable variability. Our model cohort was sampled extensively in the absorption phase and we included a transit compartment in our model. The transit compartment causes absorption delay through a chain of presystemic compartments, connecting to the central compartment by a first order absorption process.
Our model was developed in a rather homogeneous population that was sampled in both fasted and fed states. The final model was successfully externally validated in a heterogeneous population with unknown fasted and fed states. In addition, the validation patients differed significantly with respect to weight, height, BMI, pyrazinamide dose, duration of treatment and severity of disease, and the model predicted AUC24 with a mean underestimation of only 2.1%. This suggests that the popPK model could be considered in a wider population.
In this study, we developed LSSs with one to three sample time points that are easy and feasible in clinical practice with a maximum time span of 6 h postdose. In the external validation cohort, LSS0-2-6 was the only LSS with acceptable bias and precision. The other selected LSSs did not meet the preset acceptance criteria of imprecision < 15% and/or bias < 5% in the external validation (Table 6). It is striking that the LSS2 performed the worst in the external validation. This indicates that a C2 sample is insufficient to estimate AUC24 of pyrazinamide. This might be explained by the fact that Tmax ranged from 1 h to 6 h and C2 does not always capture Cmax. The LSS0-2-6 is in accordance with the best performing time point in the multiple linear regression LSS of Magis-Escurra et al. (20). Although, it is more practical than the earlier suggested LSS of 2 h, 4 h, and 8 h by our group, the latest time point of 6 h may still be considered unfeasible in clinical practice as outpatients have to wait only for a blood sample to be taken. If this latest time point is impossible to obtain, clinicians might consider only drawing a through sample, C0, taking into account the higher imprecision. Besides, as interindividual variability of PK of pyrazinamide is fairly low, one might also consider using any LSS for the combination of first-line drugs (18).
We developed the LSS using multiple linear regression next to the Bayesian approach that can be used to estimate drug exposure with straightforward calculation in a routine clinical setting (30). The LSS using the Bayesian approach provides an estimation of the AUC24 with higher precision and accuracy; however, this approach implicates the use of the modeling software.
There are some limitations for this study. First, one should be cautious using the model and the proposed LSSs in estimating AUC24 in TB patients with impaired renal function, as we were unable to incorporate renal function in our model. Second, we could not combine the two cohorts of patients to develop the popPK model of the mixed population due to the lower number of samples in the validation cohort. Third, the LSS0-2-6 underestimated AUC24 in a number of patients with delayed absorption. However, delayed absorption can be observed from the data and taken into consideration. Lastly, individual MICs were not available for the included patients. MIC determination for pyrazinamide is notoriously challenging and is dependent on the technique used, pH, and location (31, 32).
Target attainment was calculated as the percentage of the patients that achieved an AUC24/MIC > 2.79 (8), using three MIC distributions (8, 31, 33). As can be observed from Fig. 4, up to an MIC of 64 mg/L (representing 96% of MICs), 100% of the model cohort, and 96% of the validation cohort obtained an AUC24 high enough to achieve an AUC24/MIC of 2.79. From this point of view the case for standard TDM is not strong.
FIG 4.
