ABSTRACT
Pseudomonas aeruginosa is a major pathogen in burn wound infections. We present one of the first reports of small-colony variant (SCV) emergence of P. aeruginosa, taken from a patient under aminoglycosides for a persistent burn wound infection. We confirm the causative role of a single ispA mutation in SCV emergence and increased aminoglycoside resistance. IspA is involved in the synthesis of ubiquinone, providing a possible link between electron transport and SCV formation in P. aeruginosa.
KEYWORDS: SCV, antibiotic resistance, tobramycin, gentamicin, burn wound infection, Pseudomonas aeruginosa, small-colony variant, aminoglycosides
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen capable of establishing infections that are difficult to eradicate with antibiotics, and it remains the most frequent Gram-negative microorganism isolated from burn wounds (1). The refractory nature of P. aeruginosa during infection is often associated with the evolution toward host-adapted phenotypes, including biofilm production, high persister variants, conversion to mucoidy, altered expression of virulence factors, and the formation of small-colony variants (SCVs) (2–5). SCVs are characterized by their small colony size on agar plates, slow growth rate, and atypical metabolism (6). SCVs have been frequently associated with persistent and antibiotic-resistant infections caused by P. aeruginosa and other opportunistic pathogens, including Staphylococcus aureus (7), making them an important target for the development of future therapies.
While the mechanisms of SCV formation in P. aeruginosa appear diverse, often involving the secondary messenger cyclic di-GMP (8, 9) or global changes in gene expression (10, 11), SCV formation in S. aureus is comparatively conserved, with archetypal strains auxotrophic for hemin, menadione, and/or thymidine (12, 13). Heme and menadione are involved in the production of cytochrome and menaquinone, respectively, linking S. aureus SCVs to dysfunctional electron transport (14). A link between electron transport and SCV formation for P. aeruginosa, however, is yet to be described.
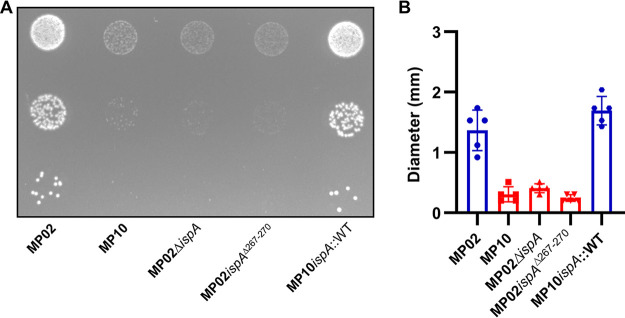
In the current study, we performed comparative genomics for a P. aeruginosa SCV and its “normal” colony counterpart (NCV). P. aeruginosa clinical isolates MP02 and MP10 were collected from the same sample taken from a hospitalized patient with severe burn wounds (see Table S1 in the supplemental material) (15). Need for informed consent and authorization for analyzing the previously collected bacterial isolates was waived by the local ethical committee. MP02 was an NCV, whereas MP10 was an SCV that produced smaller colonies on solid agar (Fig. 1A). Compared with MP02, MP10 also had a modest growth defect when grown in Luria-Bertani (LB) medium (Fig. S1). MP10 was serially propagated five times in liquid media, and colony sizes remained small when plated on agar, suggesting the phenotype was nontransient.
FIG 1.
Colony sizes of clinical P. aeruginosa isolates and engineered ispA mutants. (A) Overnight suspensions were 10-fold serially diluted. We plated 10-μL of dilutions 10−5, 10−6, and 10−7 on LB agar. Colonies were formed for 30 h at 37°C. MP02 is a normal colony variant (NCV), and MP10 is a small-colony variant (SCV). (B) Colony size quantification (n = 6). NCVs are blue, and SCVs are red.