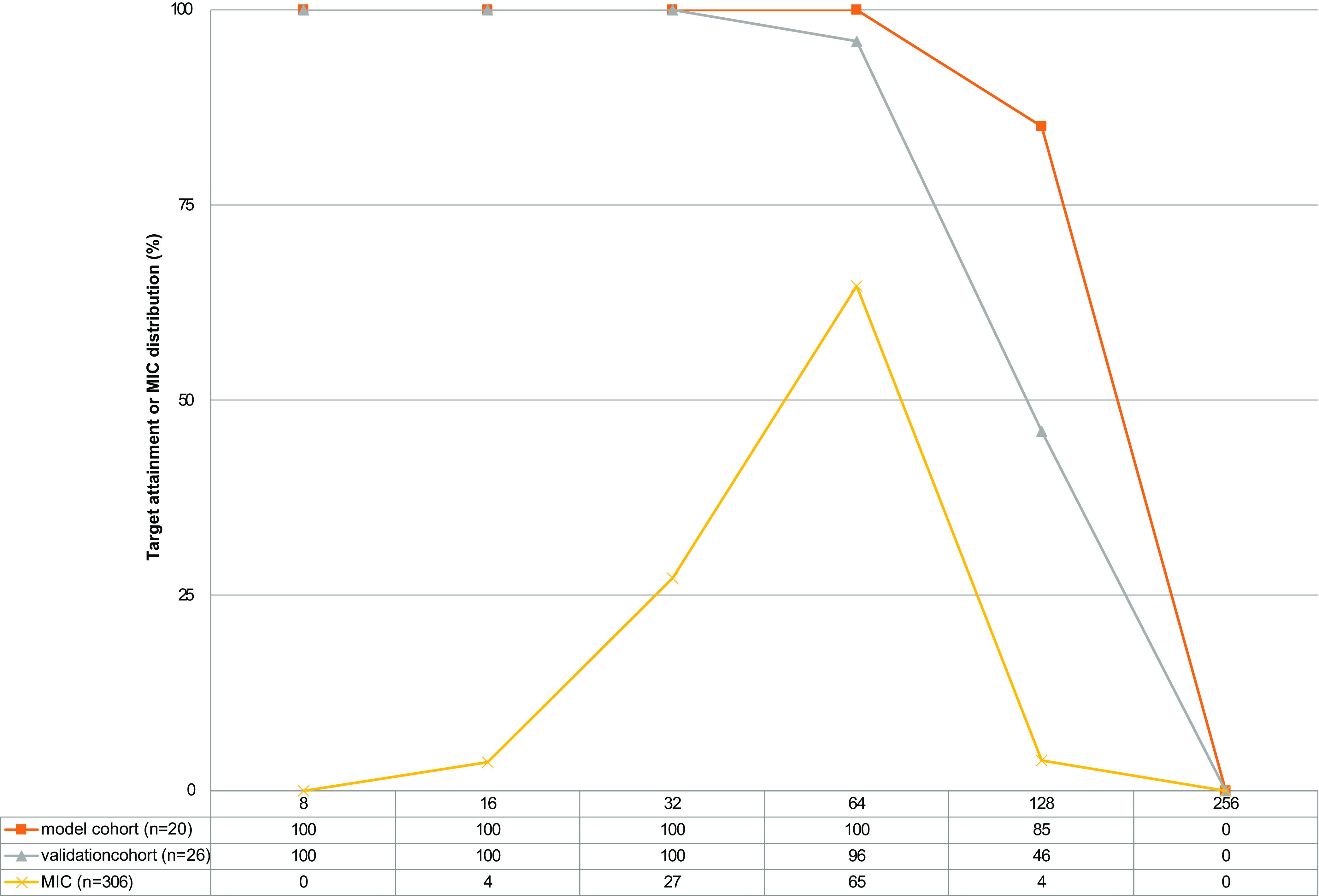
Target attainment AUC/MIC > 2.79 of model and validation cohort. MIC distributions from references are combined (8, 31, 33).
The majority of the patients (27/46, 59%) achieved a pyrazinamide exposure AUC24 >363 mg*h/L (9); however, this was mainly achieved in the model cohort (18/20, 90%) compared with only 31% (8/26) in the validation cohort. This difference could be caused by selection bias of the validation cohort, being TB patients admitted to a tertiary reference hospital. However, both numbers are higher than the earlier reported 21% and may partly be explained by the lower dose of pyrazinamide (22.7 mg/kg) compared with our study (28.6 mg/kg and 27.8 mg/kg) (10).
The AUC24 in our population ranged from 160 to 688 mg*h/L, a range that is rather similar to Alsultan et al. (10). However, this 4-fold difference between lowest and highest AUC24 is considerably less than the interindividual variability in exposure observed in, for instance, isoniazid (18-fold) and rifampicin (56-fold) at standard doses (34).
It is striking to see that, based on probability of target attainment AUC/MIC > 2.79 for 2 month culture conversion, our population performed very good (98%) and, based on the target AUC24 of > 363 mg*h/L, target attainment was only achieved in 59% of our population. In our view, this indicates that targets are still far from established. One could argue to follow the pharmacodynamic target on culture conversion as it most closely resembles outcome. However, if the difficulties with MIC determination, especially in the case of pyrazinamide, are taken into account, one would maybe prefer the PK target (35). In our view, to attain higher exposure, rather than performing TDM and adjusting doses, upfront higher dosing of pyrazinamide would be preferable. In the earlier days, the WHO recommended 35 mg/kg of pyrazinamide, based on the British Medical Research Council (BMRC) studies. For unknown reasons, the WHO lowered this to 25 mg/kg in 2003 (36–38). Moreover, doses up to 40 mg/kg seem to be tolerated rather well, not causing more hepatotoxicity (37, 39).
In this paper we showed that food slows absorption of pyrazinamide, but does not affect bioavailability, which may be very advantageous in case of nausea or vomiting in which food can be used to diminish these effects (24). Next to that, we identified a large difference in target attainment based on the chosen target, highlighting the need for development for solid targets. After defining this solid target, patient groups at risk for low exposure can be identified and these groups may have an indication for TDM. Until then, we suggest performing TDM of pyrazinamide in cases of suspicion of gastrointestinal abnormalities like malabsorption, poor response, and comorbidities like HIV and diabetes mellitus (14).
In conclusion, we successfully developed and validated a population pharmacokinetic model of pyrazinamide that can be applied for TDM in TB patients. Pyrazinamide exposure can be adequately predicted with LSS 0 h, 2 h, and 6 h in TB patients, both in patient care and future research.
MATERIALS AND METHODS
Patients.
Two data sets were used for this study. The first cohort included participants from a prospective randomized controlled PK study investigating the impact of food on the bioavailability of first-line anti-TB drugs (Fastfood, NCT02121314) (24). Study subjects were newly diagnosed treatment-naive susceptible TB patients of at least 18 years old who received 25 mg/kg pyrazinamide (24). The study was performed on the first 3 days of anti-TB treatment, so patients did not reach steady state of pyrazinamide. Subjects were randomly assigned to either drug administration on day 2 in fasted state and in fed state on day 3, or fed day 2, and fasted day 3. A PK curve was obtained during the first 3 days of treatment. Serial blood samples were taken at 11 time points: 0 h (predose), 0.5 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 4 h, 5 h, 6 h, and 8 h after dosing. Samples were centrifuged and stored at −80°C until they were analyzed for pyrazinamide using liquid-chromatography-tandem mass spectrometry (LC-MS/MS), as previously described (40).
The second cohort included TB patients aged at least 18 years old, who were treated with pyrazinamide as part of routine treatment and were admitted for a part of the intensive phase at the University Medical Center Groningen, Tuberculosis Center Beatrixoord, Haren, the Netherlands. Patients were eligible if a PK curve for pyrazinamide had been obtained in routine care and if they did not object to the use of their data. Patients were treated with 25 to 30 mg/kg pyrazinamide according to the national guideline (41). Dense PK blood sampling was performed as part of standard clinical care after at least 2 weeks of treatment; samples were taken predose, and three to nine time points between 0.5 h and 8 h postdose. Plasma was separated and stored at −20°C until analysis. Pyrazinamide concentrations were measured using the validated LC-MS/MS method (40). Demographic and medical data, including age, sex, weight, height, country of origin, ethnicity, type of TB, comorbidities, disease related malnutrition, TB drug regimen, pyrazinamide dose, renal function, liver enzymes, albumin, and presence of side effects, were collected from the electronic medical record system. This study was assessed by the Medical Ethics Review Board of the UMCG and found to be in accordance with the Dutch Law because of its retrospective nature (METc 2020-189).