Prior to isolation of MP02 and MP10, the patient had been treated with a range of antibiotics, including tobramycin, which was applied after the emergence of extensive antibiotic resistance. MP02 was defined in the clinic as intermediate susceptible to tobramycin, as its minimum inhibitory concentration (MIC) was at the susceptibility breakpoint using European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (2 μg/mL) (16). Conversely, MP10 was classified as resistant, with an Etest (bioMérieux, Switzerland) MIC of 4 μg/mL. We acquired the isolates and performed additional antibiotic susceptibility testing using broth microdilution according to Clinical and Laboratory Standards Institute guidelines and confirmed that MP10 had a modest but reproducible 2-fold increase in MIC for aminoglycosides tobramycin, gentamicin, and amikacin (17) (Table 1).
TABLE 1.
Aminoglycoside MICs as determined per CLSI guidelines using the microdilution method in Mueller-Hinton brotha
| Strain | Characteristic | MIC (μg/mL) of: |
||
|---|---|---|---|---|
| Tobramycin | Gentamicin | Amikacin | ||
| MP02 | Clinical normal colony variant | 4 | 32 | 32 |
| MP10 | Clinical small-colony variant | 8 | 64 | 64 |
| MP02ΔispA | In-frame deletion ispA mutant | 8 | 64 | 64 |
| MP02ispAΔ267-270 | MP02 engineered with the 12-base-pair deletion found in MP10 | 8 | 64 | 64 |
| MP10ispA::WT | MP10 complemented in cis with wild-type ispA | 4 | 32 | 32 |
Values were determined following 18 hours of incubation at 37°C. Experiments were performed three times in duplicates to confirm and ensure reproducibility. WT, wild-type; CLSI, Clinical and Laboratory Standards Institute.
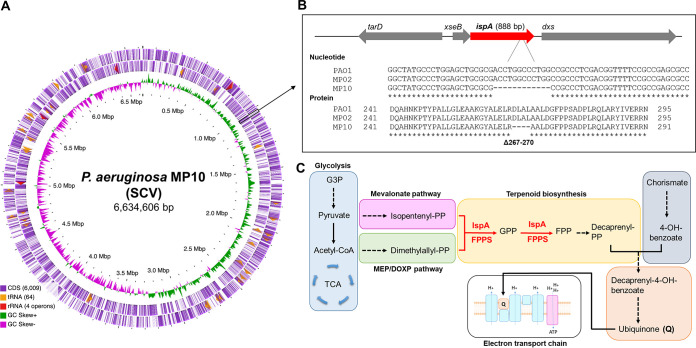
Complete, circular genome sequences of MP02 and MP10 were resolved using PacBio reads that were assembled using Flye (18) and then polished using Illumina sequencing reads (19). Each genome was annotated using the Prokaryotic Genome Annotation Pipeline (PGAP) (20) and deposited at DDBJ/ENA/GenBank (accession numbers CP063394 and CP063393, respectively). MP10 had a genome of 6,634,606 bp, average GC content of 66%, and 6,136 coding DNA sequences (CDS) (Fig. 2A). Comparative genome analysis at single nucleotide resolution identified only one mutation between MP10 and MP02; MP10 possessed a 12-bp deletion in the ispA gene, which codes for farnesyl pyrophosphate synthase (FPPS) (Fig. 2B). FPPS catalyzes the reaction required to generate geranyl and farnesyl pyrophosphate (GPP and FPP), both of which are substrates involved in the biosynthesis of the electron carrier ubiquinone (Fig. 2C). The mutation resulted in an in-frame deletion of four amino acids (from positions 267 to 270).