Cmax was defined as the highest observed concentration, with Tmax as the time to reach the Cmax. The reference AUC24, AUC24 ref, was determined by the log-linear trapezoidal rule using the Kinfit module of MWPharm version 3.81 (Mediware, Groningen, the Netherlands). Dose-corrected AUC24 was calculated by dividing AUC24 by pyrazinamide dose in mg. Noncompartmental PK parameters of model and validation population were compared using Mann-Whitney U test.
Population pharmacokinetic model.
The popPK modeling was performed using an iterative two-stage Bayesian (ITSB) of the KinPop module of Edsim++, version 1.98 (Mediware, Prague, Czech Republic). As data from the PK study subjects consisted of two full PK curves with a total 22 concentrations per subject, we decided to use these data to develop the popPK model (model cohort). Data from Beatrixoord patients was used for the external validation (validation cohort).
Various structural models, including one- and two-compartment models, with or without transit absorption and linear or nonlinear elimination were investigated as the base model. First, a simple one-compartment model with linear elimination and Bayesian estimations for clearance (CL), volume of distribution (Vd) and ka was tested. The initial values for CL, Vd, and ka were estimated based on the recent publications (13, 23, 29). Because F of pyrazinamide is almost complete and because it is administered orally only, F was fixed to 1 (42). PK parameters were assumed to be log-normally distributed. Residual errors—additive and proportional—were estimated on 0 mg/L and 0.15, respectively, and assumed to be normal distributed. Second, a one-compartment model with nonlinear elimination using three parameters (Vd, Michaelis-Menten constant, Km and maximum rate, Vmax) was tested. These two initial models were merged into a one-compartment model with combined linear and nonlinear elimination. Pyrazinamide is mainly metabolized in the liver by hydrolysis, only 2% to 14% is renally cleared (42). Therefore, the base model was designed using metabolic clearance only. Thereafter, a separate model with both metabolic and renal clearance was developed, however, the AIC value did not decline (AIC 2,209). Moreover, the renal clearance was overestimated (>24%) and lacking a significant correlation between estimated glomerular filtration rate and pyrazinamide clearance (r2 = 0.02 and P = 0.46). Therefore, the model with both metabolic and renal clearance was abandoned.
Furthermore, a model with transit absorption defined using N as the number of transit compartments, was tested. Additionally, a simple two-compartment model with fixed values of central volume of distribution (Vd), peripheral volume of distribution, peripheral clearance, and total clearance from Vd was tested.
The base model was selected using AIC, a measure for goodness of fit using likelihood penalization. An AIC decrease of 3 was considered significantly better (43, 44).
The influence of patient characteristics as possible covariates on PK parameters was investigated with the Mann-Whitney U test, chi-square test, regression, or Kruskal Wallis test. A P value ≤ 0.05 was considered statistically significant. If a statistically significant correlation was observed using Spearman correlation coefficient or Pearson correlations coefficients test, the covariate was added to the base model. Again, the final model was selected based on the AIC and an AIC decrease of 3 was considered significantly better (43, 44).
Limited sampling strategy.
Monte Carlo Simulation was used to create 10,000 virtual pharmacokinetic profiles to represent the pharmacokinetic data. A 42-year-old male with bodyweight of 42 kg, length of 146 cm, body mass index (BMI) of 19.7 kg/m2, and serum creatinine 76 μmol/L was selected as a reference patient for Monte Carlo simulation because of a well-fitting and representative pharmacokinetic curve in combination with representative patient characteristics, of the model population. LSS with different combinations of one to three sampling time points with a time resolution of 1 h were evaluated using Bayesian fitting or multiple linear regression. LSS was performed using both Bayesian estimation and multiple linear regression and maximized to a time span of 6 h instead of 24 h postdose because of clinical applicability. LSS was considered acceptable if the RMSE, as a measure of precision, was < 15%. The MPPE, as a measure of bias, was accepted if it was < 5% and the adjusted r2 >0.95 (45, 46). The best performing LSSs were validated by comparing the AUC24 predicted with LSS (AUC24, LSS) with the AUC24, ref.