FIG 2.
ispA genomic landscape. (A) Circular genome representation of MP10 (SCV) created using CGView (32). The inner ring illustrates the GC skew. The outer two rings represent coding sequences (CDS), tRNAs, and rRNAs on the reverse and forward strands, respectively. A black box is included to highlight the ispA gene. (B) Genomic localization of ispA. Nucleotide and protein alignments of PAO1 (GenPept accession number NP_250121), MP02, and MP10, showing the 12-bp deletion in MP10 and the consequent deletion of amino acids from 267 to 270. Protein and nucleotide sequence alignments were generated using Clustal Omega (33). (C) Schematic pathway displaying the involvement of IspA (also knowns as FPPS) in ubiquinone biosynthesis. Dotted arrows indicate that multiple steps have been abbreviated. FPP, farnesyl pyrophosphate; FPPS, farnesyl pyrophosphate synthase; G3P, glycerol-3-phosphate; GPP, geranyl pyrophosphate; MEP/DOXP, 2-C-methyl-d-erythritol 4-phosphate/1-deoxy-d-xylulose 5-phosphate; PP, pyrophosphate; TCA, tricarboxylic acid.
We reasoned that the SCV phenotype and increased resistance to aminoglycosides of MP10 were caused by the ispA mutation. We generated ispA mutants by bidirectional allelic exchange using the method of Hmelo et al. (21) with PCR primers listed in Table S3. The ispA mutant allele (ispAΔ267-270) was engineered into the NCV strain MP02 generating MP02ispAΔ267-270, and the full-length ispA allele (ispA::WT) was introduced into MP10 to complement in cis the mutation, generating strain MP10ispA::WT. Additionally, full-length ispA was deleted from MP02 (MP02ΔispA) (Table S1).
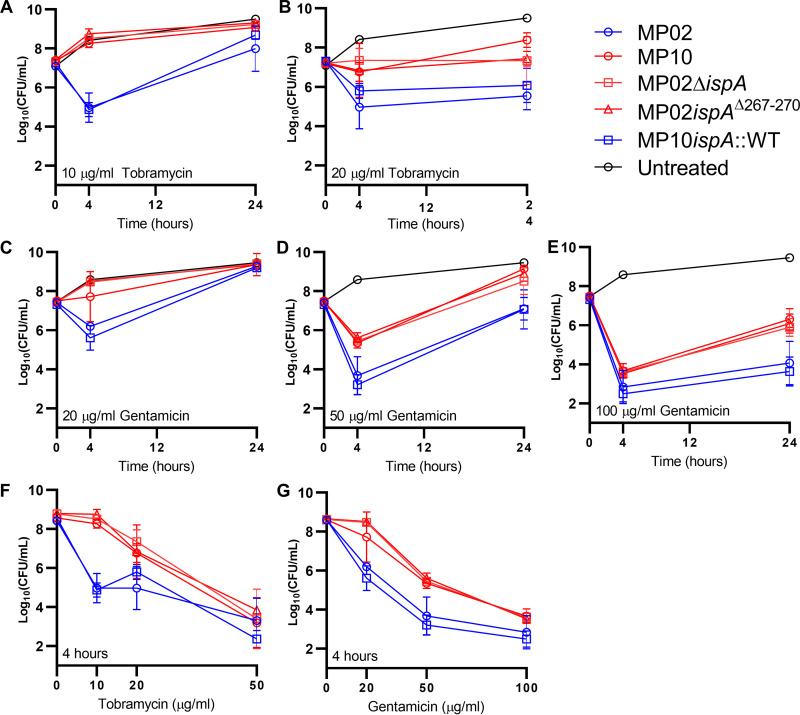
Strains with full-length ispA displayed normal colony size, whereas strains with mutated ispA were SCVs (Fig. 1). Additionally, P. aeruginosa with mutated ispA had 2-fold-higher tobramycin, gentamicin, and amikacin MICs than strains with full-length ispA (Table 1). To delineate the possible impact of this modest increase in MIC on treatment efficiency, we performed in vitro antibiotic killing assays as described elsewhere (22). NCVs and SCVs were grown in LB media at 37°C for 3 h and 4 h, respectively, to reach the same concentration of exponentially growing cells (~2 × 107 CFU/mL) prior to the addition of antibiotics. At 10 μg/mL tobramycin, which is close to the peak serum levels for burn patients treated with extended-interval tobramycin (7.4 μg/mL; range, 3.1 to 19.6) (23), >99.9% of exponentially growing MP02 cells were killed following 4 h of incubation (Fig. 3A). In contrast, MP10 grew similarly to the untreated control over a 24-h period, confirming that the difference in antibiotic susceptibility was due to enhanced resistance as opposed to enhanced tolerance (using definitions from reference 24). MP02 showed evidence of regrowth after 24 h of incubation, and this was accompanied by a 4-fold increase in MIC (4 to 16 μg/mL) for the surviving colonies. At 20 μg/mL, the emergence of resistance for MP02 was suppressed at 24 h (Fig. 3B). We performed tobramycin time-killing assays in a second