Validation.
The final popPK model was validated using the bootstrap module of Edsim++. To evaluate the performance of the developed popPK model and LSS, external validation using patient data of the Beatrixoord cohort was performed. AUC24 predicted with the popPK model (AUC24, model) or LSS (AUC24, LSS) was compared with AUC24, ref. Correlation between AUC24, ref and AUC24, model or AUC24, LSS was evaluated using Bland-Altman analysis and Passing Bablok regression in SPSS statistics, version 23 (IBM Corp., Armonk, NY, USA) and Analyze-it 4.81 (Analyze-it Software Ltd., Leeds, United Kingdom).
The external validation of the model and best performing LSS was performed according to the method described by Saktiawati et al. (45). In addition to the RMSE, the median absolute percentage prediction error (MAPE, median [100% × I(AUC24, LSS − AUC24, ref)I/AUC24, ref]) was calculated as another measure for precision. LSS was considered acceptable if bias and imprecision were smaller than 5% and 15%, respectively. A percentage prediction error (PPE), calculated as [100% × (AUC24, LSS − AUC24, ref)/AUC24, ref], was used to show the performance of the model or LSS for each individual. An acceptable PPE was considered smaller than 15%, and the number of patients with a PPE < 15% was calculated for the best performing LSS (45, 47, 48).
REFERENCES
- 1.World Health Organization (WHO). 2021. Global tuberculosis report 2021. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2017. Guidelines for the treatment of drug-susceptible tuberculosis and patient care. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int J Tuber Lung Dis 7:6–21. [PubMed] [Google Scholar]
- 4.British Thoracic Association. 1982. A controlled trial of six months chemotherapy in pulmonary tuberculosis. Second report: results during the 24 months after the end of chemotherapy. Am Rev Respir Dis 126:460–462. [DOI] [PubMed] [Google Scholar]
- 5.Steele MA, Des Prez RM. 1988. The role of pyrazinamide in tuberculosis chemotherapy. Chest 94:845–850. 10.1378/chest.94.4.845. [DOI] [PubMed] [Google Scholar]
- 6.Combs DL, O’Brien RJ, Geiter LJ. 1990. USPHS tuberculosis short-course chemotherapy trial 21: effectiveness, toxicity, and acceptability. The report of final results. Ann Intern Med 112:397–406. 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 7.Gumbo T, Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob Agents Chemother 53:3197–3204. 10.1128/AAC.01681-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng X, Bao Z, Forsman LD, Hu Y, Ren W, Gao Y, Li X, Hoffner S, Bruchfeld J, Alffenaar J-W. 2020. Drug exposure and minimum inhibitory concentration predict pulmonary tuberculosis treatment response. Clin Infect Dis 73:e3520–e3528. [DOI] [PubMed] [Google Scholar]
- 9.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsultan A, Savic R, Dooley KE, Weiner M, Whitworth W, Mac Kenzie WR, Peloquin CA, Tuberculosis Trials Consortium. 2017. Population pharmacokinetics of pyrazinamide in patients with tuberculosis. Antimicrob Agents Chemother 61:e02625-16. 10.1128/AAC.02625-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mugabo P, Mulubwa M. 2019. Population pharmacokinetic modelling of pyrazinamide and pyrazinoic acid in patients with multi-drug resistant tuberculosis. Eur J Drug Metab Pharmacokinet 44:519–530. 10.1007/s13318-018-00540-w. [DOI] [PubMed] [Google Scholar]
- 12.Sundell J, Wijk M, Bienvenu E, Äbelö A, Hoffmann K-J, Ashton M. 2021. Factors affecting the pharmacokinetics of pyrazinamide and its metabolites in patients coinfected with HIV and implications for individualized dosing. Antimicrob Agents Chemother 65. 10.1128/AAC.00046-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirehwa MT, McIlleron H, Rustomjee R, Mthiyane T, Onyebujoh P, Smith P, Denti P. 2017. Pharmacokinetics of pyrazinamide and optimal dosing regimens for drug- sensitive and -resistant tuberculosis. Antimicrob Agents Chemother 61:8–13. 10.1128/AAC.00490-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O’Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. 2016. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 63:e147–e195. 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghimire S, Bolhuis MS, Sturkenboom MGG, Akkerman OW, de Lange WCM, van der Werf TS, Alffenaar J-WC. 2016. Incorporating therapeutic drug monitoring into the World Health Organization hierarchy of tuberculosis diagnostics. Eur Respir J 47:1867–1869. 10.1183/13993003.00040-2016. [DOI] [PubMed] [Google Scholar]
- 16.van der Burgt EP, Sturkenboom MG, Bolhuis MS, Akkerman OW, Kosterink JG, de Lange WC, Cobelens FG, van der Werf TS, Alffenaar J-W. 2016. End TB with precision treatment!. Eur Respir J 47:680–682. 10.1183/13993003.01285-2015. [DOI] [PubMed] [Google Scholar]
- 17.Alffenaar J-WC, Gumbo T, Dooley KE, Peloquin CA, Mcilleron H, Zagorski A, Cirillo DM, Heysell SK, Silva DR, Migliori GB. 2020. Integrating pharmacokinetics and pharmacodynamics in operational research to end tuberculosis. Clin Infect Dis 70:1774–1780. 10.1093/cid/ciz942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sturkenboom MGG, Märtson A-G, Svensson EM, Sloan DJ, Dooley KE, van den Elsen SHJ, Denti P, Peloquin CA, Aarnoutse RE, Alffenaar J-WC. 2021. Population pharmacokinetics and Bayesian dose adjustment to advance TDM of anti-TB drugs. Clin Pharmacokinet 60:685–710. 10.1007/s40262-021-00997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alsultan A, Peloquin CA. 2014. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs 74:839–854. 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 20.Magis-Escurra C, Later-Nijland HMJ, Alffenaar JWC, Broeders J, Burger DM, van Crevel R, Boeree MJ, Donders ART, van Altena R, van der Werf TS, Aarnoutse RE. 2014. Population pharmacokinetics and limited sampling strategy for first-line tuberculosis drugs and moxifloxacin. Int J Antimicrob Agents 44:229–234. 10.1016/j.ijantimicag.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Gopal P, Grüber G, Dartois V, Dick T. 2019. Pharmacological and molecular mechanisms behind the sterilizing activity of pyrazinamide. Trends Pharmacol Sci 40:930–940. 10.1016/j.tips.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y, Davies Forsman L, Ren W, Zheng X, Bao Z, Hu Y, Bruchfeld J, Alffenaar J-W, Y Hu C. 2020. Drug exposure of first-line anti-tuberculosis drugs in China: a prospective pharmacological cohort study. 87:1347–1358. [DOI] [PubMed] [Google Scholar]
- 23.Vinnard C, Ravimohan S, Tamuhla N, Pasipanodya J, Srivastava S, Modongo C, Zetola NM, Weissman D, Gumbo T, Bisson GP. 2017. Pyrazinamide clearance is impaired among HIV/tuberculosis patients with high levels of systemic immune activation. PLoS One 12:e0187624. 10.1371/journal.pone.0187624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saktiawati AM, Sturkenboom MG, Stienstra Y, Subronto YW, Sumardi Kosterink JG, van der Werf TS, Alffenaar J-W. 2016. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: a randomized cross-over trial. J Antimicrob Chemother 71:703–710. 10.1093/jac/dkv394. [DOI] [PubMed] [Google Scholar]