medium, M9 minimal medium supplemented with 20 mM glucose, and produced similar results (Fig. S2). Time-kill data were also similar when using a second antibiotic from the aminoglycoside class, gentamicin (Fig. 3C and D); however, a higher concentration (100 μg/mL) was required to suppress resistance emergence for MP02 (Fig. 3E). Focusing on the 4-h time point, compared with MP02, the survival of MP10 was significantly higher following treatment with tobramycin 10 μg/mL and 20 μg/mL (~2,000-fold, P < 0.0001, and ~60-fold, P = 0.0002, respectively; two-way analysis of variance [ANOVA] with multiple comparisons using the method of Dunnett; Fig. 3F). No difference in survival was determined at 50 μg/mL. Similarly, compared with MP02, survival of MP10 was significantly higher at 20 μg/mL, 50 μg/mL, and 100 μg/mL of gentamicin (~30-fold, P < 0.0001, ~50-fold, P < 0.0001; and ~7-fold, P < 0.05, respectively; Fig. 3G). Likewise, at the 24-h time point, MP10 showed statistically higher survival than MP02 following treatment with tobramycin and gentamicin (P < 0.01 and P < 0.0001, respectively, two-way ANOVA with Dunnett’s test; two-way ANOVA; Fig. S3).
FIG 3.
Mutation to ispA results in decreased aminoglycoside killing. Bacteria were grown to mid-exponential phase (~2 × 107 CFU/mL) in LB at 37°C and then treated with different concentrations of tobramycin or gentamicin. Time-dependent (A to E) and concentration-dependent (F and G) curves are shown. Normal colony variants are blue; small-colony variants are red. The limit of detection was 102 CFU/mL.
Concentration-dependent killing and time-dependent killing phenotypes of engineered mutants were determined by the ispA allele. Strains with full-length ispA revealed phenotypes similar to MP02, and those with mutated ispA (either deletion or the clinical variant, ispAΔ267-270) were similar to MP10, confirming the causative role for ispA mutation in reduced aminoglycoside susceptibility (Fig. 3).
IspA is conserved across diverse bacteria. Disruption of ispA reduced growth yield in Escherichia coli (25), reduced spreading for Shigella flexneri (26), and produced an SCV-like phenotype in laboratory-generated mutants of S. aureus (27). Further, aminoglycoside exposure in vitro produced ispA mutants of E. coli and P. aeruginosa PA14 that had enhanced gentamicin resistance (28, 29) suggesting ispA may be a broad evolutionary target for SCV formation and/or reduced susceptibility to aminoglycosides.
IspA is a key enzyme in the synthesis of the electron carrier ubiquinone; E. coli ispA mutants that evolved in vitro had limited ubiquinone pools (30), and strains engineered to overexpress ispA produced more ubiquinone (31). Dysfunctional electron transport is a frequently described mechanism of SCV formation for S. aureus; the current report provides the first evidence linking SCV formation to electron transport for P. aeruginosa. Future studies are warranted to determine the effect of ispA mutation upon bioenergetics and to determine whether this is a convergent mechanism across diverse bacterial pathogens.
Data availability.
Genomes of MP02 and MP10 were deposited into DDBJ/ENA/GenBank (accession numbers CP063394 and CP063393, respectively).
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance of Sandra Nansoz, Severin Jung, and Viola Grünenfelder and would like to thank the Next Generation Sequencing Platform of the University of Bern for performing the high-throughput sequencing experiments.
The study was funded by a research grants from the Stiftung für die Forschung in Anästhesiologie und Intensivmedizin (no. 32/2019 awarded to D.R.C.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ladhani HA, Yowler CJ, Claridge JA. 2021. Burn wound colonization, infection, and sepsis. Surg Infect (Larchmt) 22:44–48. 10.1089/sur.2020.346. [DOI] [PubMed] [Google Scholar]
- 2.Schick A, Kassen R. 2018. Rapid diversification of Pseudomonas aeruginosa in cystic fibrosis lung-like conditions. Proc Natl Acad Sci USA 115:10714–10719. 10.1073/pnas.1721270115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher JC, Yu H, Mudd MH, Deretic V. 1997. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect Immun 65:3838–3846. 10.1128/iai.65.9.3838-3846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogardt M, Heesemann J. 2010. Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int J Med Microbiol 300:557–562. 10.1016/j.ijmm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Bartell JA, Cameron DR, Mojsoska B, Haagensen JAJ, Pressler T, Sommer LM, Lewis K, Molin S, Johansen HK. 2020. Bacterial persisters in long-term infection: emergence and fitness in a complex host environment. PLoS Pathog 16:e1009112. 10.1371/journal.ppat.1009112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 7.Proctor RA, Peters G. 1998. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin Infect Dis 27:419–422. 10.1086/514706. [DOI] [PubMed] [Google Scholar]
- 8.Drenkard E, Ausubel FM. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740–743. 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 9.Malone JG, Jaeger T, Manfredi P, Dotsch A, Blanka A, Bos R, Cornelis GR, Haussler S, Jenal U. 2012. The YfiBNR signal transduction mechanism reveals novel targets for the evolution of persistent Pseudomonas aeruginosa in cystic fibrosis airways. PLoS Pathog 8:e1002760. 10.1371/journal.ppat.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irvine S, Bunk B, Bayes HK, Sproer C, Connolly JPR, Six A, Evans TJ, Roe AJ, Overmann J, Walker D. 2019. Genomic and transcriptomic characterization of Pseudomonas aeruginosa small colony variants derived from a chronic infection model. Microb Genom 5:e000262. 10.1099/mgen.0.000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schniederjans M, Koska M, Haussler S. 2017. Transcriptional and mutational profiling of an aminoglycoside-resistant Pseudomonas aeruginosa small-colony variant. Antimicrob Agents Chemother 61:e01178-17. 10.1128/AAC.01178-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Gotz F. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J Bacteriol 179:4706–4712. 10.1128/jb.179.15.4706-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates DM, von Eiff C, McNamara PJ, Peters G, Yeaman MR, Bayer AS, Proctor RA. 2003. Staphylococcus aureus menD and hemB mutants are as infective as the parent strains, but the menadione biosynthetic mutant persists within the kidney. J Infect Dis 187:1654–1661. 10.1086/374642. [DOI] [PubMed] [Google Scholar]
- 14.McNamara PJ, Proctor RA. 2000. Staphylococcus aureus small colony variants, electron transport and persistent infections. Int J Antimicrob Agents 14:117–122. 10.1016/S0924-8579(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 15.Tissot F, Blanc DS, Basset P, Zanetti G, Berger MM, Que YA, Eggimann P, Senn L. 2016. New genotyping method discovers sustained nosocomial Pseudomonas aeruginosa outbreak in an intensive care burn unit. J Hosp Infect 94:2–7. 10.1016/j.jhin.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 16.The European Committee on Antimicrobial Susceptibility Testing . 2022. Breakpoint tables for interpretation of MICs and zone diameters, version 12.0.