- 25.Zent C, Smith P. 1995. Study of the effect of concomitant food on the bioavailability of rifampicin, isoniazid and pyrazinamide. Tuber Lung Dis 76:109–113. 10.1016/0962-8479(95)90551-0. [DOI] [PubMed] [Google Scholar]
- 26.Lin MY, Lin SJ, Chan LC, Lu YC. 2010. Impact of food and antacids on the pharmacokinetics of anti-tuberculosis drugs: systematic review and meta-analysis. Int J Tuber Lung Dis 14:806–818. [PubMed] [Google Scholar]
- 27.Verbeeck RK, Günther G, Kibuule D, Hunter C, Rennie TW. 2016. Optimizing treatment outcome of first-line anti-tuberculosis drugs: the role of therapeutic drug monitoring. Eur J Clin Pharmacol 72:905–916. 10.1007/s00228-016-2083-4. [DOI] [PubMed] [Google Scholar]
- 28.Rockwood N, Meintjes G, Chirehwa M, Wiesner L, McIlleron H, Wilkinson RJ, Denti P. 2016. HIV-1 coinfection does not reduce exposure to rifampin, isoniazid, and pyrazinamide in South African tuberculosis outpatients. Antimicrob Agents Chemother 60:6050–6059. 10.1128/AAC.00480-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denti P, Jeremiah K, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, Castel S, Wiesner L, Hagen CM, Christiansen M, Changalucha J, McIlleron H, Friis H, Andersen AB. 2015. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One 10:e0141002. 10.1371/journal.pone.0141002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting LSL, Villeneuve E, Ensom MH. 2006. Beyond cyclosporine: a systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit 28:419–430. 10.1097/01.ftd.0000211810.19935.44. [DOI] [PubMed] [Google Scholar]
- 31.Gumbo T, Chigutsa E, Pasipanodya J, Visser M, van Helden PD, Sirgel FA, McIlleron H. 2014. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 69:2420–2425. 10.1093/jac/dku136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Association of Public Health Laboratories. 2016. Issues in mycobacterium tuberculosis complex (MTBC) drug susceptibility testing: pyrazinamide (PZA). https://www.aphl.org/aboutAPHL/publications/Documents/ID-MTBC_DrugSusceptibility_0216.pdf. Accessed March 6, 2022.
- 33.Werngren J, Sturegård E, Juréen P, Ängeby K, Hoffner S, Schön T. 2012. Reevaluation of the critical concentration for drug susceptibility testing of Mycobacterium tuberculosis against pyrazinamide using wild-type MIC distributions and pncA gene sequencing. Antimicrob Agents Chemother 56:1253–1257. 10.1128/AAC.05894-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturkenboom MG, Akkerman OW, Van Altena R, De Lange WC, Kosterink JG, Van Der Werf TS, Alffenaar JW. 2016. Dosage of isoniazid and rifampicin poorly predicts drug exposure in tuberculosis patients. Eur Respir J 48:1237–1239. 10.1183/13993003.00986-2016. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO). 2018. Technical manual for drug susceptibility testing of medicines used in the treatment of tuberculosis 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 36.American Review of Respiratory Disease. 1978. Controlled trial of 6-month and 8-month regimens in the treatment of pulmonary tuberculosis. First report. Am Rev Respir Dis 118:219–227. [DOI] [PubMed] [Google Scholar]
- 37.Pasipanodya JG, Gumbo T. 2010. Clinical and toxicodynamic evidence that high-dose pyrazinamide is not more hepatotoxic than the low doses currently used. Antimicrob Agents Chemother 54:2847–2854. 10.1128/AAC.01567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang N, Savic RM, Boeree MJ, Peloquin CA, Weiner M, Heinrich N, Bliven-Sizemore E, Phillips PPJ, Hoelscher M, Whitworth W, Morlock G, Posey J, Stout JE, Mac Kenzie W, Aarnoutse R, Dooley KE. 2021. Optimising pyrazinamide for the treatment of tuberculosis. Eur Respir J 58:2002013. 10.1183/13993003.02013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekqvist D, Bornefall A, Augustinsson D, Sönnerbrandt M, Nordvall MJ, Fredrikson M, Carlsson B, Sandstedt M, Simonsson USH, Alffenaar J-WC, Paues J, Niward K. 2022. Safety and pharmacokinetics-pharmacodynamics of a shorter tuberculosis treatment with high-dose pyrazinamide and rifampicin: a study protocol of a phase II clinical trial (HighShort-RP). BMJ Open 12:e054788. 10.1136/bmjopen-2021-054788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sturkenboom MGG, van der Lijke H, Jongedijk EM, Kok WT, Greijdanus B, Uges DRA, Alffenaar J-WC, van der Lijke H, Jongedijk EM, Kok WT, Greijdanus B, Uges DRA, Alffenaar J-WC. 2015. Quantification of isoniazid, pyrazinamide and ethambutol in serum using liquid chromatography-tandem mass spectrometry. J Appl Bioanal 1:89–98. 10.17145/jab.15.015. [DOI] [Google Scholar]
- 41.Bakker M, Boeree M, de Lange WCM, Van Loenhout-Rooyackers J, D Vries G. 2014. Richtlijn Medicamenteuze behandeling van tuberculose. The Netherlands. https://www.nvalt.nl/kwaliteit/richtlijnen/infectieziekten//Infectieziekten/Richtlijn-medicamenteuze-behandeling-van-TBC%20Oktober%202014.pdf. Accessed March 06, 2022. [Google Scholar]
- 42.Thomson Reuters. 2021. Micromedex 2.0 (electronic version). Thomson Reuters (Healthcare), Greenwood Village, Colorado, USA. Available at: http://www.thomsonhc.com. [Google Scholar]
- 43.Proost JH, Eleveld DJ. 2006. Performance of an iterative two-stage Bayesian technique for population pharmacokinetic analysis of rich data sets. Pharm Res 23:2748–2759. 10.1007/s11095-006-9116-0. [DOI] [PubMed] [Google Scholar]
- 44.Burnham KP, Anderson DR. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304. 10.1177/0049124104268644. [DOI] [Google Scholar]
- 45.Saktiawati AMI, Harkema M, Setyawan A, Subronto YW, Sumardi Stienstra Y, Aarnoutse RE, Magis-Escurra C, Kosterink JGW, van der Werf TS, Alffenaar J-WC, Sturkenboom MGG. 2019. Optimal sampling strategies for therapeutic drug monitoring of first-line tuberculosis drugs in patients with tuberculosis. Clin Pharmacokinet 58:1445–1454. 10.1007/s40262-019-00763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sturkenboom MG, Mulder LW, de Jager A, van Altena R, Aarnoutse RE, de Lange WCM, Proost JH, Kosterink JGW, van der Werf TS, Alffenaar J-WC. 2015. Pharmacokinetic modeling and optimal sampling strategies for therapeutic drug monitoring of rifampin in patients with tuberculosis. Antimicrob Agents Chemother 59:4907–4913. 10.1128/AAC.00756-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David OJ, Johnston A. 2001. Limited sampling strategies for estimating cyclosporin area under the concentration-time curve: review of current algorithms. Ther Drug Monit 23:100–114. 10.1097/00007691-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Meier-Kriesche HU, Kaplan B, Brannan P, Kahan BD, Portman RJ. 1998. A limited sampling strategy for the estimation of eight-hour neoral areas under the curve in renal transplantation. Ther Drug Monit 20:401–407. 10.1097/00007691-199808000-00009. [DOI] [PubMed] [Google Scholar]