- 17.CLSI . 2020. Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute. Wayne, PA.
- 18.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37:540–546. 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 19.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9:e112963. 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. 2016. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hmelo LR, Borlee BR, Almblad H, Love ME, Randall TE, Tseng BS, Lin C, Irie Y, Storek KM, Yang JJ, Siehnel RJ, Howell PL, Singh PK, Tolker-Nielsen T, Parsek MR, Schweizer HP, Harrison JJ. 2015. Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat Protoc 10:1820–1841. 10.1038/nprot.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron DR, Shan Y, Zalis EA, Isabella V, Lewis K. 2018. A genetic determinant of persister cell formation in bacterial pathogens. J Bacteriol 200:e00303-18. 10.1128/JB.00303-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bracco D, Landry C, Dubois MJ, Eggimann P. 2008. Pharmacokinetic variability of extended interval tobramycin in burn patients. Burns 34:791–796. 10.1016/j.burns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo JM, Hardt WD, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan MW, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisaki S, Takahashi I, Hara H, Horiuchi K, Nishino T, Nishimura Y. 2005. Disruption of the structural gene for farnesyl diphosphate synthase in Escherichia coli. J Biochem 137:395–400. 10.1093/jb/mvi049. [DOI] [PubMed] [Google Scholar]
- 26.Mac Siomoin RA, Nakata N, Murai T, Yoshikawa M, Tsuji H, Sasakawa C. 1996. Identification and characterization of ispA, a Shigella flexneri chromosomal gene essential for normal in vivo cell division and intracellular spreading. Mol Microbiol 19:599–609. 10.1046/j.1365-2958.1996.405941.x. [DOI] [PubMed] [Google Scholar]
- 27.Krute CN, Carroll RK, Rivera FE, Weiss A, Young RM, Shilling A, Botlani M, Varma S, Baker BJ, Shaw LN. 2015. The disruption of prenylation leads to pleiotropic rearrangements in cellular behavior in Staphylococcus aureus. Mol Microbiol 95:819–832. 10.1111/mmi.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roemhild R, Gokhale CS, Dirksen P, Blake C, Rosenstiel P, Traulsen A, Andersson DI, Schulenburg H. 2018. Cellular hysteresis as a principle to maximize the efficacy of antibiotic therapy. Proc Natl Acad Sci USA 115:9767–9772. 10.1073/pnas.1810004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lázár V, Pal Singh G, Spohn R, Nagy I, Horváth B, Hrtyan M, Busa-Fekete R, Bogos B, Méhi O, Csörgő B, Pósfai G, Fekete G, Szappanos B, Kégl B, Papp B, Pál C. 2013. Bacterial evolution of antibiotic hypersensitivity. Mol Syst Biol 9:700. 10.1038/msb.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujisaki S, Nishino T, Katsuki H, Hara H, Nishimura Y, Hirota Y. 1989. Isolation and characterization of an Escherichia coli mutant having temperature-sensitive farnesyl diphosphate synthase. J Bacteriol 171:5654–5658. 10.1128/jb.171.10.5654-5658.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samoudi M, Omid Yeganeh N, Shahbani Zahiri H, Shariati P, Hajhosseini R. 2015. Inhibition of coenzyme Qs accumulation in engineered Escherichia coli by high concentration of farnesyl diphosphate. Avicenna J Med Biotechnol 7:113–120. [PMC free article] [PubMed] [Google Scholar]
- 32.Grant JR, Stothard P. 2008. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res 36:W181–W184. 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S3 and Fig. S1 to S3. Download aac.00621-22-s0001.pdf, PDF file, 0.5 MB (513.5KB, pdf)
Data Availability Statement
Genomes of MP02 and MP10 were deposited into DDBJ/ENA/GenBank (accession numbers CP063394 and CP063393, respectively).